Abstract
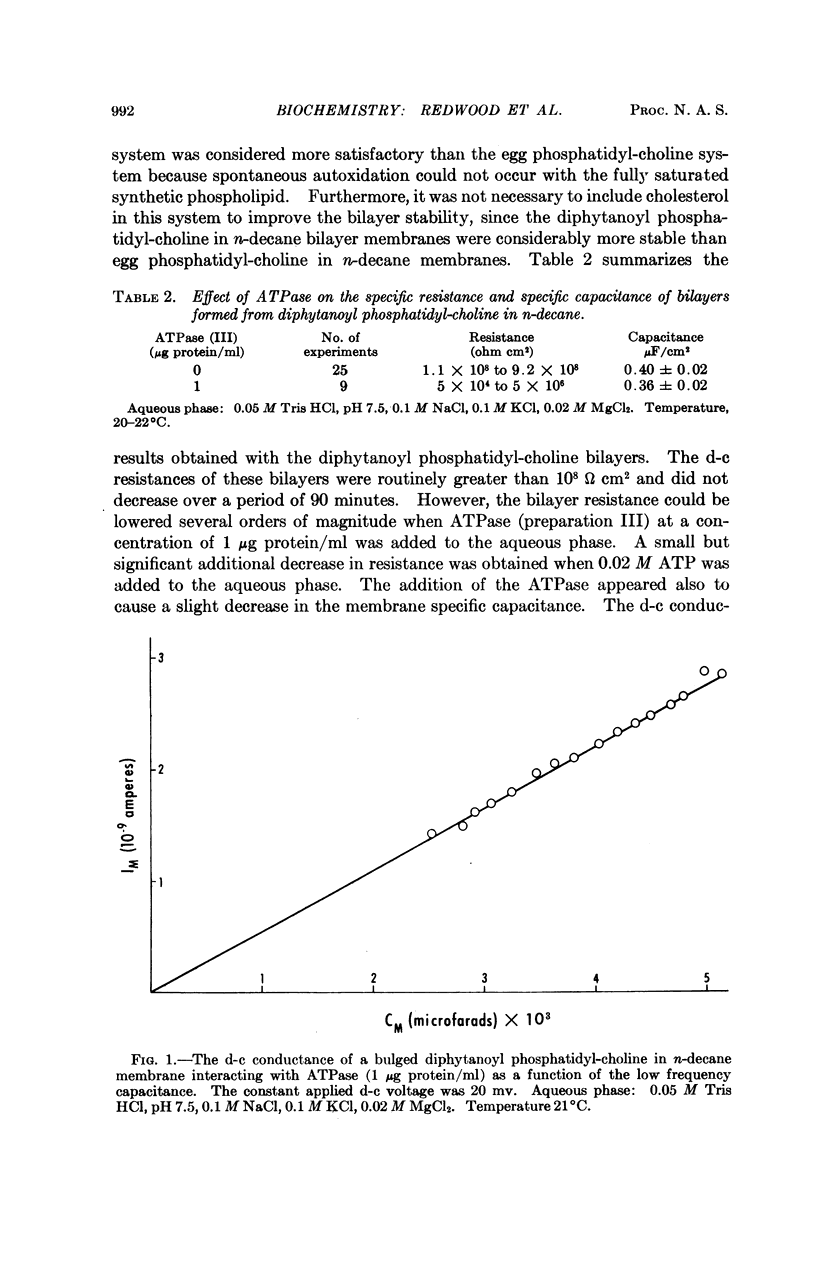
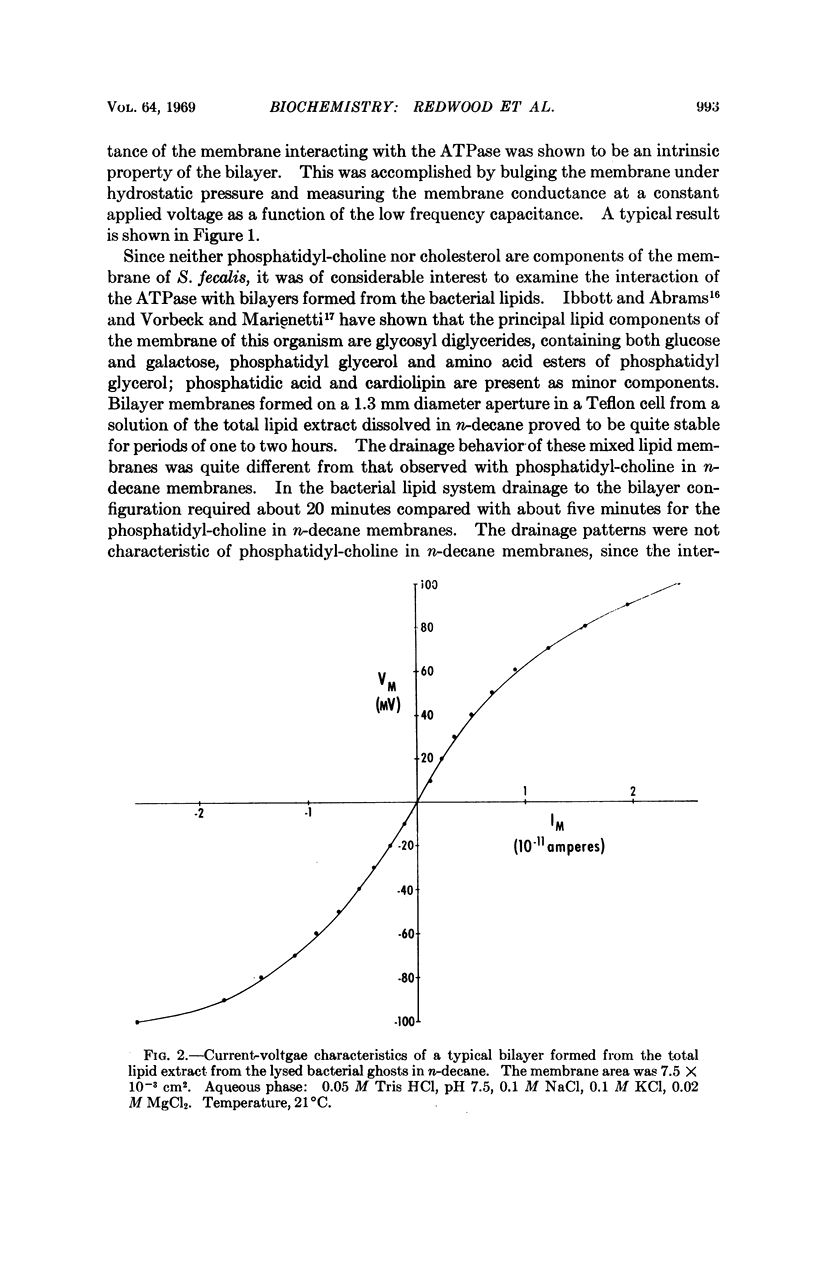
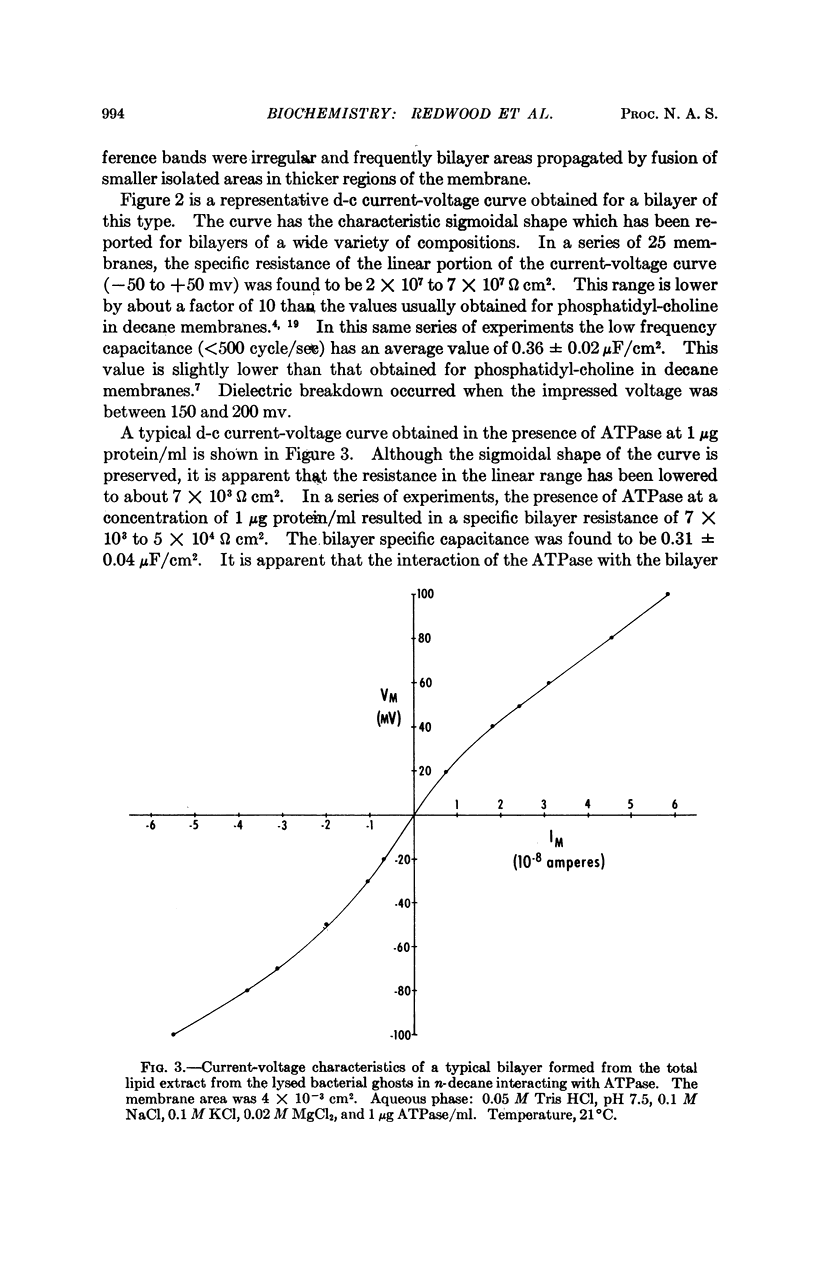
A solubilized nonparticulate adenosine triphosphatase from Streptococcus fecalis spheroplast membranes (Abrams, A., and C. Baron, Biochemistry, 7,501 (1968)) interacts at pH 7.5 with lipid bilayer membranes to produce a 102- to 104-fold increase in the electric conductance of the bilayer. In addition, a small decrease in the electrical capacitance of the interactant system is also observed. Interaction is obtained with bilayers formed from a solution in n-decane of either purified egg phosphatidyl-choline, synthetic diphytanoyl phosphatidyl-choline, or a total extract of the membrane lipid of S. fecalis. The magnitude of the increase in conductance is dependent on the presence of Mg++ and upon the concentrations of both Na+ and K+ in the range of 10-2 to 10-1M. An additional tenfold increase in conductance is obtained when ATP is added to the ambient aqueous phase surrounding the bilayer ATPase interactant system. No conductance change is obtained with ATPase which has been treated with pronase. ATPase activity is dependent upon Mg++ and upon the concentrations of Na+ and K+ in the region of 10-1M. The function of the ATPase in the intact bacterial membrane is apparently associated with the active transport of cations. The increased conductance in the bilayer which results from its interaction with the ATPase, together with the similarity between the dependence of this interaction and the dependence of ATPase activity on Mg++, ATP, and the Na+, K+ concentrations suggests that the bilayer-ATPase interactant complex may be similar in structure and properties to the membrane-ATPase complex in the intact organism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Baron C. The isolation and subunit structure of streptococcal membrane adenosine triphosphatase. Biochemistry. 1967 Jan;6(1):225–229. doi: 10.1021/bi00853a035. [DOI] [PubMed] [Google Scholar]
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Bangham A. D., Standish M. M., Watkins J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- HUANG C., WHEELDON L., THOMPSON T. E. THE PROPERTIES OF LIPID BILAYER MEMBRANES SEPARATING TWO AQUEOUS PHASES: FORMATION OF A MEMBRANE OF SIMPLE COMPOSITION. J Mol Biol. 1964 Jan;8:148–160. doi: 10.1016/s0022-2836(64)80155-8. [DOI] [PubMed] [Google Scholar]
- Hanai T., Haydon D. A., Taylor J. The influence of lipid composition and of some adsorbed proteins on the capacitance of black hydrocarbon membranes. J Theor Biol. 1965 Nov;9(3):422–432. doi: 10.1016/0022-5193(65)90041-x. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- IBBOTT F. A., ABRAMS A. THE PHOSPHOLIPIDS IN MEMBRANE GHOSTS FROM STREPTOCOCCUS FAECALIS PROTOPLASTS. Biochemistry. 1964 Dec;3:2008–2012. doi: 10.1021/bi00900a039. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]



