Abstract
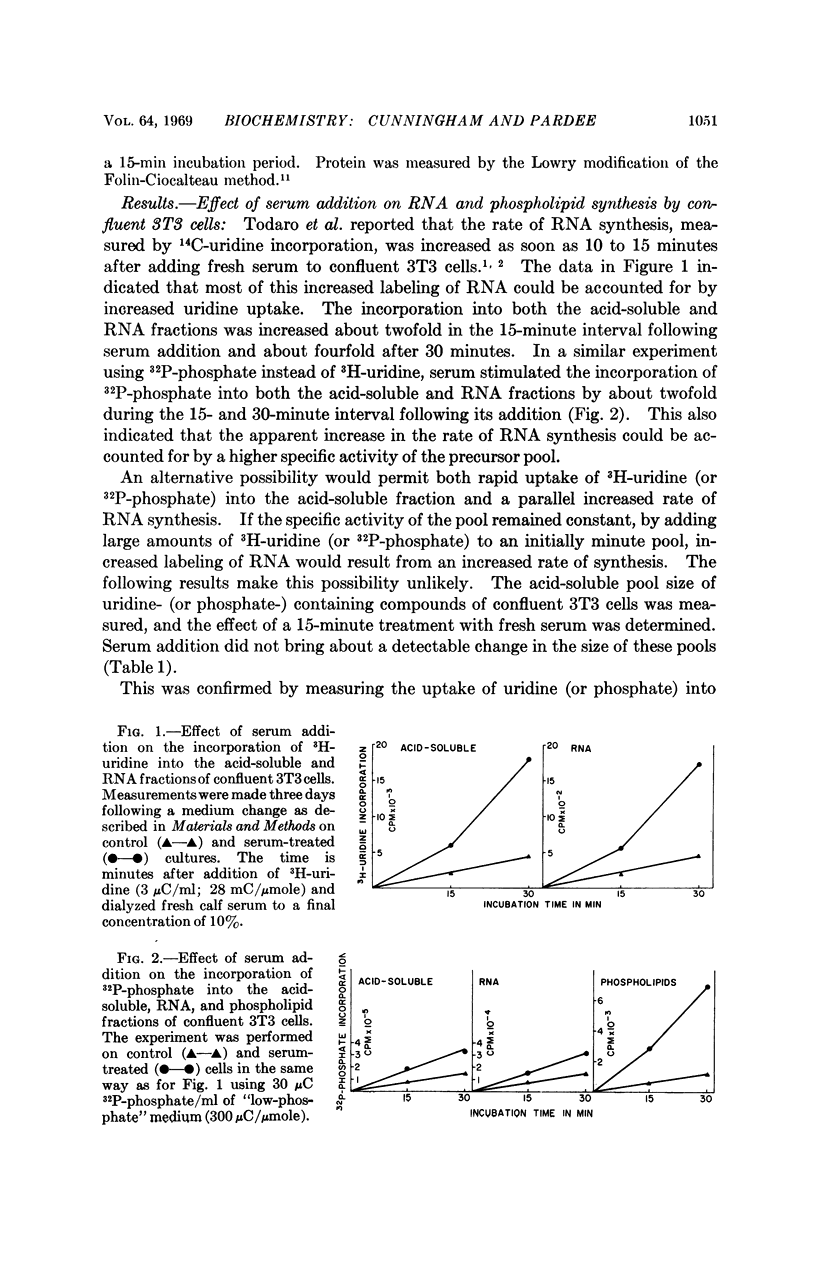
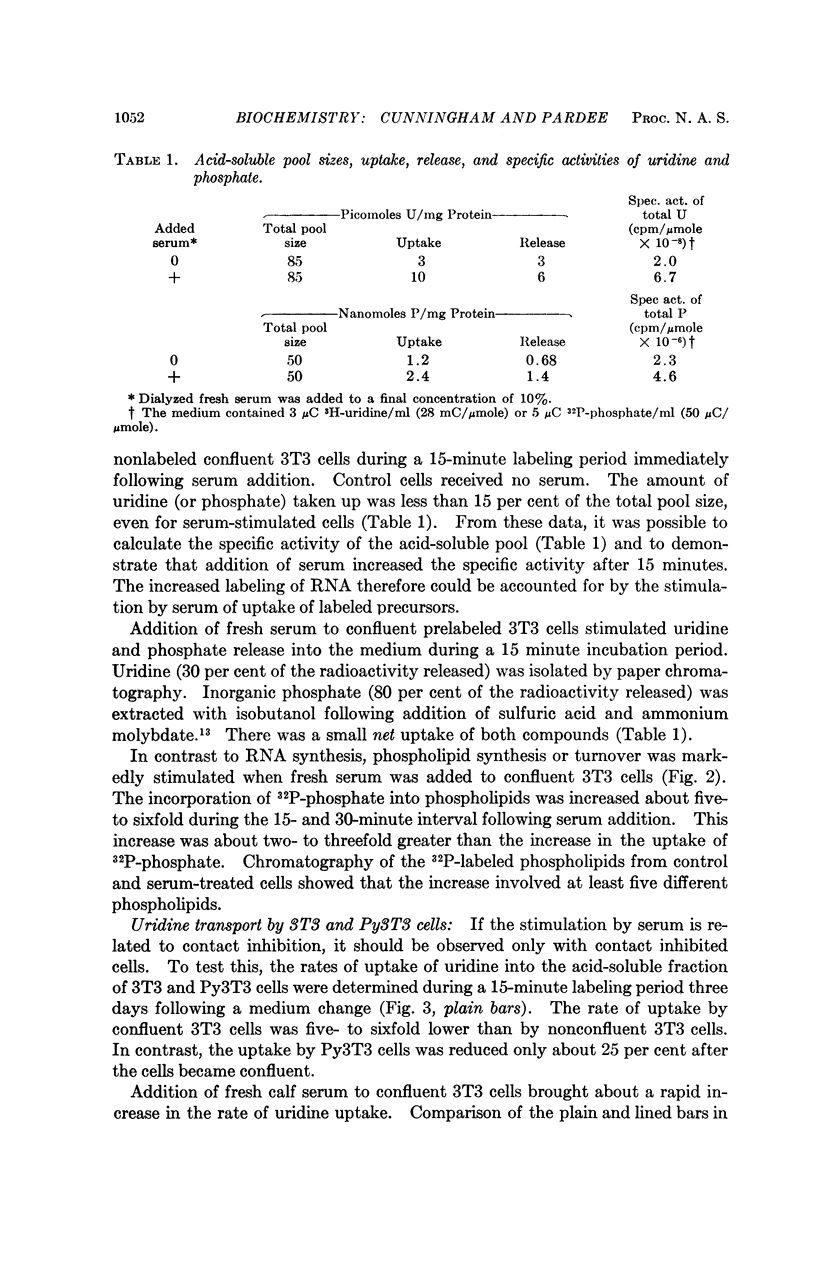
Two- to fourfold increases in uridine and phosphate uptake were brought about within 10 to 15 minutes after adding fresh serum to confluent 3T3 cells. The stimulation of transport was specific, since no serum effect was observed for 3-O-methyl-D-glucose or amino acids. The early increase in RNA labeling, previously identified by 14C-uridine incorporation, could be accounted for by this transport effect. Increased labeling with 32P-phosphate of at least five phospholipids occurred soon after adding serum. This increase, however, could not be accounted for by increased transport. Both of these results suggest that specific early membrane changes are involved in “contact inhibition.”
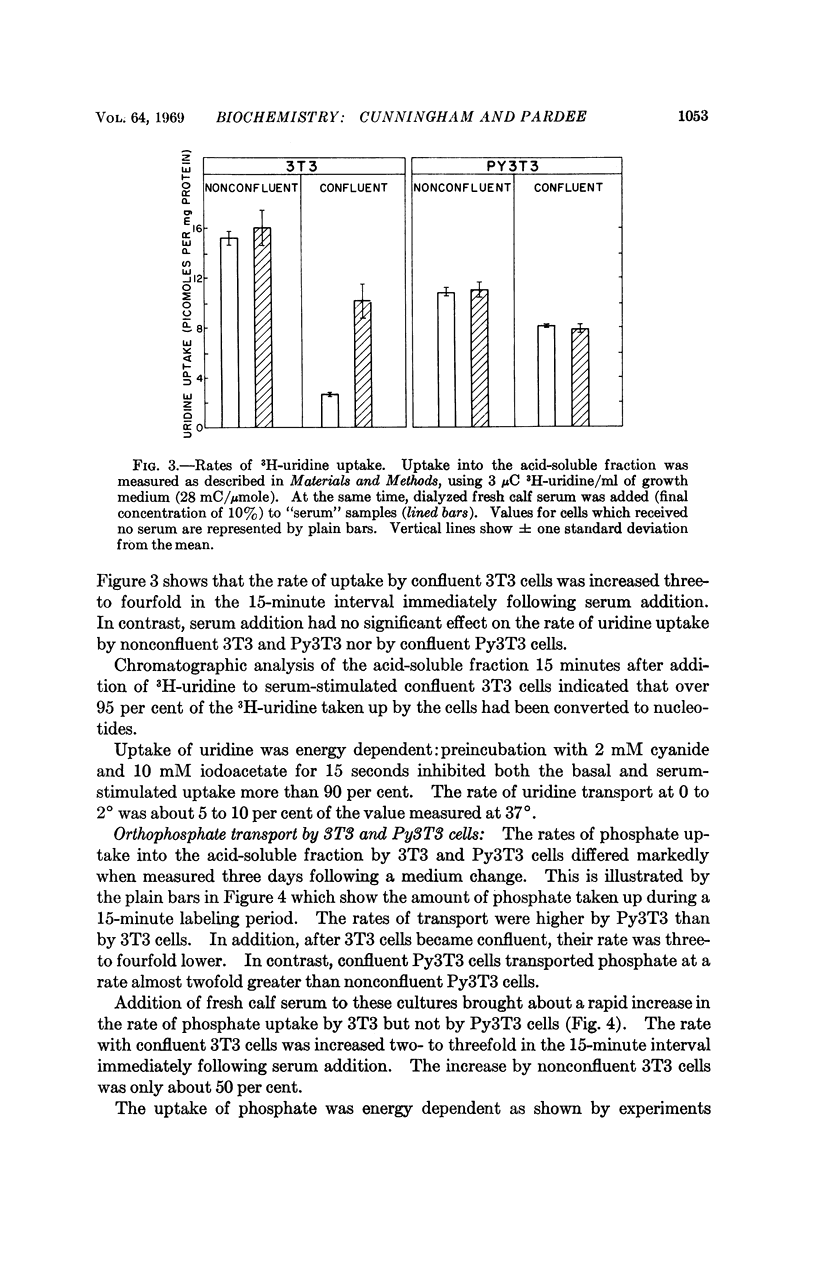
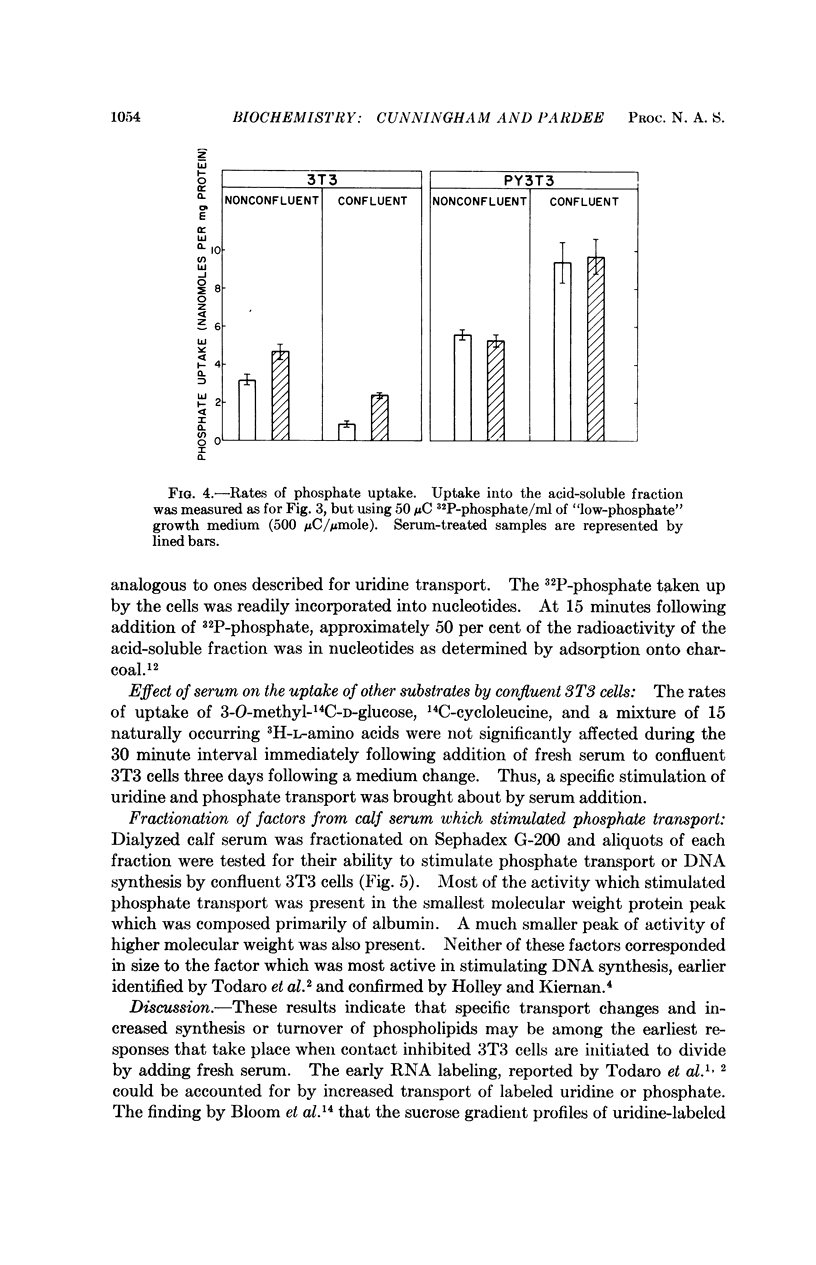
The following results are consistent with this suggestion. Uptake of uridine and phosphate by nonconfluent 3T3 cells was higher than by confluent cells and was only slightly increased by fresh serum. In contrast, uptake of these substrates by Polyoma virus-transformed 3T3 cells, which are not subject to “contact inhibition,” was not significantly decreased after the cells became confluent, and serum addition had no effect on transport by either nonconfluent or confluent Polyoma virus-transformed 3T3 cells.
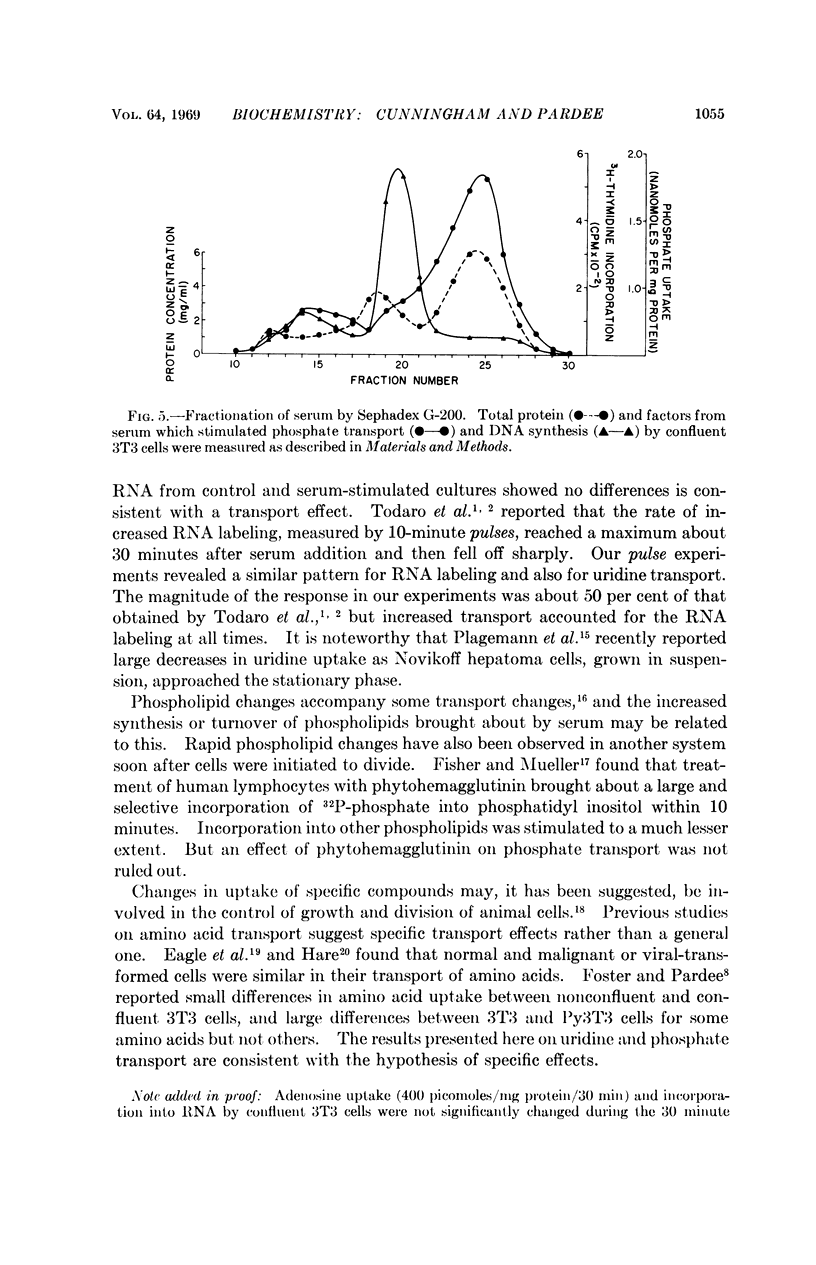
Fractionation of serum on Sephadex G-200 demonstrated that the factor which stimulated phosphate transport was different from the previously identified factor which stimulates DNA synthesis by confluent 3T3 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S., Todaro G. J., Green H. RNA synthesis during preparation for growth in a resting population of mammalian cells. Biochem Biophys Res Commun. 1966 Aug 12;24(3):412–417. doi: 10.1016/0006-291x(66)90175-6. [DOI] [PubMed] [Google Scholar]
- CABIB E., LELOIR L. F., CARDINI C. E. Uridine diphosphate acetylglucosamine. J Biol Chem. 1953 Aug;203(2):1055–1070. [PubMed] [Google Scholar]
- EAGLE H., PIEZ K. A., LEVY M. The intracellular amino acid concentrations required for protein synthesis in cultured human cells. J Biol Chem. 1961 Jul;236:2039–2042. [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Hare J. D. Location and characteristics of the phenylalanine transport mechanism in normal and polyoma-transformed hamster cells. Cancer Res. 1967 Dec;27(12):2357–2363. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE E. M., BECKER Y., BOONE C. W., EAGLE H. CONTACT INHIBITION, MACROMOLECULAR SYNTHESIS, AND POLYRIBOSOMES IN CULTURED HUMAN DIPLOID FIBROBLASTS. Proc Natl Acad Sci U S A. 1965 Feb;53:350–356. doi: 10.1073/pnas.53.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARINETTI G. V., ERBLAND J., KOCHEN J. Quantitative chromatography of phosphatides. Fed Proc. 1957 Sep;16(3):837–844. [PubMed] [Google Scholar]
- PARDEE A. B. CELL DIVISION AND A HYPOTHESIS OF CANCER. Natl Cancer Inst Monogr. 1964 May;14:7–20. [PubMed] [Google Scholar]
- Plagemann P. G., Ward G. A., Mahy B. W., Korbecki M. Relationship between uridine kinase activity and rate of incorporation of uridine into acid-soluble pool and into RNA during growth cycle of rat hepatoma cells. J Cell Physiol. 1969 Jun;73(3):233–249. doi: 10.1002/jcp.1040730308. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Control by factors in serum of multiplication of uninfected cells and cells infected and converted by avian sarcoma viruses. Wistar Inst Symp Monogr. 1967;7:103–116. [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]


