Abstract
In the Drosophila, a single copy of the phosphoprotein Dishevelled (Dsh) is found. In the genomes of higher organism (including mammals), three genes encoding isoforms of Dishevelled (Dvl1, Dvl2, and Dvl3) are present. In the fly, Dsh functions in the Wnt-sensitive stabilization of intracellular β-catenin and activation of the Lef/Tcf-sensitive transcriptional response known as the Wnt “canonical” pathway. In the current work we explore the expression of Dishevelleds in mammalian cells and provide an estimate of the relative cellular abundance of each Dvl. In mouse F9 cells, all three Dvls are expressed. Dvl2 constitutes more than 95% of the total pool, the sum of Dvl1 and Dvl3 constituting the remainder. Similarly, Dvl2 constitutes more than 80% of the Dvl1-3 pool in mouse P19 and human HEK 293 cells. siRNA-induced knockdown of individual Dvls was performed using Wnt3a-sensitive canonical pathway in F9 cells as the read-out. Activation of the canonical signaling pathway by Wnt3a was dependent upon the presence of Dvl1, Dvl2, and Dvl3, but to a variable extent. Wnt3a-sensitive canonical transcription was suppressible, by knockdown of Dvl1, Dvl2, or Dvl3. Conversely, the overexpression of any one of the three Dvls individually was found to be capable of promoting Lef/Tcf-sensitive transcriptional activation, in the absence of Wnt3a, i.e., overexpression of Dvl1, Dvl2, or Dvl3 is Wnt3a-mimetic. Graded suppression of individual Dvl isoforms by siRNA was employed to test if the three Dvls could be distinguished from one another with regard to mediation of the canonical pathway. Canonical signaling was most sensitive to changes in the abundance of either Dvl3 or Dvl1. Changes in expression of Dvl2, the most abundant of the three isoforms, resulted in the least effect on canonical signaling. Dvl-based complexes were isolated by pull-downs from whole-cell extracts with isoform-specific antibodies and found to include all three Dvl isoforms. Rescue experiments were conducted in which depletion of either Dvl3 or Dvl1 suppresses Wnt3a activation of the canonical pathway and the ability of a Dvl isoform to rescue the response evaluated. Rescue of Wnt3a-stimulated transcriptional activation in these siRNA-treated cells occurred only by the expression of the very same Dvl isoform depleted by the siRNA. Thus, Dvls appear to function cooperatively as well as uniquely with respect to mediation of Wnt3a-stimulated canonical signaling. The least abundant (Dvl3) plays the most obvious role, whereas the most abundant (Dvl2) plays the least obvious role, suggesting that individual Dvl isoforms in mammals may operate as a network with some features in common and others rather unique.
Keywords: Wnt, canonical, β-catenin, Lef/Tcf-sensitive transcription, Dishevelled-1, Dishevelled-2, Dishevelled-3
Introduction
Wnts are secreted, glycosylated, palmitoylated ligands that bind to member of Frizzled class of heptihelical, G protein-coupled receptors to regulate fundamental aspects of development and growth [1–8]. The “canonical” Wnt pathway operates to activate Lef/Tcf-sensitive gene transcription through the stabilization and accumulation of cellular β-catenin [9]. Wnts (e.g., Wnt3a), acting via Frizzled-1 leads to the suppression of phosphorylation of β-catenin that provokes its destabilization and loss by an Axin-based degradation complex that includes the product of the adenomatous polyposis coli (APC) tumor suppressor gene, glycogen synthase kinase-3β, phosphophosphatase-2A, other proteins, as well as the phosphoprotein Dishevelled (Dvl) [10–12]. Dishevelled functions in Wnt signaling downstream to the canonical (i.e., Wnt/Frizzled-1/β-catenin/Lef-Tcf-sensitive transcription), non-canonical pathway (i.e., Wnt5a/Frizzled-2/cGMP/Ca2+), and planar cell polarity (i.e., Wnt/small GTPase/MEKK/MKK/JNK) pathways [13–15]. In Drosophila, a single Dishevelled is expressed and proper development requires its presence [16; 17]. In mammalian species, three Dishevelled isoforms are expressed, termed Dvl1, Dvl2, and Dvl3 [18–22]. Three conserved domains provide the major landmarks of Dvls: a DIshevelled and AXin (DIX) binding domain at the N-terminus; a Post-synaptic density-95, Discs-large and Zonula occludens-1 (PDZ) domain in the mid region of Dvl; and a Dishevelled, Egl-10, Pleckstrin (DEP) domain located about midway between the PDZ domain and the C-terminus of Dvl [23;24]. The DIX domain enables the possible dimerization of Dvl with other members of the Dvl family as well as with Axin, which itself is a scaffold protein functioning downstream of β-catenin to organize the multiprotein complex responsible for degrading β-catenin [25]. The PDZ domain provides a docking site for a large number of proteins that includes protein kinases [e.g., casein kinase-1 (CK1) [26], -2 (CK2) [27], and p21-activated kinase [28]], phosphatases (e.g., serine/threoine- protein phosphatase-2C family members [29]), adaptor proteins such as β-arrestin [30], and importantly Frizzleds with a C-terminal PDZ ligand structure [31]. An N-terminally located binding site for Diego [32] and two C-terminal regions of basic residues bracket the PDZ domain and these regions appear to bind the Par-1 kinase [33]and the product of the naked cuticle gene [34]. These regions also may function in targeting Dvls to the inner leaflet of the cell membrane, attracted to the negative charges there. The organization of the N-terminal basic residues in these sequences have structural similarity to the Membrane Effector Domain (MED) of the myristoylated alanine-rich C-kinase substrate (MARCKS) protein [35], providing the possibility of a dynamic, reversible association with the lipid bilayer. Finally, the DEP domain enables protein-protein interactions between Dvl and Daam1 [36], linking Dvl to the small molecular weight GTPases like RhoA, as well as between Dvl and protein kinases like the muscle-specific kinase, MuSK[28].
The three isoforms of Dvl observed in mammals display high sequence homology, beyond that reflecting the conserved DIX, PDZ, and DEP domains. The expression of three isoforms suggests some level of redundancy, a tenet supported by gene interruption studies of either Dvl1 or Dvl2, or of both Dvl1 and Dvl2 in mice [37–39]. At least for Dvl1 and Dvl2, any redundancy is far from complete and deficiency in either one alone does impact fertility, signaling, and behavior [37–39]. We have made use of mouse embryonal F9 teratocarcinoma cells as a model system for detailed analysis of Wnt signaling. Clones of these totipotent F9 cells expressing Frizzled-1 respond to stimulation by purified Wnt3a and go on to form primitive endoderm [40;41]. We have illuminated key facets of the role of signaling in development using these cells to probe Wnt actions on the canonical, non-canonical, and planar cell polarity pathways [40–49]. Based upon the existing knowledge of the mouse F9 cells and their utility in the study of Wnt signaling we adopted this model for analysis of the roles of individual mammalian Dvl1, Dvl2, and Dvl3 in the Wnt/β-catenin/Lef-Tcf-sensitive gene activation or “canonical” pathway. We demonstrate both common as well as unique roles of individual Dvl isoforms in the mediation of Wnt3a-stimulated canonical signaling.
Experimental procedures
Cell Culture
The mouse F9 teratocarcinoma (F9) cells and the mouse embryonal P19 teratocarcinoma (P19) cells (from ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% heat-inactivated fetal bovine serum (FBS) (Hyclone, South Logan, UT) at 37°C in a 5% CO2 incubator. Human embryonic kidney HEK 293 cells (from ATCC, Manassas, VA) were grown in DMEM supplemented with 10% FBS at 37°C in a 5% CO2 incubator. F9 cells were transiently transfected to express empty vector (EV), or rat Frizzled-1 (Fz1), or rat Frizzled-2 (Fz2), as described elsewhere [43;44]. For overexpression studies, hemagglutinin (HA)- and green fluorescence protein (GFP)-tagged mouse Dvl isoforms, as well as enhanced GFP (eGFP)-tagged human Dvl isoforms were expressed by transient transfection by using LipofectAMINE Plus reagent as per the instructions of the manufacturer (Invitrogen, Calsbad, CA).
Immunoprecipitation and Immunolotting
F9 and the other cells grown in monolayers were serum-starved for 8 hr prior to stimulation by either Wnt3a (10 ng/ml) or Wnt5a (50 ng/ml) for 6 h. Cells were harvested in lysis buffer containing protease and phosphatase inhibitors (20 mM Tris pH 7.4, 150 mM NaCl, 1% TritonX-100, 200 μM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 mM NaF, 1mM Na3VO4, and 100 nM okadaic acid). Whole-cell lysates and samples from immunoprecipitation were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the separated proteins were transferred to nitrocellulose blots electrophoretically. The blots were blocked with 10% bovine serum albumin and probed with antibody against either Dvl1, Dvl2, Dvl3 (Santa Cruz Biotechnology, Santa Cruz, CA). Blots of samples from derived from cells that were transiently transfected to express an HA-tagged Dvl isoform were stained with anti-HA antibodies (Roche). Immune complexes were detected by enhanced chemiluminescence method, as per the instructions of the manufacturer (Amersham).
Lef/Tcf-sensitive Reporter Gene Assay
F9 clones transiently transfected with either reporter plasmid Super8XTOPFLASH or mutated reporter plasmid Super8X FOPFLASH (gifts from Dr. Randall Moon, HHMI, University of Washington, Seattle,WA); and cotransfected with 0.05–0.8 μg of expression vector harboring either Dvl1, Dvl2 or Dvl3 (dually tagged with HA- and GFP-at the C-terminus of Dvl, i.e., Dvl-HA-GFP) for 24 hr. Cells were harvested in reporter lysis buffer (Promega, Madison, WI) following exposure to serum-free DMEM for 8 hr and luciferase activity was assayed. Briefly, 20 μl of cell lysates were incubated for 10 sec with 100 μl of reaction mixture containing 0.67 mM luciferin, 0.27 mM Coenzyme A, 0.1 mM EDTA, 1.1 mM MgCO3, 4 mM MgSO4 and 20 mM Tricine (pH 7.8) and the intensity of luminenscence was immediately measured using a luminometer (Lumat LB 9507, Berthold Technologies, Oak Ridge, TN). Samples were assayed in triplicate and the luciferase activity was normalized based to protein content of the sample.
Knock-down of Dvl Isoforms siRNA
At least 3 pairs of sense and antisense oligonucleotides were designed and synthesized by Ambion (Austin, TX) for each of the following molecules: mouse Dishevelled-1; mouse Dishevelled-2; and, mouse Dishevelled-3. Each pair of oligos was annealed in 200μl of annealing buffer (100 mM potassium acetate, 2 mM magnesium acetate and 30 mM HEPES-KOH, pH 7.4) at 37°C for 1 hr. F9 cells were treated with 100 nM of annealed siRNA by using LipofectAMINE 2000. The efficiency of the knockdown for each Dvl isoform was determined by immunoblotting, staining with an antibody against each individual isoform. The sequences of siRNA oligos, which are the most effective in knockdown of targeted proteins, are listed as follows: CCAGGAUAUUGGCUUGACAtt and UGUCAAGCCAAUAUCCUGGtg target mouse Dishevelled-1, GGAAGAGAUCUCCGAUGACtt and GUCAUCGGAGAUCUCUUCCtt target mouse Dishevelled-2, and GGAAGAGAUCUCGGACGACtt and GUCGUCCGAGAUCUCUUCCtt target mouse Dishevelled-3. For the “rescue” experiments of cells treated with siRNA designed to knockdown a specific Dvl isoform, expression of human Dvl isoforms (resistant to the mouse-based siRNA) with an eGFP-tag fused to the C-terminus of each human Dvl isoform was necessary. Cells were treated with siRNA for 48 h. Twenty four hours after the initial administration of siRNA, cells were transiently transfected with expression vector harboring eGFP-tagged human Dvl isoform by using LipofectAMINE. Cell lysates were collected and the Lef/Tcf-sensitive luciferase reporter gene activity was determined. The efficiency of the siRNA-mediated knock down as well as the expression of exogenous Dvl-isoform (Dvl-eGFP) were established by immunoblotting.
Quantification of Stained Proteins
Exposed films were scanned by calibrated Umax 1000 absorbance scanner equipped with SilverFastAi software (LaserSoft Imaging Inc. Longboat Key, FL). The bands were quantified by use of Aida software (Raytest, Germany).
Statistical Analysis
In each experiment, triplicates were performed in the sampling. All of the data displayed are expressed as mean values ± S.E.M. for at least 3 separate experiments. Comparisons of data among groups were performed with one-way analysis of variance followed by the Newman-Keuls test. Statistical significance (p value of ≤ 0.05) is denoted with asterisks or pound symbles.
Results
Mouse Embryonal F9 Teratocarcinoma Cells Express Dvl1, Dvl2, and Dvl3 Isoforms
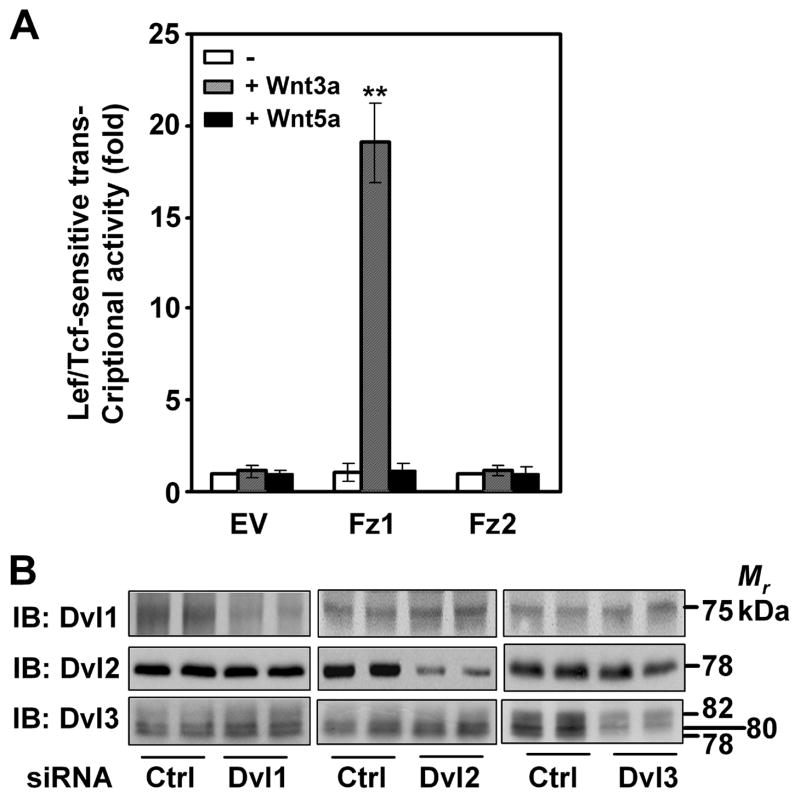
For these experiments we made use of mouse F9 cells that are transfected to express the rat Frizzle-1 (Fz1) and have been shown to form primitive endoderm in response to stimulation with purified Wnt3a [49]. Using the Super8XTOPFLASH luciferase reporter to assay the Lef/Tcf-sensitive transcriptional response to Wnt stimulation, we demonstrated that purified Wnt3a stimulates gene activation of the canonical pathway in F9 cells expressing Fz1(fig. 1A). Stimulation of the canonical pathway was observed in response to purified Wnt3a, but not to purified Wnt5a, in these same cells. F9 cells transfected with either empty vector (EV) or expression vector harboring rat Frizzled-2 (Fz2), in contrast, displayed no stimulation of the canonical pathway in response to Wnt3a. For the F9 cells expressing the Frizzled-1 and stimulated with Wnt3a under these conditions, the canonical transcriptional response was stimulated robustly.
Figure 1. Fz1-expressing mouse totipotent F9 teratocarcinoma cells display canonical activation by Wnt3a and express Dv1, Dvl2, and Dvl3.
A, mouse F9 cells transfected to express Fz1 or Fz2 or empty vector (EV) were treated for 6 hr without or with purified Wnt3a (10 ng/ml) or Wnt5a (50 ng/ml) and the activation of the Wnt-sensitive canonical pathway monitored by the use of the Lef/Tcf-sensitive luciferase gene reporter. Data presented are mean values ± S.E.M. from three or more independent experiments. **, p ≤ 0.001 for the difference from control. B, Whole cell lysates from prepared F9 cells either untreated (Control) or treated with siRNA targeting either mDvl1, or mDvl2, or mDvl3. Samples of the lysates were subjected to SDS-PAGE and transfer of the resolved proteins to nitrocellulose blots. The blots were stained with primary antibodies against either Dvl1, or Dvl2, or Dvl3. A blot, representative of three or more individual, separate experiments is displayed. The tabulated data from these multiple experiments are presented as mean values ± S.E.M. from three or more independent experiments. **, p ≤ 0.01 for the difference from control can be found in Table 1.
Using immunoblotting, we probed the expression of each of the Dvl isoforms in the mouse F9 cells (fig. 1B). Antibodies raised to synthetic peptide fragments unique to Dvl1, Dvl2, and Dvl3 were employed to stain samples of whole-cell lysates subjected to SDS-PAGE and transfer of the resolved proteins to blots. F9 cells express all three isoforms of Dvl: Dvl1 (Mr = 75 kDa); Dvl2 (Mr = 78 kDa); and Dvl3 (Mr = 78, 80, and 82 kDa; likely differentially phosphorylated forms). We next subjected the F9 cells to treatment with siRNAs designed specifically to target each Dvl isoform individually. siRNAs designed to suppress the expression of a specific Dvl isoform successfully suppressed the targeted Dvl, while not suppressing the expression of the other non-targeted Dvls (Table 1). These initial studies demonstrate the specificity of both the siRNA reagents as well as of the isoform-specific anti-Dvl antibodies employed throughout these studies. Under these conditions, the extent of the siRNA-induced suppression ranged from about 70–85% (Table 1).
Table 1. siRNA-induced knock down/depletion of Dvl1, Dvl2, or Dvl3 in mammalian cells.
The cellular abundance of each Dvl isoform in mouse F9 cells was measured by immunoblotting. Blots were stained with Dvl isoform-specific antibodies. The abundance of the Dvl isoform in untreated cells was quantified and is set to a value of “1.0”. The results are presented as mean values ± S.E.M. from three or more separate experiments.
| siRNA | Control | Dvl1 | Dvl2 | Dvl3 |
|---|---|---|---|---|
| Protein | ||||
| Dvl1 | 1.00 ± 0.09 | 0.21 ± 0.04* | 1.09 ± 0.02 | 1.11 ± 0.09 |
| Dvl2 | 1.00 ± 0.02 | 0.94 ± 0.03 | 0.14 ± 0.04* | 0.98 ± 0.02 |
| Dvl3 | 1.00 ± 0.03 | 1.11 ± 0.08 | 1.07 ± 0.06 | 0.28 ± 0.03* |
, p < 0.001 for the difference from the values observed for untreated and control siRNA-treated cells.
Knockdown of Dvl1, Dvl2, or Dvl3 Suppresses Wnt3a-stimulated Canonical Signaling
The first and most straightforward experimental approach was to knockdown the expression of each Dvl individually and then to measure the impact of the loss of the Dvl on the ability of purified Wnt3a to activate Lef/Tcf-sensitive gene expression (fig. 2A). Our initial experiments suggested that knockdown of either Dvl1, Dvl2, or Dvl3 is capable of attenuating the ability of Wnt3a to stimulate the canonical pathway. The reductions of Wnt3a-stimulated Lef/Tcf-sensitive gene expression for siRNA-treated F9 cells with Dvl isoform-specific knockdowns (KD) were as follows: 75% for KD-Dvl1; 60% for Dvl2; and 85% for Dvl3. Since the knockdown under these conditions provided only a single determination, we examined the effects of a dose-response to graded amounts of siRNAs on the functional read-out. We plotted the extent of the knockdown from immunoblotting data (see fig. 1B) versus the Lef/Tcf-sensitive transcriptional activation in response to Wnt3a, setting the maximal response as “100%” (fig. 2B). The results of the studies were quite revealing, as they identified differences in the character by which the loss of a specific Dvl impacted the Lef/Tcf-sensitive response to purified Wnt3a. On one extreme were the data derived from use of siRNA targeting Dvl3, in which 50% of the canonical response was lost when the amount of Dvl3 expression was suppressed by less than 20%. For Dvl2, in sharp contrast, 50% of the canonical response to Wnt3a was lost only when more than 50% of intracellular complement of Dvl2 was lost. The relationship between knockdown and canonical response to Wnt3a for F9 cells treated with increasing amounts of siRNA targeting Dvl1 was very similar to that of the same studies performed with siRNA targeting Dvl3, but not that of Dvl2. Although optimized empirically for the conditions for siRNA-induced knockdown of each Dvl isoforms (e.g., sequence and concentration of siRNA employed, length of exposure to siRNA, etc.), we were unable to suppress the cellular content of each targeted Dvl by more than 70–85%. We speculate that some level of each individual Dvl isoform or the sum total of all three Dvls may be required for cell viability.
Figure 2. siRNA-induced knockdown of Dvl1, Dvl2, or Dvl3 attenuated Wnt3a-stimulated canonical signaling.
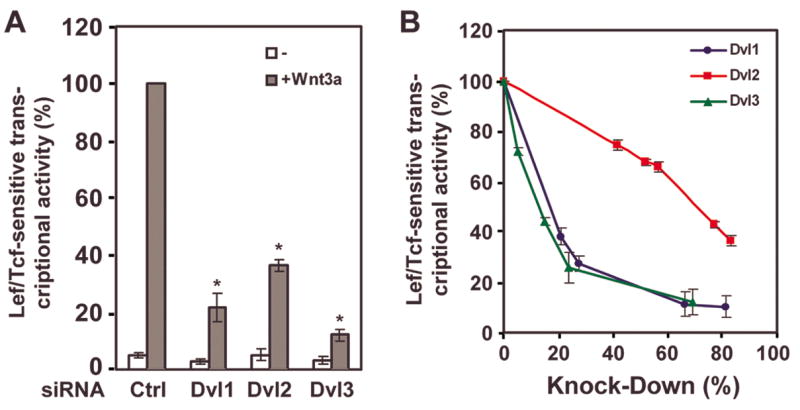
Mouse F9 cells expressing Fz1were treated with maximal (panel A) or variable (panel B) amounts of siRNAs targeting one of the three Dvls (Dvl1, Dvl2, or Dvl3) and the activation of the Wnt-sensitive canonical pathway in response to Wnt3a (6 hr) and monitored by the use of the Lef/Tcf-sensitive luciferase gene reporter. The assay of the canonical pathway was performed at 6 hr following stimulation without or with purified Wnt3a. Data presented are mean values ± S.E.M. from three or more independent experiments. *, p ≤ 0.01 for the difference from control. In panel A, the data are plotted as % of maximal Wnt3a-stimulated canonical response versus treatment with a maximal amount of siRNA reagent. In panel B, the data are plotted as “% of maximal” of the Wnt3a-sensitive canonical response versus the extent of knockdown in Dvl isoform protein produced by treatment increasing amounts of siRNA targeting a given Dvl isoform.
Estimation of the Relative Expression of Dvl1, Dvl2, and Dvl3
Although the antibodies employed to identify each Dvl isoform were specific (fig. 1), the immunoblotting approach with three different antibodies was unable to provide information on the relative contribution of each Dvl isoform to the total pool of Dishevelleds in these mammalian cells. Our attempts to produce an antibody to any one of several short regions common to all of the three Dvls (while avoiding well-known protein motifs and domains, such as DIX, PDZ, and DEP that would not be unique to only the Dishevelleds) were unsuccessful. To obviate these obstacles, we adopted an alternative strategy that used these anti-isoform-specific antibodies in tandem with antibodies that recognize the haemagglutin antigen (HA) and green fluorescent protein (GFP)-tagged versions of each Dvl expressed exogenously (fig. 3A). The F9 cells were transfected with increasing amounts of plasmid DNA of an expression vector harboring a C-terminally HA- and GFP-tagged fusion protein of each Dvl isoform. Immunoblotting of samples derived from whole-cell lysates from F9 cells were stained under the same conditions, with an both an antibody specific to the individual Dvl isoform and that to the HA-tag. Since all three exogenously-expressed HA-tagged Dvl isoforms are recognized by the anti-HA antibody, we could relate the HA-tag derived expression to a comparison with the immunoblotting data for blots stained with the antibodies to the individual Dvl isoforms (fig. 3A). Using this approach we were able to quantify the relative amounts of the individual Dvl isoforms expressed endogenously in F9 cells, setting the total pool (i.e., Dvl1 + Dvl2 + Dvl3) to “100%”. The relative levels of expression of each Dvl isoform expressed endogenously was as follows: Dvl1, 0.7±0.3% ; Dvl2, 97.8±0.3 %; and Dvl3, 1.5±0.4% (fig. 3B). Thus, the expression of Dvl2 provides to more than 95% of the total Dvl pool, whereas at the other extreme the expression of Dvl1 contributes the least, less than 1%. We sought to probe the extent to which the rank order of Dvl isoform abundance in mouse F9 cells would approximate the same determination in other cells. The same analysis was performed using mouse P19 teratocarcinoma (P19) and human embryonic kidney (HEK) 293 cells. The rank order of endogenous expression of Dvl isoforms in P19 and HEK293 cells was similar to that established in the F9 cells (fig. 3B). In P19 and HEK293 cells, the relative abundance of Dvls was calculated as follows: for P19 cells, Dvl1, 3.1±1.1%; Dvl2, 90.2±5.1%; and Dvl3, 6.7±2.1%; for HEK293 cells, Dvl1, 1.5±0.5%; Dvl2, 81.2±0.4%; and Dvl3, 17.3±2.3%. Thus, Dvl2 is the most abundant Dvl isoform expressed in each of these mammalian cell lines, with Dvl1 and Dvl3 making up a relatively small component of the total pool. Each Dvl, however, contributes individually to the overall Wnt3a-stimulated canonical signaling (fig. 2).
Figure 3. Determination of the relative abundance of Dvl1, Dvl2, and Dvl3 in mouse F9, mouse P19, and human HEK 293 cells.
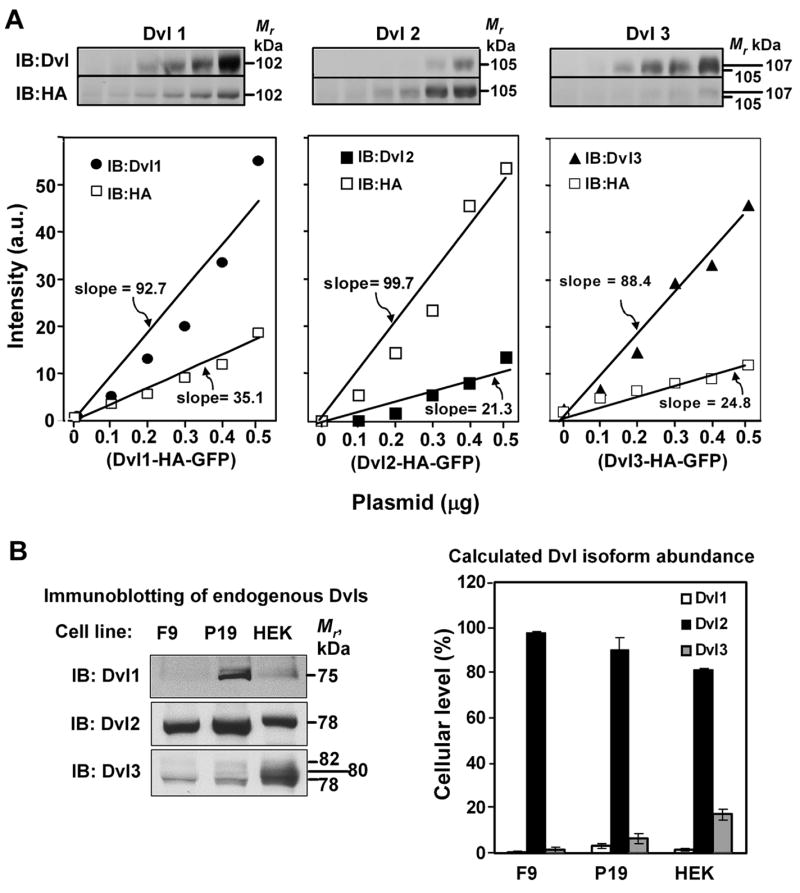
A, mouse F9 cells expressing Fz1 were co-transfected without or with increasing amounts of plasmid DNA (expression vector) harboring a single Dvl isoform tagged with both HA and GFP at the C-terminus of the molecule, as described. After 48 hr, the F9 cells were disrupted and sample of whole-cell lysates was subjected to SDS-PAGE and transfer of the resolved proteins to nitrocellulose blots that were then stained with primary antibodies against either Dvl1, or Dvl2, Dvl3, or the HA-antigen tag. A set of representative blots obtained from exogenously expressed Dvl isoforms (upper panels) and plots of expression of these isoforms (lower panels). B, analyses of the relative abundance of Dvl1, Dvl2, and Dvl3 in F9, P19, and HEK 293 cells. Samples (0.1 mg protein/SDS-PAGE lane) of whole-cell lysates were subjected to SDS-PAGE and transfer of the resolved proteins to nitrocellulose blots. The blots were stained with primary antibodies against Dvl1, or Dvl2, or Dvl3. A set of representative blots of endogenously expressed Dvl isoforms is displayed shown (left panels). These data were analyzed and the relative amount of Dvl isoform expressed in each cell calculated (right panel). *, p ≤ 0.01; **, p ≤ 0.001 for the difference from Dvl1 expression.
Expression of Individual Dvls Activate Lef/Tcf-sensitive Transcription
By siRNA-mediated knockdown we demonstrated that Dvl1, Dvl2, and Dvl3 each contribute individually to mediating Wnt3a-stimulated activation of Lef/Tcf-sensitive transcription. We sought to analyze Dvl biology further using the converse approach, i.e., “over”expression of Dvl1, Dvl2, and Dvl3 individually in F9 cells. Cells were transiently transfected with increasing amounts of plasmid DNA harboring the HA-tagged fusions of Dvl1, Dvl2, or Dvl3 and then the Lef/Tcf-sensitive luciferase reporter activity measured. Overexpression of either HA-tagged Dvl1, -Dvl2, or –Dvl3 was observed to activate Lef/Tcf-sensitive transcription, in the absence of stimulation by Wnt3a (fig. 4B). Relative small changes in the expression of Dvl3 abundance was observed to yield a more profound enhancement of the Lef/Tcf-sensitive transcription than did substantial greater increases in either Dvl1 or Dvl2. It should be noted that under the conditions employed in which the same cells were transiently transfected with equivalent amounts of input DNA of the same expression vector, the amount of Dvl3 expressed was substantially lower than that yielded by expression vectors harboring either Dvl1 or Dvl2. We speculate that whatever the factors operating to maintain the relative levels of expression of endogenous Dvl isoforms in F9 cells (rank order Dvl2≫Dvl1>Dvl3) also are limiting the amount of tagged isoform exogenously expressed under identical conditions of transfection. Whatever this/these factor(s) is/are, the data demonstrate that when expressed in the normal “background” of Dvl1, Dvl2, and Dvl3, expression of any individual isoform of Dvl promotes Lef/Tcf-dependent transcription in the absence of Wnt3a stimulation. The exogenous expression of Dvl3 yields the greatest impact on Lef/Tcf-sensitive transcriptional activity (fig. 4B).
Figure 4. Effects of “over”expression of Dvl1, Dvl2, or Dvl3 on the activation of Lef/Tcf-sensitive transcriptional activation, in the absence of Wnt3a stimulation.
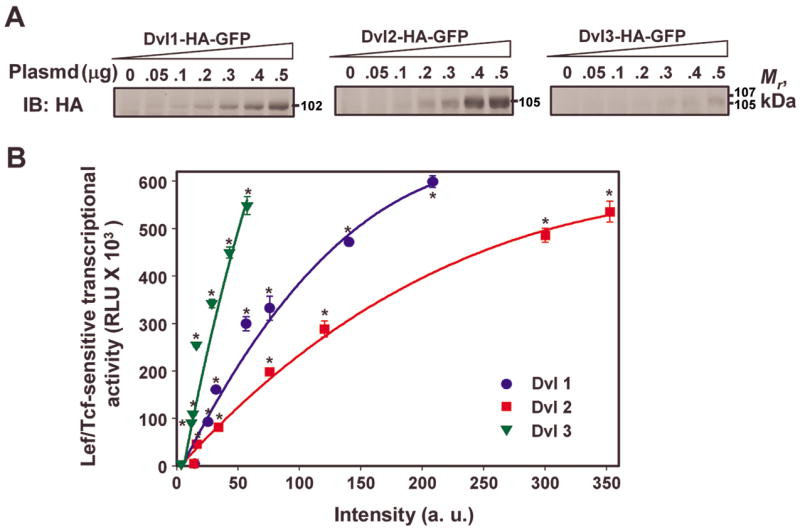
Fz1-expressing F9 cells were transiently co-transfected with SuperX8TOPFLASH reporter gene in the absence or presence of increasing amounts of input plasmid DNA (expression vector) harboring either Dvl1, or Dvl2, or Dvl3 which had been tagged on the C-terminus with HA. After 48 hr, the cells were disrupted and whole-cell lysates prepared. A, sample of whole-cell lysates was subjected to SDS-PAGE and transfer of the resolved proteins to nitrocellulose blots. The blots were stained with primary anti-HA antibody and their relative levels of expression compared. B, the activation of the Lef/Tcf-sensitive transcription was assayed by using SuperX8TOPFLASH luciferase gene reporter. The luciferase reporter activities were plotted versus the amount of immunoreactive staining for the HA-tag (in arbitrary unit; a.u.) quantified in the blots obtained from the same cell lysates. Data presented are mean values ± S.E.M. from three or more independent experiments. *, p ≤ 0.01 for the difference from the “Control” cells that were treated with empty vector that lacks a Dvl isoform cDNA.
Heterogeneity of Isoforms in Dvl-based complexes
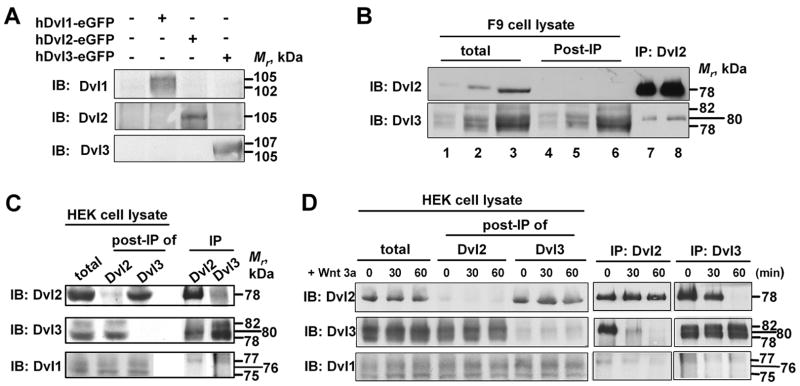
We sought to establish if the cellular Dvl-based complexes were heterogeneous with respect to their composition and Dvl isoforms. The approach, to perform “pull-downs” of a specific Dvl isoform followed by analysis of the presence of other isoforms in the complex, required very high quality antibodies with essentially no cross reactivity for other isoforms. To interrogate the existence of cross-reactivity among the Dvl isoform-specific antibodies, we prepared cells that overexpressed one of the three Dvl isoforms, prepared whole-cell extracts, and performed immunoblotting of each overexpressing cell line with each of the Dvl isoform-specific antibodies (fig. 5A). No significant cross-reactivity was observed for the antibodies employed in these studies, even when overexpressing cells were used as a source of the blots. We first performed pull-downs (i.e., immunoprecipitations) from F9 cells using antibody against Dvl2 (fig. 5B). Pull-downs performed with anti-Dvl2 antibodies appeared quantitative, as no immunoreactive material was detected in the supernatant from post-immunoprecipitation (post-IP, fig. 5B). Analysis of Dvl2-based complexes isolated in this manner Dvl2 (as expected) as well as Dvl3 (fig. 5B). Dvl3 immunoreactivity is detected largely in the supernatant fraction (post-IP) in Dvl2-based pull-downs, indicating that only a small portion of Dvl3 has been sequestered in the Dvl2-based complex (fig. 5B). Dvl1 was not detected under the conditions.
Figure 5. Dvl-based complexes are heterogeneous with respect to Dvl isoforms.
A, Dvl isoform-specific antibodies were tested for cross reactivity by immunoblotting of whole-cell lysates obtained from cell “over”expressing a single Dvl isoform. Immunoblots of cell lines that were “over”expressing a single Dvl isoform then were stained with individual antibodies against each of the Dvl isoforms. B, immune precipitation-based “pull-downs” from whole-cell lysates of F9 cells were performed using antibodies that recognize only Dvl2. The Dvl-based complexes isolated in the pull-downs were subjected to SDS-PAGE and the resolved proteins transferred to nitrocellulose blots. The blots were stained with antibodies specific for each Dvl isoform, seeking to detect Dvl1, DVl2, and Dvl3 that may be present in the complex. C, immune precipitation-based “pull-downs” from whole-cell lysates of HEK293 cells were performed using antibodies that recognize only either Dvl2 or Dvl3. Whole-cell lysates (“total”), pull-down complexes (“IP”) as well as supernatant fraction following the immune precipitation reaction (“post-IP”) were subjected to SDS-PAGE and the resolved proteins transferred to nitrocellulose blots. The blots were stained for each Dvl isoform. D, HEK293 cells expressing Fz1 were treated without and with purified Wnt3a for up to 60 min and the composition of Dvl2-based as well as Dvl3-based complexes analyzed for the presence of all three isoforms in the complex performed by immunoblotting. Immune precipitation-based “pull-downs” were performed using antibodies that recognize either Dvl2 or Dvl3. Whole-cell lysates (total), pull-down complexes (IP) as well as supernatant from post-immunoprecipitated fraction (post-IP) were subjected to SDS-PAGE and the resolved proteins transferred to nitrocellulose blots and stained for each Dvl isoform. The data presented in these panels are blots representative of three or more independent analyses for each protocol. The data from each of the experiments are in agreement.
We also analyzed the profile of isoforms in Dvl2-based pull-downs in HEK293, which display a significantly great relative abundance of Dvl1 and Dvl3 (fig. 3B). In Dvl2-based pull-downs from HEK 293 cells we detected the presence of all three Dvl isoforms (fig. 5C). Similarly, pull-downs performed with antibodies against Dvl3, revealed the presence of Dvl1, Dvl2, and of course Dvl3 (fig. 5C). Pull-downs performed with antibodies to Dvl1, in contrast, were too weak to be of value (data not shown).
Finally, we questioned whether or not activation of the canonical pathway by Wnt3a would alter the apparent composition of the Dvl-based complexes prepared from HEK 293 cells (fig. 5D). HEK293 cells expressing Fz1 were stimulated for 60 min without and with purified Wnt3a. Dvl2- and Dvl3-based pull-downs were performed from whole-cell extracts and the complexes screened for the presence of all three isoforms. The amounts of Dvl1 and Dvl3 found in the Dvl2-based complex were reduced in response to Wnt3a stimulation. No Dvl3 and little Dvl1 was detected in the Dvl2-based complexes at 60 min post stimulation with Wnt3a. All three isoforms (Dvl3, Dvl1, and Dvl 2) were detected in the complexes subject to pull-down with the antibody to Dvl3 (fig. 5D). Similar to our observations obtained with Dvl2-based complexes isolated from cells treated with purified Wnt3a, the composition of the Dvl3-based complexes showed a Wnt3a-induced reduction in Dvl2 and Dvl1 within 30 min. With 60 min of stimulation with Wnt3a, the content of Dvl1 and Dvl2 in the Dvl3-based complexes declined to below detection.
Rescue of Dvl3 Knockdown by Overexpression of Other Dvl Isoforms
We wondered if the attenuation of the Wnt3a-stimulated canonical pathway by depletion of either Dvl3 or Dvl1 could be “rescued” by expression of other Dvl isoforms? The strategy for a rescue experiment required some modifications. Since the siRNA reagents were prepared specifically to knockdown mouse Dvl1, Dvl2, or Dvl3, we could not perform the “rescue” with the mouse Dvls, but rather with the expression of the human counterparts. In addition, since overexpression of Dvl isoforms (fig. 4) can lead to activation of the canonical pathway in the absence of Wnt3a, we were restricted to employing the highest amount of exogenous expression that did not significantly increase the basal levels of the reporter gene activity. We achieved both of these parameters and were able to test the hypothesis that the expression of one Dvl isoform might rescue the function of another isoform, once depleted. As shown above, treating the Fz1-expressing cells F9 cells with siRNA targeting Dvl3 effectively suppressed the expression of Dvl3 as well as the ability of Wnt3a to stimulate the Lef/Tcf-sensitive transcriptional response (fig. 6A). Exogenous expression of eGFP-tagged human Dvl3 clearly rescues fully the ability of Wnt3a to stimulate the activation of the canonical pathway. In sharp contrast, the expression of either Dvl1 or Dvl2 was largely unable to rescue the Wnt3a-stimulated response in the Dvl3-depleted cells. A similar experiment was performed in Fz1-expressing F9 cells that were depleted of Dvl1 by siRNA treatment (fig. 6B). Whereas expression of the human Dvl1 restored the Wnt3a-stimulated canonical response to approximately 75% of control levels, expression of neither human Dvl2 nor Dvl3 was able to rescue the Wnt3a-stimulated canonical response.
Figure 6. Rescue of Wnt3a-stimulated Lef/Tcf-sensitive transcriptional activation of Dvl3-or Dvl1-depleted mouse F9 cells using exogenous expression of hDvl1, hDvl2, or hDvl3.
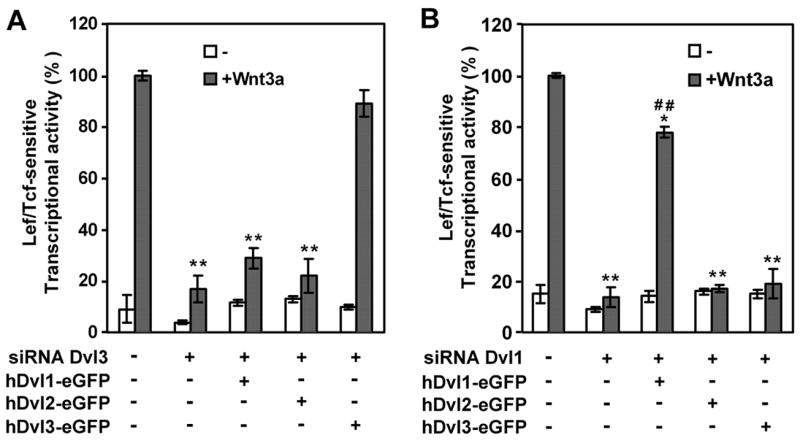
Fz1-expressing mouse F9 cells were treated with siRNA targeting either mouse Dvl1 (A) or Dvl3 (B). At 24 hr post addition of the siRNA, the cells were co-transfected with an expression vector harboring hDvl1, hDvl2, or Dvl3 which were tagged with eGFP at its C-terminus as well as with the SuperX8TOPFLASH luciferase reporter gene construct and the culture extended for another 24 hr. The assay of the Wnt3a-sensitive canonical pathway was performed by the assay of the SuperX8TOPFLASH luciferase reporter at 6 hr following stimulation without or with purified Wnt3a. A, rescue of Wnt3a-stimulated activation of the canonical pathway in Dvl3-depleted F9 cells by exogenous expression of human Dvl1, Dvl2, or Dvl3. B, rescue of Wnt3a-stimulated activation of the canonical pathway in Dvl1-depleted F9 cells by exogenous expression of human Dvl1, Dvl2, or Dvl3. The human Dvl isoforms are resistant to suppression by siRNAs targeting the mouse homologues. Data presented are mean values ± S.E.M. from three or more independent experiments. *, p ≤ 0.01; **, p ≤ 0.001 for the difference from control, +Wnt3a group. ##, ≤ 0.001 for the difference from group treated with siRNA alone.
Discussion
The phosphoprotein Dishevelled is a multifunctional protein harboring docking sites for more than 20 suspected binding partners[14;15]. Prominent domains found in Dishevelled, such as DIX, PDZ, and DEP, are highly conserved from the single fly Dsh to the mammalian Dvl1, Dvl2, Dvl3. The primary question addressed in this study is why are there three Dvls expressed in mammals and what specific role(s), if any, do the Dvl isoforms play in the well-known Wnt-stimulated stabilization of intracellular β-catenin that is responsible for activation of Lef/Tcf-sensitive transcription of genes key in development? The current work demonstrates in several different cell lines, including mouse (F9 and P19 embryonal teratocarcinoma) and human (HEK 293), that all express the three isoforms of Dvl. The next question is are the relative levels of expression of Dvl1, Dvl2, and Dvl3 comparable?, or do differences exist among the three Dvls with respect to cellular content? Through the use of both Dvl isoform-specific antibodies and antibodies targeting the protein HA-tag fused onto the C-terminus of exogenously expressed Dvl isoforms, it was possible to make a first-approximation determination of the relative levels of expression of each of the Dvl1, Dvl2, and Dvl3. The results are clear and the expression of Dvl2 dominates the pool of Dvl isoforms expressed in these cells. The expression of Dvl2 accounts more than 95 % of the total pool of Dvls (i.e., Dvl1 + Dvl2 + Dvl3) in F9 cells, whereas Dvl3 and Dvl1 each account less than 2 %. Similar studies performed on the mouse P19 and human HEK 293 cells suggest that same rank order of cellular abundance for Dvl2≫Dvl1=Dvl3. Finally, it is of keen interest that when exogenously expressed, the relative levels of Dvl isoforms mirror those among three native Dvl isoforms. Little is known about the regulation of Dvl expression at the levels of translation and protein stability, but certainly interesting differences must exist among how these three highly-homologous mammalian Dvls are processed to account for such disparity between the expression of Dvl2 (very high) and that of either Dvl1 or Dvl3 (very low).
Considering the differences in relative abundance and high-homology among the three Dvls, the ability of the siRNA-induced depletion of any one of the Dvl isoforms to impact the ability of Wnt3a to stimulate the canonical pathway was unexpected. Although studies making use of gene interruption to knock-out the expression of Dvl1, Dvl2, or both [37] in mice [38;39]reveal some level of redundancy, the mouse knock-outs do display unique differences in aspects of signaling that also seem apparent in the current study [37–39]. If all three mammalian Dvls were truly redundant, it seems unlikely that the knockdown of the expression of Dvl1 or Dvl3 (which together constitute ~5% of the total Dvl pool in F9 cells) would have an impact on ability of Wnt3a to stimulate Lef/Tcf-sensitive transcriptional activation. Suppression of either Dvl1 or Dvl3 individually, attenuated signaling in the Wnt-sensitive canonical pathway. Likewise, exogenous expression of individual Dvl isoforms increases the amount of Lef/Tcf-sensitive luciferase reporter gene [50]. Expression of either Dvl1 or Dvl3 individually yields a greater increase in the transcriptional response than that due to expression of Dvl2.
Combining the data obtained from study of Wnt3a-stimulated activation of the Lef/Tcf-sensitive transcriptional response with the relative levels of expression of either exogenously expressed (HA-tagged) or of the sum of the endogenous and exogenously expressed Dvl isoforms together, it was possible to speculate about the relative roles of each Dvl isoform and their impact in signaling of the Wnt canonical pathway. Changes in the cellular levels of Dvl1 and Dvl3 appear to impact the function of the Wnt-sensitive canonical pathway far more than those of Dvl2. The data obtained from the pull-downs of Dvl2-based and Dvl3-based complexes demonstrate that the complexes are not homogenous, but rather heterogeneous, with respect to composition of Dvl isoforms. Attempts to rescue the Wnt3a-stimulated canonical response in Dvl3-depleted cells were successful only when performed with exogenously expressed Dvl3, yet no other isoform. Similar experiments performed in cells depleted of Dvl1 yield the same observation, exogenous expression of the Dvl isoform depleted (and no other) is able to rescue the Wnt3a-stimulated Lef/Tcf-sensitive transcriptional response. The experiments in knock-out mice, the current work in mammalian cells in culture, and recent work on the possible dynamic oligomerization of Dvl-based complexes [51;52] suggest that each mammalian Dvl plays a critical role in the Wnt-sensitive canonical pathway, though not one that is independent of the other Dvls (fig. 7). What remains to be determined is whether the spatial and temporal localization of Dvl1, Dvl2, and Dvl3 may be uniquely regulated by stimulation of the canonical pathway by Wnt3a.
Figure 7. A schematic presentation of Dvl complex containing Dvl1, Dvl2 and Dvl3 in Frizzled-1/β-catenin signaling pathway.
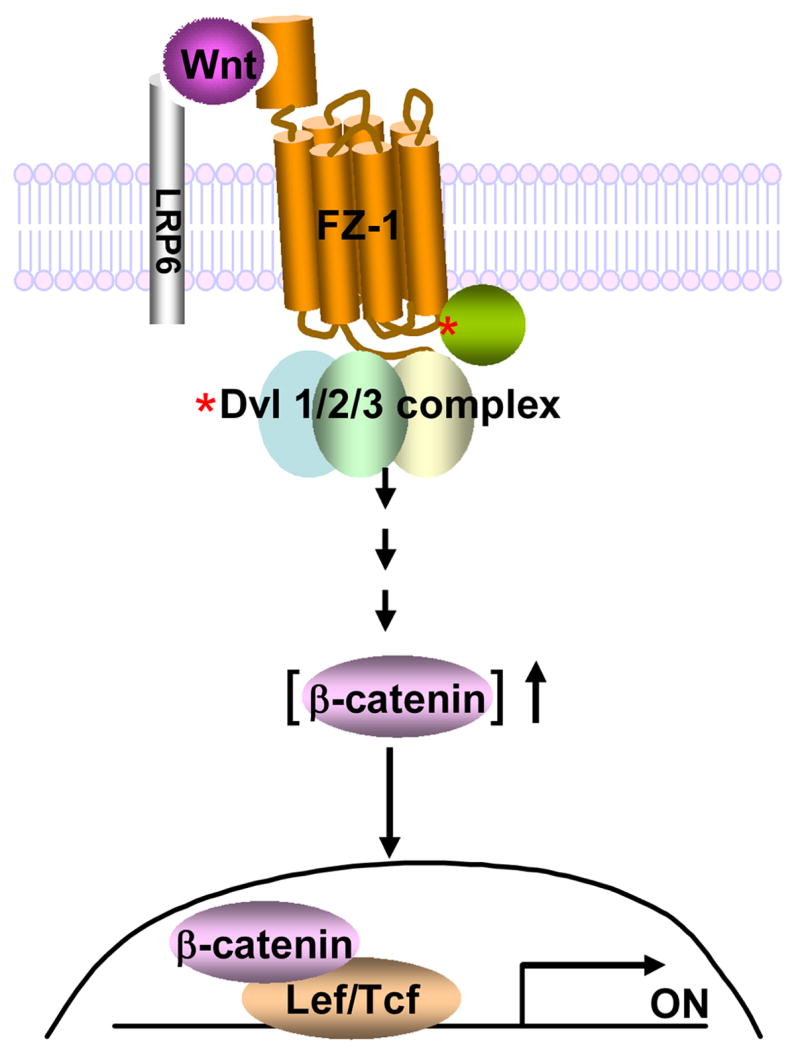
*, denotes “activation” in response to Wnt3a.
Acknowledgments
This work was supported by a United States Public Health Service grant award GM69375 from the National Institute of General Medical Sciences, the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 2.Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Malbon CC. G proteins in development. Nat Rev Mol Cell Biol. 2005;6:689–701. doi: 10.1038/nrm1716. [DOI] [PubMed] [Google Scholar]
- 5.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 6.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 10.Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart MJ, de los S, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 12.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 13.Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 1907;72:153–166. doi: 10.1016/S0070-2153(05)72002-0. [DOI] [PubMed] [Google Scholar]
- 14.Wharton KAJ. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 15.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 16.Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 17.Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- 18.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 19.Semenov MV, Snyder M. Human dishevelled genes constitute a DHR-containing multigene family. Genomics. 1997;42:302–310. doi: 10.1006/geno.1997.4713. [DOI] [PubMed] [Google Scholar]
- 20.Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- 21.Pizzuti A, Amati F, Calabrese G, Mari A, Colosimo A, Silani V, Giardino L, Ratti A, Penso D, Calza L, Palka G, Scarlato G, Novelli G, Dallapiccola B. cDNA characterization and chromosomal mapping of two human homologues of the Drosophila dishevelled polarity gene. Hum Mol Genet. 1996;5:953–958. doi: 10.1093/hmg/5.7.953. [DOI] [PubMed] [Google Scholar]
- 22.Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Dev Dyn. 1996;207:253–262. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Strovel ET, Sussman DJ. Transient overexpression of murine dishevelled genes results in apoptotic cell death. Exp Cell Res. 1999;253:637–648. doi: 10.1006/excr.1999.4700. [DOI] [PubMed] [Google Scholar]
- 24.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 25.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT. Casein kinase iepsilon in the wnt pathway: regulation of beta-catenin function. Proc Natl Acad Sci U S A. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 28.Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 29.Strovel ET, Wu D, Sussman DJ. Protein phosphatase 2Calpha dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem. 2000;275:2399–2403. doi: 10.1074/jbc.275.4.2399. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci U S A. 2001;98:14889–14894. doi: 10.1073/pnas.211572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- 33.Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- 34.Rousset R, Mack JA, Wharton KAJ, Axelrod JD, Cadigan KM, Fish MP, Nusse R, Scott MP. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 2001;15:658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin S, Hangyas-Mihalyne G, Zaitseva I, Golebiewska U. Reversible - through calmodulin - electrostatic interactions between basic residues on proteins and acidic lipids in the plasma membrane. Biochem Soc Symp. 2005:189–198. doi: 10.1042/bss0720189. [DOI] [PubMed] [Google Scholar]
- 36.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 37.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 38.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T, Lee YN, Malbon CC, Wang HY. Activation of the beta-catenin/Lef-Tcf pathway is obligate for formation of primitive endoderm by mouse F9 totipotent teratocarcinoma cells in response to retinoic acid. J Biol Chem. 2002;277:30887–30891. doi: 10.1074/jbc.M203852200. [DOI] [PubMed] [Google Scholar]
- 41.Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem. 2006;281:30990–31001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- 42.Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- 43.Ma L, Wang HY. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J Biol Chem. 2007;282:28980–28990. doi: 10.1074/jbc.M702840200. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Wang HY. Casein kinase 2 Is activated and essential for Wnt/beta-catenin signaling. J Biol Chem. 2006;281:18394–18400. doi: 10.1074/jbc.M601112200. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Lee Y, Malbon CC. PDE6 is an effector for the Wnt/Ca2+/cGMP-signalling pathway in development. Biochem Soc Trans. 2004;32:792–796. doi: 10.1042/BST0320792. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Malbon CC, Wang HY. Gene profiling of Frizzled-1 and Frizzled-2 signaling: expression of G-protein-coupled receptor chimeras in mouse F9 teratocarcinoma embryonal cells. Mol Pharmacol. 2004;65:45–55. doi: 10.1124/mol.65.1.45. [DOI] [PubMed] [Google Scholar]
- 47.Liu T, Liu X, Wang H, Moon RT, Malbon CC. Activation of rat frizzled-1 promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J Biol Chem. 1999;274:33539–33544. doi: 10.1074/jbc.274.47.33539. [DOI] [PubMed] [Google Scholar]
- 48.Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y, Wang HY. Inositol Pentakisphosphate Mediates Wnt/beta-Catenin Signaling. J Biol Chem. 2007;282:26490–26502. doi: 10.1074/jbc.M702106200. [DOI] [PubMed] [Google Scholar]
- 50.Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 1907;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–5277. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]