Abstract
The prototypical xanthine oxidase (XO) inhibitor allopurinol, has been the cornerstone of the clinical management of gout and conditions associated with hyperuricemia for several decades. More recent data indicate that XO also plays an important role in various forms of ischemic and other types of tissue and vascular injuries, inflammatory diseases, and chronic heart failure. Allopurinol and its active metabolite oxypurinol showed considerable promise in the treatment of these conditions both in experimental animals and in small-scale human clinical trials. Although some of the beneficial effects of these compounds may be unrelated to the inhibition of the XO, the encouraging findings rekindled significant interest in the development of additional, novel series of XO inhibitors for various therapeutic indications. Here we present a critical overview of the effects of XO inhibitors in various pathophysiological conditions and also review the various emerging therapeutic strategies offered by this approach.
I. Introduction, Historical Background
Allopurinol, or 1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one, was one of the crown jewels of the venerable drug discovery program at Burroughs Wellcome that started in 1940s and culminated by the awarding of a 1988 Nobel Prize in Physiology and Medicine to Gertrude B. Elion and George H. Hitchings, shared with British scientist James W. Black, for “discoveries of important principles for drug treatment.” An excellent overview of this effort, which yielded, in addition to the xanthine oxidase (XO)1 inhibitor allopurinol, block-buster drugs such as acyclovir, trimethoprim, and the early antineoplastic compounds thioguanidine and 6-mercaptopurine (6-MP), can be found in the Nobel lectures by Elion and Hitchings and elsewhere [Hitchings and Elion, 1963; Elion, 1988 (http://nobelprize.org/medicine/laureates/1988/elion-lecture.pdf), 1993]. As they recount, the program grew up out of a very general notion that synthetic analogs of the purine and pyrimidine bases can interfere with nucleic acid biosynthesis. They soon found antibacterial activity for multiple compounds, some of which were tested at Sloan-Kettering Institute for their anticancer properties. 6-MP emerged as having very high activity against leukemia. These compounds rapidly progressed into clinical trials and culminated in regulatory approval in 1953, propelled by a desperate need for new treatments, especially for acute leukemia in children, which were limited at the time to methotrexate and steroids. This project ultimately led also to the discovery of allopurinol, when thiouric acid was identified as a major 6-MP metabolite generated by XO.
Inhibition of XO was thought to inhibit oxidation of 6-MP and potentiate the antitumor properties. Because XO was one of the test enzymes in the early experiments in the laboratory, several nontoxic XO inhibitors, including a hypoxanthine analog, allopurinol, were available to directly confirm this suggestion. It was known that XO is involved in formation of uric acid from xanthine and hypoxanthine (Fig. 1). Thus, subsequent experiments showed effective control of serum and urinary uric acid by allopurinol (along with the major metabolite, oxypurinol) and its future potential for the treatment of hyperuricemia (although it took a number of years to establish the detailed mechanism of drug action and its safety profile). Although the XO inhibitor discovery programs at Burroughs Wellcome continued for many years, none of the subsequent compounds could surpass allopurinol for in vivo efficacy and tolerance. Allopurinol was approved by the Food and Drug Administration in 1966 for treatment of gout and remains a mainstay in the therapy of primary and secondary hyperuricemia. The mechanism relates to the inhibition of XO-catalyzed formation of uric acid from hypoxanthine and xanthine.
Fig. 1.
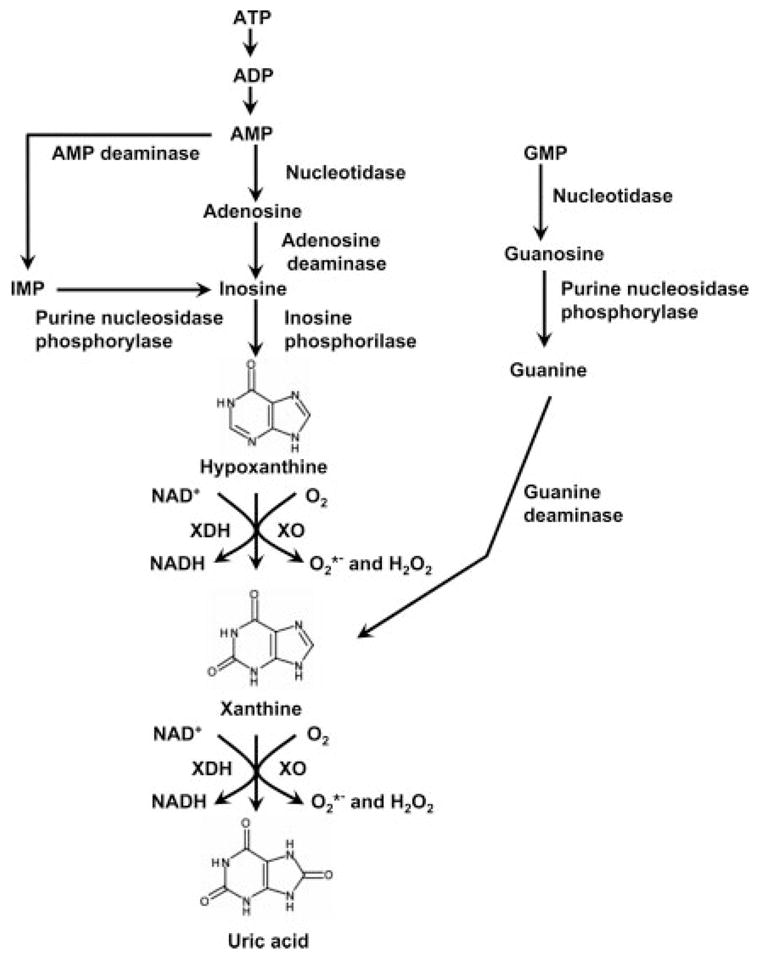
Schematic diagram of the purine degradation pathway.
An increasing number of researchers during the past decade have also suggested that XO plays and important role in various forms of ischemic and other types of tissue and vascular injuries, inflammatory diseases, and chronic heart failure (reviewed in Harrison, 2002, 2004; Berry and Hare, 2004) (Tables 1–4). Allopurinol and its active metabolite oxypurinol showed beneficial effects in the treatment of these conditions both in experimental animal models and in small-scale human clinical trials. Although some of the beneficial effects of these compounds go beyond inhibition of XO, these studies generated renewed interest in the development of additional, novel series of XO inhibitors for various therapeutic indications (overview in Borges et al., 2002). The goal of this article is to give a critical overview of the effects of XO inhibitors in various forms of tissue injury and also to review the novel emerging therapeutic strategies offered by this promising approach.
TABLE 1.
Effects of XO inhibitors in myocardial ischemia-reperfusion injury
| Model | Disease or Trigger | Mode of XO Inhibition | Effects of XO Inhibition | Reference |
|---|---|---|---|---|
| Rat | Myocardial infarction- induced by isoproterenol | Allopurinol | Reduced myocardial damage | Wexler and McMurthy (1981) |
| Rat | Myocardial I/R | Allopurinol | Decreased reperfusion-induced arrhythmias | Manning et al. (1984) |
| Rat heart | Myocardial I/R | Oxypurinol | Reduction of myocardial damage and decrease of myocardial vascular resistance | Badylak et al. (1987) |
| Rat heart | Hypothermic cardioplegia, myocardial I/R | Allopurinol | Improved cardiac function | Bergsland et al. (1987) |
| Rat | Myocardial I/R | Allopurinol | Reduction of myocardial infarct size | Montor et al. (1987) |
| Rat/rabbit heart | Myocardial I | Allopurinol | Improved postischemic function in rats but not in rabbits | Grum et al. (1987) |
| Rat heart | Myocardial I/R | Allopurinol | Improved function during reperfusion and higher ATP levels | Lasley et al. (1988) |
| Rat heart | Myocardial I/R | Allopurinol | Better ventricular function after I/R | Brown et al. (1988) |
| Rat heart | Myocardial I/R | Allopurinol | Better recovery of ventricular function and decreased incidence and duration of reperfusion-induced arrhythmias without effects on recovery of ATP, creatine and inorganic phosphates, and H+ | Headrick et al. (1990) |
| Rat | Myocardial I | Allopurinol | No effect on the infarct size and failure to improve cardiac function | Boucher and de Leiris (1991)* |
| Rat heart | Myocardial I/R | Allopurinol | Improved postischemic recovery of cardiac function, but this result is attributed to an unrelated effect to XO inhibition | Chambers et al. (1992) |
| Rat heart | Myocardial I/R | Allopurinol | Increased XO activity during I/R in interstitial cells, coronary vessel endothelium, and smooth muscle cells detected by immunocytochemistry, which was reduced by allopurinol | Ashraf and Samra (1993) |
| Rat heart | Myocardial I/R | Allopurinol | Intermittent infusion of allopurinol during global myocardial ischemia resulted in improved myocardial functional recovery and improved preservation of high-energy phosphates | Sakakibara (1993) |
| Rat heart | Myocardial I/R | Allopurinol | Reduced the incidence and severity of reperfusion arrhythmias and increased the tissue ascorbate levels | Yang et al. (1995) |
| Rat heart, right ventricular trabeculae | Myocardial I/R | Allopurinol, Oxypurinol | Ca2+-sensitizing effect of allopurinol and oxypurinol underlying the preservation of contractility in a model of stunned myocardium | Perez et al. (1998) |
| Rat heart | Myocardial I/R | Allopurinol | Reduced accumulation of mRNA for heat shock proteins (HSP70 and HSP90) after repetitive ischemia/reperfusion | Nishizawa et al. (1999) |
| Rat heart | Myocardial I/R | Allopurinol | Allopurinol significantly inhibited myocardial xanthine oxidase activity, improved left ventricular dysfunction after ischemia, and decreased myocardial lipid peroxidation and superoxide formation | King et al (1998)* |
| Guinea pig isolated right ventricular segment | Myocardial I/R | Allopurinol | Reduced the incidence and severity of reperfusion arrhythmias | Li and Ferrier (1992) |
| Rabbit | Myocardial I/R | Allopurinol | Decreased reperfusion-induced arrhythmias and protected against ultrastructural damage | Godin et al. (1986) |
| Rabbit | Myocardial I/R | Allopurinol | Protection of the I/R myocardium to t- butylhydroperoxide induced glutathione depletion and production of thiobarbituric acid reactive substances, which was not associated with any significant alterations in tissue ATP levels or in the activities of the myocardial antioxidant enzymes catalase, Cu,Zn-superoxide dismutase, or glutathione peroxidase, suggesting that allopurinol may exert its effects by direct radical scavenging or by some other mechanism unrelated to xanthine oxidase inhibition. | Godin and Garnett (1989) |
| Rabbit | Myocardial I/R | Allopurinol | Better preservation of cellular ATP levels and mitochondrial ATP generation during ischemia and prevention of the decrease in left ventricular pressure, sodium and calcium accumulation, and decreases in sarcolemmal Na+,K+-stimulated and sarcoplasmic reticulum K+,Ca2+-stimulated ATPase activities | Godin and Bhimji (1987) |
| Rabbit heart | Hypothermic cardioplegia, myocardial I/R | Allopurinol | Improved cardiac function | Myers et al. (1986) |
| Rabbit heart | Myocardial I/R | Allopurinol | No detectable XO activity and reduction of the infarct size | Downey et al. (1987)* |
| Rabbit heart | Myocardial I/R | Allopurinol | Decreased XO activity and improved ventricular developed pressure, peak systolic pressure, and coronary flow | Terada et al. (1991) |
| Dog | Myocardial I | Allopurinol | No reduction of the infarct size | Shatney et al. (1976)* |
| Dog | Myocardial I/R | Allopurinol | Reduction of infarct size | Chambers et al. (1985) |
| Dog | Myocardial I | Allopurinol | Reduction of infarct size | Akizuki et al. (1985) |
| Dog | Myocardial I/R | Allopurinol | No reduction of the infarct size | Reimer and Jennings (1985)* |
| Dog | Hypothermic cardioplegia, myocardial I/R | Allopurinol | Improved left ventricular function | Stewart et al. (1985) |
| Dog | Myocardial I/R | Allopurinol | Reduction of infarct size | Werns et al. (1986) |
| Dog | Myocardial I/R | Allopurinol | Better recovery of systolic contractile function following reperfusion | Charlat et al. (1987) |
| Dog | Myocardial I/R | Allopurinol | No decrease in I/R-induced arrhythmias | Parratt and Wainwright, (1987)* |
| Dog | Myocardial I | Allopurinol | Reduction of infarct size | Kingma et al. (1987, 1989) |
| Dog | Myocardial I/R | Oxypurinol | Improved regional ventricular function after reperfusion, but failed to reduce infarct size | Puett et al. (1987) |
| Dog | Myocardial I | Allopurinol | Delayed but did not prevent infarction in the permanent occlusion model | Miura et al. (1988) |
| Dog | Myocardial I/R | Oxypurinol | No reduction of the infarct size | Kinsman et al. (1988)* |
| Dog | Myocardial I | Allopurinol | No reduction of the infarct size if allopurinol was given 1 min postocclusion | Kingma et al. (1988)* |
| Dog | Myocardial I/R | Allopurinol, Oxypurinol | Oxypurinol but not allopurinol given before reperfusion reduced the infarct size but not ventricular arrhythmias | Matsuki et al. (1990) |
| Dog | Myocardial I/R | Oxypurinol, Amflutizole | Xanthine oxidase inhibition was demonstrated in each of the drug treatment groups, but only oxypurinol limited the extent of myocardial injury | Werns et al. (1991) |
| Dog | Myocardial I/R | Allopurinol | Reduction of infarct size | Motoe and Yoshida (1991) |
| Dog | Myocardial I/R | Allopurinol | Improved endothelium-dependent coronary vascular relaxation in vitro | Sobey et al. (1992) |
| Dog | Myocardial I/R | Allopurinol | Improved contractility, decreased lipid peroxidation | Konya et al. (1993) |
| Dog | Myocardial I/R | Allopurinol | Improved endothelium-dependent coronary vascular relaxation in vivo | Sobey et al. (1993) |
| Dog | Myocardial I/R | Allopurinol | Inhibition of XO activity by allopurinol resulted in a dose-dependent increase in cardiac interstitial fluid hypoxanthine and xanthine levels and a decrease in uric acid | Kuzmin et al. (1995) |
| Newborn lamb | Low O2 ventilation and blood volume reduction-induced hypoxic injury | Allopurinol | Allopurinol exerted a beneficial effect on the pump function by afterload reduction but not by changes in contractility and also inhibited uric acid formation with a consequent decrease in antioxidative capacity | Shahid et al. (1999) |
| Pig heart | Myocardial I/R | Allopurinol, Oxypurinol | Decreased reperfusion injury, free radical scavenging effect | Das et al. (1987) |
| Pig heart and lung | I/R associated with heart-lung transplantation | Allopurinol | Better recovery of cardiac and pulmonary function and generalized alterations in tissue antioxidant status | Qayumi et al. (1993) |
| Pig | Myocardial I/R | Allopurinol | Better recovery of cardiac function and decreased propensity of reperfusion arrhythmias | Hopson et al. (1995) |
| Human (169 patients) | Coronary bypass surgery | Allopurinol | Decreased hospital mortality rate, increased cardiac index | Johnson et al. (1991) |
| Human (90 patients) | Coronary bypass surgery | Allopurinol | Reduced arrhythmias, need for inotropes and perioperative myocardial infarction in patients | Rashid and William- Olsson (1991) |
| Human (140 patients) | Myocardial I | Allopurinol | Increased incidence of infarct extensions in the treatment group | Parmley et al. (1992)* |
| Human (80 patients) | Ischemic heart disease | Allopurinol + erinit | Decreases in serum and daily urinary levels of uric acid and lipid peroxidation antioxidative system and an improvement of central hemodynamics | Kaliakin and Mit’kin (1993) |
| Human (50 patients) | Coronary bypass surgery | Allopurinol | Improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass grafting | Coghlan et al. (1994) |
| Human (20 patients) | Coronary bypass surgery | Allopurinol | Failed to demonstrate a cardioprotective effect of allopurinol in patients with good left ventricular function undergoing elective coronary artery surgery | Taggart et al. (1994)* |
| Human (20 patients) | Coronary bypass surgery | Allopurinol | Allopurinol suppressed the reaction rate of xanthine oxidase; therefore, the levels of intermediates, hypoxanthine and xanthine, were high, and the level of the final product, uric acid, was low—however, allopurinol had no efficacy for the level of lactate, pyruvate, CK, and CK-MB | Yamazaki et al. (1995)* |
| Human (33 patients) | Coronary bypass surgery | Allopurinol | Better recovery of cardiac output and left ventricular stroke work after bypass surgery and reduction of plasma XO activity and concentrations of uric acid | Castelli et al. (1995) |
| Human (52 patients) | Coronary bypass surgery | Allopurinol | Allopurinol failed to improve left ventricular stroke work after cardiopulmonary bypass surgery | Coetzee et al. (1996)* |
| Human (38 patients) | Percutaneous transluminal coronary angioplasty in patients with acute myocardial infarction | Allopurinol | Allopurinol pretreatment was effective in inhibiting generation of oxygen-derived radicals during reperfusion and in the recovery of left ventricular function | Guan et al. (2003) |
I/R, ischemia-reperfusion; I, ischemia; CK, creatine kinase.
Studies concluded with negative results.
TABLE 4.
Effects of XO inhibitors on vascular dysfunction associated with hypercholesterolemia, atherosclerosis, hypertension, diabetes, and chronic heart failure
| Model | Disease or Trigger | Mode of XO Inhibition | Effects of XO Inhibition | Reference |
|---|---|---|---|---|
| Rabbit | Hypercholesterolemia | Oxypurinol | Decreased vascular free radical production | Ohara et al. (1993); Mugge et al. (1994); White et al. (1996) |
| Human (60 patients) | Hypercholesterolemia and hypertension | Oxypurinol | Oxypurinol improved in vivo endothelial-dependent vasorelaxation in hypercholesterolemic but not hypertensive patients | Cardillo et al. (1997) |
| Rat | HHcy-induced by methhionine feeding | Oxypurinol | Impaired flow-mediated vascular responses of HHcy arterioles were prevented by oxypurinol | Bagi et al. (2002) |
| Rat | SHRs | N.A. | Increased XO activity in the hearts of hypertensive rats | Janssen et al. (1993) |
| Rat | SHRs | Tungsten | Increased XO activities in the mesenteric tissues of SHRs, and normalization of endothelial function and blood pressure in these animals after inhibition of XO activity by tungsten | Suzuki et al. (1998) |
| Rat | SHRs | Oxypurinol | Significantly higher uric acid levels in SHRs than in normal rats; oxypurinol decreased the blood pressure in SHRs but not in normal rats | Nakazono et al. (1991) |
| Rat | Dahl salt-sensitive hypertensive rats | Tungsten | Tungsten-rich diet lowered blood pressure in Dahl salt-sensitive hypertensive rats but not in salt-resistant rats | Swei et al. (1999) |
| Rat | SHRs | Allopurinol | Renal XO activity was increased and correlated with systolic blood pressure during growth in SHRs but not in WKY rats; allopurinol had a negligible effect on blood pressure but prevented hypertension-induced left ventricular and renal hypertrophy in SHRs | Laakso et al. (1998, 2004) |
| Rat | Oxonic acid-induced hypertension | Allopurinol | Allopurinol or uricosuric agent (benziodarone) prevented uricase inhibitor (oxonic acid)-induced hypertension | Mazzali et al. (2001) |
| Rat | Hypertensive double-transgenic rats harboring human renin and angiotensinogen genes (dTGR) | Oxypurinol | Preincubation with oxypurinol improved impaired endothelium-dependent vascular relaxation in rats with elevated angiotensin II levels | Mervaala et al. (2001) |
| Rat | Dexamethasone-induced hypertension | Allopurinol | Allopurinol reduced blood pressure in hypertensive rats and attenuated the increased XO levels in cremaster muscle | Wallwork et al. (2003) |
| Human (19 and 11 patients) | CHF | Allopurinol | Allopurinol improved endothelial function | Doehner et al. (2002); Farquharson et al. (2002) |
| Human (24 patients) | CHF | N.A. | Endothelium-bound xanthine-oxidase activity was increased by >200% in patients with CHF and inversely correlated with endothelium-dependent vasodilation | Landmesser et al. (2002) |
| Human (32 patients) | Hyperuricemia-induced | Allopurinol | 3-month therapy with allopurinol improved impaired endothelium-dependent flow-mediated dilation in hyperuricemic subjects | Mercuro et al. (2004) |
N.A., not available; HHcy, hyperhomocysteinemia; WKY, Wistar-Kyoto.
II. The Structure and Molecular Biochemistry of Xanthine Oxidoreductase
Xanthine oxidase (EC 1.1.3.22) and xanthine dehydrogenase (XDH) (EC 1.17.1.4) are interconvertible forms of the same enzyme, known as xanthine oxidoreductase (XOR). The enzymes are molybdopterin-containing flavoproteins that consist of two identical subunits of approximately 145 kDa. The enzyme from mammalian sources, including man, is synthesized as the dehydrogenase form, but it can be readily converted to the oxidase form by oxidation of sulfhydryl residues or by proteolysis. Mechanistic studies of XO and related work on the electron transfer processes has been reviewed (Hille, 1996; Harrison, 2002, 2004); the latter topic also remains a very active area of investigation (Canne et al., 1999; Caldeira et al., 2000; Eger et al., 2000; Enroth et al., 2000; Truglio et al., 2002; Kuwabara et al., 2003; Okamoto et al., 2004). A majority of the recent reaction pathways for XO catalytic transformations are based on the crystal structure of the active site of aldehyde oxidoreductase, as determined by Huber and coworkers (Romao et al., 1995). Both enzymes belong to a molybdenum hydroxylase family as mentioned above, are structurally similar, and have identical X-ray absorption spectra indicative of the same ligand environment and coordination geometry around molybdenum center. The crystal structures of XOR in the two forms, dehydrogenase and oxidase, have been solved after successful crystallization of both forms of the enzyme, to clarify the structure-based mechanism of conversion (Eger et al., 2000; Enroth et al., 2000; Truglio et al., 2002; Kuwabara et al., 2003; Okamoto et al., 2004; Godber et al., 2005) (Fig. 2). The active form of the enzyme is a homodimer of molecular mass 290 kDa, with each of the monomers acting independently in catalysis. Each subunit molecule is composed of an N-terminal 20-kDa domain containing two iron sulfur centers, a central 40-kDa FAD domain, and a C-terminal 85-kDa molybdopterin-binding domain with the four redox centers aligned in an almost linear fashion (Fig. 2). The hydroxylation of xanthine takes place at the molybdopterin center, and the electrons thus introduced are rapidly transferred to the other linearly aligned redox centers. For a more detailed overview of the structure and function of XOR see Harrison (2002, 2004).
Fig. 2.
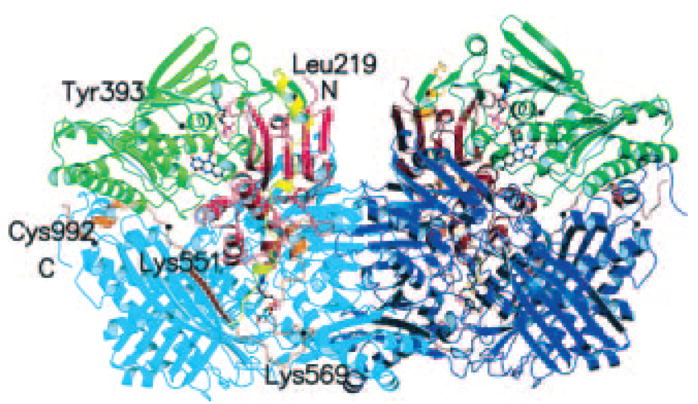
Crystal structure of the xanthine dehydrogenase dimer divided into the three major domains and two connecting loops. The two monomers have symmetry related domains in the same colors, in lighter shades for the monomer on the left and in darker shades for the monomer on the right. From the N to the C terminus, the domains are the iron/sulfur-center domain (residues 3–165; red), the FAD domain (residues 226–531; green), and the molybdopterin center (Mo-pt) domain (residues 590–1331; blue). The loop connecting the iron/sulfur domain with the FAD domain (residues 192–225) is shown in yellow, the one connecting the FAD domain with the Mo-pt domain (residues 537–589) is in brown, and the N and C termini are labeled. The FAD cofactor, the two iron/sulfur centers, the molybdopterin cofactor, and the salicylate also are included (Enroth et al., 2000). Copyright © 2000 National Academy of Sciences (Washington, DC). Reproduced with permission.
XOR is widely distributed throughout various organs including the liver, gut, lung, kidney, heart, and brain as well as the plasma. It is generally accepted that the enzyme normally is present in vivo as an NAD-dependent cytosolic dehydrogenase (XDH), incapable of reactive oxygen species production. Most investigators agree that XDH activity converts by sulfhydryl oxidation or limited proteolysis to an oxidase that produces superoxide and hydrogen peroxide. It is worthwhile to note, nevertheless, that both XO and XDH can oxidize NADH, with the concomitant formation of reactive oxygen species (Zhang et al., 1998a;b; Sanders and Massey, 1999). Physiologically, XO and XDH participate in a variety of biochemical reactions including the hydroxylation of various purines, pterins, and aromatic heterocycles, as well as aliphatic and aromatic aldehydes, thereby contributing to the detoxification or activation of endogenous compounds and xenobiotics. One of XOR’s primary roles is the conversion of hypoxanthine to xanthine and xanthine to uric acid (see Fig. 1). Inherited XOR deficiency leads to xanthinuria and a characteristic multiple organ failure syndrome characterized by the deposition of xanthine in various tissues. The detailed biochemistry of XO and XDH conversion of XDH to XO has been subject to several reviews and research papers (Nishino, 1994; Nishino et al., 1997, 2005; Pritsos, 2000; Borges et al., 2002; Ilich and Hille, 2002; Mc-Manaman and Bain, 2002; Meneshian and Bulkley, 2002). It is interesting to note that recent work has shown that XO is regulated on the transcriptional and post-transcriptional levels (Hoidal et al., 1997; Terada et al., 1997; Hassoun et al., 1998; Page et al., 1998; Xu et al., 2000; Ghio et al., 2002). For instance, XO is phosphorylated in hypoxic endothelial cells via p38 MAP kinase and casein kinase II, and this process may be necessary for the activation of the enzyme during hypoxia (Kayyali et al., 1998). In a recent study McNally et al. (2005) demonstrated that the endothelial xanthine oxidoreductase protein expression is regulated by hydrogen peroxide and calcium.
In the mid-1970s, it was observed that various hepatotoxic agents, such as halothane and alcohol, induce the systemic release of XO from the liver (Giler et al., 1976, 1977; Ghio et al., 1977; Zima et al., 1993). Initially, it was considered that circulating XO could be used as a sensitive marker to quantitate liver injury. Subsequent work by Freeman and colleagues demonstrated that circulating XO is not only a marker of hepatic and intestinal damage, but it can also act as a circulating mediator that is responsible for remote organ injury in a variety of pathophysiological conditions including hepatic ischemia and reperfusion, hemorrhagic shock, atherosclerosis, and sickle cell disease (Yokoyama et al., 1990; Tan et al., 1993b, 1995b, 1998; White et al., 1996; Radi et al., 1997; Houston et al., 1999; Aslan et al., 2001). Circulating XO activity has also been detected by other groups during thoracoabdominal surgery, intestinal ischemia-reperfusion, skin burn, liver transplantation, and hind limb ischemia and reperfusion (Terada et al., 1992; Poggetti et al., 1992; Nielsen et al., 1994; Burton et al., 1995; Pesonen et al., 1998). Circulating XO has also been demonstrated in human ischemia-reperfusion injury, during an aortic cross-clamp procedure (Tan et al., 1995). It appears that circulating XO binds to glycosaminoglycans on the surface of endothelial cells, where it acquires somewhat modified kinetic characteristics (Radi et al., 1997). Endothelial cell-bound XO continues to produce oxidants, which can trigger endothelial dysfunction, and thereby contribute to organ injury in remote organs such as the lung (Radi et al., 1997). This circulating and depositing XO appears to be more important in the pathogenesis of endothelial injury compared with the XO constitutively contained in the endothelial cells, which appears to be quite low (Panus et al., 1992; Radi et al., 1997).
It is important to note that many differences exist between the XDH/XO system of various experimental animals and the XDH/XO system present in humans (Sarnesto et al., 1996; Kinnula et al., 1997; Rouquette et al., 1998; Linder et al., 1999; Pritsos,2000). Nevertheless, both the conversion of XDH to XO, and the presence of circulating XO have been confirmed in human studies. It will be important, throughout the current article, to keep in mind that findings obtained in experimental models do not always or necessarily transfer to the human conditions (see also below, for examples of specific disease conditions).
III. Xanthine Oxidase-Derived Superoxide as Part of a Complex Oxidant and Antioxidant System
XO-derived superoxide exerts its actions in the overall context of various endogenous oxidant and antioxidant systems. For example, nitric oxide (NO) can act as an endogenous suppressor of XO activity (Rinaldo et al., 1994; Fukahori et al., 1994; Hassoun et al., 1995; Ichimori et al., 1999; Godber et al., 2000; Kinugawa et al., 2005; see also section V.A. for more details). Because in many pathophysiological conditions there is an impairment of endogenous NO production (e.g., atherosclerosis reduces endothelial NO production), a reduced level of the tonic, NO-mediated suppression of XO may actually lead to increased superoxide generation and pathophysiological positive feed-forward cycles. At the same time, there may also be an XO-derived superoxide dependent tonic suppression of NO synthase activity, which may lead to an enhancement of NO production in response to XO inhibition in vivo (Terada et al., 1997a,b).
There is some evidence that XO can catalyze the reduction of nitrite and nitrate (normally the decomposition products of NO) back to NO (Millar et al., 1998; Zhang et al., 1998b; Godber et al., 2000; Millar et al., 2002). These activities are more prominent under acidic conditions and may contribute to a pathophysiological enhancement of NO generation in ischemic or hypoxic tissues.
The time course of XO-derived superoxide production may also depend on the nature of the chemical environment. For instance, there is some in vitro evidence that oxidative stress can inactivate the activity of XO (Anderson et al., 1995; Houston et al., 1998), but the significance of this mechanism (if any) in vivo is unclear.
XO-derived superoxide can rapidly react with NO or nitrosothiols in its vicinity to form the cytotoxic oxidant species peroxynitrite (Trujillo et al., 1998), which can lead to a feedback inhibition of the enzyme (Houston et al., 1998; Godber et al., 2001), as well as to a variety of oxidative and nitrosative injury to proteins, lipids, and DNA in the vicinity of XO (Trujillo et al., 1998; Sawa et al., 2000). It is conceivable that after binding of circulating XO to the endothelium, XO-derived superoxide can combine with endothelially derived NO (produced by the endothelial NO synthase), and the subsequent formation of peroxynitrite and the activation of downstream pathways of cell injury can lead to endothelial and tissue injury in various pathophysiological conditions (White et al., 1996; Radi et al., 1997; Pesonen et al., 1998; Aslan et al., 2001; Desco et al., 2002; Szabó et al., 2002a,b, 2004; Pacher et al., 2002a,b; 2003a,b; 2005a;b; Obrosova et al., 2005; Ungvári et al., 2005).
We must also put uric acid into our equations: this product of XO is a known antioxidant that can neutralize certain reactivities of superoxide and peroxynitrite (Ames et al., 1981; Kooy et al., 1994). In fact, uric acid and its precursors have been used in protecting against the oxidative neuronal damage in experimental allergic encephalomyelitis, a model of multiple sclerosis, and, in fact, patients with gout have a markedly reduced likelihood of developing multiple sclerosis (Hooper et al., 1998). Thus, in some pathophysiological conditions, a paradoxical adverse effect of XO inhibition, via reduction of plasma uric acid levels, must also be considered. It is noteworthy that uric acid can actually inhibit XO and thereby act as a feedback inhibitor of the enzyme, although its inhibitory effect was associated with increased superoxide formation (Radi et al., 1992; Tan et al., 1993a). Uric acid can also act as a chelator of iron in extracellular fluids, and this action has been shown to regulate the expression of XO in the lung (Ghio et al., 2002).
From the above data, it appears that one must view XO-derived superoxide generation in the overall complexity of the cellular environment. It is not easy to delineate the relevance of some of these reactions in vivo, but we can assume that some of the above-described interactions and pathways may be relevant in various disease conditions. Overall, it is safe to conclude that inhibition of XO, by reducing superoxide production, is beneficial in most pathophysiological states (see also below). However, the relative contribution of XO-derived superoxide formation (as opposed to other cellular and extracellular sources of superoxide) is unclear and, again, may be dependent on many factors, including the species, the cell type, the tissue, and the type and stage of the particular disease (reviewed in Berry and Hare, 2004; Griendling, 2004; Brandes and Kreuzer, 2005).
IV. Xanthine Oxidase Inhibitors
A. Allopurinol and Oxypurinol
As noted in the Introduction, allopurinol (1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) (Fig. 3) was initially synthesized as an attempt to produce new antineoplastic agents in the mid-1950s by Falco, but it was found to have inhibitory activity on XO, reducing both urinary and serum uric acid levels [Elion, 1988 (http://nobelprize.org/medicine/laureates/1988/elion-lecture.pdf), 1993]. Allopurinol was approved by Food and Drug Administration in 1966 for treatment of gout and remains a cornerstone in the therapy of primary and secondary hyperuricemia (Rott and Agudelo, 2003; Terkeltaub, 2003; Bieber and Terkeltaub, 2004; Schlesinger, 2004; Pea, 2005; Wortmann, 2005). Allopurinol is rapidly oxidized by XO in vivo to its active metabolite oxypurinol (both isosteres of hypoxanthine and xanthine, respectively), which also inhibits XO. At low concentrations, allopurinol is a substrate for and competitive inhibitor of the enzyme; at higher concentrations, it is a noncompetitive inhibitor. Oxypurinol is a noncompetitive inhibitor of the enzyme; the formation of this compound, together with its long persistence in tissues, is responsible for much of the pharmacological activity of allopurinol.
Fig. 3.
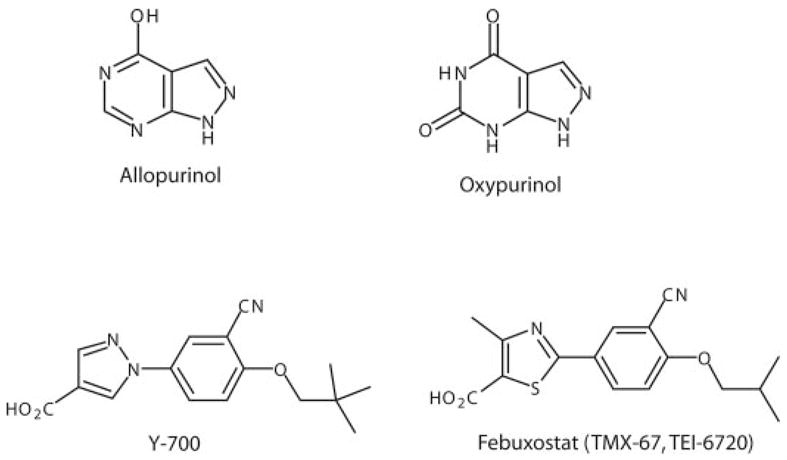
Chemical structures of selected xanthine oxidase inhibitors.
In terms of pharmacokinetics, allopurinol is rapidly absorbed, reaching peak plasma concentrations within 30 to 60 min, following oral administration. Oxypurinol has lower oral bioavailability than allopurinol. Allopurinol has relatively short half-life in plasma (2–3 h), whereas the half-life of oxypurinol is much longer (14–30 h) due to renal reabsorption (Pea, 2005).
The most common adverse effects of allopurinol are gastrointestinal distress, hypersensitivity reactions, and skin rash. The hypersensitivity reaction may occur even after months or years of medication. These effects generally occur in individuals with decreased renal functions, for whom the dosage of allopurinol was not reduced. Allopurinol may increase the effects of cyclo-phosphamide and inhibits the metabolism of oral coagulants and probenecid. Symptoms of allopurinol toxicity include fever, rash, vasculitis, eosinophilia, and worsening of renal function, which can lead to a fatal outcome especially in elderly patients with renal insufficiency taking thiazide diuretics (Rott and Agudelo, 2003; Terkeltaub, 2003; Bieber and Terkeltaub, 2004; Schlesinger, 2004; Pea, 2005; see also section V.A.).
By lowering the uric acid concentration in plasma below its limit of solubility, allopurinol facilitates the dissolution of tophi and prevents the development and progression of chronic gouty arthritis (Rott and Agudelo, 2003; Terkeltaub, 2003; Bieber and Terkeltaub, 2004; Schlesinger, 2004; Pea, 2005). The formation of uric acid stones gradually disappears with therapy, and this prevents the development of nephropathy. In addition to the gout and hyperuricemia, there are numerous potential therapeutic applications for allopurinol and oxypurinol in various forms of ischemic and other types of tissue and vascular injuries, inflammatory diseases, and chronic heart failure (see section V. and Tables 1–4).
B. Novel Xanthine Oxidase Inhibitors
Although allopurinol is a very efficient drug, it is a relatively weak XO inhibitor in in vitro assays, with reported IC50 values of 0.2 to 50 μM. Ironically, for many contemporary drug discovery programs, this would have probably been well above the threshold numbers for a lead to qualify even for early selection rounds.
The early search for novel XO inhibitors, fueled by the success of allopurinol, focused on synthetic purine and pyrimidine derivatives. However, the drug structural framework based on the purine and pyrimidine motifs is responsible for some side effect caused by allopurinol, i.e., rashes, which are sometimes severe (occurring in 2–8% patients). The rashes result from the metabolic conversion of the drugs to corresponding nucleotides through the action of phosphoribosyl transferase. This prompted a search for new XO inhibitors that are structurally distinct from purines (reviewed in Borges et al., 2002).
A somewhat unexpected but beneficial turn occurred in the 1960s when Fridovich and his colleagues (Hodgson and Fridovich, 1973; McCord and Fridovich, 1968) elucidated the role of XO in free radical production. They later introduced an ingenious assay for generation and detection of superoxide radicals based on the XO/xanthine production of , inducing chemiluminescence of the lucigenin dye (McCord and Fridovich, 1969). As the quest for medicinal free radical scavengers intensified, this assay was routinely used for screening of the free radical quenching activity of the extracts and natural compounds derived from plants and other sources. Serendipitously, a variety of novel XO inhibitors of varying structures and activity (often low) has been discovered when the validity of the assay was verified. Some known and novel antioxidants and nonantioxidants: phenolic compounds, coumarins, flavonoids, and steroids were found to have relatively high activity and in few instances the initially discovered leads became a subject of more detailed structure/activity studies. Such promiscuity in binding preferences also extends to a wealth of substrates oxidized by XO that includes not only purine derivatives but simple aliphatic, aromatic, and heteroaromatic aldehydes as well (Xia et al., 1999).
During the past decade, definite progress has also been achieved in the understanding of the XO enzyme structure, and rational drug development approaches led to the discovery of new powerful XO inhibitors of various classes, including purine analogs, imidazole and triazole derivatives, and flavonoids among many others (reviewed in Borges et al., 2002). Two of these very potent new compounds, febuxostat [2-[3-cyano-4-(2-methylpropoxy)phenyl)-4-methylthiazole-5-carboxylic acid; TMX-67, TEI-6720] and Y-700 (Fig. 3) are reported to have a favorable toxicology profile, high bioavailability, and more potent and longer-lasting hypouricemic action than allopurinol. These new compounds are currently in human clinical trials for the treatment of hyperuricemia and gout (Okamoto et al., 2003; Yamamoto, 2003; Becker et al., 2004; Fukunari et al., 2004; Hoshide et al., 2004; Komoriya et al., 2004; Yamada et al., 2004; Hashimoto et al., 2005; Mayer et al., 2005; Takano et al., 2005).
Thus, it appears that the hegemony of allopurinol, as a drug in its own league, which essentially had no competitors in clinical use for half a century, is about to change. There are a few structural classes of compounds that are many hundreds times more potent than allopurinol in vitro (both of a purine and nonpurine types). Several drug candidates are either in the development phase or are moving toward clinical testing (Borges et al., 2002; Naito et al., 2000; Okamoto et al., 2003; Nivorozhkin et al., 2003a,b; Yamamoto, 2003; Mabley et al., 2003; Becker et al., 2004; Fukunari et al., 2004; Hoshide et al., 2004; Komoriya et al., 2004; Yamada et al., 2004; Hashimoto et al., 2005; Mayer et al., 2005; Takano et al., 2005). It was clearly established that a xanthine-like structural framework is not a prerequisite for high inhibitory activity. Several recent reports on crystal structure of the different forms of XO, also with bound inhibitors, are indicative of the fact that crystallization techniques have advanced enough to help characterize in the near future protein binding of any lead compound of interest.
V. Therapeutic Areas Relevant for Xanthine Oxidase Inhibitors
A. Xanthine Oxidase Inhibitors in the Treatment of Gout and Tumor Lysis Syndrome
As already mentioned in the previous section, currently, the main indication for XO inhibitors is the treatment of hyperuricemia and gout. Gout is a common disease with prevalence of >2% in men older than 30 years and in woman older than 50 years according to the Third National Health and Nutrition Examination Survey (1988–1994) (Kramer and Curhan, 2002; Choi and Curhan, 2005). The prevalence increases with increasing age, reaching 9% in men and 6% in women older than 80 years (reviewed in Choi and Curhan, 2005). Furthermore, recent epidemiological studies indicate that the overall disease burden of gout is increasing (reviewed in Choi and Curhan, 2005). Indeed, a recent study based on a managed care population in the United States has suggested that the overall prevalence of gout or hyperuricemia increased by 80% from 1990 to 1999 (Wallace et al., 2004).
Gout occurs in individuals who have high serum uric acid levels, in response to precipitation of monosodium urate monohydate crystals in various tissues, followed by an inflammatory response. Typical symptoms of gout include acute recurrent gouty arthritis, a tophinodular collection of monosodium urate crystals and uric acid urolithiasis. The cornerstone of the prevention and treatment of gout is antihyperuricemic therapy, either by uricosuric drugs or by XO inhibitors, such as allopurinol. The area of gout therapy has been reviewed in multiple recent articles (McCarthy et al., 1991; Star and Hochberg, 1993; Terkeltaub, 1993; Davis, 1999; Pal et al., 2000; Pascual, 2000; Rott and Agudelo, 2003; Terkeltaub, 2003; Bieber and Terkeltaub, 2004; Schlesinger 2004; Pea, 2005; Wortmann, 2005), as well as in various textbooks, and is not discussed herein in detail.
An additional, important indication for the clinical use of allopurinol and possibly future XO inhibitors is tumor lysis syndrome associated with tumor chemotherapy (Maurer et al., 1988; Smalley et al., 2000). It is clear that in both indications, XO inhibition is very effective. Allopurinol, which yields its active metabolite oxypurinol in vivo, irreversibly inhibits XO. Although the therapeutic dose of allopurinol is rather high, the administration of a high dose, on its own, would not necessarily represent a problem. The problems and, therefore, the need for new, improved XO inhibitors are mainly related to the relatively high incidence of adverse effects seen with allopurinol in patients with gout. These effects include acute problems (fever and rash), as well as progressively developing leukocytosis, eosinophilia, vasculitis, aseptic meningitis, nephritis and renal dysfunction, and hepatic dysfunction (Jarzobski et al., 1970; Boyer et al., 1977; Wolkenstein and Revuz, 1995; Parra et al., 1995; Duchene et al., 2000; Khoo and Leow, 2000; Greenberg et al., 2001). The so-called “allopurinol hypersensitivity syndrome” can sometimes result in fatal outcome (Arellano and Sacristan, 1993).
The alternatives to allopurinol are rather limited. Therapeutic options in those patients in whom traditional uricosuric drugs are contraindicated, ineffective, or poorly tolerated include slow oral desensitization to allopurinol and cautious administration of oxypurinol. Allopurinol desensitization is useful particularly in those for whom other treatment modalities have failed. If available, benzbromarone may be effective in patients with gout and mild-to-moderate renal insufficiency. Recombinant urate oxidase is not widely available, but it certainly has shown promise for the short-term prophylaxis and treatment of chemotherapy-associated hyperuricemia in patients with lymphoproliferative and myeloproliferative disorders (Fam, 2001).
B. Xanthine Oxidase and Ischemia-Reperfusion Injury
In the early 1980s Granger et al. (1981, 1986) demonstrated that ischemic bowel injury occurred only on reperfusion and was attenuated by superoxide dismutase. On the basis of this observation, the hypothesis was put forward that XO-derived reactive oxygen species (ROS) contribute to the ischemic injury via ATP catabolism during hypoxia and increased electron acceptor availability on reperfusion (Fig. 4). Since the introduction of the concept of ischemia-reperfusion injury (Granger et al., 1981; McCord, 1985), several lines of evidence support the role of XO-derived ROS generation and beneficial effects of XO inhibitors against ischemic damage of the heart, brain, intestine, liver, kidney, lung and other tissues were shown (see below and Tables 1–4).
Fig. 4.
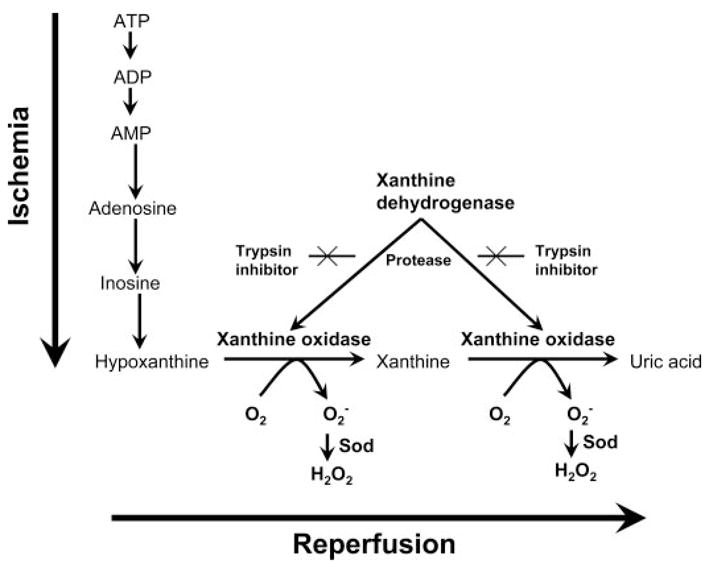
Ischemia-reperfusion injury hypothesis. During the course of ischemia, transmembrane ion gradients are dissipated, allowing cytosolic concentrations of calcium to rise, which in turn, activates protease that irreversibly converts XDH, predominant in vivo, into XO. At the same time, cellular ATP is catabolized to hypoxanthine, which accumulates. During the reperfusion, XO using readmitted oxygen and hypoxanthine generates superoxide and hydrogen peroxide. Scheme derived from Granger et al. (1981, 1986) and McCord (1985).
1. Xanthine Oxidase and Myocardial Ischemia-Reperfusion Injury
One of the longest studied and most controversial research areas in the field of XO is related to the pathogenetic role of XO in myocardial infarction. A detailed analysis of the findings in this experimental model will help illustrate the difficulties and controversies related to investigations into the pathophysiological role of XO.
Studies more than two decade ago reported that the administration of allopurinol has salutary effects on ischemic and reperfused hearts (overviews in McCord, 1985; Schrader, 1985; Berry and Hare 2004) (see also Table 1). For example, Manning et al, (1984; reviewed in Hearse et al., 1986) demonstrated that reperfusion-induced arrhythmias and infarct size are reduced by allopurinol in rats. Similar findings were reported by a variety of other authors using allopurinol or oxypurinol in diverse rat, rabbit, canine, and pig models (Wexler and McMurtry, 1981; Akizuki et al., 1985; Chambers et al., 1985; Stewart et al., 1985; Godin et al., 1986; Myers et al., 1986; Werns et al., 1986; Badylak et al., 1987; Charlat et al., 1987; Godin and Bhimji, 1987; Grum et al., 1987; Brown et al., 1988). At the same time, there were a number of groups that could not confirm the protective effect of allopurinol in various models of myocardial ischemia and reperfusion, especially in response to more severe periods of ischemia (Reimer and Jennings, 1985; Parratt and Wainwright, 1987; overview in Richard et al., 1990).
Relatively early on, the following question arose: Are the effects of allopurinol, indeed, related to the inhibition of XO, or are they related to other pharmacological actions of this agent? Another, related question that arose was the following: Is there XO or an increase in XO in the reperfused heart? With respect to the specificity of allopurinol, Das and colleagues reported in 1987 that both allopurinol and oxypurinol exerted direct free radical scavenging effects in isolated hearts, and exerted cardioprotective effects despite no detectable XO activities in the preparations used (Das et al., 1987; Downey et al., 1987; Hopson et al., 1995). These findings were in stark contrast with other studies demonstrating detectable XO activities in reperfused hearts and its inhibition by allopurinol (Chambers et al., 1985; Brown et al., 1988; Terada et al., 1991; Werns et al., 1991; Ashraf and Samra 1993). From these findings, one conclusion was clear: even if there is XO activity in the reperfused heart and even if allopurinol or oxypurinol is able to inhibit this activity, the additional, nonselective actions of these compounds [such as free radical scavenging effects as well as other nonspecific effects such as copper chelation (Malkiel et al., 1993), superoxide scavenging (Arai et al., 1998), inhibition of lipid peroxidation (Coghlan et al., 1994; Belboul et al., 2001; Kinugasa et al., 2003) and heat shock factor expression (Nishizawa et al., 1999), Ca2+-sensitizing (Perez et al., 1998), and effects on the antioxidant status of the cells (Qayumi et al., 1993; Yang et al., 1995)] will make the interpretation of the data difficult. Nevertheless, it is now generally accepted that XO is present in the heart (including human heart) (MacGowan et al., 1995; Berry and Hare, 2004), and substrate accumulation does occur during ischemia, which, ultimately, results in the production of free radicals during the reperfusion stage (Xia and Zweier, 1995).
Although the mechanism of action remained questionable (or, in the very least, multiple), follow-up work confirmed the cardioprotective effects of allopurinol and extended them to hypoxic storage and cardioplegia conditions (Bergsland et al., 1987; Vinten-Johansen et al., 1988), cardiopulmonary surgery (Coghlan et al., 1994; Castelli et al., 1995), and acute cardiac allograft rejection (Akizuki et al., 1985).
The cardioprotective effects of allopurinol are clinically exploitable (even if not all of these effects are related to XO inhibition). In a randomized trial conducted in 169 patients, allopurinol significantly decreased early hospital mortality rate after coronary bypass surgery and improved cardiac performance, scored by cardiac index and the need for inotropic or mechanical support (Johnson et al., 1991). In another study (conducted in 90 patients), allopurinol reduced arrhythmias, the need for inotropes, and perioperative myocardial infarction in patients undergoing elective coronary artery bypass grafting (Rashid and William-Olsson, 1991). Allopurinol was also found to reduce lipid peroxidation during coronary artery bypass grafting (50 patients) (Coghlan et al., 1994) and facilitated the recovery of cardiac output and left ventricular stroke work, while reducing plasma uric acid levels in 33 patients (Castelli et al., 1995). In patients with stable angina (60 patients) combination therapy with erinit and allopurinol led to a significant decrease in serum and daily urinary levels of uric acid and lipid peroxidation antioxidative system and an improvement of central hemodynamics (Kaliakin and Mit’kin, 1993). However, in the above referenced Coghlan et al. (1994) study as well as in other small studies by Taggart et al. (1994) (20 patients), Coetzee et al. (1996) (52 patients), no improvements in cardiac function were seen in the patients. Further complicating the picture, in a double-blind, randomized therapy study of 140 patients with ischemic heart disease, allopurinol surprisingly increased the incidence of myocardial infarct extension, suggesting that it may actually be contraindicated in patients with ischemic heart disease (Parmley et al., 1992).
Thus, the data are rather conflicting, with no large-scale multicenter trial data available. Clearly, larger randomized studies are required to determine the ultimate utility of allopurinol in acute myocardial reperfusion conditions.
Although allopurinol’s efficacy has not been directly proven, another area in which the agent is widely used in cardioprotection is cold storage: allopurinol is a constituent of the University of Wisconsin (UW) storage solution (Okada et al., 1995; Askenasy and Navon, 1998; Hegge et al., 2001) (see below for more detail).
2. Xanthine Oxidase and Stroke
By the analogy of the cardioprotective effects of allopurinol, the compound has also been tested in various models of ischemic and reperfused brain (Table 2). Allopurinol was found to be protective against mortality and neurological impairment in a stroke model in spontaneously hypertensive rats (Itoh et al., 1986). In a gerbil model of stroke, tungsten-mediated inhibition of XO, but not allopurinol, was shown to protect against neurological deficits (Patt et al., 1988). In a permanent middle cerebral artery occlusion model in Sprague-Dawley rats, allopurinol pretreatment reduced infarct size by approximately 35% (Martz et al., 1989). Similar to allopurinol in a rat model of transient middle cerebral artery occlusion, oxypurinol reduced the ischemic cerebral damage and attenuated the neurological deficits (Lin and Phillis, 1991, 1992; Phillis and Lin, 1991). Allopurinol was also effective in a rabbit model of focal cerebral ischemia (Akdemir et al., 2001) and in hypoxic-ischemic injury models in rat pups, as well as in newborn lambs, even if its administration was delayed to the beginning of the reperfusion period (Palmer et al., 1990, 1993; Shadid et al., 1998). Allopurinol, at relatively high doses, was also effective in transient middle cerebral occlusion models (Lindsay et al., 1991). In a relatively mild model of stroke (20 min of ischemia and 10–90 min of reperfusion), oxypurinol produced improvements in cellular ATP levels (Phillis et al., 1995). It must also be mentioned that in some studies oxypurinol was without significant protective effects (Arai et al., 1998; Nakashima et al., 1999), and the reasons for these differences between the outcome of these various studies are unclear.
TABLE 2.
Effects of XO inhibitors in cerebral, intestinal, liver, kidney and lung ischemia-reperfusion injury
| Model | Disease or Trigger | Mode of XO Inhibition | Effects of XO Inhibition | Reference |
|---|---|---|---|---|
| Cerebral I/R | ||||
| Gerbil | Temporary unilateral carotid artery occlusion | Tungsten, Allopurinol | Tungsten and allopurinol protected against neurological deficits and damage | Patt et al. (1988); Phillis and Lin (1991) |
| Gerbil | Global brain ischemia induced by the ligation of both common carotid arteries | Oxypurinol | Oxypurinol failed to protect against ischemic brain damage | Arai et al. (1998)* |
| Rat | Occlusion of bilateral common carotid arteries in SHRs | Allopurinol | Allopurinol pretreatment reduced infarct size and prevented mortality | Itoh et al. (1986) |
| Rat | Permanent middle cerebral artery occlusion | Allopurinol | Allopurinol pretreatment reduced infarct size | Martz et al. (1989) |
| Rat | Transient MCA occlusion | Oxypurinol | Oxypurinol reduced the ischemic cerebral damage, increased cellular ATP levels, and attenuated the neurological deficits | Lin and Phillis (1991, 1992); Phillis and Lin (1991); Phillis et al. (1995) |
| Rat | Transient MCA occlusion | Oxypurinol | Oxypurinol failed to show any preventive effect on the infarction in contrast to other free radical scavengers | Nakashima et al. (1999)* |
| Rat | Permanent MCA occlusion | Allopurinol | Only very high doses of allopurinol pretreatment reduced the infarct size, which was independent of XO inhibition | Lindsay et al. (1991) |
| Newborn rats and lambs | Ligation of the right common carotid artery | Allopurinol | Allopurinol reduced both cerebral edema and the extent of perinatal hypoxic-ischemic brain damage | Palmer et al. (1990, 1993); Shadid et al. (1998) |
| Rabbit | Focal cerebral ischemia | Allopurinol | Allopurinol pretreatment protected neural tissue in the early period after arterial occlusion and prevented cerebral injury in the late period and also decreased uric acid levels | Akdemir et al. (2001) |
| Human | Severely asphyxiated infants | Allopurinol | Allopurinol tended to improve survival and exerted beneficial effects on free radical formation, cerebral blood flow volume, and electrical brain activity | Van Bel et al. (1998) |
| Splanchnic I/R | ||||
| Mouse | Intestinal I/R | Allopurinol | Allopurinol reduced the colonic leukocyte infiltration, rolling and adhesion, levels of lipid peroxidation, and inflammatory chemokines | Riaz et al. (2002, 2003) |
| Rat | Intestinal ischemia or I/R | Allopurinol, tungsten | Allopurinol reduced I/R-induced neutrophil infiltration and bacterial translocation | Grisham et al. (1986); Deitsch et al. (1988); Vaughan et al. (1992) |
| Rat | Intestinal I/R | Allopurinol | Allopurinol decreased mucosal injury and reduced mortality | Megison et al. (1990) |
| Rat | Intestinal I/R | Allopurinol | Allopurinol-induced increased survival in intestinal ischemia in rats was attributed to an effect unrelated to XO inhibition | Garcia Garcia et al. (1990) |
| Rat | Intestinal I/R | Tungsten | Protection against gut reperfusion injury | Pitt et al. (1991) |
| Rat | Intestinal I/R | Allopurinol | Administration of allopurinol before reperfusion preserved intestinal motility | Hakguder et al. (2002) |
| Cat | Intestinal ischemia | Allopurinol | Allopurinol attenuated the necrosis of villus and crypt epithelium and the pathologically increased vascular permeability | Parks et al. (1982); Parks and Granger (1983); Granger et al. (1986); Nilsson et al. (1994); Kulah et al. (2004) |
| Dog | Intestinal I/R | Allopurinol | Allopurinol reduced histamine release in the reperfused gut | Boros et al. (1989) |
| Dog | Intestinal I/R | Allopurinol | Allopurinol significantly elevated the postischemic 6-keto prostaglandin 1α/thromboxane B2 ratio | Boros et al. (1991) |
| Liver I/R | ||||
| Mouse | Liver I/R | BOF-4272 | BOF-4272 attenuated hepatic damage indicated by decreased hepatic enzyme release and lipid peroxidation during hypoxia reoxygenation | Kakita et al. (2002) |
| Rat | Liver ischemia or I/R | NA | Up-regulation of XO, the conversion of XO to XDH during ischemia | Engerson et al. (1987); McKelvey et al. (1988); Frederiks and Bosch (1996) |
| Rat | Liver I/R | BOF-4272 | BOF-4272 reduced the release of chemoattractant in response to oxygen radicals reducible by Kupffer cells | Matsumura et al. (1998) |
| Rat | Liver I/R | Allopurinol | Allopurinol decreased I/R-induced lipid peroxidation and nuclear factor-κB activation | Matsui et al. (2000) |
| Rat | Liver I/R | Allopurinol | Allopurinol attenuated hepatic damage indicated by decreased hepatic enzyme release | Yildirim et al. (2002) |
| Kidney I/R | ||||
| Rat, rat kidney | Renal I/R | Allopurinol | Allopurinol improved morphology and renal function | Linas et al. (1990); Hestin and Johns (1999); Rhoden et al. (2000b) |
| Rat kidney | Repetitive renal I/R | Allopurinol | Allopurinol improved renal function after repetitive brief I/R in the isolated perfused rat kidney | Willgoss et al. (2003) |
| Dog | Renal I/R induced by transplantation | Allopurinol | Protection against kidney transplantation damage | Owens et al. (1974) |
| Lung I/R | ||||
| Rat lung | Lung I/R | Lodoxamide, allopurinol | Lodoxamide (1 mM) caused significant attenuation of postischemic lung injury in isolated rat lung model | Lynch et al. (1988); Okuda et al. (1993) |
| Rabbit lung | Lung I/R | Allopurinol | Allopurinol attenuated lung injury | Aiba et al. (1992) |
I/R, ischemia-reperfusion; MCA, middle cerebral artery.
Studies concluded with negative results.
As in the case of myocardial infarction, the multiple potential modes of allopurinol’s protective action must always be kept in mind when one interprets the results of the stroke studies. Nevertheless, in vitro studies clearly demonstrate that, at least in rodents, hypoxia and reoxygenation increase the activity of XO and XDH, possibly due to a direct action of excitatory amino acids such as kainate (Battelli et al., 1995, 1998; Sermet et al., 2000). In addition, there is some evidence that the hypoxic and reoxygenation injury to the cerebral vasculature, especially to the endothelial cells, is dependent on XO (Beetsch et al., 1998; Wakatsuki et al., 1999).
So far, the human therapeutic experience with allopurinol is limited to one study conducted in 22 severely asphyxiated infants (Van Bel et al., 1998). In this study, allopurinol tended to improve survival, and exerted beneficial effects on free radical formation, cerebral blood flow volume, and electrical brain activity. Larger follow-up studies are required to expand on these pilot findings.
Interestingly, a recent study has shown increased XO activity being partially responsible for the generation of oxygen free radicals in a rat model of traumatic brain injury, suggesting that inhibition of XO may be of potential therapeutic benefit in neurotrauma (Solaroglu et al., 2005).
3. Xanthine Oxidase and Splanchnic Ischemia-Reperfusion
Allopurinol pretreatment is protective in the ischemic and reperfused gut and beneficially affects the changes in vascular permeability (Parks et al., 1982; Parks and Granger, 1983; Granger et al., 1986; Kulah et al., 2004), neutrophil infiltration (Grisham et al., 1986; Riaz et al., 2002), bacterial translocation (Deitsch et al., 1988; Vaughan et al., 1992), intestinal inflammatory chemokine levels (Riaz et al., 2003), motility (Hakguder et al., 2002), and mortality (Megison et al., 1990). Most of the early studies are reviewed in Schoenberg and Beger (1993). XO-derived superoxide may trigger histamine release in the reperfused gut (Boros et al., 1989). Protection against gut reperfusion injury can also be achieved by inhibition of XO by tungsten (Pitt et al., 1991).
Similar to the situation in the reperfused heart, doubts have been raised with respect to the specificity of allopurinol’s action in protecting the ischemic gut (Garcia Garcia et al., 1990; Boros et al., 1991; Nilsson et al., 1994). There are also disagreements with respect to the time course and importance of the conversion of XO to xanthine dehydrogenase during the course of intestinal ischemia (Parks et al., 1988; Vatistas et al., 1998).
Other than reports demonstrating increases in human gut XO activity in response to reperfusion (Wilkins et al., 1993), the clinical experience with respect to XO, allopurinol, and gut is limited to the use of the allopurinol-containing University of Wisconsin preservation solution during colon surgery (Tesi et al., 1996; Kawashima et al., 1999).
4. Xanthine Oxidase and Ischemia-Reperfusion of Liver, Kidney, Lung, and Other Organs
There is significant experimental evidence on the role of XO in the ischemic and reperfused liver and kidney. In the liver, multiple studies demonstrated both the up-regulation of XO, the conversion of XO to XDH during ischemia (Engerson et al., 1987; McKelvey et al., 1988; Frederiks and Bosch, 1996), as well as the protective effects of allopurinol, tungsten, or BOF-4272 in terms of improved morphology and renal function or hepatic enzyme release during reperfusion (Linas et al., 1990; Saugstad, 1996; Rhoden et al., 2000a,b; Kakita et al., 2002; Yildirim et al., 2002; Willgoss et al., 2003) in most, but not all (Metzger et al., 1988), studies. XO-derived reactive oxygen species have been proposed to act as mediators of inflammatory signal transduction pathways and proinflammatory gene expression (Matsumura et al., 1998; Matsui et al., 2000). An important feature of XO release from the damaged liver is the fact that this enzyme can, in turn, act as a circulating mediator and induce remote organ injury.
Concerning reperfusion injury to the kidney, almost three decades ago, Owens et al. (1974) reported significant protection against kidney transplantation damage by allopurinol. Although the evidence in human kidney preservation and storage was less convincing (Toledo-Pereyra et al., 1977), allopurinol became a standard constituent of the widely used UW organ storage solution. Based on cold ischemic damage studies in rat kidney, allopurinol was, in fact, confirmed as an active ingredient of the UW solution (Biguzas et al., 1990; Gulian et al., 1992; Booster et al., 1994). Interestingly, in a recent study propofol (an anesthetic) attenuated liver ischemia-reperfusion injury in patients undergoing liver surgery by reducing superoxide dismutase and XO activity (Lin et al., 2004).
Allopurinol may also have beneficial effects in ischemic and reperfused kidneys in situ. Hestin and Johns (1999) found that in a rat model of kidney ischemia and reperfusion, allopurinol ameliorated the decrease in kidney hemodynamic and excretory function, at least for the initial few hours of reperfusion. In addition, Rhoden et al. (2000b) demonstrated that allopurinol suppressed the oxidative damage seen in a renal ischemia-reperfusion model in uninephrectomized rats. Pretreatment with allopurinol also improved renal function after repetitive brief ischemia-reperfusion in the isolated perfused rat kidney (Willgoss et al., 2003). In addition to the above-mentioned reports, there is good evidence for the protective effect of XO inhibition against the ischemia-reperfusion injury in the lung (Lynch et al., 1988; Aiba et al., 1992; Okuda et al., 1993) but apparently not in skeletal muscle (Dorion et al., 1993).
C. Xanthine Oxidase and Circulatory Shock
Most of the experimental experience with respect to XO, allopurinol, and circulatory shock is related to hemorrhagic shock models. This is not surprising, as hemorrhagic shock presents many parallels with various forms of ischemia-reperfusion and in fact is considered by many investigators as a form of whole-body ischemia-reperfusion. In the late 1960s and early 1970s Smith and colleagues, Lazarus and colleagues, and other groups demonstrated that allopurinol treatment protects against mortality and organ injury associated with various models of severe hemorrhagic shock (Crowell et al., 1969; Baker, 1972; Lazarus et al., 1974; Hopkins et al., 1975). Allopurinol protected against hepatic damage, reduced the degree of DNA injury, and maintained tissue high energy phosphate levels (Lazarus et al., 1974; Hopkins et al., 1975; Cunningham and Keaveny, 1978). Additional protective modes of allopurinol’s action were found to include protection against vascular injury and progressive hemodynamic decompensation (Parks et al., 1983, Allan et al., 1986; Bond et al., 1988; Flynn et al., 1997, 1999) and reduction in intestinal bacterial translocation (Deitch et al., 1988). Similar to the various forms of ischemia-reperfusion injury models (see above), a conversion of XDH to XO has been proposed to occur in hemorrhagic shock. Circulating XO appears to be involved in the generation of remote organ injury associated with hemorrhagic shock (see below).
Some experimental evidence also exists with respect to increased XO expression and limited beneficial effects of allopurinol in other forms of shock (endotoxic, septic, traumatic, anaphylactic, and burn-induced) (Parker and Smith, 1972; Shatney et al., 1980; Saez et al., 1984; McKechnie et al., 1986; Novotny et al., 1988; Lochner et al., 1989; Ahn et al., 1990; Castillo et al., 1991; Ward et al., 1992; Mainous et al., 1993; Xu et al., 1993; Takeyama et al., 1996; Cetinkale et al., 1999; Khadour et al., 2002; Wang et al., 2002).
Unfortunately, the above listed basic observations did not translate into the clinic. According to a recent randomized study by Wijnen et al. (2002), a multiantioxidant supplementation regimen (containing allopurinol, as well as vitamins E and C, mannitol, and N-acetylcysteine) failed to affect the changes in gut permeability after lower torso ischemia.
D. Xanthine Oxidase and Chronic Heart Failure
Recent work has indicated a role for XO and XO-related oxidant species in the pathogenesis of chronic heart failure (CHF) (overviews in Landmesser and Drexler, 2002; Berry and Hare, 2004; Doehner and Anker, 2005a; Kittleson and Hare, 2005; Pacher et al., 2005b; Ungvari et al., 2005) (Table 3). In vitro studies in isolated hearts have demonstrated that the progressive development of heart failure is associated with increased myocardial XO levels, which contribute to an enhancement of oxidative stress in the heart (Ferdinandy et al., 1999, 2000). In a heart failure model induced by pacing in the dog, a 4-fold increase in myocardial XO activity or levels was found, with subsequent increases in oxidative stress in the heart (Ekelund et al., 1999; Saavedra et al., 2002; Amado et al., 2005). In a ligation-induced CHF model in the rat, an approximately 50% increase was noted (de Jong et al., 2000). In patients with CHF, elevated circulating uric acid levels have been noted as well as an increase in myocardial XO activity (Leyva et al., 1998; Cappola et al., 2001) (Fig. 5). There was a strong correlation between the levels of uric acid and the severity of chronic inflammation, as evaluated by plasma measurements of soluble intercellular adhesion molecule-1, tumor necrosis factor-α, soluble tumor necrosis factor receptor 2, and E-selectin (Leyva et al., 1998).
TABLE 3.
Effects of XO inhibitors in chronic heart failure
| Model | Disease or Trigger | Mode of XO Inhibition | Effects of XO Inhibition | Reference |
|---|---|---|---|---|
| Mouse | Coronary ligation-induced HF | Allopurinol | Allopurinol doubled the survival and improved contractility and response to isoproterenol both in vivo and in isolated muscle; allopurinol also reduced the elevated XO activity in mice with heart failure | Stull et al. (2004) |
| Mouse | Coronary ligation-induced HF | Allopurinol | Allopurinol improved left ventricular contractile function, decreased ROS generation, attenuated LV cavity dilation, and reduced myocardial hypertrophy and fibrosis | Engberding et al. (2004) |
| Mouse | Transgenic model of cardiomyopathy | Allopurinol | Chronic treatment prevented myofibrillar protein oxidation and preserved cardiac function | Duncan et al. (2005) |
| Mouse | Acute heart failure | Allopurinol, oxypurinol | XO inhibitors improved ventricular function | Naumova et al. (2005) |
| Rat | Monocrotaline-induced right ventricular hypertrophy and failure model and HF- induced by coronary ligation | N.A. | Increase in myocardial XO levels | de Jong et al. (2000) |
| Rat | Coronary ligation-induced HF | Oxypurinol | Oxypurinol improved cardiac contractility and mechanoenergetic coupling in HF without affecting resting tension and intracellular Ca2+ transients | Kögler et al. (2003) |
| Rat | Coronary ligation-induced HF | Allopurinol | Both acute (5-day) and chronic (10-week) treatment with allopurinol improved LV hemodynamics; chronic treatment also prevented LV remodeling | Mellin et al. (2005) |
| Rat | Spontaneously hypertensive heart failure rat | Oxypurinol | 4-week treatment restored cardiac structure and function | Minhas et al. (2006) |
| Dog | Pacing-induced HF | Allopurinol | Allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced HF; increased myocardial XO activity or levels and subsequent increases in oxidative stress in the failing hearts | Ekelund et al. (1999); Saavedra et al. (2002) |
| Dog | Pacing-induced HR | Allopurinol | Allopurinol had no effects on LV contractile function or adrenergic responsiveness in normal conscious animals; in pacing-induced CHF, allopurinol improved LV systolic function at rest and during adrenergic stimulation and exercise | Ukai et al. (2001) |
| Dog | Pacing-induced HF | Allopurinol | Allopurinol ameliorated increases in afterload and reductions in myocardial contractility during evolving HF, thereby preserving ventricular- vascular coupling | Amado et al. (2005) |
| Human (56 patients) | CHF | N.A. | In patients with CHF, elevated circulating uric acid levels correlate with inflammatory markers (e.g., TNF-α and ICAM-1) | Leyva et al. (1998) |
| Human (9 patients) | Idiopathic dilated cardiomyopathy | Allopurinol | Intracoronary administration of allopurinol resulted in an acute improvement in myocardial efficiency by diminishing oxygen consumption in the presence of standard supportive therapy | Cappola et al. (2001) |
| Human (19 patients) | CHF | Allopurinol | Allopurinol improved endothelial function | Doehner et al. (2002); Farquharson et al. (2002) |
| Human (1760 patients) | CHF | Allopurinol | High-dose treatment with allopurinol was found to beneficially affect survival, whereas low-dose allopurinol treatment actually appeared to increase mortality | Struthers et al. (2002) |
| Human (50 patients) | CHF | Allopurinol | 3-month allopurinol treatment had no effect on excerise capacity but reduced B-type natriuretic peptide, a surrogate marker for prognosis in CHF | Gavin and Struthers (2005) |
| Human (405 patients multicenter) | CHF | Oxypurinol | Failed to show significant benefits | Cardiome Pharma Corp. (Vancouver, BC, Canada) |
HF, heart failure; N.A., not applicable; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1.
Fig. 5.
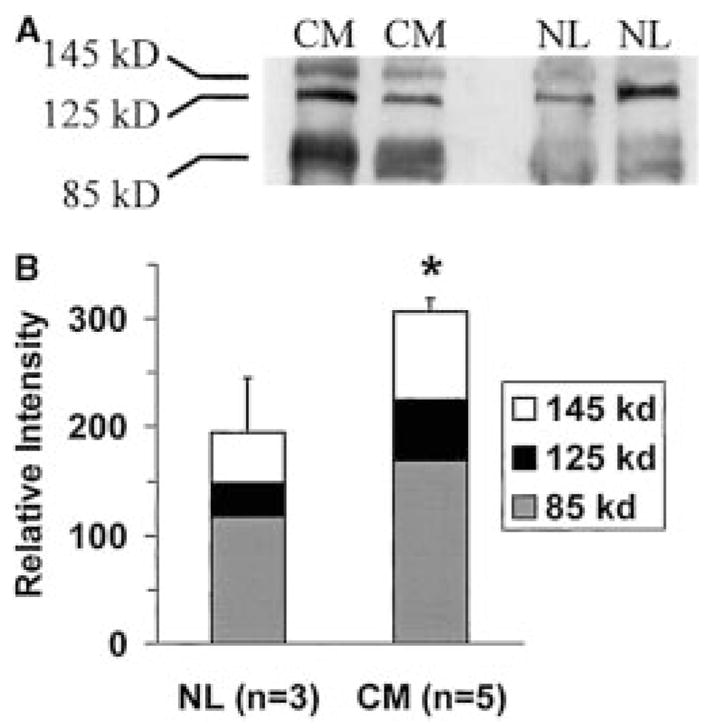
XOR is up-regulated in patients with heart failure. A, representative Western blot of myocardial extracts from patients with end-stage idiopathic dilated cardiomyopathy (CM) and patients with normal cardiac function (NL) probed with monoclonal anti-XDH antibody. Bands corresponding to both XDH (145 kDa) and XO (125 and 85 kDa). B, densitometry depicting the average XDH/XO signal from all patients. The total XDH/XO signal is increased by 60% in idiopathic dilated cardiomyopathy. *, P < 0.05 by Student’s unpaired t test. Reprinted from Cappola et al. (2001), with permission from Lippincott Williams & Wilkins (Philadelphia, PA).
The above observations triggered interventional studies with allopurinol. In a study in pacing-induced CHF in the dog, allopurinol induced a decrease in myocardial oxygen consumption and an increase in cardiac contractility and mechanical efficiency at rest (Ekelund et al., 1999) as well as during dobutamine-induced β-adrenergic stimulation and exercise (Ukai et al., 2001). In addition, allopurinol ameliorated increases in afterload and reductions in myocardial contractility during evolving heart failure, thereby preserving ventricular-vascular coupling (Amado et al., 2005). Remarkably, the benefits of allopurinol and ascorbate in dogs with heart failure could be prevented by nitric-oxide synthase (NOS) inhibition, suggesting that XO-derived superoxide may interfere with NO regulation of myocardial energetics (Saavedra et al., 2002). Because XO in failing myocardium is elevated (Ekelund et al., 1999; Ferdinandy et al., 1999, 2000; de Jong et al., 2000; Cappola et al., 2001), the normal interaction between NO and XO may be disrupted in CHF. Interestingly, in a recent study Khan et al. (2004) demonstrated that neuronal NOS (nNOS) and the superoxide-generating enzyme XOR are in physical proximity (coimmunoprecipitate and colocalize) in the sarcoplasmic reticulum of the cardiac myocytes of mice. Deficiency of neuronal NOS but not endothelial NOS was associated with profound increases in XOR-mediated superoxide production, which in turn depressed myocardial excitation-contraction coupling in a manner reversible by XOR inhibition with allopurinol (reviewed in Hare, 2003; Berry and Hare, 2004). These data suggest that nNOS exerts tonic inhibition of XOR-mediated superoxide production that protects the cardiac myocytes from contractile depression. Therefore, nNOS not only regulates the sarcoplasmic reticulum Ca2+ cycle (Khan and Hare, 2003; Khan et al., 2003), but also represents an important antioxidant system, inhibiting XOR activity (Bonaventura and Gow, 2004; Khan et al., 2004). In a recent study by Kögler et al. (2003) oxypurinol boosted the cardiac contractility and improved mechanoenergetic coupling in a rat model of heart failure. Importantly, the inotropic actions of oxypurinol were more pronounced in failing rat myocardium, a tissue that exhibits enhanced XOR activity. Furthermore, oxypurinol did not affect resting tension and intracellular Ca2+ transients; thus myocardial function is not impaired (Kögler et al., 2003), which is crucially important from the point of potential therapeutic use of oxypurinol in patients with CHF (Freudenberger et al., 2004). These and similar other preclinical studies have encouraged the clinical testing of oxypurinol for CHF. Despite high hopes for it, allopurinol had no effects on exercise capacity in CHF in 50 patients, although it reduced the B-type natriuretic peptide, an important prognostic marker of CHF (Gavin and Struthers, 2005). After encouraging preliminary results, a phase II multicenter trial evaluating the effects of oxypurinol in 405 patients with CHF failed to show significant benefit in the primary composite endpoints of the study (conducted by Cardioma Pharma Corp.).
In a recent mouse model of heart failure, allopurinol doubled the survival, decreased pathologically elevated XO activity, and improved contractility and response to isoproterenol both in vivo and in isolated cardiac muscle (Stull et al., 2004). Consistent with these results in other recent murine and rat heart failure studies (Engberding et al., 2004; Duncan et al., 2005; Mellin et al., 2005; Minhas et al., 2006; Naumova et al., 2006), investigators have demonstrated reduction of reactive oxygen species production and decreased myocardial dysfunction following allopurinol treatment. Importantly, in addition to the beneficial effect of the drug on left ventricular contractile function, allopurinol treatment also attenuated left ventricular cavity dilation and reduced myocardial hypertrophy and intestinal fibrosis (Engberding et al., 2004; Mellin et al., 2005).
In patients with idiopathic dilated cardiomyopathy, intracoronary administration of allopurinol resulted in an acute, significant improvement in myocardial efficiency by diminishing oxygen consumption in the presence of standard supportive therapy (Cappola et al., 2001). CHF also results in an impairment of peripheral vascular reactivity (possibly via circulating XO, see section V.E. and Table 4). Acute intravenous infusion of allopurinol or chronic treatment with the drug for 1 month improved endothelial function in patients, as evaluated by the measurement of acetylcholine-induced flow responses (Doehner et al., 2002; Farquharson et al., 2002). In a retrospective study in 1760 patients, high-dose treatment with allopurinol was found to beneficially affect survival, whereas low-dose allopurinol treatment actually appeared to increase mortality (Struthers et al., 2002). On the other hand, the degree of autonomic dysfunction was unaffected by allopurinol (Shehab et al., 2001). Long-term prospective evaluation of the possible benefits of allopurinol treatment in CHF is currently lacking.
Considering that the sources of reactive oxygen and nitrogen species in the failing heart are multiple (Sorescu and Griendling, 2002), it is likely that the beneficial effects of inhibition of XO can be enhanced when XO inhibitors are used as part of combination therapy approaches. It must also be kept in mind that some of the effects of allopurinol may be related to free radical scavenging independent of inhibition of XO (as discussed above) and therefore may not always be reproducible with new, more potent XO inhibitors, which may lack allopurinol’s additional antioxidant effects. Nevertheless, XO inhibitor therapy in myocardial infarction and chronic heart failure is an appealing possibility for various reasons. First, there is evidence that increased levels of uric acid strongly correlate with mortality rates in congestive heart failure (Fig. 6) (Alderman, 2002; Cicoira et al., 2002; Doehner et al., 2002; Anker et al., 2003; Alderman and Aiyer, 2004), and XO inhibitors exert certain beneficial effects both in animals and humans with heart failure (see Table 3). Second, allopurinol and its active metabolite oxypurinol are well known and relatively safe drugs that have been used for decades to treat gout. Third, the mechanism of action is unique and thus would be expected to potentiate the beneficial effects of conventional therapeutic agents (e.g., β-blockers and angiotensin-converting enzyme inhibitors).
Fig. 6.
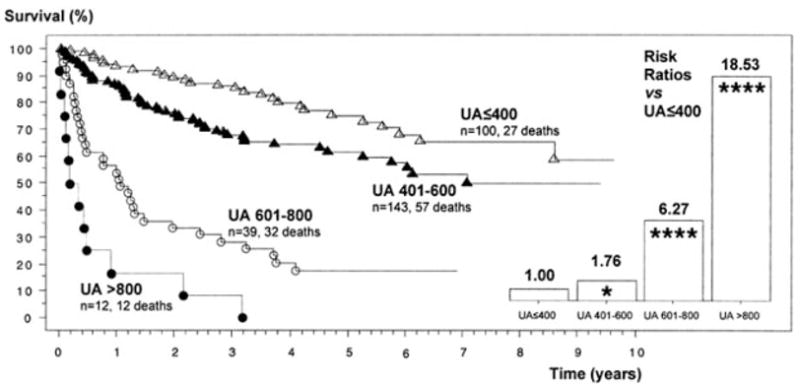
Serum uric acid (UA) levels and survival in CHF patients. A recent study in 294 chronic heart failure patients indicates a graded relationship between serum uric acid levels and survival. The plots show Kaplan-Meier survival curves and hazard ratios for different serum uric acid levels. Patients with normal UA (400 μM) had best survival (at 12 months, 93%) compared with patients with UA between 401 and 600 μM [87%, risk ratio [RR] 1.76 (1.11 to 2.78)], patients with UA between 601 and 800 μM [RR 6.27 (3.73 to 10.54)], and patients with UA >800 μM [17%, RR 18.53 (9.18 to 37.42)]. Reprinted from Anker et al. (2003), with permission from Lippincott William & Wilkins.
E. Xanthine Oxidase and Vascular Disease: Hypertension, Hypercholesterolemia, Atherosclerosis, and Diabetes
A substantial body of experimental and epidemiological evidence suggests that serum uric acid is an important, independent risk factor for cardiovascular and renal disease, especially in patients with heart failure, diabetes, and hypertension (overviews in Alderman, 2002; Alderman and Aiyer, 2004; Doehner and Anker 2005b). In patients with coronary artery disease, heart failure, or diabetes, elevated serum uric acid levels are highly predictive of mortality (Fig. 6). Although the mechanisms by which uric acid may play a pathogenetic role in cardiovascular disease are poorly understood, hyperuricemia is associated with deleterious effects on vascular function. It has recently been demonstrated that patients with hyperuricemia had impaired flow-mediated dilation, which was normalized by 3 months of therapy with the XO inhibitor allopurinol (Mercuro et al., 2004). Furthermore, uric acid was found to inhibit both basal and vascular endothelial growth factor-induced nitric oxide production in bovine endothelial cells (Khosla et al., 2005).
Endothelial dysfunction represents a predominant early feature of atherosclerosis, diabetes, hypertension, and heart failure and makes this population prone to cardiovascular complications and microthrombus formation. It has clearly been established that the endothelial dysfunction associated with these disorders is related to the local formation of reactive oxygen and nitrogen species in the vicinity of the vascular endothelium (overview in Li and Shah, 2004). There is accumulating evidence suggesting that XO-derived superoxide significantly contributes to the vascular disease in some of the above-mentioned conditions and the inhibition of XO may exert beneficial effects on impaired vascular function (overview in Berry and Hare, 2004) (see also below and Table 4).
As mentioned in the previous section, there is clinical evidence that allopurinol improves endothelium-dependent vascular function in patients with chronic heart failure (Doehner et al., 2002; Farquharson et al., 2002), in whom endothelium-bound xanthine oxidase activity is increased and inversely correlates with the endothelium-dependent vasodilation (Landmesser and Drexler, 2002). XO inhibition with allopurinol also reverses NO-dependent endothelial dysfunction in heavy smokers (Guthikonda et al., 2003, 2004). Interestingly, tobacco smoke condensate up-regulates XO and increases its activity in pulmonary endothelial cells (Kayyali et al., 2003).
XO has also been implicated in the pathogenesis of endothelial dysfunction associated with hypercholesterolemia and atherosclerosis. Oscillatory shear stress, which occurs at sites of the circulation that are vulnerable to atherosclerosis-induced superoxide production in endothelial cells, was reported to depend on XO activity (McNally et al., 2003). In the vasculature of hypercholesterolemic rabbits, oxypurinol treatment resulted in a decrease in vascular free radical production (Ohara et al., 1993; Mugge et al., 1994; White et al., 1996). XO activity in the vasculature is due to the deposition of circulating XO to sulfated glucosaminoglycans in the intimal surface (White et al., 1996). The original source of the circulating XO in hypercholesterolemia is unclear at present. Also in patients, oxypurinol treatment was found to improve the endothelium-dependent vasorelaxant responses in some (Cardillo et al., 1997) but not other (O’Driscoll et al., 1999) studies. As native and minimally oxidized low-density lipoprotein increases allopurinol-inhibitable superoxide generation in isolated vascular rings, there may be an additional role for local up-regulation of vascular (endothelial) XO levels (Stepp et al., 2002), although the findings may equally be interpretable through the direct oxidant scavenging effect of allopurinol. Interestingly, the local concentration of uric acid is elevated in atherosclerotic plaques from carotid endarterectomy specimens and XO and cholesterol are colocalized (Patetsios et al., 2001).
There is some recent evidence for the role of XO in the pathogenesis of vascular dysfunction associated with diabetes: there is an elevation in the plasma and liver XO levels in type I diabetic patients (Desco et al., 2002). In aortae from alloxan-induced diabetic rabbits, endothelial superoxide formation is increased and can be blocked by the XO inhibitor allopurinol (Desco et al., 2002). Heparin, which releases bound XO from the endothelial cells, also decreases superoxide production by aortic rings from diabetic rabbits (Desco et al., 2002). Diabetes also causes an increase of XO activity in the liver of rats, and XO is released from the liver of these animals (Desco et al., 2002). Plasma XO activity is therefore increased in diabetic mice and correlated with the degree of superoxide generation 2 weeks after the onset of diabetes and could be normalized by pretreatment with allopurinol or oxypurinol (Matsumoto et al., 2003). In type I diabetic patients allopurinol reduced the degree of oxidative stress (hemoglobin glycation, glutathione oxidation, and lipid peroxidation) (Desco et al., 2002) whereas in type II diabetic patients with mild hypertension, prolonged treatment with allopurinol resulted in significant improvements in peripheral endothelium-dependent vasorelaxant function (Butler et al., 2000). Collectively, it appears that XO plays an important role in the generation of free radicals in diabetes. Given the importance of oxidative stress in the development of diabetic complications XO inhibitors could be of significant therapeutic benefit in diabetic patients.
There is also evidence implicating XO in the pathogenesis of hypertension. In the hearts of hypertensive rats, Janssen et al. (1993) reported increased XO activities. Suzuki et al. (1998) reported increased XO activities in the mesenteric tissues of spontaneously hypertensive rats (SHRs) and normalization of endothelial function and blood pressure in these animals after inhibition of XO activity by tungsten. A tungsten-rich diet also successfully lowered blood pressure in Dahl salt-sensitive hypertensive rats but had no effect on Dahl salt-resistant rats, indicating a potential role for XO-generated ROS in salt-induced hypertension (Swei et al., 1999). Oxypurinol was also shown to lower blood pressure in SHRs but not in controls (Nakazono et al., 1991). Similarly, the mean blood pressure in a dexamethasone-induced hypertension model was also reduced by allopurinol. Furthermore, allopurinol reduced the increased XO level in cremaster muscle of dexamethasone-treated rats but not in controls (Wallwork et al., 2003). In other studies Laakso et al. (1998; 2004) demonstrated that regardless of sodium intake, renal XO activity increased 2-fold during growth in SHRs, but not in Wistar-Kyoto rats. Furthermore renal XO activity correlated with systolic blood pressure in SHRs. Allopurinol exerted negligible effects on blood pressure but prevented hypertension-induced left ventricular and renal hypertrophy in SHRs (Laakso et al., 1998, 2004).
Preincubation with oxypurinol improved the endothelium-dependent vascular relaxation in transgenic rats with elevated angiotensin II levels (Mervaala et al., 2001) and also improved the flow-mediated vascular responses in hyperhomocysteinemic rats (Bagi et al., 2002). There is evidence that elevated uric acid levels correlate with the increase in blood pressure in rats (Mazzali et al., 2001). Furthermore, elevated serum uric acid levels are associated with increased cardiovascular risk in hypertensive patients (Alderman, 1999; Alderman et al., 1999).
In mild gestational hypertension, an indirect measure of XO was increased (Nemeth et al., 2002), and in mild hypertension associated with type II diabetes, chronic treatment with allopurinol improved peripheral vascular function (Butler et al., 2000). However, in another study, endothelium-dependent relaxations of hypertensive patients were unaffected by acute oxypurinol treatment (Cardillo et al., 1997). Thus, either there appears to be no major role for XO in the pathogenesis of vascular injury in hypertensive (nondiabetic) humans or the endothelial dysfunction that develops is XO-dependent but irreversible.
Although physiological aging is known to be associated with increased oxidative stress in the vasculature (van der Loo et al., 2000; Pacher et al., 2002b; Csiszar et al., 2002), increased XO does not appear to play a major role in the elevated oxidative stress (Csiszar et al., 2002). Interestingly, a recent study has shown that in cultured vascular smooth muscle cells XO is capable of activating promatrix metalloproteinase-2 through non-free radical-dependent mechanisms (Liu et al., 2004).
In conclusion, the above data suggest that XO may play an important role in various forms of vascular dysfunction and injury, and pharmacological inhibitors of the enzyme may represent important new additions to the therapeutic arsenal to treat these conditions, if proven in larger scale clinical trials.
F. Xanthine Oxidase and Inflammatory Bowel Disease and Other Inflammatory Diseases
There is significant evidence for the pathogenetic role of XO in some (Keshavarzian et al., 1990; Siems et al., 1992; Ben-Hamida et al., 1998) but not all (Clark et al., 1988) murine experimental models of colitis and inflammatory bowel disease and duodenal ulceration. Allopurinol has been shown to be an effective addition to standard 5-aminosalicylic acid therapy in human trials (Salim, 1992; Jarnerot et al., 2000). However, in one study, the increased chemiluminescence seen in the colonic mucosa patients with colitis was not inhibitable by allopurinol coincubation in vitro (Sedghi et al., 1993), whereas in another study, no increases in colonic XO activity were demonstrated in human colonic samples (Reynolds et al., 1996). It is possible that basal levels of XO (when converted from XDH), in the presence of other sources of oxidative and nitrosative stress in colitis, contribute to the development of the disease, even in the absence of a clear up-regulation of XO in patients. Alternatively, it is also possible that the beneficial effects of allopurinol in colitis are unrelated to XO inhibition and are actually related to oxyradical scavenging. The latter proposal may be supported by an experimental study by Keshavarzian and colleagues (1990), in which XO depletion with tungsten was ineffective in reducing the symptoms of experimental colitis in rats, whereas allopurinol was effective in the same model. The novel nonpurine XO inhibitor AN-01-24, however, was effective in a murine model of dextran sulfate-induced colitis (Nivorozhkin et al., 2003a).
There is evidence for increased circulating levels of XO in human plasma samples from patients with rheumatoid arthritis (Miesel and Zuber, 1993). Allopurinol ameliorates the symptoms of arthritis in animal models of rheumatoid arthritis (Miesel et al., 1994; Yossif et al., 1995); at least some of these effects are likely related to the antioxidant actions of the compound. (The above referenced models of arthritis do not involve uric acid deposition into the joints. Obviously, by the prevention of hyperuricemia and urate crystal deposition, allopurinol and other XO inhibitors will have a clear and pronounced effect in preventing the development of gouty arthritis, as demonstrated in human clinical practice.)
There is also some evidence for the role of XO in the pathogenesis of sickle cell disease, a disease with a variety of components including inflammation, ischemia-reperfusion, and also a high incidence of hyperuricemia and gout (Reynolds, 1983). There is a significant degree of conversion of XDH to XO and release of XO into the systemic circulation (Osarogiagbon et al., 2000; Aslan et al., 2001). As discussed above, this increased circulating XO activity deposits at the vascular intima and leads to the development of endothelial dysfunction via the local formation of peroxynitrite in the vicinity of endothelial cells (Aslan et al., 2001). Allopurinol also appears to reduce the oxidative stress in sickle cell erythrocytes and in a murine model of sickle cell disease via a direct scavenging mechanism (Sertac et al., 1997; Osarogiagbon et al., 2000).
Additional inflammatory diseases in which XO has been implicated or the beneficial effect of XO inhibitors have been reported, include pneumonia (Akaike et al., 1990; Ikeda et al., 1992; Miyakawa et al., 2002; Wright et al., 2004), acute respiratory distress syndrome (Mabley et al., 2003), chronic obstructive pulmonary disease (Komaki et al., 2005), nephritis (Roberts et al., 1990; Gwinner et al., 1999), pancreatitis (Niederau et al., 1992; Czako et al., 2000; Folch et al., 2001; Zeki et al., 2002), peritonitis and peritoneal adhesions (Rijhwani et al., 1995; Cavallari et al., 2000), uveitis (Augustin et al., 1994, 1999) and dermatitis (Deliconstantinos et al., 1996). It must be kept in mind, however, that efficacy of allopurinol does not necessarily mean that XO activation is involved. For example, in a study by Augustin et al. (1994) in uveitis, a dose of allopurinol that was effective in blocking XO activity failed to affect the course of the disease, whereas higher doses of the compound (at which antioxidant effects become more prominent) proved effective in reducing the inflammation. The attempts to use allopurinol for anti-inflammatory purposes at the bedside have also failed so far: in a 300-patient study on pancreatitis developing as a complication of endoscopic retrograde cholangiopancreatography, allopurinol was found to be clinically ineffective (Budzynska et al., 2001).
There is some evidence that XO-derived reactive oxygen species play a role in antimicrobial defense (reviewed in Vorbach et al., 2003; Martin et al., 2004). Allopurinol and AHPP each exacerbated mortality in a Salmonella typhimurium model of infection in mice (Umezawa et al., 1997). In the absence of functional NADPH oxidase, the allopurinol-related impairment of antibacterial defense was prominent (Segal et al., 2000). Allopurinol has also been shown to increase ear swelling and mortality in a contact hypersensitivity model (Horiuchi et al., 1999a). It is noteworthy that the clinical use of allopurinol is normally not associated with reduced antibacterial defense, although it is possible that this issue has not been systematically investigated. On the other hand, the effects on contact hypersensitivity seen in the murine model do correlate with the clinical experience (hypersensitivity reactions to allopurinol in a significant fraction of the patients).
G. Xanthine Oxidase and Various Forms of Toxic Organ Injury
XO has been implicated and allopurinol treatment has demonstrated effectiveness in a variety of toxic organ injury models. These models include various forms of liver injury, i.e., ones induced by ionizing radiation (Srivastava and Kale, 1999; Srivastava et al., 2002), ethanol (Oei et al., 1982; Lieber, 1997; Kono et al., 2000; Zima et al., 2001), cocaine (Aoki et al., 1997), thioacetamide (Ali et al., 2001), acetaminophen (Knight et al., 2001), and aluminum (Moumen et al., 2001). In the case of ethanol, aluminum, and radiation, enhancement of hepatic XO levels and/or spillage of XO into the circulation were detected (Oei et al., 1982; Zima et al., 1993; Moumen et al., 2001; Srivastava et al., 2002). In the case of paracetamol, it is interesting to note that XO actually participates in the metabolism of the drug, with the generation of toxic by-products (Van Steveninck et al., 1989). Similar oxidant-yielding reductive activation by XO and XDH has been also reported in the case of doxorubicin, streptonigrin, and menadione (Yee and Pritsos, 1997). It is noteworthy that in the acetaminophen hepatotoxicity model, lower doses of allopurinol (sufficient to block XO activity) failed to show protection, whereas higher (antioxidant) levels were effective, indicating that the mode of allopurinol’s action is its antioxidant nature in this particular study (Knight et al., 2001). In many other investigations, low and high doses of allopurinol were not compared, but the doses of the compound used were generally sufficient to produce significant antioxidant effects. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine- and manganese-induced neurotoxicity (Miele et al., 1995; Desole et al., 1996; Obata et al., 2001), cisplatin-induced ototoxicity and nephrotoxicity (Lynch et al., 2005), paraquat-and nitrofurantoin-induced lung injury (Kitazawa et al., 1991; Bernard et al., 1997), and renal contrast nephropathy (Katholi et al., 1998) have also been suppressed by allopurinol in murine and rat studies.
VI. Future Development of Xanthine Oxidase Inhibitors
From the small number of current examples of the development of novel XO inhibitor compounds that have entered a clinical phase (Becker et al., 2004, 2005; Fukunari et al., 2004; Komoriya et al., 2004; Yamada et al., 2004; Hashimoto et al., 2005; Mayer et al., 2005; Takano et al., 2005), it appears that hyperuricemia and gout remain the main indications for the development of novel XO inhibitors, with additional growing interest in cardiac indications, such as chronic heart failure. Novel XO inhibitors must preferably be more potent, more effective, and possess better pharmacodynamic profile than allopurinol/oxypurinol, which is expected to translate in the clinical practice to lower daily doses and/or less frequent daily administration of the drug. Considering current tools for small molecule design and development, achievement of such goals does not appear to be too ambitious, although one must note that oxypurinol, as an irreversible inhibitor of XO, may have advantages over novel, ultrapotent competitive inhibitors of XO. The irreversible inhibition of XO by oxypurinol, in fact, can result in situations, in which new XO inhibitors, that appear to be several orders of magnitude more potent that allopurinol in vitro lose much of their potency advantage over allopurinol in vivo (Horiuchi et al., 1999b,c; Naito et al., 2000; Ishibuchi et al., 2001).
It is important to note that XO inhibitors, including allopurinol, although inhibiting the activity of the enzyme, can actually reduce the enzyme by transfer of an electron to oxygen, thus generating superoxide (Miyamoto et al., 1996). Other XO inhibitors, such as AHPP, do not share this oxidant-generating ability of allopurinol. Although it is unclear whether this finding is relevant for in vivo situations, it is probably preferable to develop future XO inhibitors that do not exert pro-oxidant effects.
A more important area in which XO inhibitors clearly need improvement is the reduction of their side effects. As reviewed above, allopurinol does have a number of serious side effects, and the cellular and molecular mechanisms of these side effects are incompletely understood. Some recent data indicate that the renal toxicity of allopurinol is related to impairment of pyrimidine metabolism (Horiuchi et al., 2000). There are no reliable or rapid screening tools that would predict the safety profile of novel XO inhibitors in terms of hypersensitivity reactions or organ toxicity; contact hypersensitivity mouse ear models and toxicity studies in rodents are being used to predict such side effects (Horiuchi et al., 1999a). Intuitively, one would predict that novel XO inhibitors that would move away from the purine-based inhibitor structure may have fewer of the allopurinol-like side effects (of course, they may introduce new types of side effects or toxicities). One must also be cautious with widely used long-term safety trials, especially in rodents, as rodents and primates have different biochemical pathways for handling purines: urate oxidase is an essential enzyme in rodents that converts uric acid into allantoin, which subsequently metabolizes to allantoate and then glioxylate and urea (Wu et al., 1994).
With respect to the utility of novel XO inhibitors for the experimental therapy of pathophysiological conditions other than gout (reperfusion, inflammation, and toxic organ injury), the first three interrelated questions to be addressed are the following: 1) Is there up-regulation of XO in human disease? 2) Does oxidant generation from XO substantially contribute to the pathogenesis of the disease? and 3) How much of the previously reported effects of allopurinol are actually related to XO inhibition, as opposed to non-XO related additional pharmacological effects of the compound? These issues have been reviewed, in some detail, in the preceding section. For questions 1 and 2, it appears that in many pathophysiological conditions, there is an up-regulation of XO in humans sometimes coupled with a deposition of circulating XO to the vasculature (as reviewed above). Under these conditions, it does make sense to counteract with the XO-derived oxidant generation. Whether or not XO represents the major source of oxidants in various pathophysiological conditions, as opposed to one of many sources, would determine the right approach to be taken (i.e., XO inhibition versus a more broadly based antioxidant strategy). With respect to question 3, the answer is unknown. In some studies, in which a dose-response relationship with allopurinol has been carefully investigated (as in uveitis and in some forms of toxic liver injury), low doses of allopurinol, which would be expected to inhibit XO, failed to affect the pathogenesis of the disease, whereas high doses become effective (Augustin et al., 1994, Knight et al., 2001). In some other models of disease (stroke and colitis), depletion of XO with tungsten was compared with allopurinol, and, frequently, allopurinol but not tungsten was found to be effective (Patt et al., 1988; Keshavarzan et al., 1990). Based on these studies, one can conclude that non-XO-related actions of allopurinol can be responsible for at least some of (or possibly much of?) the protective effects in disease models. If, indeed, this latter possibility proves to be the case, it would not invalidate the clinical efficacy of allopurinol or oxypurinol in various pathophysiological conditions. Indeed, there are some indications that allopurinol is effective in some forms of human disease (such as CHF, myocardial infarction, and also possibly in inflammatory bowel disease); after all, what ultimately and clinically matters is that the outcome of the disease improves in the patients, regardless of the precise mode of action. Nevertheless, pilot (phase II type) studies need to be confirmed with large phase III type trials. Because allopurinol and oxypurinol are not protected by particularly strong intellectual property positions (although use patents exist, for example, the use of oxypurinol to treat patients with heart failure) and these compounds are not protected by structure-of-the-matter patents and can be made relatively cheaply and simply, it is unlikely that large pharmaceutical firms would be interested in sponsoring such phase III trials. Nevertheless, qualified investigators, in collaboration with granting agencies, nonprofit foundations, or government bodies may be able to organize such testing. At any rate, it is unclear whether the development of allopurinol for its antioxidant properties would be competitive compared with the development of so-called catalytic antioxidant molecules (e.g., low-molecule superoxide dismutase mimics or peroxynitrite decomposition catalysts), which are likely to be substantially more effective than allopurinol in neutralizing oxidants in the same disease conditions, as they would interfere with oxidants derived not only from XO, but also from a variety of other sources (mitochondria, NADPH oxidase, nitric-oxide synthase, etc.).
As with most preclinical studies, it also remains to be determined whether preclinical data observed in somewhat artificial animal models of disease are actually applicable to the human condition, especially in light of the differences between the XDH/XO systems in primates versus lower species. It is noteworthy that, so far, practically all published efficacy data associated with the novel XO inhibitor compounds relate to the area of gout, and researchers have not tested or reported efficacy of these compounds in preclinical models of inflammation or reperfusion or toxic organ injury (Okamoto et al., 2003; Yamamoto, 2003; Becker et al., 2004; Fukunari et al., 2004; Hoshide et al., 2004; Komoriya et al., 2004; Yamada et al., 2004; Hashimoto et al., 2005; Mayer et al., 2005; Takano et al., 2005). The XO inhibitor pterin-6-aldehyde is a known superoxide scavenger (Mori et al., 1998) and probably so are the 2-alkyloxyalkylthiohypoxanthines as well (Biagi et al., 2001). BOF-4272 and AHPP appear to reduce oxidative stress in the liver and lung in vivo (Matsumura et al., 1998; Miyakawa et al., 2002). There are only a few reports to investigate whether or not the other classes of novel XO inhibitor compounds share some of the antioxidant or non-XO-related activities of allopurinol. Even if they do have such effects, one would expect that the therapeutic dose of the novel, more potent XO inhibitors would be much lower than that of allopurinol. Thus, antioxidant effects would be expected to contribute less to the in vivo actions of the new XO inhibitors.
Taken together, allopurinol remains the cornerstone of current clinical management of hyperuricemia and gout, despite its problematic side effect profile. There is room for the development of novel XO inhibitors for the experimental therapy of hyperuricemia and gout, which are major medical indications and major drug markets worldwide. It is currently unclear whether novel XO inhibitors will be effective (and/or competitive with other antioxidant approaches) for the experimental therapy of ischemic conditions, inflammatory diseases, CHF, and various forms of organ injury. Several series of novel XO inhibitors have entered clinical testing, and, undoubtedly, there is interest for the development of additional, novel series of XO inhibitors. It will be interesting to see how the efficacy and safety profiles of these novel agents compare to those of allopurinol.
Acknowledgments
We are grateful to Dr. Thomas Spector for sharing historical information on the XO inhibitor program carried out over the years at Burroughs Wellcome Laboratories, Dr. Sandor Batkai and Lucja Katarzyna Flis for carefully reading the manuscript and for providing valuable comments and corrections, and Carol Oesch for help in preparing the references.
This work was supported by Intramural Research of National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (to P.P.).
Footnotes
Abbreviations: XO, xanthine oxidase; 6-MP, 6-mercaptopurine; XDH, xanthine dehydrogenase; XOR, xanthine oxidoreductase; NO, nitric oxide; ROS, reactive oxygen species; UW, University of Wisconsin; CHF, chronic heart failure; NOS, nitric-oxide synthase; nNOS, neuronal NOS; SHR, spontaneously hypertensive rat; AHPP, 4-amino-6-hydroxypyrazolo[3,4-d]pyrimidine; BOF-4272, (+/−)-8-(3-methoxy-4-phenylsulfinylphenyl) pyrazolo[1,5-a]-1,3,5-triazine-4(1H)-one; Y-700, 1-[3-cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid.
References
- Ahn SY, Sugi K, Talke P, Theissen JL, Linares HA, Traber LD, Herndon DN, Traber DL. Effects of allopurinol on smoke inhalation in the ovine model. J Appl Physiol. 1990;68:228–234. doi: 10.1152/jappl.1990.68.1.228. [DOI] [PubMed] [Google Scholar]
- Aiba M, Yokoyama Y, Snow TR, Novitzky D, McKeown PP. Effects of allopurinol pretreatment with pulmonary flush on lung preservation. J Heart Lung Transplant. 1992;11:1025–1030. [PubMed] [Google Scholar]
- Akaike T, Ando M, Oda T, Doi T, Ijiri S, Araki S, Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Investig. 1990;85:739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdemir H, Asik Z, Pasaoglu H, Karakucuk I, Oktem IS, Koc RK. The effect of allopurinol on focal cerebral ischaemia: an experimental study in rabbits. Neurosurg Rev. 2001;24:131–135. doi: 10.1007/pl00012397. [DOI] [PubMed] [Google Scholar]
- Akizuki S, Yoshida S, Chambers DE, Eddy LJ, Parmley LF, Yellon DM, Downey JM. Infarct size limitation by the xanthine oxidase inhibitor, allopurinol, in closed-chest dogs with small infarcts. Cardiovasc Res. 1985;19:686–692. doi: 10.1093/cvr/19.11.686. [DOI] [PubMed] [Google Scholar]
- Alderman M. Uric acid in hypertension and cardiovascular disease. Can J Cardiol. 1999;15(Suppl F):20–22. [PubMed] [Google Scholar]
- Alderman M, Aiyer KJ. Uric acid: role in cardiovascular disease and effects of losartan. Curr Med Res Opin. 2004;20:369–379. doi: 10.1185/030079904125002982. [DOI] [PubMed] [Google Scholar]
- Alderman MH. Uric acid and cardiovascular risk. Curr Opin Pharmacol. 2002;2:126–130. doi: 10.1016/s1471-4892(02)00143-1. [DOI] [PubMed] [Google Scholar]
- Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. doi: 10.1161/01.hyp.34.1.144. [DOI] [PubMed] [Google Scholar]
- Ali S, Diwakar G, Pawa S, Siddiqui MR, Abdin MZ, Ahmad FJ, Jain SK. Xanthine oxidase-derived reactive oxygen metabolites contribute to liver necrosis: protection by 4-hydroxypyrazolo[3,4-d]pyrimidine. Biochim Biophys Acta. 2001;1536:21–30. doi: 10.1016/s0925-4439(01)00030-8. [DOI] [PubMed] [Google Scholar]
- Allan G, Cambridge D, Lee-Tsang-Tan L, Van Way CW, Whiting MV. The protective action of allopurinol in an experimental model of haemorrhagic shock and reperfusion. Br J Pharmacol. 1986;89:149–155. doi: 10.1111/j.1476-5381.1986.tb11130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado LC, Saliaris AP, Raju SV, Lehrke S, St John M, Xie J, Stewart G, Fitton T, Minhas KM, Brawn J, et al. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure. J Mol Cell Cardiol. 2005;39:531–536. doi: 10.1016/j.yjmcc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RF, Hille R, Patel KB. Inactivation of xanthine oxidase by oxidative radical attack. Int J Radiat Biol. 1995;68:535–541. doi: 10.1080/09553009514551521. [DOI] [PubMed] [Google Scholar]
- Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- Aoki K, Ohmori M, Takimoto M, Ota H, Yoshida T. Cocaine-induced liver injury in mice is mediated by nitric oxide and reactive oxygen species. Eur J Pharmacol. 1997;336:43–49. doi: 10.1016/s0014-2999(97)01230-2. [DOI] [PubMed] [Google Scholar]
- Arai T, Mori H, Ishii H, Adachi T, Endo N, Makino K, Mori K. Oxypurinol, a xanthine oxidase inhibitor and a superoxide scavenger, did not attenuate ischemic neuronal damage in gerbils. Life Sci. 1998;63:107–112. doi: 10.1016/s0024-3205(98)00312-9. [DOI] [PubMed] [Google Scholar]
- Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–343. doi: 10.1177/106002809302700317. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Samra ZQ. Subcellular distribution of xanthine oxidase during cardiac ischemia and reperfusion: an immunocytochemical study. J Submicrosc Cytol Pathol. 1993;25:193–201. [PubMed] [Google Scholar]
- Askenasy N, Navon G. Intracellular volumes and membrane permeability in rat hearts during prolonged hypothermic preservation with St Thomas and University of Wisconsin solutions. J Mol Cell Cardiol. 1998;30:1329–1339. doi: 10.1006/jmcc.1998.0695. [DOI] [PubMed] [Google Scholar]
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin AJ, Boker T, Blumenroder SH, Lutz J, Spitznas M. Free radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitis. Investig Ophthalmol Vis Sci. 1994;35:3897–3904. [PubMed] [Google Scholar]
- Augustin AJ, Loeffler KU, Sekundo W, Grus FH, Lutz J. Effects of systemically applied allopurinol and prednisolone on experimental autoimmune uveitis. Graefes Arch Clin Exp Ophthalmol. 1999;237:508–512. doi: 10.1007/s004170050270. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Simmons A, Turek J, Babbs CF. Protection from reperfusion injury in the isolated rat heart by postischaemic deferoxamine and oxypurinol administration. Cardiovasc Res. 1987;21:500–506. doi: 10.1093/cvr/21.7.500. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Ungvari Z, Koller A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: possible role of peroxynitrite. Arterioscler Thromb Vasc Biol. 2002;22:28–33. doi: 10.1161/hq0102.101127. [DOI] [PubMed] [Google Scholar]
- Baker CH. Protection against irreversible hemorrhagic shock by allopurinol. Proc Soc Exp Biol Med. 1972;141:694–698. doi: 10.3181/00379727-141-36854. [DOI] [PubMed] [Google Scholar]
- Battelli MG, Buonamici L, Abbondanza A, Virgili M, Contestabile A, Stirpe F. Excitotoxic increase of xanthine dehydrogenase and xanthine oxidase in the rat olfactory cortex. Brain Res Dev Brain Res. 1995;86:340–344. doi: 10.1016/0165-3806(95)00012-3. [DOI] [PubMed] [Google Scholar]
- Battelli MG, Buonamici L, Virgili M, Abbondanza A, Contestabile A. Simulated ischaemia-reperfusion conditions increase xanthine dehydrogenase and oxidase activities in rat brain slices. Neurochem Int. 1998;32:17–21. doi: 10.1016/s0197-0186(97)00052-1. [DOI] [PubMed] [Google Scholar]
- Becker MA, Kisicki J, Khosravan R, Wu J, Mulford D, Hunt B, MacDonald P, Joseph-Ridge N. Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids. 2004;8–9:1111–1116. doi: 10.1081/NCN-200027372. [DOI] [PubMed] [Google Scholar]
- Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- Beetsch JW, Park TS, Dugan LL, Shah AR, Gidday JM. Xanthine oxidase-derived superoxide causes reoxygenation injury of ischemic cerebral endothelial cells. Brain Res. 1998;786:89–95. doi: 10.1016/s0006-8993(97)01407-8. [DOI] [PubMed] [Google Scholar]
- Belboul A, Roberts D, Borjesson R, Johnsson J. Oxygen free radical generation in healthy blood donors and cardiac patients: the protective effect of allopurinol. Perfusion. 2001;16:59–65. doi: 10.1177/026765910101600109. [DOI] [PubMed] [Google Scholar]
- Ben-Hamida A, Man WK, McNeil N, Spencer J. Histamine, xanthine oxidase generated oxygen-derived free radicals and Helicobacter pylori in gastroduodenal inflammation and ulceration. Inflamm Res. 1998;47:193–199. doi: 10.1007/s000110050317. [DOI] [PubMed] [Google Scholar]
- Bergsland J, LoBalsamo L, Lajos P, Feldman MJ, Mookerjee B. Allopurinol in prevention of reperfusion injury of hypoxically stored rat hearts. J Heart Transplant. 1987;6:137–140. [PubMed] [Google Scholar]
- Bernard CE, Magid AA, Yen TS, Hoener BA. Mitigation of nitrofurantoin-induced toxicity in the perfused rat lung. Hum Exp Toxicol. 1997;16:727–732. doi: 10.1177/096032719701601206. [DOI] [PubMed] [Google Scholar]
- Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol (Lond) 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi G, Giorgi I, Pacchini F, Livi O, Scartoni V. 2-Alkyloxyalkylthiohypoxanthines as new potent inhibitors of xanthine oxidase. Farmaco. 2001;56:809–813. doi: 10.1016/s0014-827x(01)01160-0. [DOI] [PubMed] [Google Scholar]
- Bieber JD, Terkeltaub RA. Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis Rheum. 2004;50:2400–2414. doi: 10.1002/art.20438. [DOI] [PubMed] [Google Scholar]
- Biguzas M, Jablonski P, Howden BO, Thomas AC, Walls K, Scott DF, Marshall VC. Evaluation of UW solution in rat kidney preservation. II. The effect of pharmacological additives. Transplantation. 1990;49:1051–1055. doi: 10.1097/00007890-199006000-00005. [DOI] [PubMed] [Google Scholar]
- Bonaventura J, Gow A. NO and superoxide: opposite ends of the seesaw in cardiac contractility. Proc Natl Acad Sci USA. 2004;101:16403–16404. doi: 10.1073/pnas.0405859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond RF, Haines GA, Johnson G., 3rd The effect of allopurinol and catalase on cardiovascular hemodynamics during hemorrhagic shock. Circ Shock. 1988;25:139–151. [PubMed] [Google Scholar]
- Booster MH, van der Vusse GJ, Wijnen RM, Yin M, Stubenitsky BM, Kootstra G. University of Wisconsin solution is superior to histidine tryptophan ketoglutarate for preservation of ischemically damaged kidneys. Transplantation. 1994;58:979–984. doi: 10.1097/00007890-199411150-00001. [DOI] [PubMed] [Google Scholar]
- Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem. 2002;9:195–217. doi: 10.2174/0929867023371229. [DOI] [PubMed] [Google Scholar]
- Boros M, Bako L, Nagy S. Effect of antioxidant therapy on cyclooxygenase-derived eicosanoid release during intestinal ischemia-reperfusion. Eur Surg Res. 1991;23:141–150. doi: 10.1159/000129146. [DOI] [PubMed] [Google Scholar]
- Boros M, Kaszaki J, Nagy S. Oxygen free radical-induced histamine release during intestinal ischemia and reperfusion. Eur Surg Res. 1989;21:297–304. doi: 10.1159/000129042. [DOI] [PubMed] [Google Scholar]
- Boucher F, de Leiris J. Chronic administration of allopurinol fails to exert any cardioprotective effect in rats submitted to permanent coronary artery ligation. Basic Res Cardiol. 1991;86:227–235. doi: 10.1007/BF02190602. [DOI] [PubMed] [Google Scholar]
- Boyer TD, Sun N, Reynolds TB. Allopurinol-hypersensitivity vasculitis and liver damage. West J Med. 1977;126:143–147. [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65(1):16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Investig. 1988;81:1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766–772. doi: 10.1055/s-2001-16520. [DOI] [PubMed] [Google Scholar]
- Burton LK, Velasco SE, Patt A, Terada LS, Repine JE. Xanthine oxidase contributes to lung leak in rats subjected to skin burn. Inflammation. 1995;19:31–38. doi: 10.1007/BF01534378. [DOI] [PubMed] [Google Scholar]
- Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- Caldeira J, Belle V, Asso M, Guigliarelli B, Moura I, Moura JJG, Bertrand P. Analysis of the electron paramagnetic resonance properties of the [2Fe-2S]1+ centers in molybdenum enzymes of the xanthine oxidase family: assignment of signals I and II. Biochemistry. 2000;39:2700–2707. doi: 10.1021/bi9921485. [DOI] [PubMed] [Google Scholar]
- Canne C, Lowe DJ, Fetzner S, Adams B, Smith AT, Kappl R, Bray RC, Huttermann J. Kinetics and interactions of molybdenum and iron-sulfur centers in bacterial enzymes of the xanthine oxidase family: mechanistic implications. Biochemistry. 1999;38:14077–14087. doi: 10.1021/bi991089s. [DOI] [PubMed] [Google Scholar]
- Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- Castelli P, Condemi AM, Brambillasca C, Fundaro P, Botta M, Lemma M, Vanelli P, Santoli C, Gatti S, Riva E. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. J Cardiovasc Pharmacol. 1995;125:119–125. doi: 10.1097/00005344-199501000-00019. [DOI] [PubMed] [Google Scholar]
- Castillo M, Toledo-Pereyra LH, Gutierrez R, Prough D, Shapiro E. Peritonitis after cecal perforation: an experimental model to study the therapeutic role of antibiotics associated with allopurinol and catalase. Am Surg. 1991;57:313–316. [PubMed] [Google Scholar]
- Cavallari N, Polistena A, Cavallaro A. Inability of University of Wisconsin solution to reduce postoperative peritoneal adhesions in rats. Eur J Surg. 2000;166:650–653. doi: 10.1080/110241500750008330. [DOI] [PubMed] [Google Scholar]
- Cetinkale O, Senel O, Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999;25:113–118. doi: 10.1016/s0305-4179(98)00124-7. [DOI] [PubMed] [Google Scholar]
- Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Chambers DJ, Takahashi A, Humphrey SM, Harvey DM, Hearse DJ. Allopurinol-enhanced myocardial protection does not involve xanthine oxidase inhibition or purine salvage. Basic Res Cardiol. 1992;87:227–238. doi: 10.1007/BF00804332. [DOI] [PubMed] [Google Scholar]
- Charlat MI, O’Neill PG, Egan JM, Abernethy DR, Michael LH, Myers ML, Roberts R, Bolli R. Evidence for a pathogenetic role of xanthine oxidase in the “stunned” myocardium. Am J Physiol. 1987;252:H566–H577. doi: 10.1152/ajpheart.1987.252.3.H566. [DOI] [PubMed] [Google Scholar]
- Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17:341–345. [PubMed] [Google Scholar]
- Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Zeni P, Zardini P. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am Heart J. 2002;143:1107–1111. doi: 10.1067/mhj.2002.122122. [DOI] [PubMed] [Google Scholar]
- Clark DA, Fornabaio DM, McNeill H, Mullane KM, Caravella SJ, Miller MJ. Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am J Pathol. 1988;130:537–542. [PMC free article] [PubMed] [Google Scholar]
- Coetzee A, Roussouw G, Macgregor L. Failure of allopurinol to improve left ventricular stroke work after cardiopulmonary bypass surgery. J Cardiothorac Vasc Anesth. 1996;10:627–633. doi: 10.1016/s1053-0770(96)80141-8. [DOI] [PubMed] [Google Scholar]
- Coghlan JG, Flitter WD, Clutton SM, Panda R, Daly R, Wright G, Ilsley CD, Slater TF. Allopurinol pretreatment improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;107:248–256. [PubMed] [Google Scholar]
- Crowell JW, Jones CE, Smith EE. Effect of allopurinol on hemorrhagic shock. Am J Physiol. 1969;216:744–748. doi: 10.1152/ajplegacy.1969.216.4.744. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Cunningham SK, Keaveny TV. Effect of a xanthine oxidase inhibitor on adenine nucleotide degradation in hemorrhagic shock. Eur Surg Res. 1978;10:305–313. doi: 10.1159/000128020. [DOI] [PubMed] [Google Scholar]
- Czako L, Takacs T, Varga IS, Tiszlavicz L, Hai DQ, Hegyi P, Matkovics B, Lonovics J. Oxidative stress in distant organs and the effects of allopurinol during experimental acute pancreatitis. Int J Pancreatol. 2000;27:209–216. doi: 10.1385/IJGC:27:3:209. [DOI] [PubMed] [Google Scholar]
- Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun. 1987;148:314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- Davis JC., Jr A practical approach to gout: Current management of an ‘old’ disease. Postgrad Med. 1999;106:115–116. 119–123. doi: 10.3810/pgm.1999.10.1.706. [DOI] [PubMed] [Google Scholar]
- de Jong JW, Schoemaker RG, de Jonge R, Bernocchi P, Keijzer E, Harrison R, Sharma HS, Ceconi C. Enhanced expression and activity of xanthine oxidoreductase in the failing heart. J Mol Cell Cardiol. 2000;32:2083–2089. doi: 10.1006/jmcc.2000.1240. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Bridges W, Baker J, Ma JW, Ma L, Grisham MB, Granger DN, Specian RD, Berg R. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery. 1988;104:191–198. [PubMed] [Google Scholar]
- Deliconstantinos G, Villiotou V, Stavrides JC. Alterations of nitric oxide synthase and xanthine oxidase activities of human keratinocytes by ultraviolet B radiation: potential role for peroxynitrite in skin inflammation. Biochem Pharmacol. 1996;51:1727–1738. doi: 10.1016/0006-2952(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- Desole MS, Esposito G, Fresu L, Migheli R, Sircana S, Delogu R, Miele M, Miele E. Further investigation of allopurinol effects on MPTP-induced oxidative stress in the striatum and brain stem of the rat. Pharmacol Biochem Behav. 1996;54:377–383. doi: 10.1016/0091-3057(95)02161-2. [DOI] [PubMed] [Google Scholar]
- Doehner W, Anker SD. Xanthine oxidase inhibition for chronic heart failure: is allopurinol the next therapeutic advance in heart failure? Heart. 2005a;91:707–709. doi: 10.1136/hrt.2004.057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner W, Anker SD. Uric acid in chronic heart failure. Semin Nephrol. 2005b;25:61–66. doi: 10.1016/j.semnephrol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- Dorion D, Zhong A, Chiu C, Forrest CR, Boyd B, Pang CY. Role of xanthine oxidase in reperfusion injury of ischemic skeletal muscles in the pig and human. J Appl Physiol. 1993;75:246–255. doi: 10.1152/jappl.1993.75.1.246. [DOI] [PubMed] [Google Scholar]
- Downey JM, Miura T, Eddy LJ, Chambers DE, Mellert T, Hearse DJ, Yellon DM. Xanthine oxidase is not a source of free radicals in the ischemic rabbit heart. J Mol Cell Cardiol. 1987;19:1053–1060. doi: 10.1016/s0022-2828(87)80350-4. [DOI] [PubMed] [Google Scholar]
- Duchene DA, Smith CP, Goldfarb RA. Allopurinol induced meningitis. J Urol. 2000;164:2028. [PubMed] [Google Scholar]
- Duncan JG, Ravi R, Stull LB, Murphy AM. Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse model of cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H1512–H1528. doi: 10.1152/ajpheart.00168.2005. [DOI] [PubMed] [Google Scholar]
- Eger BT, Okamoto K, Enroth C, Sato M, Nishino T, Pai EF, Nishino T. Purification, crystallization and preliminary X-ray diffraction studies of xanthine dehydrogenase and xanthine oxidase isolated from bovine milk. Acta Crystallogr D Biol Crystallogr. 2000;56:1656–1658. doi: 10.1107/s0907444900012890. [DOI] [PubMed] [Google Scholar]
- Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- Elion GB. Nobel lecture. Burroughs Wellcome Co; Research Triangle Park, NC: 1988. The purine path to chemotherapy. [Google Scholar]
- Elion GB. The quest for a cure. Annu Rev Pharmacol Toxicol. 1993;33:1–23. doi: 10.1146/annurev.pa.33.040193.000245. [DOI] [PubMed] [Google Scholar]
- Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, Fuchs M, Hilfiker-Kleiner D, Hornig B, Drexler H, et al. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110:2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder SJ, Jones HP. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Investig. 1987;79:1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF. Crystal structure of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci USA. 2000;97:10723–10728. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam AG. Difficult gout and new approaches for control of hyperuricemia in the allopurinol-allergic patient. Curr Rheumatol Rep. 2001;3:29–35. doi: 10.1007/s11926-001-0048-8. [DOI] [PubMed] [Google Scholar]
- Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Panas D, Schulz R. Peroxynitrite contributes to spontaneous loss of cardiac efficiency in isolated working rat hearts. Am J Physiol. 1999;276:H1861–H1867. doi: 10.1152/ajpheart.1999.276.6.H1861. [DOI] [PubMed] [Google Scholar]
- Flynn WJ, Jr, Pilati D, Hoover EL. Xanthine oxidase inhibition prevents mesenteric blood flow deficits after resuscitated hemorrhagic shock by preserving endothelial function. J Surg Res. 1997;68:175–180. doi: 10.1006/jsre.1997.5016. [DOI] [PubMed] [Google Scholar]
- Flynn WJ, Jr, Pilati D, Hoover EL. Effect of allopurinol on venous endothelial dysfunction after resuscitated hemorrhagic shock. Int J Surg Investig. 1999;1:11–18. [PubMed] [Google Scholar]
- Folch E, Gelpi E, Rosello-Catafau J, Closa D. Free radicals generated by xanthine oxidase mediate pancreatitis-associated organ failure. Dig Dis Sci. 2001;276:14359–14365. doi: 10.1023/a:1026617812283. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Bosch KS. The proportion of xanthine oxidase activity of total xanthine oxidoreductase activity in situ remains constant in rat liver under various (patho)physiological conditions. Hepatology. 1996;24:1179–1184. doi: 10.1002/hep.510240533. [DOI] [PubMed] [Google Scholar]
- Freudenberger RS, Schwarz RP, Jr, Brown J, Moore A, Mann D, Givertz MM, Colucci WS, Hare JM. Rationale, design and organisation of an efficacy and safety study of oxypurinol added to standard therapy in patients with NYHA class III–IV congestive heart failure. Expert Opin Investig Drugs. 2004;13:1509–1516. doi: 10.1517/13543784.13.11.1509. [DOI] [PubMed] [Google Scholar]
- Fukahori M, Ichimori K, Ishida H, Nakagawa H, Okino H. Nitric oxide reversibly suppresses xanthine oxidase activity. Free Radic Res. 1994;21:203–212. doi: 10.3109/10715769409056572. [DOI] [PubMed] [Google Scholar]
- Fukunari A, Okamoto K, Nishino T, Eger BT, Pai EF, Kamezawa M, Yamada I, Kato N. Y-700 [1-[3-cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid]: a potent xanthine oxidoreductase inhibitor with hepatic excretion. J Pharmacol Exp Ther. 2004;311:519–528. doi: 10.1124/jpet.104.070433. [DOI] [PubMed] [Google Scholar]
- Garcia Garcia J, Martin Rollan C, Refoyo Enrinquez MA, Holgado Madruga M, Marino Hernandez E, Macias Nunez JF, Gomez Alonso A. Improved survival in intestinal ischemia by allopurinol not related to xanthine-oxidase inhibition. J Surg Res. 1990;48:144–146. doi: 10.1016/0022-4804(90)90206-h. [DOI] [PubMed] [Google Scholar]
- Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–753. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Kennedy TP, Stonehuerner J, Carter JD, Skinner KA, Parks DA, Hoidal JR. Iron regulates xanthine oxidase activity in the lung. Am J Physiol. 2002;283:L563–L572. doi: 10.1152/ajplung.00413.2000. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Kennedy TP, Stonehuerner J, Carter JD, Skinner KA, Parks DA, Hoidal JR, Giler S, Eshel Y, Pinkhas J, et al. Elevation of serum xanthine oxidase activity following halothane anesthesia in man. Experientia (Basel) 1977;33:1356–1358. doi: 10.1007/BF01920178. [DOI] [PubMed] [Google Scholar]
- Giler S, Eshel Y, Pinkhas J, Ventura E, Levy E, Urea I, Sperling O, de Vries A. Elevation of serum xanthine oxidase activity following halothane anesthesia in man. Experientia. 1977;33:1356–1358. doi: 10.1007/BF01920178. [DOI] [PubMed] [Google Scholar]
- Giler S, Ventura E, Levy E, Urca I, Sperling O, de Vries A. Elevation of serum xanthine oxidase following halothane anesthesia in the rat. Experientia (Basel) 1976;32:620–621. doi: 10.1007/BF01990198. [DOI] [PubMed] [Google Scholar]
- Godber BL, Doel JJ, Goult TA, Eisenthal R, Harrison R. Suicide inactivation of xanthine oxidoreductase during reduction of inorganic nitrite to nitric oxide. Biochem J. 2001;358:325–333. doi: 10.1042/0264-6021:3580325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- Godber BL, Schwarz G, Mendel RR, Lowe DJ, Bray RC, Eisenthal R, Harrison R. Molecular characterization of human xanthine oxidoreductase: the enzyme is grossly deficient in molybdenum and substantially deficient in iron-sulphur centres. Biochem J. 2005;388:501–508. doi: 10.1042/BJ20041984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin DV, Bhimji S. Effects of allopurinol on myocardial ischemic injury induced by coronary artery ligation and reperfusion. Biochem Pharmacol. 1987;36:2101–2107. doi: 10.1016/0006-2952(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Godin DV, Garnett ME. Altered antioxidant status in the ischemic/reperfused rabbit myocardium: effects of allopurinol. Can J Cardiol. 1989;5:365–371. [PubMed] [Google Scholar]
- Godin DV, Bhimji S, McNeill JH. Effects of allopurinol pretreatment on myocardial ultrastructure and arrhythmias following coronary artery occlusion and reperfusion. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52:327–341. doi: 10.1007/BF02889975. [DOI] [PubMed] [Google Scholar]
- Granger DN, McCord JM, Parks DA, Hollwarth ME. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986;90:80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- Greenberg LE, Nguyen T, Miller SM. Suspected allopurinol-induced aseptic meningitis. Pharmacotherapy. 2001;21:1007–1009. doi: 10.1592/phco.21.11.1007.34526. [DOI] [PubMed] [Google Scholar]
- Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Grum CM, Ketai LH, Myers CL, Shlafer M. Purine efflux after cardiac ischemia: relevance to allopurinol cardioprotection. Am J Physiol. 1987;252:H368–H373. doi: 10.1152/ajpheart.1987.252.2.H368. [DOI] [PubMed] [Google Scholar]
- Guan W, Osanai T, Kamada T, Hanada H, Ishizaka H, Onodera H, Iwasa A, Fujita N, Kudo S, Ohkubo T, et al. Effect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarction. J Cardiovasc Pharmacol. 2003;41:699–705. doi: 10.1097/00005344-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Gulian JM, Dalmasso C, Desmoulin F, Scheiner C, Cozzone PJ. Twenty-four-hour hypothermic preservation of rat liver with Euro-Collins and UW solutions: a comparative evaluation by 31P NMR spectroscopy, biochemical assays and light microscopy. Transplantation. 1992;54:599–603. doi: 10.1097/00007890-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Woods K, Sinkey CA, Haynes WG. Role of xanthine oxidase in conduit artery endothelial dysfunction in cigarette smokers. Am J Cardiol. 2004;93:664–668. doi: 10.1016/j.amjcard.2003.11.046. [DOI] [PubMed] [Google Scholar]
- Gwinner W, Plasger J, Brandes RP, Kubat B, Schulze M, Regele H, Kerjaschki D, Olbricht CJ, Koch KM. Role of xanthine oxidase in passive Heymann nephritis in rats. J Am Soc Nephrol. 1999;10:538–544. doi: 10.1681/ASN.V103538. [DOI] [PubMed] [Google Scholar]
- Hakguder G, Akgur FM, Ates O, Olguner M, Aktug T, Ozer E. Short-term intestinal ischemia-reperfusion alters intestinal motility that can be preserved by xanthine oxidase inhibition. Dig Dis Sci. 2002;47:1279–1283. doi: 10.1023/a:1015314312730. [DOI] [PubMed] [Google Scholar]
- Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol. 2003;35:719–729. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev. 2004;36:363–375. doi: 10.1081/dmr-120037569. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Fukunari A, Yamada I, Yanaka N, Chen D, Kato N. Y-700, a novel inhibitor of xanthine oxidase, suppresses the development of colon aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine-treated mice. Biosci Biotechnol Biochem. 2005;69:209–211. doi: 10.1271/bbb.69.209. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, Yu FS, Cote CG, Zulueta JJ, Sawhney R, Skinner KA, Skinner HB, Parks DA, Lanzillo JJ. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1 and hypoxia: role in acute lung injury. Am J Respir Crit Care Med. 1998;158:299–305. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, Yu FS, Zulueta JJ, White AC, Lanzillo JJ. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am J Physiol. 1995;268:L809–L817. doi: 10.1152/ajplung.1995.268.5.L809. [DOI] [PubMed] [Google Scholar]
- Headrick JP, Armiger LC, Willis RJ. Behaviour of energy metabolites and effect of allopurinol in the “stunned” isovolumic rat heart. J Mol Cell Cardiol. 1990;22:1107–1116. doi: 10.1016/0022-2828(90)90074-c. [DOI] [PubMed] [Google Scholar]
- Hearse DJ, Manning AS, Downey JM, Yellon DM. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Hegge JO, Southard JH, Haworth RA. Preservation of metabolic reserves and function after storage of myocytes in hypothermic UW solution. Am J Physiol. 2001;281:C758–C772. doi: 10.1152/ajpcell.2001.281.3.C758. [DOI] [PubMed] [Google Scholar]
- Hestin D, Johns EJ. The influence of allopurinol on kidney haemodynamic and excretory responses to renal ischaemia in anaesthetized rats. Br J Pharmacol. 1999;128:255–261. doi: 10.1038/sj.bjp.0702789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R. The mononuclear molybdenum enzymes. Chem Rev. 1996;96:2757–2816. doi: 10.1021/cr950061t. [DOI] [PubMed] [Google Scholar]
- Hitchings GH, Elion GB. Chemical suppression of the immune response. Pharmacol Rev. 1963;15:365–405. [PubMed] [Google Scholar]
- Hodgson EK, Fridovich I. The role of O2− in the chemiluminescence of luminol. Photochem Photobiol. 1973;18:451–455. doi: 10.1111/j.1751-1097.1973.tb06449.x. [DOI] [PubMed] [Google Scholar]
- Hoidal JR, Xu P, Huecksteadt T, Sanders KA, Pfeffer K. Transcriptional regulation of human xanthine dehydrogenase/xanthine oxidase. Biochem Soc Trans. 1997;25:796–799. doi: 10.1042/bst0250796. [DOI] [PubMed] [Google Scholar]
- Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RW, Abraham J, Simeone FA, Damewood CA. Effects of allopurinol on hepatic adenosine nucleotides in hemorrhagic shock. J Surg Res. 1975;19:381–390. doi: 10.1016/0022-4804(75)90067-0. [DOI] [PubMed] [Google Scholar]
- Hopson SB, Lust RM, Sun YS, Zeri RS, Morrison RF, Otaki M, Chitwood WR., Jr Allopurinol improves myocardial reperfusion injury in a xanthine oxidase-free model. J Natl Med Assoc. 1995;87:480–484. [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Ota M, Kaneko H, Kasahara Y, Ohta T, Komoriya K. Nephrotoxic effects of allopurinol in dinitrofluorobenzene-sensitized mice: comparative studies on TEI-6720. Res Commun Mol Pathol Pharmacol. 1999c;104:293–305. [PubMed] [Google Scholar]
- Horiuchi H, Ota M, Kitahara S, Ohta T, Kiyoki M, Komoriya K. Allopurinol increases ear swelling and mortality in a dinitrofluorobenzene-induced contact hypersensitivity mouse model. Biol Pharm Bull. 1999a;22:810–815. doi: 10.1248/bpb.22.810. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Ota M, Kobayashi M, Kaneko H, Kasahara Y, Nishimura S, Kondo S, Komoriya K. A comparative study on the hypouricemic activity and potency in renal xanthine calculus formation of two xanthine oxidase/xanthine dehydrogenase inhibitors: TEI-6720 and allopurinol in rats. Res Commun Mol Pathol Pharmacol. 1999b;104:307–319. [PubMed] [Google Scholar]
- Horiuchi H, Ota M, Nishimura S, Kaneko H, Kasahara Y, Ohta T, Komoriya K. Allopurinol induces renal toxicity by impairing pyrimidine metabolism in mice. Life Sci. 2000;66:2051–2070. doi: 10.1016/s0024-3205(00)00532-4. [DOI] [PubMed] [Google Scholar]
- Hoshide S, Takahashi Y, Ishikawa T, Kubo J, Tsuchimoto M, Komoriya K, Ohno I, Hosoya T. PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairment. Nucleosides Nucleotides Nucleic Acids. 2004;23:1117–1118. doi: 10.1081/NCN-200027377. [DOI] [PubMed] [Google Scholar]
- Houston M, Chumley P, Radi R, Rubbo H, Freeman BA. Xanthine oxidase reaction with nitric oxide and peroxynitrite. Arch Biochem Biophys. 1998;355:1–8. doi: 10.1006/abbi.1998.0675. [DOI] [PubMed] [Google Scholar]
- Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium: kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- Ichimori K, Fukahori M, Nakazawa H, Okamoto K, Nishino T. Inhibition of xanthine oxidase and xanthine dehydrogenase by nitric oxide: nitric oxide converts reduced xanthine-oxidizing enzymes into the desulfo-type inactive form. J Biol Chem. 1999;274:7763–7768. doi: 10.1074/jbc.274.12.7763. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Shimokata K, Daikoku T, Fukatsu T, Tsutsui Y, Nishiyama Y. Pathogenesis of cytomegalovirus-associated pneumonitis in ICR mice: possible involvement of superoxide radicals. Arch Virol. 1992;127:11–24. doi: 10.1007/BF01309571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilich P, Hille R. Oxo, sulfido and tellurido Moenedithiolate models for xanthine oxidase: understanding the basis of enzyme reactivity. J Am Chem Soc. 2002;124:6796–6797. doi: 10.1021/ja011957k. [DOI] [PubMed] [Google Scholar]
- Ishibuchi S, Morimoto H, Oe T, Ikebe T, Inoue H, Fukunari A, Kamezawa M, Yamada I, Naka Y. Synthesis and structure-activity relationships of 1-phenylpyrazoles as xanthine oxidase inhibitors. Bioorg Med Chem Lett. 2001;11:879–882. doi: 10.1016/s0960-894x(01)00093-2. [DOI] [PubMed] [Google Scholar]
- Itoh T, Kawakami M, Yamauchi Y, Shimizu S, Nakamura M. Effect of allopurinol on ischemia and reperfusion-induced cerebral injury in spontaneously hypertensive rats. Stroke. 1986;17:1284–1287. doi: 10.1161/01.str.17.6.1284. [DOI] [PubMed] [Google Scholar]
- Janssen M, de Jong JW, Pasini E, Ferrari R. Myocardial xanthine oxidoreductase activity in hypertensive and hypercholesterolemic rats. Cardioscience. 1993;4:25–29. [PubMed] [Google Scholar]
- Jarnerot G, Strom M, Danielsson A, Kilander A, Loof L, Hultcrantz R, Lofberg R, Floren C, Nilsson A, Brostrom O. Allopurinol in addition to 5-aminosalicylic acid based drugs for the maintenance treatment of ulcerative colitis. Aliment Pharmacol Ther. 2000;14:1159–1162. doi: 10.1046/j.1365-2036.2000.00821.x. [DOI] [PubMed] [Google Scholar]
- Jarzobski J, Ferry J, Wombolt D, Fitch DM, Egan JD. Vasculitis with allopurinol therapy. Am Heart J. 1970;79:116–121. doi: 10.1016/0002-8703(70)90401-1. [DOI] [PubMed] [Google Scholar]
- Johnson WD, Kayser KL, Brenowitz JB, Saedi SF. A randomized controlled trial of allopurinol in coronary bypass surgery. Am Heart J. 1991;121:20–24. doi: 10.1016/0002-8703(91)90950-m. [DOI] [PubMed] [Google Scholar]
- Kakita T, Suzuki M, Takeuchi H, Unno M, Matsuno S. Significance of xanthine oxidase in the production of intracellular oxygen radicals in an in-vitro hypoxia-reoxygenation model. J Hepatobiliary Pancreat Surg. 2002;9:249–255. doi: 10.1007/s005340200027. [DOI] [PubMed] [Google Scholar]
- Kaliakin IE, Mit’kin AF. Effects of allopurinol on uric acid metabolism and lipid peroxidation in ischemic heart disease patients with stable angina. Kardiologiia. 1993;33:15–17. [PubMed] [Google Scholar]
- Katholi RE, Woods WT, Jr, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, McCann WP. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64–71. doi: 10.1053/ajkd.1998.v32.pm9669426. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Takeyoshi I, Furukawa H, Lee RG, Starzl TE, Todo S. Cold preservation of the human colon and ileum with University of Wisconsin solution. Clin Transplant. 1999;13:420–425. doi: 10.1034/j.1399-0012.1999.130508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayyali US, Budhiraja R, Pennella CM, Cooray S, Lanzillo JJ, Chalkley R, Hassoun PM. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol Appl Pharmacol. 2003;188:59–68. doi: 10.1016/s0041-008x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem. 1998;43:2405–2410. doi: 10.1074/jbc.M010100200. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990;31:786–790. doi: 10.1136/gut.31.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadour FH, Panas D, Ferdinandy P, Schulze C, Csont T, Lalu MM, Wildhirt SM, Schulz R. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol. 2002;283:H108–H115. doi: 10.1152/ajpheart.00549.2001. [DOI] [PubMed] [Google Scholar]
- Khan SA, Hare JM. The role of nitric oxide in the physiological regulation of Ca2+ cycling. Curr Opin Drug Discov Devel. 2003;6:658–666. [PubMed] [Google Scholar]
- Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fradley M, Shoukas AA, Berkowitz DE, Hare JM. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- Khoo BP, Leow YH. A review of inpatients with adverse drug reactions to allopurinol. Singapore Med J. 2000;41:156–160. [PubMed] [Google Scholar]
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia -induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Kingma JG, Jr, Denniss AR, Hearse DJ, Downey JM, Yellon DM. Limitation of infarct size for 24 hours by combined treatment with allopurinol plus verapamil during acute myocardial infarction in the dog. Circulation. 1987;75:V25–V33. [PubMed] [Google Scholar]
- Kingma JG, Jr, Denniss AR, Yellon DM. Influence of allopurinol plus verapamil treatment on myocardial tissue necrosis during permanent coronary occlusion in canine hearts with small infarcts. J Cardiovasc Pharmacol. 1989;14:6–13. doi: 10.1097/00005344-198907000-00003. [DOI] [PubMed] [Google Scholar]
- Kingma JG, Jr, Miura T, Downey JM, Hearse DJ, Yellon DM. Myocardial salvage with allopurinol during 24 h of permanent coronary occlusion: importance of pretreatment. Can J Cardiol. 1988;4:360–365. [PubMed] [Google Scholar]
- Kinnula VL, Sarnesto A, Heikkila L, Toivonen H, Mattila S, Raivio KO. Assessment of xanthine oxidase in human lung and lung transplantation. Eur Respir J. 1997;10:676–680. [PubMed] [Google Scholar]
- Kinsman JM, 3rd, Murry CE, Richard VJ, Jennings RB, Reimer KA. The xanthine oxidase inhibitor oxypurinol does not limit infarct size in a canine model of 40 minutes of ischemia with reperfusion. J Am Coll Cardiol. 1988;12:209–217. doi: 10.1016/0735-1097(88)90376-2. [DOI] [PubMed] [Google Scholar]
- Kinugasa Y, Ogino K, Furuse Y, Shiomi T, Tsutsui H, Yamamoto T, Igawa O, Hisatome I, Shigemasa C. Allopurinol improves cardiac dysfunction after ischemia-reperfusion via reduction of oxidative stress in isolated perfused rat hearts. Circ J. 2003;67:781–787. doi: 10.1253/circj.67.781. [DOI] [PubMed] [Google Scholar]
- Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–362. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Matsubara M, Takeyama N, Tanaka T. The role of xanthine oxidase in paraquat intoxication. Arch Biochem Biophys. 1991;288:220–224. doi: 10.1016/0003-9861(91)90187-n. [DOI] [PubMed] [Google Scholar]
- Kittleson MM, Hare JM. Xanthine oxidase inhibitors: an emerging class of drugs for heart failure. Eur Heart J. 2005;26:1458–1460. doi: 10.1093/eurheartj/ehi321. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Kögler H, Fraser H, McCune S, Altschuld R, Marban E. Disproportionate enhancement of myocardial contractility by the xanthine oxidase inhibitor oxypurinol in failing rat myocardium. Cardiovasc Res. 2003;59:582–592. doi: 10.1016/s0008-6363(03)00512-1. [DOI] [PubMed] [Google Scholar]
- Komaki Y, Sugiura H, Koarai A, Tomaki M, Ogawa H, Akita T, Hattori T, Ichinose M. Cytokine-mediated xanthine oxidase upregulation in chronic obstructive pulmonary disease’s airways. Pulm Pharmacol Ther. 2005;18:297–302. doi: 10.1016/j.pupt.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Komoriya K, Hoshide S, Takeda K, Kobayashi H, Kubo J, Tsuchimoto M, Nakachi T, Yamanaka H, Kamatani N. Pharmacokinetics and pharmacodynamics of febuxostat (TMX-67), a non-purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NPSIXO) in patients with gout and/or hyperuricemia. Nucleosides Nucleotides Nucleic Acids. 2004;23:1119–1122. doi: 10.1081/NCN-200027381. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Bradford BU, Connor HD, Mason RP, Thurman RG. Allopurinol prevents early alcohol-induced liver injury in rats. J Pharmacol Exp Ther. 2000;293:296–303. [PubMed] [Google Scholar]
- Konya L, Kekesi V, Nagy SJ, Feher J. Effect of antioxidant treatment on the myocardium during reperfusion in dogs. Acta Physiol Hung. 1993;81:219–228. [PubMed] [Google Scholar]
- Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994;16:149–156. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Kidney Dis. 2002;40:37–42. doi: 10.1053/ajkd.2002.33911. [DOI] [PubMed] [Google Scholar]
- Kulah B, Besler HT, Akdag M, Oruc T, Altinok G, Kulacoglu H, Ozmen MM, Coskun F. The effects of verapamil vs. allopurinol on intestinal ischemia/reperfusion injury in rats: “an experimental study”. Hepatogastroenterology. 2004;51:401–407. [PubMed] [Google Scholar]
- Kuwabara Y, Nishino T, Okamoto K, Matsumura T, Eger BT, Pai EF, Nishino T. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc Natl Acad Sci USA. 2003;100:8170–8175. doi: 10.1073/pnas.1431485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin AI, Tskitishvili OV, Serebryakova LI, Kapelko VI, Majorova IV, Medvedev OS. Allopurinol: kinetics, inhibition of xanthine oxidase activity and protective effect in ischemic-reperfused canine heart as studied by cardiac microdialysis. J Cardiovasc Pharmacol. 1995;25:564–571. [PubMed] [Google Scholar]
- Laakso J, Mervaala E, Himberg JJ, Teravainen TL, Karppanen H, Vapaatalo H, Lapatto R. Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension. 1998;32:902–906. doi: 10.1161/01.hyp.32.5.902. [DOI] [PubMed] [Google Scholar]
- Laakso JT, Teravainen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. 2004;22:1333–1340. doi: 10.1097/01.hjh.0000125441.28861.9f. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Drexler H. Allopurinol and endothelial function in heart failure: future or fantasy? Circulation. 2002;106:173–175. doi: 10.1161/01.cir.0000024270.37833.f9. [DOI] [PubMed] [Google Scholar]
- Lasley RD, Ely SW, Berne RM, Mentzer RM., Jr Allopurinol enhanced adenine nucleotide repletion after myocardial ischemia in the isolated rat heart. J Clin Investig. 1988;81:16–20. doi: 10.1172/JCI113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus HM, Owens ML, Hopfenbeck A. Allopurinol protection of hepatic nuclear function during hemorrhagic shock. Surg Forum. 1974;25:10–12. [PubMed] [Google Scholar]
- Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole-Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–1822. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- Li GR, Ferrier GR. Effects of allopurinol on reperfusion arrhythmias in isolated ventricles. Am J Physiol. 1992;263:H341–H348. doi: 10.1152/ajpheart.1992.263.2.H341. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601–628. doi: 10.1016/s1054-3589(08)61001-7. [DOI] [PubMed] [Google Scholar]
- Lin LN, Wang WT, Wu JZ, Hu ZY, Xie KJ. Protective effect of propofol on liver during ischemia-reperfusion injury in patients undergoing liver surgery. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:42–44. [PubMed] [Google Scholar]
- Lin Y, Phillis JW. Oxypurinol reduces focal ischemic brain injury in the rat. Neurosci Lett. 1991;126:187–190. doi: 10.1016/0304-3940(91)90550-d. [DOI] [PubMed] [Google Scholar]
- Lin Y, Phillis JW. Deoxycoformycin and oxypurinol: protection against focal ischemic brain injury in the rat. Brain Res. 1992;571:272–280. doi: 10.1016/0006-8993(92)90665-v. [DOI] [PubMed] [Google Scholar]
- Linas SL, Whittenburg D, Repine JE. Role of xanthine oxidase in ischemia/reperfusion injury. Am J Physiol. 1990;258:F711–F716. doi: 10.1152/ajprenal.1990.258.3.F711. [DOI] [PubMed] [Google Scholar]
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab Investig. 1999;79:967–974. [PubMed] [Google Scholar]
- Lindsay S, Liu TH, Xu JA, Marshall PA, Thompson JK, Parks DA, Freeman BA, Hsu CY, Beckman JS. Role of xanthine dehydrogenase and oxidase in focal cerebral ischemic injury to rat. Am J Physiol. 1991;261:H2051–H2057. doi: 10.1152/ajpheart.1991.261.6.H2051. [DOI] [PubMed] [Google Scholar]
- Liu W, Rosenberg GA, Shi H, Furuichi T, Timmins GS, Cunningham LA, Liu KJ. Xanthine oxidase activates promatrix metalloproteinase-2 in cultured rat vascular smooth muscle cells through non-free radical mechanisms. Arch Biochem Biophys. 2004;426:11–17. doi: 10.1016/j.abb.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Lochner F, Sangiah S, Burrows G, Shawley R, McNew R, Walker J. Effects of allopurinol in experimental endotoxin shock in horses. Res Vet Sci. 1989;47:178–184. [PubMed] [Google Scholar]
- Lynch ED, Gu R, Pierce C, Kil J. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res. 2005;201:81–89. doi: 10.1016/j.heares.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lynch MJ, Grum CM, Gallagher KP, Bolling SF, Deeb GM, Morganroth ML. Xanthine oxidase inhibition attenuates ischemic-reperfusion lung injury. J Surg Res. 1988;44:538–544. doi: 10.1016/0022-4804(88)90159-x. [DOI] [PubMed] [Google Scholar]
- Mabley JG, Nivorozhkin A, Southan GJ, Szabó C, Salzman AL. Pathogenetic role of xanthine oxidase in a murine model of acute lung injury. FASEB J. 2003;17:A247. [Google Scholar]
- MacGowan SW, Regan MC, Malone C, Sharkey O, Young L, Gorey TF, Wood AE. Superoxide radical and xanthine oxidoreductase activity in the human heart during cardiac operations. Ann Thorac Surg. 1995;60:1289–1293. doi: 10.1016/0003-4975(95)00616-S. [DOI] [PubMed] [Google Scholar]
- Mainous MR, Xu D, Deitch EA. Role of xanthine oxidase and prostaglandins in inflammatory-induced bacterial translocation. Circ Shock. 1993;40:99–104. [PubMed] [Google Scholar]
- Malkiel S, Harel R, Schwalb H, Uretzky G, Borman JB, Chevion M. Interaction between allopurinol and copper: possible role in myocardial protection. Free Radic Res Commun. 1993;18:7–15. doi: 10.3109/10715769309149909. [DOI] [PubMed] [Google Scholar]
- Manning AS, Coltart DJ, Hearse DJ. Ischemia and reperfusion-induced arrhythmias in the rat: effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984;55:545–548. doi: 10.1161/01.res.55.4.545. [DOI] [PubMed] [Google Scholar]
- Martin HM, Hancock JT, Salisbury V, Harrison R. Role of xanthine oxidoreductase as an antimicrobial agent. Infect Immun. 2004;72:4933–4939. doi: 10.1128/IAI.72.9.4933-4939.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz D, Rayos G, Schielke GP, Betz AL. Allopurinol and dimethylthiourea reduce brain infarction following middle cerebral artery occlusion in rats. Stroke. 1989;20:488–494. doi: 10.1161/01.str.20.4.488. [DOI] [PubMed] [Google Scholar]
- Matsui N, Satsuki I, Morita Y, Inaizumi K, Kasajima K, Kanoh R, Fukuishi N, Akagi M. Xanthine oxidase-derived reactive oxygen species activate nuclear factor κB during hepatic ischemia in rats. Jpn J Pharmacol. 2000;84:363–366. doi: 10.1254/jjp.84.363. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Shirato C, Cohen MV, Downey JM. Oxypurinol limits myocardial infarct size in closed chest dogs without pretreatment. Can J Cardiol. 1990;6:123–129. [PubMed] [Google Scholar]
- Matsumoto S, Koshiishi I, Inoguchi T, Nawata H, Utsumi H. Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice. Free Radic Res. 2003;37:767–772. doi: 10.1080/1071576031000107344. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Yamaguchi Y, Goto M, Ichiguchi O, Akizuki E, Matsuda T, Okabe K, Liang J, Ohshiro H, Iwamoto T, et al. Xanthine oxidase inhibition attenuates kupffer cell production of neutrophil chemoattractant following ischemia-reperfusion in rat liver. Hepatology. 1998;28:1578–1587. doi: 10.1002/hep.510280618. [DOI] [PubMed] [Google Scholar]
- Maurer HS, Steinherz PG, Gaynon PS, Finklestein JZ, Sather HN, Reaman GH, Bleyer WA, Hammond GD. The effect of initial management of hyperleukocytosis on early complications and outcome of children with acute lymphoblastic leukemia. J Clin Oncol. 1988;6:1425–1432. doi: 10.1200/JCO.1988.6.9.1425. [DOI] [PubMed] [Google Scholar]
- Mayer MD, Khosravan R, Vernillet L, Wu JT, Joseph-Ridge N, Mulford DJ. Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther. 2005;12:22–34. doi: 10.1097/00045391-200501000-00005. [DOI] [PubMed] [Google Scholar]
- Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- McCarthy GM, Barthelemy CR, Veum JA, Wortmann RL. Influence of antihyperuricemic therapy on the clinical and radiographic progression of gout. Arthritis Rheum. 1991;34:1489–1494. doi: 10.1002/art.1780341203. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;17(312):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- McKechnie K, Furman BL, Parratt JR. Modification by oxygen free radical scavengers of the metabolic and cardiovascular effects of endotoxin infusion in conscious rats. Circ Shock. 1986;19:429–439. [PubMed] [Google Scholar]
- McKelvey TG, Hollwarth ME, Granger DN, Engerson TD, Landler U, Jones HP. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988;254:G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- McManaman JL, Bain DL. Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. J Biol Chem. 2002;277:21261–21268. doi: 10.1074/jbc.M200828200. [DOI] [PubMed] [Google Scholar]
- McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol. 2005;25:1623–1628. doi: 10.1161/01.ATV.0000170827.16296.6e. [DOI] [PubMed] [Google Scholar]
- Megison SM, Horton JW, Chao H, Walker PB. High dose versus low dose enteral allopurinol for prophylaxis in mesenteric ischemia. Circ Shock. 1990;30:323–329. [PubMed] [Google Scholar]
- Mellin V, Isabelle M, Oudot A, Vergely-Vandriesse C, Monteil C, Di Meglio B, Henry JP, Dautreaux B, Rochette L, Thuillez C, et al. Transient reduction in myocardial free oxygen radical levels is involved in the improved cardiac function and structure after long-term allopurinol treatment initiated in established chronic heart failure. Eur Heart J. 2005;15:1544–1550. doi: 10.1093/eurheartj/ehi305. [DOI] [PubMed] [Google Scholar]
- Meneshian A, Bulkley GB. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, Rosano GM. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94:93293–93295. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Mervaala EM, Cheng ZJ, Tikkanen I, Lapatto R, Nurminen K, Vapaatalo H, Muller DN, Fiebeler A, Ganten U, Ganten D, et al. Endothelial dysfunction and xanthine oxidoreductase activity in rats with human renin and angiotensinogen genes. Hypertension. 2001;37:414–418. doi: 10.1161/01.hyp.37.2.414. [DOI] [PubMed] [Google Scholar]
- Metzger J, Dore SP, Lauterburg BH. Oxidant stress during reperfusion of ischemic liver: no evidence for a role of xanthine oxidase. Hepatology. 1988;8:580–584. doi: 10.1002/hep.1840080324. [DOI] [PubMed] [Google Scholar]
- Miele M, Esposito G, Migheli R, Sircana S, Zangani D, Fresu GL, Desole MS. Effects of allopurinol on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurochemical changes in the striatum and in the brainstem of the rat. Neurosci Lett. 1995;3:155–159. doi: 10.1016/0304-3940(94)11138-9. [DOI] [PubMed] [Google Scholar]
- Miesel R, Zuber M. Elevated levels of xanthine oxidase in serum of patients with inflammatory and autoimmune rheumatic diseases. Inflammation. 1993;17:551–561. doi: 10.1007/BF00914193. [DOI] [PubMed] [Google Scholar]
- Miesel R, Zuber M, Sanocka D, Graetz R, Kroeger H. Effects of allopurinol on in vivo suppression of arthritis in mice and ex vivo modulation of phagocytic production of oxygen radicals in whole human blood. Inflammation. 1994;18:597–612. doi: 10.1007/BF01535258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar TM, Kanczler JM, Bodamyali T, Blake DR, Stevens CR. Xanthine oxidase is a peroxynitrite synthase: newly identified roles for a very old enzyme. Redox Rep. 2002;7:65–70. doi: 10.1179/135100002125000280. [DOI] [PubMed] [Google Scholar]
- Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006 doi: 10.1161/01.RES.0000200181.59551.71. in press. [DOI] [PubMed] [Google Scholar]
- Miura T, Yellon DM, Kingma J, Downey JM. Protection afforded by allopurinol in the first 24 hours of coronary occlusion is diminished after 48 hours. Free Radic Biol Med. 1988;4:25–30. doi: 10.1016/0891-5849(88)90007-x. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Sato K, Shinbori T, Okamoto T, Gushima Y, Fujiki M, Suga M. Effects of inducible nitric oxide synthase and xanthine oxidase inhibitors on SEB-induced interstitial pneumonia in mice. Eur Respir J. 2002;19:447–457. doi: 10.1183/09031936.02.00265902. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Akaike T, Yoshida M, Goto S, Horie H, Maeda H. Potentiation of nitric oxide-mediated vasorelaxation by xanthine oxidase inhibitors. Proc Soc Exp Biol Med. 1996;211:366–373. doi: 10.3181/00379727-211-43982. [DOI] [PubMed] [Google Scholar]
- Montor SG, Thoolen MJ, Mackin WM, Timmermans PB. Effect of azapropazone and allopurinol on myocardial infarct size in rats. Eur J Pharmacol. 1987;140:203–207. doi: 10.1016/0014-2999(87)90806-5. [DOI] [PubMed] [Google Scholar]
- Mori H, Arai T, Ishii H, Adachi T, Endo N, Makino K, Mori K. Neuroprotective effects of pterin-6-aldehyde in gerbil global brain ischemia: comparison with those of α-phenyl-N-tert-butyl nitrone. Neurosci Lett. 1998;241:99–102. doi: 10.1016/s0304-3940(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Motoe M, Yoshida S. A useful canine model of ischemic myocardium with coronary retrograde flow diversion and its application for the study of allopurinol on myocardial infarct size. Jpn Circ J. 1991;55:490–499. doi: 10.1253/jcj.55.490. [DOI] [PubMed] [Google Scholar]
- Moumen R, Ait-Oukhatar N, Bureau F, Fleury C, Bougle D, Arhan P, Neuville D, Viader F. Aluminium increases xanthine oxidase activity and disturbs antioxidant status in the rat. J Trace Elem Med Biol. 2001;15:89–93. doi: 10.1016/S0946-672X(01)80049-3. [DOI] [PubMed] [Google Scholar]
- Mugge A, Brandes RP, Boger RH, Dwenger A, Bode-Boger S, Kienke S, Frolich JC, Lichtlen PR. Vascular release of superoxide radicals is enhanced in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 1994;24:994–998. doi: 10.1097/00005344-199424060-00019. [DOI] [PubMed] [Google Scholar]
- Myers CL, Weiss SJ, Kirsh MM, Shepard BM, Shlafer M. Effects of supplementing hypothermic crystalloid cardioplegic solution with catalase, superoxide dismutase, allopurinol, or deferoxamine on functional recovery of globally ischemic and reperfused isolated hearts. J Thorac Cardiovasc Surg. 1986;91:281–289. [PubMed] [Google Scholar]
- Naito S, Nishimura M, Tamao Y. Evaluation of the pharmacological actions and pharmacokinetics of BOF-4272, a xanthine oxidase inhibitor, in mouse liver. J Pharm Pharmacol. 2000;52:173–179. doi: 10.1211/0022357001773823. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Niwa M, Iwai T, Uematsu T. Involvement of free radicals in cerebral vascular reperfusion injury evaluated in a transient focal cerebral ischemia model of rat. Free Radic Biol Med. 1999;26:722–729. doi: 10.1016/s0891-5849(98)00257-3. [DOI] [PubMed] [Google Scholar]
- Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol. 2006;290:H837–H843. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- Nemeth I, Talosi G, Papp A, Boda D. Xanthine oxidase activation in mild gestational hypertension. Hypertens Pregnancy. 2002;21:1–11. doi: 10.1081/PRG-120002905. [DOI] [PubMed] [Google Scholar]
- Niederau C, Niederau M, Borchard F, Ude K, Luthen R, Strohmeyer G, Ferrell LD, Grendell JH. Effects of antioxidants and free radical scavengers in three different models of acute pancreatitis. Pancreas. 1992;7:4864–4896. doi: 10.1097/00006676-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Nielsen VG, Weinbroum A, Tan S, Samuelson PN, Gelman S, Parks DA. Xanthine oxidoreductase release after descending thoracic aorta occlusion and reperfusion in rabbits. J Thorac Cardiovasc Surg. 1994;107:1222–1227. [PubMed] [Google Scholar]
- Nilsson UA, Schoenberg MH, Aneman A, Poch B, Magadum S, Beger HG, Lundgren O. Free radicals and pathogenesis during ischemia and reperfusion of the cat small intestine. Gastroenterology. 1994;106:629–636. doi: 10.1016/0016-5085(94)90695-5. [DOI] [PubMed] [Google Scholar]
- Nishino T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J Biochem (Tokyo) 1994;116:1–6. doi: 10.1093/oxfordjournals.jbchem.a124480. [DOI] [PubMed] [Google Scholar]
- Nishino T, Nakanishi S, Okamoto K, Mizushima J, Hori H, Iwasaki T, Nishino T, Ichimori K, Nakazawa H. Conversion of xanthine dehydrogenase into oxidase and its role in reperfusion injury. Biochem Soc Trans. 1997;25:783–786. doi: 10.1042/bst0250783. [DOI] [PubMed] [Google Scholar]
- Nishino T, Okamoto K, Kawaguchi Y, Hori H, Matsumura T, Eger BT, Pai EF, Nishino T. Mechanism of conversion of xanthine dehydrogenase to xanthine oxidase: Identification of the two cysteine disulfide bonds and crystal structure of a non-convertible rat liver xanthine dehydrogenase mutant. J Biol Chem. 2005;280:24888–24894. doi: 10.1074/jbc.M501830200. [DOI] [PubMed] [Google Scholar]
- Nishizawa J, Nakai A, Matsuda K, Komeda M, Ban T, Nagata K. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation. 1999;99:934–941. doi: 10.1161/01.cir.99.7.934. [DOI] [PubMed] [Google Scholar]
- Nivorozhkin A, Mabley J, Pacher P, Southan GJ, Szabó C, Salzman AL. A novel xanthine oxidase inhibitor, AN-01-24, protects against development of colitis in mice. FASEB J. 2003a;17:A265. [Google Scholar]
- Nivorozhkin A, Mabley JG, Pacher P, Li H, Southan GJ, Szabó C, Salzman AL. A novel class of potent non-purine based xanthine oxidase inhibitors: antihyperuricemic and hepatoprotective effects. FASEB J. 2003b;17:A253. [Google Scholar]
- Novotny MJ, Laughlin MH, Adams HR. Evidence for lack of importance of oxygen free radicals in Escherichia coli endotoxemia in dogs. Am J Physiol. 1988;254:H954–H962. doi: 10.1152/ajpheart.1988.254.5.H954. [DOI] [PubMed] [Google Scholar]
- Obata T, Kubota S, Yamanaka Y. Allopurinol suppresses paranonylphenol and 1-methyl-4-phenylpyridinium ion (MPP+)-induced hydroxyl radical generation in rat striatum. Neurosci Lett. 2001;1–2:9–12. doi: 10.1016/s0304-3940(01)01828-6. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Pacher P, Szabó C, Zsengeller Z, Hirooka H, Stevens MJ, Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005;1:234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll JG, Green DJ, Rankin JM, Taylor RR. Nitric oxide-dependent endothelial function is unaffected by allopurinol in hypercholesterolaemic subjects. Clin Exp Pharmacol Physiol. 1999;26:779–783. doi: 10.1046/j.1440-1681.1999.03125.x. [DOI] [PubMed] [Google Scholar]
- Oei HH, Stroo WE, Burton KP, Schaffer SW. A possible role of xanthine oxidase in producing oxidative stress in the heart of chronically ethanol treated rats. Res Commun Chem Pathol Pharmacol. 1982;38:453–461. [PubMed] [Google Scholar]
- Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Investig. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Yamashita C, Okada M, Okada M. Successful 24-hour rabbit heart preservation by hypothermic continuous coronary microperfusion with oxygenated University of Wisconsin solution. Ann Thorac Surg. 1995;60:1723–1728. doi: 10.1016/0003-4975(95)00761-x. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, Nishino T. An extremely potent inhibitor of xanthine oxidoreductase: crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem. 2003;278:1848–1855. doi: 10.1074/jbc.M208307200. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Matsumoto K, Hille R, Eger BT, Pai EF, Nishino T. The crystal structure of xanthine oxidoreductase during catalysis: implications for reaction mechanism and enzyme inhibition. Proc Natl Acad Sci USA. 2004;101:7931–7936. doi: 10.1073/pnas.0400973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M, Furuhashi K, Nakai Y, Muneyuki M. Decrease of ischaemia-reperfusion related lung oedema by continuous ventilation and allopurinol in rat perfusion lung model. Scand J Clin Lab Investig. 1993;53:625–631. [PubMed] [Google Scholar]
- Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- Owens ML, Lazarus HM, Wolcott MW, Maxwell JG, Taylor JB. Allopurinol and hypoxanthine pretreatment of canine kidney donors. Transplantation. 1974;17:424–427. doi: 10.1097/00007890-197404000-00015. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabó E, Ungvari Z, Wolin MS, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Mabley J, Komjati K, Szabó C. Pharmacologic inhibition of poly(adenosine diphosphateribose) polymerase may represent a novel therapeutic approach in chronic heart. J Am Coll Cardiol. 2002a;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabó C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002b;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Obrosova IG, Mabley JG, Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications: emerging new therapeutical strategies. Curr Med Chem. 2005a;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Schulz R, Liaudet L, Szabó C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005b;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S, Powell D, Benboubetra M, Stevens CR, Blake DR, Selase F, Wolstenholme AJ, Harrison R. Xanthine oxidoreductase in human mammary epithelial cells: activation in response to inflammatory cytokines. Biochim Biophys Acta. 1998;1381:191–202. doi: 10.1016/s0304-4165(98)00028-2. [DOI] [PubMed] [Google Scholar]
- Pal B, Foxall M, Dysart T, Carey F, Whittaker M. How is gout managed in primary care? A review of current practice and proposed guidelines. Clin Rheumatol. 2000;19:21–25. doi: 10.1007/s100670050005. [DOI] [PubMed] [Google Scholar]
- Palmer C, Towfighi J, Roberts RL, Heitjan DF. Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr Res. 1993;33:405–411. doi: 10.1203/00006450-199304000-00018. [DOI] [PubMed] [Google Scholar]
- Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res. 1990;27:332–336. doi: 10.1203/00006450-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Panus PC, Wright SA, Chumley PH, Radi R, Freeman BA. The contribution of vascular endothelial xanthine dehydrogenase/oxidase to oxygen-mediated cell injury. Arch Biochem Biophys. 1992;294:695–702. doi: 10.1016/0003-9861(92)90743-g. [DOI] [PubMed] [Google Scholar]
- Park DA, Bulkley GB, Granger DN. Role of oxygen free radicals in shock, ischemia and organ preservation. Surgery. 1983;94:428–432. [PubMed] [Google Scholar]
- Parker JC, Smith EE. Effects of xanthine oxidase inhibition in cardiac arrest. Surgery. 1972;71:339–344. [PubMed] [Google Scholar]
- Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982;82:9–15. [PubMed] [Google Scholar]
- Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983;245:G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988;254:G768–G774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- Parmley LF, Mufti AG, Downey JM. Allopurinol therapy of ischemic heart disease with infarct extension. Can J Cardiol. 1992;8:280–286. [PubMed] [Google Scholar]
- Parra E, Gota R, Gamen A, Moros M, Azuara M. Granulomatous interstitial nephritis secondary to allopurinol treatment. Clin Nephrol. 1995;43:350. [PubMed] [Google Scholar]
- Parratt JR, Wainwright CL. Failure of allopurinol and a spin trapping agent N-t-butyl-α-phenyl nitrone to modify significantly ischaemia and reperfusion-induced arrhythmias. Br J Pharmacol. 1987;91:49–59. doi: 10.1111/j.1476-5381.1987.tb08982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual E. Gout update: from lab to the clinic and back. Curr Opin Rheumatol. 2000;12:213–218. doi: 10.1097/00002281-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol. 2001;88:188–191. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- Patt A, Harken AH, Burton LK, Rodell TC, Piermattei D, Schorr WJ, Parker NB, Berger EM, Horesh IR, Terada LS, et al. Xanthine oxidase-derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J Clin Investig. 1988;81:1556–1562. doi: 10.1172/JCI113488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pea F. Pharmacology of drugs for hyperuricemia: mechanisms, kinetics and interactions. Contrib Nephrol. 2005;147:35–46. doi: 10.1159/000082540. [DOI] [PubMed] [Google Scholar]
- Perez NG, Gao WD, Marban E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ Res. 1998;83:423–430. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- Pesonen EJ, Linder N, Raivio KO, Sarnesto A, Lapatto R, Hockerstedt K, Makisalo H, Andersson S. Circulating xanthine oxidase and neutrophil activation during human liver transplantation. Gastroenterology. 1998;14:1009–1015. doi: 10.1016/s0016-5085(98)70321-x. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Lin Y. Oxypurinol reduces ischemic brain injury in the gerbil and rat. Adv Exp Med Biol. 1991;309A:343–346. doi: 10.1007/978-1-4899-2638-8_77. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Perkins LM, Smith-Barbour M, O’Regan MH. Oxypurinol-enhanced postischemic recovery of the rat brain involves preservation of adenine nucleotides. J Neurochem. 1995;64:2177–2184. doi: 10.1046/j.1471-4159.1995.64052177.x. [DOI] [PubMed] [Google Scholar]
- Pitt RM, McKelvey TG, Saenger JS, Shah AK, Jones HP, Manci EA, Powell RW. A tungsten-supplemented diet delivered by transplacental and breast-feeding routes lowers intestinal xanthine oxidase activity and affords cytoprotection in ischemia-reperfusion injury to the small intestine. J Pediatr Surg. 1991;26:930–935. doi: 10.1016/0022-3468(91)90839-l. [DOI] [PubMed] [Google Scholar]
- Poggetti RS, Moore FA, Moore EE, Koeike K, Banerjee A. Simultaneous liver and lung injury following gut ischemia is mediated by xanthine oxidase. J Trauma. 1992;32:723–727. doi: 10.1097/00005373-199206000-00008. [DOI] [PubMed] [Google Scholar]
- Pritsos CA. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem Biol Interact. 2000;129:195–208. doi: 10.1016/s0009-2797(00)00203-9. [DOI] [PubMed] [Google Scholar]
- Puett DW, Forman MB, Cates CU, Wilson BH, Hande KR, Friesinger GC, Virmani R. Oxypurinol limits myocardial stunning but does not reduce infarct size after reperfusion. Circulation. 1987;76:678–686. doi: 10.1161/01.cir.76.3.678. [DOI] [PubMed] [Google Scholar]
- Qayumi AK, Godin DV, Jamieson WR, Ko KM, Poostizadeh A. Correlation of red cell antioxidant status and heart-lung function in swine pretreated with allopurinol (a model of heart-lung transplantation) Transplantation. 1993;56:37–43. doi: 10.1097/00007890-199307000-00007. [DOI] [PubMed] [Google Scholar]
- Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glycosaminoglycans: kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Arch Biochem Biophys. 1997;339:125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- Radi R, Tan S, Prodanov E, Evans RA, Parks DA. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta. 1992;1122:178–182. doi: 10.1016/0167-4838(92)90321-4. [DOI] [PubMed] [Google Scholar]
- Rashid MA, William-Olsson G. Influence of allopurinol on cardiac complications in open heart operations. Ann Thorac Surg. 1991;52:127–130. doi: 10.1016/0003-4975(91)91433-v. [DOI] [PubMed] [Google Scholar]
- Reimer KA, Jennings RB. Failure of the xanthine oxidase inhibitor allopurinol to limit infarct size after ischemia and reperfusion in dogs. Circulation. 1985;71:1069–1075. doi: 10.1161/01.cir.71.5.1069. [DOI] [PubMed] [Google Scholar]
- Reynolds MD. Gout and hyperuricemia associated with sickle-cell anemia. Semin Arthritis Rheum. 1983;12:404–413. doi: 10.1016/0049-0172(83)90020-3. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Rhenius ST, Hunter JO. Xanthine oxidase activity is not increased in the colonic mucosa of ulcerative colitis. Aliment Pharmacol Ther. 1996;10:737–741. doi: 10.1046/j.1365-2036.1996.57199000.x. [DOI] [PubMed] [Google Scholar]
- Rhoden E, Pereira-Lima L, Lucas M, Mauri M, Rhoden C, Pereira-Lima JC, Zettler C, Petteffi L, Bello-Klein A. The effects of allopurinol in hepatic ischemia and reperfusion: experimental study in rats. Eur Surg Res. 2000a;32:215–222. doi: 10.1159/000008767. [DOI] [PubMed] [Google Scholar]
- Rhoden E, Teloken C, Lucas M, Rhoden C, Mauri M, Zettler C, Bello-Klein A, Barros E. Protective effect of allopurinol in the renal ischemia-reperfusion in uninephrectomized rats. Gen Pharmacol. 2000b;35:189–193. doi: 10.1016/s0306-3623(01)00105-7. [DOI] [PubMed] [Google Scholar]
- Riaz AA, Schramm R, Sato T, Menger MD, Jeppsson B, Thorlacius H. Oxygen radical-dependent expression of CXC chemokines regulate ischemia/reperfusion-induced leukocyte adhesion in the mouse colon. Free Radic Biol Med. 2003;35:782–789. doi: 10.1016/s0891-5849(03)00405-2. [DOI] [PubMed] [Google Scholar]
- Riaz AA, Wan MX, Schafer T, Dawson P, Menger MD, Jeppsson B, Thorlacius H. Allopurinol and superoxide dismutase protect against leucocyte-endothelium interactions in a novel model of colonic ischaemia-reperfusion. Br J Surg. 2002;89:1572–1580. doi: 10.1046/j.1365-2168.2002.02279.x. [DOI] [PubMed] [Google Scholar]
- Richard VJ, Murry CE, Jennings RB, Reimer KA. Oxygen-derived free radicals and postischemic myocardial reperfusion: therapeutic implications. Fundam Clin. 1990;4:85–103. doi: 10.1111/j.1472-8206.1990.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Rijhwani A, Sen S, Gunasekaran S, Ponnaiya J, Balasubramanian KA, Mammen KE. Allopurinol reduces the severity of peritoneal adhesions in mice. J Pediatr Surg. 1995;30:533–537. doi: 10.1016/0022-3468(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Rinaldo JE, Clark M, Parinello J, Shepherd VL. Nitric oxide inactivates xanthine dehydrogenase and xanthine oxidase in interferon-γ-stimulated macrophages. Am J Respir Cell Mol Biol. 1994;11:625–630. doi: 10.1165/ajrcmb.11.5.7524568. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Kaack MB, Baskin G. Treatment of experimental pyelonephritis in the monkey. J Urol. 1990;143:150–154. doi: 10.1016/s0022-5347(17)39900-7. [DOI] [PubMed] [Google Scholar]
- Romao MJ, Archer M, Moura I, Moura JJG, LeGall J, Engh R, Schneider M, Hof P, Huber R. Crystal structure of the xanthine oxidase-related aldehyde oxidoreductase from D. gigas. Science (Wash DC) 1995;270:1170–1176. doi: 10.1126/science.270.5239.1170. [DOI] [PubMed] [Google Scholar]
- Rott KT, Agudelo CA. Gout. J Am Med Assoc. 2003;289:2857–2860. doi: 10.1001/jama.289.21.2857. [DOI] [PubMed] [Google Scholar]
- Rouquette M, Page S, Bryant R, Benboubetra M, Stevens CR, Blake DR, Whish WD, Harrison R, Tosh D. Xanthine oxidoreductase is asymmetrically localised on the outer surface of human endothelial and epithelial cells in culture. FEBS Lett. 1998;426:397–401. doi: 10.1016/s0014-5793(98)00385-8. [DOI] [PubMed] [Google Scholar]
- Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- Saez JC, Ward PH, Gunther B, Vivaldi E. Superoxide radical involvement in the pathogenesis of burn shock. Circ Shock. 1984;12:229–239. [PubMed] [Google Scholar]
- Sakakibara Y. Evaluation of the effectiveness of 5′-nucleotidase inhibitor and allopurinol in myocardial ischemia. Jpn Circ J. 1993;57:809–816. doi: 10.1253/jcj.57.809. [DOI] [PubMed] [Google Scholar]
- Salim AS. Role of oxygen-derived free radical scavengers in the management of recurrent attacks of ulcerative colitis: a new approach. J Lab Clin Med. 1992;119:710–717. [PubMed] [Google Scholar]
- Sanders SA, Massey V. The thermodynamics of xanthine oxidoreductase catalysis. Antioxid Redox Signal. 1999;1:371–379. doi: 10.1089/ars.1999.1.3-371. [DOI] [PubMed] [Google Scholar]
- Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Investig. 1996;74:48–56. [PubMed] [Google Scholar]
- Saugstad OD. Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics. 1996;98:103–107. [PubMed] [Google Scholar]
- Sawa T, Akaike T, Maeda H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J Biol Chem. 2000;275:32467–32474. doi: 10.1074/jbc.M910169199. [DOI] [PubMed] [Google Scholar]
- Schlesinger N. Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs. 2004;64:2399–2416. doi: 10.2165/00003495-200464210-00003. [DOI] [PubMed] [Google Scholar]
- Schoenberg MH, Beger HG. Reperfusion injury after intestinal ischemia. Crit Care Med. 1993;21:376–386. doi: 10.1097/00003246-199309000-00023. [DOI] [PubMed] [Google Scholar]
- Schrader J. Mechanisms of ischemic injury in the heart. Basic Res Cardiol. 1985;80(Suppl 2):135–139. [PubMed] [Google Scholar]
- Sedghi S, Fields JZ, Klamut M, Urban G, Durkin M, Winship D, Fretland D, Olyaee M, Keshavarzian A. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut. 1993;34:1191–1197. doi: 10.1136/gut.34.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BH, Sakamoto N, Patel M, Maemura K, Klein AS, Holland SM, Bulkley GB. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47(phox−/−) mouse model of chronic granulomatous disease. Infect Immun. 2000;68:2374–2378. doi: 10.1128/iai.68.4.2374-2378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermet A, Tasdemir N, Deniz B, Atmaca M. Time-dependent changes in superoxide dismutase, catalase, xanthine dehydrogenase and oxidase activities in focal cerebral ischaemia. Cytobios. 2000;102:157–172. [PubMed] [Google Scholar]
- Sertac A, Bingol F, Aydin S, Uslu A. Peroxidative damage in sickle-cell erythrocyte ghosts: protective effect of allopurinol. Gen Pharmacol. 1997;28:427–428. doi: 10.1016/s0306-3623(96)00297-2. [DOI] [PubMed] [Google Scholar]
- Shadid M, Moison R, Steendijk P, Hiltermann L, Berger HM, van Bel F. The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism and electrical activity of the newborn brain. Pediatr Res. 1998;44:119–124. doi: 10.1203/00006450-199807000-00019. [DOI] [PubMed] [Google Scholar]
- Shadid M, Van Bel F, Steendijk P, Dorrepaal CA, Moison R, Van der Velde ET, Baan J. Pretreatment with allopurinol in cardiac hypoxic-ischemic reperfusion injury in newborn lambs exerts its beneficial effect through afterload reduction. Basic Res Cardiol. 1999;94:23–30. doi: 10.1007/s003950050123. [DOI] [PubMed] [Google Scholar]
- Shatney CH, MacCarter DJ, Lillehei RC. Effects of allopurinol, propranolol and methylprednisolone on infarct size in experimental myocardial infarction. Am J Cardiol. 1976;37:572–580. doi: 10.1016/0002-9149(76)90398-2. [DOI] [PubMed] [Google Scholar]
- Shatney CH, Toledo-Pereyra LH, Lillehei RC. Experiences with allopurinal in canine endotoxin shock. Adv Shock Res. 1980;4:119–137. [PubMed] [Google Scholar]
- Shehab AM, Butler R, MacFadyen RJ, Struthers AD. A placebo-controlled study examining the effect of allopurinol on heart rate variability and dysrhythmia counts in chronic heart failure. Br J Clin Pharmacol. 2001;51:329–334. doi: 10.1046/j.1365-2125.2001.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siems WG, Grune T, Werner A, Gerber G, Buntrock P, Schneider W. Protective influence of oxypurinol on the trinitrobenzene sulfonic acid (TNB) model of inflammatory bowel disease in rats. Cell Mol Biol. 1992;38:189–199. [PubMed] [Google Scholar]
- Smalley RV, Guaspari A, Haase-Statz S, Anderson SA, Cederberg D, Hohneker JA. Allopurinol: intravenous use for prevention and treatment of hyperuricemia. J Clin Oncol. 2000;18:1758–1763. doi: 10.1200/JCO.2000.18.8.1758. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Dalipram RA, Dusting GJ, Woodman OL. Impaired endothelium-dependent relaxation of dog coronary arteries after myocardial ischaemia and reperfusion: prevention by amlodipine, propranolol and allopurinol. Br J Pharmacol. 1992;105:557–562. doi: 10.1111/j.1476-5381.1992.tb09018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Dalipram RA, Woodman OL. Allopurinol and amlodipine improve coronary vasodilatation after myocardial ischaemia and reperfusion in anaesthetized dogs. Br J Pharmacol. 1993;108:342–347. doi: 10.1111/j.1476-5381.1993.tb12807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaroglu I, Okutan O, Kaptanoglu E, Beskonakli E, Kilinc K. Increased xanthine oxidase activity after traumatic brain injury in rats. J Clin Neurosci. 2005;12:273–275. doi: 10.1016/j.jocn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Sorescu D, Griendling KK. Reactive oxygen species, mitochondria and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Chandra D, Kale RK. Modulation of radiation-induced changes in the xanthine oxidoreductase system in the livers of mice by its inhibitors. Radiat Res. 2002;157:290–297. doi: 10.1667/0033-7587(2002)157[0290:morici]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Kale RK. Effect of radiation on the xanthine oxidoreductase system in the liver of mice. Radiat Res. 1999;152:257–264. [PubMed] [Google Scholar]
- Star VL, Hochberg MC. Prevention and management of gout. Drugs. 1993;45:212–222. doi: 10.2165/00003495-199345020-00004. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Ou J, Ackerman AW, Welak S, Klick D, Pritchard KA., Jr Native LDL and minimally oxidized LDL differentially regulate superoxide anion in vascular endothelium in situ. Am J Physiol. 2002;283:H750–H759. doi: 10.1152/ajpheart.00029.2002. [DOI] [PubMed] [Google Scholar]
- Stewart JR, Crute SL, Loughlin V, Hess ML, Greenfield LJ. Prevention of free radical-induced myocardial reperfusion injury with allopurinol. J Thorac Cardiovasc Surg. 1985;90:68–72. [PubMed] [Google Scholar]
- Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart. 2002;87:229–234. doi: 10.1136/heart.87.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res. 2004;95:1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, Suematsu M, Zweifach BW, Schmid-Schonbein GW. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci USA. 1998;95:4754–4759. doi: 10.1073/pnas.95.8.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A, Lacy F, Delano FA, Parks DA, Schmid-Schonbein GW. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation. 1999;6:179–187. [PubMed] [Google Scholar]
- Szabó C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, et al. Part I: pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2002a;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- Szabó C, Pacher P, Zsengeller Z, Vaslin A, Komjati K, Benko R, Chen M, Mabley JG, Kollai M. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10:28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002b;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- Taggart DP, Young V, Hooper J, Kemp M, Walesby R, Magee P, Wright JE. Lack of cardioprotective efficacy of allopurinol in coronary artery surgery. Br Heart J. 1994;71:177–181. doi: 10.1136/hrt.71.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–1847. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Takeyama N, Shoji Y, Ohashi K, Tanaka T. Role of reactive oxygen intermediates in lipopolysaccharide-mediated hepatic injury in the rat. J Surg Res. 1996;60:258–262. doi: 10.1006/jsre.1996.0040. [DOI] [PubMed] [Google Scholar]
- Tan S, Gelman S, Wheat JK, Parks DA. Circulating xanthine oxidase in human ischemia reperfusion. South Med J. 1995;88:479–482. doi: 10.1097/00007611-199504000-00021. [DOI] [PubMed] [Google Scholar]
- Tan S, McAdams M, Royall J, Freeman BA, Parks DA. Endothelial injury from a circulating mediator following rat liver ischemia. Free Radic Biol Med. 1998;24:427–434. doi: 10.1016/s0891-5849(97)00274-8. [DOI] [PubMed] [Google Scholar]
- Tan S, Radi R, Gaudier F, Evans RA, Rivera A, Kirk KA, Parks DA. Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr Res. 1993a;34:303–307. doi: 10.1203/00006450-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Tan S, Yokoyama Y, Dickens E, Cash TG, Freeman BA, Parks DA. Xanthine oxidase activity in the circulation of rats following hemorrhagic shock. Free Radic Biol Med. 1993b;15:407–414. doi: 10.1016/0891-5849(93)90040-2. [DOI] [PubMed] [Google Scholar]
- Terada LS, Dormish JJ, Shanley PF, Leff JA, Anderson BO, Repine JE. Circulating xanthine oxidase mediates lung neutrophil sequestration after intestinal ischemia-reperfusion. Am J Physiol. 1992;263:L394–L401. doi: 10.1152/ajplung.1992.263.3.L394. [DOI] [PubMed] [Google Scholar]
- Terada LS, Piermattei D, Shibao GN, McManaman JL, Wright RM. Hypoxia regulates xanthine dehydrogenase activity at pre- and posttranslational levels. Arch Biochem Biophys. 1997a;348:163–168. doi: 10.1006/abbi.1997.0367. [DOI] [PubMed] [Google Scholar]
- Terada LS, Radisavljevic Z, Mahr NN, Jacobson ED. Xanthine oxidase decreases production of gut wall nitric oxide. Proc Soc Exp Biol Med. 1997b;216:410–413. doi: 10.3181/00379727-216-44190. [DOI] [PubMed] [Google Scholar]
- Terada LS, Rubinstein JD, Lesnefsky EJ, Horwitz LD, Leff JA, Repine JE. Existence and participation of xanthine oxidase in reperfusion injury of ischemic rabbit myocardium. Am J Physiol. 1991;260:H805–H810. doi: 10.1152/ajpheart.1991.260.3.H805. [DOI] [PubMed] [Google Scholar]
- Terkeltaub RA. Gout and mechanisms of crystal-induced inflammation. Curr Opin Rheumatol. 1993;5:510–516. doi: 10.1097/00002281-199305040-00017. [DOI] [PubMed] [Google Scholar]
- Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349:1647–1655. doi: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- Tesi RJ, Jaffe BM, McBride G, Haque S. Human small bowel preservation injury in University of Wisconsin solution. Transplant Proc. 1996;28:2609–2610. [PubMed] [Google Scholar]
- Toledo-Pereyra LH, Simmons RL, Olson LC, Najarian JS. Clinical effect of allopurinol on preserved kidneys: a randomized double-blind study. Ann Surg. 1977;185:128–131. doi: 10.1097/00000658-197701000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truglio JJ, Theis K, Leimkuhler S, Rappa R, Rajagopalan KV, Kisker C. Crystal structure of the active and alloxanthine-inhibited forms of xanthine dehydrogenase from Rhodobacter capsulatus. Structure. 2002;10:115–125. doi: 10.1016/s0969-2126(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Trujillo M, Alvarez MN, Peluffo G, Freeman BA, Radi R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J Biol Chem. 1998;273:7828–7834. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation. 2001;103:750–755. doi: 10.1161/01.cir.103.5.750. [DOI] [PubMed] [Google Scholar]
- Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvári Z, Gupte SA, Recchia FA, Bátkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vas Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel F, Shadid M, Moison RM, Dorrepaal CA, Fontijn J, Monteiro L, Van De Bor M, Berger HM. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics and electrical brain activity. Pediatrics. 1998;101:185–193. doi: 10.1542/peds.101.2.185. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steveninck J, Koster JF, Dubbelman TM. Xanthine oxidase-catalysed oxidation of paracetamol. Biochem J. 1989;259:633–637. doi: 10.1042/bj2590633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatistas NJ, Snyder JR, Nieto J, Hildebrand SV, Woliner MJ, Harmon FA, Barry SJ, Drake C. Morphologic changes and xanthine oxidase activity in the equine jejunum during low flow ischemia and reperfusion. Am J Vet Res. 1998;59:772–776. [PubMed] [Google Scholar]
- Vaughan WG, Horton JW, Walker PB. Allopurinol prevents intestinal permeability changes after ischemia-reperfusion injury. J Pediatr Surg. 1992;27:968–972. doi: 10.1016/0022-3468(92)90542-f. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J, Chiantella V, Faust KB, Johnston WE, McCain BL, Hartman M, Mills SA, Hester TO, Cordell AR. Myocardial protection with blood cardioplegia in ischemically injured hearts: reduction of reoxygenation injury with allopurinol. Ann Thorac Surg. 1988;45:319–326. doi: 10.1016/s0003-4975(10)62472-1. [DOI] [PubMed] [Google Scholar]
- Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003;24:512–517. doi: 10.1016/s1471-4906(03)00237-0. [DOI] [PubMed] [Google Scholar]
- Wakatsuki A, Okatani Y, Izumiya C, Ikenoue N. Effect of ischemia-reperfusion on xanthine oxidase activity in fetal rat brain capillaries. Am J Obstet Gynecol. 1999;181:731–735. doi: 10.1016/s0002-9378(99)70520-x. [DOI] [PubMed] [Google Scholar]
- Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–1587. [PubMed] [Google Scholar]
- Wallwork CJ, Parks DA, Schmid-Schonbein GW. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res. 2003;66:30–37. doi: 10.1016/s0026-2862(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Ward PH, Maldonado M, Vivaldi E. Oxygen-derived free radicals mediate liver damage in rats subjected to tourniquet shock. Free Radic Res Commun. 1992;17:313–325. doi: 10.3109/10715769209079524. [DOI] [PubMed] [Google Scholar]
- Werns SW, Grum CM, Ventura A, Hahn RA, Ho PP, Towner RD, Fantone JC, Schork MA, Lucchesi BR. Xanthine oxidase inhibition does not limit canine infarct size. Circulation. 1991;83:995–1005. doi: 10.1161/01.cir.83.3.995. [DOI] [PubMed] [Google Scholar]
- Werns SW, Shea MJ, Mitsos SE, Dysko RC, Fantone JC, Schork MA, Abrams GD, Pitt B, Lucchesi BR. Reduction of the size of infarction by allopurinol in the ischemic-reperfused canine heart. Circulation. 1986;73:518–524. doi: 10.1161/01.cir.73.3.518. [DOI] [PubMed] [Google Scholar]
- Wexler BC, McMurtry JP. Allopurinol amelioration of the pathophysiology of acute myocardial infarction in rats. Atherosclerosis. 1981;39:71–87. doi: 10.1016/0021-9150(81)90090-3. [DOI] [PubMed] [Google Scholar]
- White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen MH, Vader HL, Roumen RM. Multi-antioxidant supplementation does not prevent an increase in gut permeability after lower torso ischemia and reperfusion in humans. Eur Surg Res. 2002;34:300–305. doi: 10.1159/000063072. [DOI] [PubMed] [Google Scholar]
- Wilkins EG, Rees RS, Smith D, Cashmer B, Punch J, Till GO, Smith DJ., Jr Identification of xanthine oxidase activity following reperfusion in human tissue. Ann Plast Surg. 1993;31:60–65. [PubMed] [Google Scholar]
- Willgoss DA, Zhang B, Gobe GC, Kadkhodaee M, Endre ZH. Repetitive brief ischemia: intermittent reperfusion during ischemia ameliorates the extent of injury in the perfused kidney. Ren Fail. 2003;25:379–395. doi: 10.1081/jdi-120021164. [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Revuz J. Drug-induced severe skin reactions: incidence, management and prevention. Drug Saf. 1995;13:56–68. doi: 10.2165/00002018-199513010-00007. [DOI] [PubMed] [Google Scholar]
- Wortmann RL. Recent advances in the management of gout and hyperuricemia. Curr Opin Rheumatol. 2005;17:319–324. doi: 10.1097/01.bor.0000162060.25895.a5. [DOI] [PubMed] [Google Scholar]
- Wright RM, Ginger LA, Kosila N, Elkins ND, Essary B, McManaman JL, Repine JE. Mononuclear phagocyte xanthine oxidoreductase contributes to cytokine-induced acute lung injury. Am J Respir Cell Mol Biol. 2004;30:479–490. doi: 10.1165/rcmb.2003-0309OC. [DOI] [PubMed] [Google Scholar]
- Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C, Jr, Jones P, Bradley A, Caskey CT. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci USA. 1994;91:742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Dempski R, Hille R. The reductive half-reaction of xanthine oxidase: reaction with aldehyde substrates and identification of the catalytically labile oxygen. J Biol Chem. 1999;274:3323–3330. doi: 10.1074/jbc.274.6.3323. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zweier JL. Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J Biol Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- Xu D, Qi L, Guillory D, Cruz N, Berg R, Deitch EA. Mechanisms of endotoxin-induced intestinal injury in a hyperdynamic model of sepsis. J Trauma. 1993;34:676–682. doi: 10.1097/00005373-199305000-00010. [DOI] [PubMed] [Google Scholar]
- Xu P, LaVallee P, Hoidal JR. Repressed expression of the human xanthine oxidoreductase gene: E-box and TATA-like elements restrict ground state transcriptional activity. J Biol Chem. 2000;275:5918–5926. doi: 10.1074/jbc.275.8.5918. [DOI] [PubMed] [Google Scholar]
- Yamada I, Fukunari A, Osajima T, Kamezawa M, Mori H, Iwane J. Pharmacokinetics/pharmacodynamics of Y-700, a novel xanthine oxidase inhibitor, in rats and man. Nucleosides Nucleotides Nucleic Acids. 2004;23:1123–1125. doi: 10.1081/NCN-200027384. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Development antihyperuricemic candidates. Nippon Rinsho. 2003;61(Suppl 1):209–212. [PubMed] [Google Scholar]
- Yamazaki I, Soma T, Ichikawa Y, Iwai Y, Kondo J, Matsumoto A. Usefulness of allopurinol for prevention of myocardial reperfusion injury in open heart surgery. Nippon Kyobu Geka Gakkai Zasshi. 1995;43:26–31. [PubMed] [Google Scholar]
- Yang CS, Tsai PJ, Chou ST, Niu YL, Lai JS, Kuo JS. The roles of reactive oxygen species and endogenous opioid peptides in ischemia-induced arrhythmia of isolated rat hearts. Free Radic Biol Med. 1995;18:593–598. doi: 10.1016/0891-5849(94)00153-b. [DOI] [PubMed] [Google Scholar]
- Yee SB, Pritsos CA. Comparison of oxygen radical generation from the reductive activation of doxorubicin, streptonigrin and menadione by xanthine oxidase and xanthine dehydrogenase. Arch Biochem Biophys. 1997;347:235–241. doi: 10.1006/abbi.1997.0340. [DOI] [PubMed] [Google Scholar]
- Yildirim S, Tok H, Koksal H, Erdem L, Baykan A. Allopurinol plus pentoxifilline in hepatic ischaemia/reperfusion injury. Asian J Surg. 2002;25:149–153. doi: 10.1016/S1015-9584(09)60164-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Beckman JS, Beckman TK, Wheat JK, Cash TG, Freeman BA, Parks DA. Circulating xanthine oxidase: potential mediator of ischemic injury. Am J Physiol. 1990;258:G564–G570. doi: 10.1152/ajpgi.1990.258.4.G564. [DOI] [PubMed] [Google Scholar]
- Yossif AM, Ibrahim TM, Salem HA, Gamil NM, el-Sayed LM. Effect of high lipid diet and allopurinol on the development of experimentally induced arthritis in rats. Pharmacology. 1995;51:160–164. doi: 10.1159/000139330. [DOI] [PubMed] [Google Scholar]
- Zeki S, Miura S, Suzuki H, Watanabe N, Adachi M, Yokoyama H, Horie Y, Saito H, Kato S, Ishii H. Xanthine oxidase-derived oxygen radicals play significant roles in the development of chronic pancreatitis in WBN/Kob rats. J Gastroenterol Hepatol. 2002;17:606–616. doi: 10.1046/j.1440-1746.2002.02733.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Blake DR, Stevens CR, Kanczler JM, Winyard PG, Symons MC, Benboubetra M, Harrison R. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic Res. 1998a;28:151–164. doi: 10.3109/10715769809065801. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MC. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998b;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- Zima T, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, Stipek S, Mikulikova L, Popov P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci. 2001;8:59–70. doi: 10.1007/BF02255972. [DOI] [PubMed] [Google Scholar]
- Zima T, Novak L, Stipek S. Plasma xanthine oxidase level and alcohol administration. Alcohol Alcohol. 1993;28:693–694. [PubMed] [Google Scholar]


