Abstract
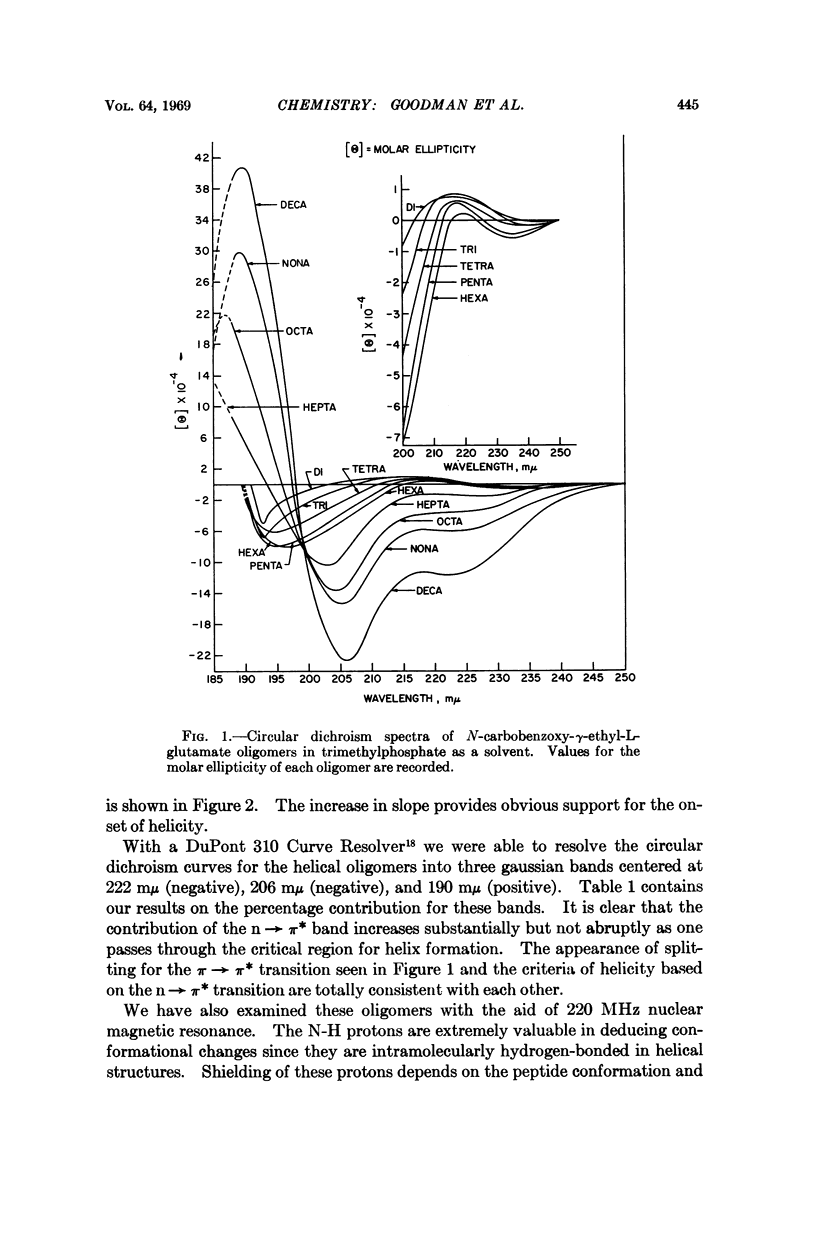
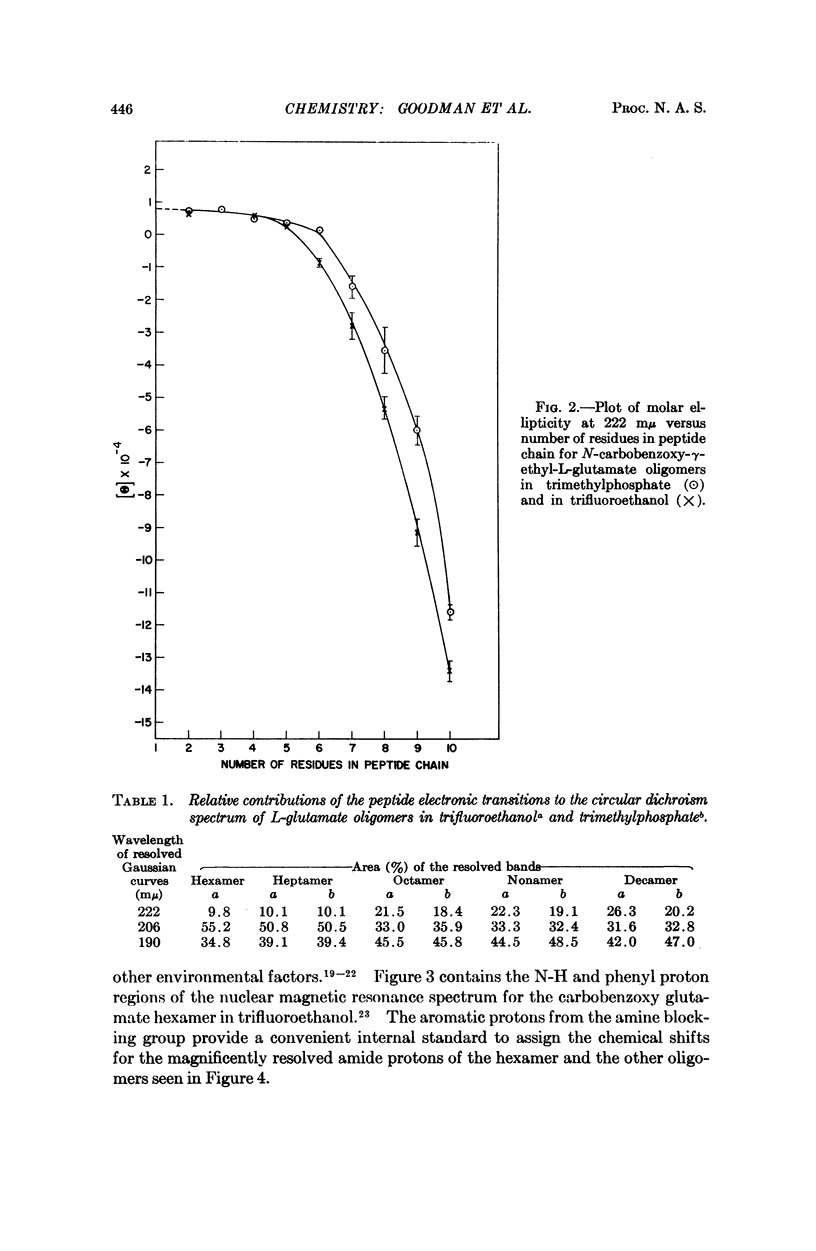
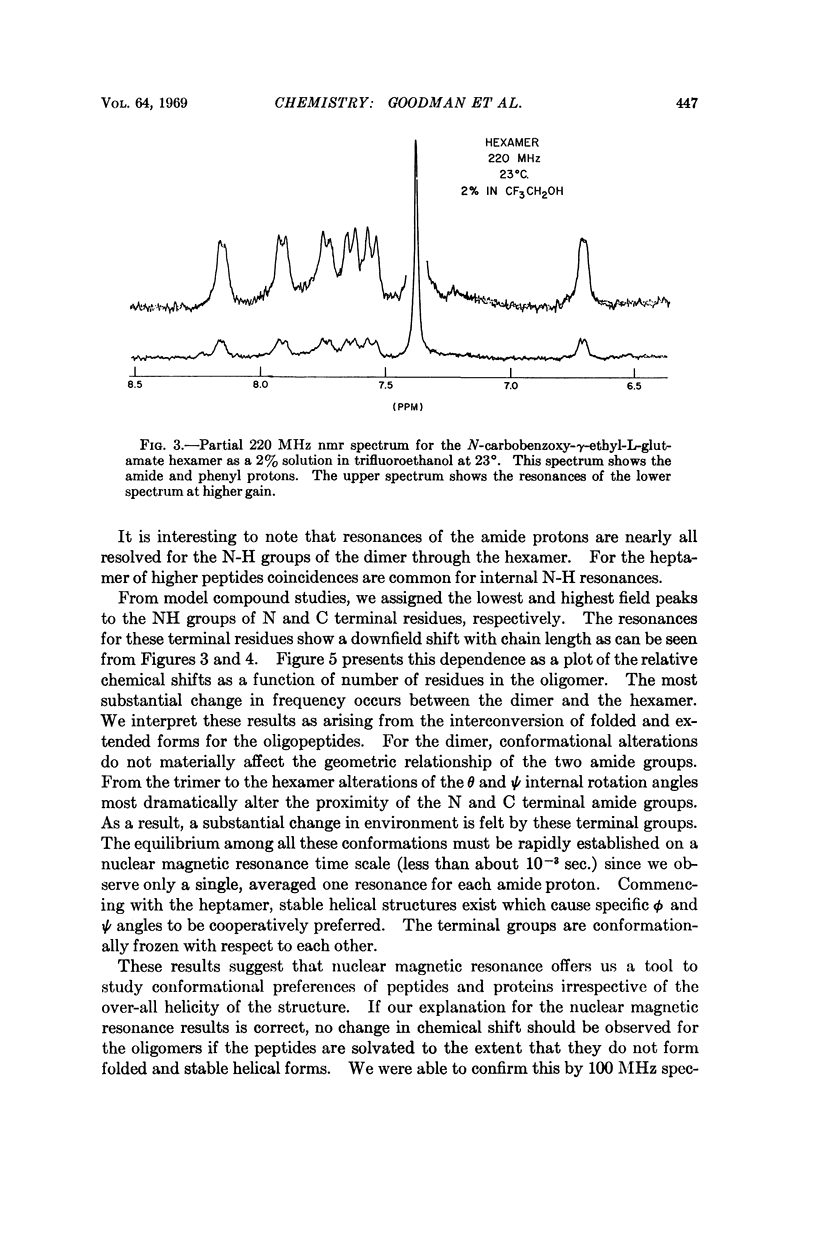
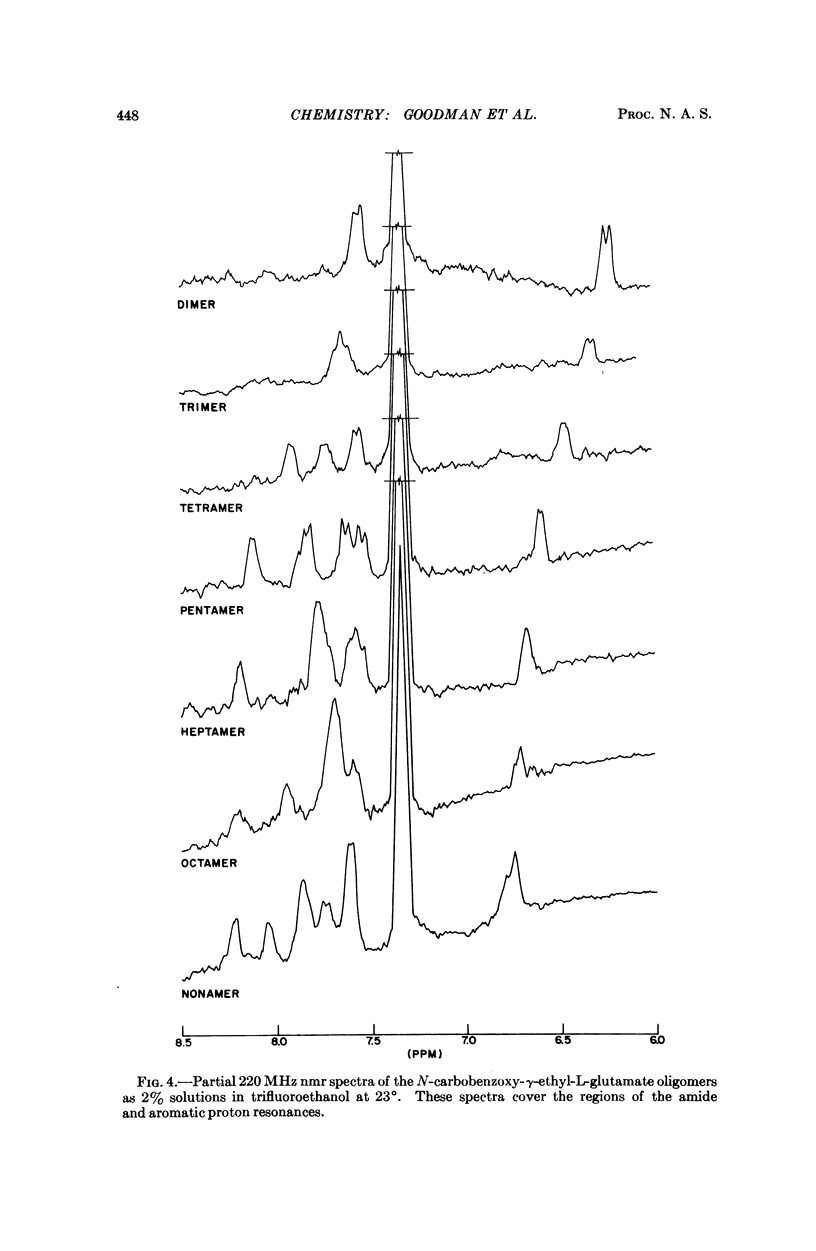
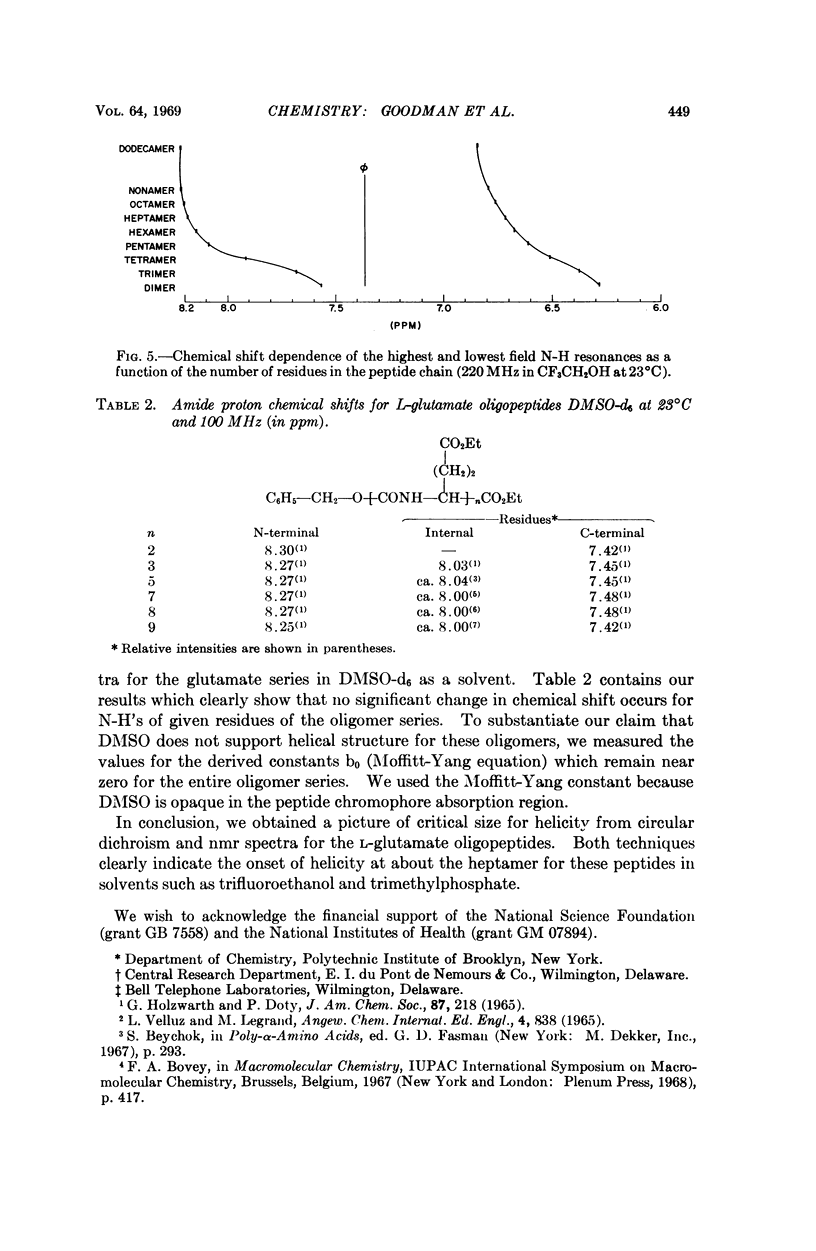
We studied the conformation of a series of γ-ethyl-L-glutamate oligopeptides by circular dichroism and 220 MHz nuclear magnetic resonance spectroscopy. By use of the first technique we noted enhancement of the n → π* and splitting of the π → π* transitions commencing with the heptamer in trimethylphosphate and trifluoroethanol. With the second method we found changes in chemical shifts for the amide protons consistent with the onset of helicity at the heptamer in the solvents noted above. When DMSO-d6 is used as a solvent, no such chemical shift changes occur because the oligopeptides do not assume helical conformations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beecham A. F., Ham N. S. Proton magnetic resonance spectra of some peptides of L-leucine and glycine. Tetrahedron. 1968 Mar;24(6):2773–2781. doi: 10.1016/s0040-4020(01)82549-7. [DOI] [PubMed] [Google Scholar]
- Beecham A. F. The Ord of glycine peptides with a C-terminal or N-terminal L-leucine residue. Tetrahedron. 1967 Nov;23(11):4481–4486. doi: 10.1016/s0040-4020(01)88846-3. [DOI] [PubMed] [Google Scholar]
- Ferretti J. A., Paolillo L. Nuclear magnetic resonance investigation of the helix in random coil transformation in poly-alpha-amino acids. I. Biopolymers. 1969;7(2):155–171. doi: 10.1002/bip.1969.360070203. [DOI] [PubMed] [Google Scholar]
- HOLZWARTH G., DOTY P. THE ULTRAVIOLET CIRCULAR DICHROISM OF POLYPEPTIDES. J Am Chem Soc. 1965 Jan 20;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- MANDEL M. PROTON MAGNETIC RESONANCE SPECTRA OF SOME PROTEINS. I. RIBONUCLEASE, OXIDIZED RIBONUCLEASE, LYSOZYME, AND CYTOCHROME C. J Biol Chem. 1965 Apr;240:1586–1592. [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D., Lazar J. Nuclear magnetic resonance determination of thymine nearest neighbor base frequency ratios in deoxyribonucleic acid. J Am Chem Soc. 1967 Aug 2;89(16):4166–4170. doi: 10.1021/ja00992a035. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Manifestations of the tertiary structures of proteins in high-frequency nuclear magnetic resonance. J Am Chem Soc. 1967 Nov 22;89(24):6332–6341. doi: 10.1021/ja01000a061. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Jardetzky O. Systematic analysis of chemical shifts in the nuclear magnetic resonance spectra of peptide chains. II. Oligoglycines. Biochemistry. 1968 Mar;7(3):1226–1230. doi: 10.1021/bi00843a045. [DOI] [PubMed] [Google Scholar]
- Shields J. E., McDowell S. T. Conformation of small peptides. I. Secondary structure in a tetrapeptide. J Am Chem Soc. 1967 May 10;89(10):2499–2500. doi: 10.1021/ja00986a055. [DOI] [PubMed] [Google Scholar]
- Weinryb I., Steiner R. F. The properties of some phenylalanyl peptides in ethylene glycol-aqueous buffer solvent. Arch Biochem Biophys. 1969 Apr;131(1):263–271. doi: 10.1016/0003-9861(69)90130-1. [DOI] [PubMed] [Google Scholar]


