Abstract
The interaction of ryanodine and derivatives of ryanodine with the high affinity binding site on the ryanodine receptor (RyR) channel brings about a characteristic modification of channel function. In all cases, channel open probability increases dramatically and single-channel current amplitude is reduced. The amplitude of the ryanoid-modified conductance state is determined by structural features of the ligand. An investigation of ion handling in the ryanodine-modified conductance state has established that reduced conductance results from changes in both the affinity of the channel for permeant ions and the relative permeability of ions within the channel (Lindsay, A.R.G., A. Tinker, and A.J. Williams. 1994. J. Gen. Physiol. 104:425–447). It has been proposed that these alterations result from a reorganization of channel structure induced by the binding of the ryanoid. The experiments reported here provide direct evidence for ryanoid-induced restructuring of RyR. TEA+ is a concentration- and voltage-dependent blocker of RyR in the absence of ryanoids. We have investigated block of K+ current by TEA+ in the unmodified open state and modified conductance states of RyR induced by 21-amino-9α-hydroxyryanodine, 21-azido-9α-hydroxyryanodine, ryanodol, and 21-p-nitrobenzoylamino-9α-hydroxyryanodine. Analysis of the voltage dependence of block indicates that the interaction of ryanoids with RyR leads to an alteration in this parameter with an apparent relocation of the TEA+ blocking site within the voltage drop across the channel and an alteration in the affinity of the channel for the blocker. The degree of change of these parameters correlates broadly with the change in conductance of permeant cations induced by the ryanoids, indicating that modification of RyR channel structure by ryanoids is likely to underlie both phenomena.
Keywords: ryanodine receptor, sarcoplasmic reticulum, ryanodine, ryanoids, block
INTRODUCTION
The specific, high affinity interaction of the plant alkaloid ryanodine with one class of intracellular membrane Ca2+ release channel defines these channels as ryanodine receptors (RyRs). On binding, ryanodine and derivatives of ryanodine (ryanoids) induce profound alterations in RyR channel function. Single-channel open probability (P o) rises dramatically and rates of ion translocation are reduced, giving rise to the occurrence of characteristic subconductance states (Rousseau et al. 1987; Ashley and Williams 1990). Ryanodine-induced modification of permeant ion translocation in RyR cannot be explained by a simple blocking mechanism; rather, the interaction of ryanodine with RyR results in alterations to many of the processes that underlie single-channel conductance. Alterations occur in the affinity of the conduction pathway of the channel for some permeant ions and the relative permeability of some ions is changed. Altered mechanisms of ion handling and the resultant, ryanodine-induced conductance vary with the nature of the permeant ion. These observations led to the suggestion that the ryanodine-induced modification of ion handling in RyR results from a conformational change of the channel protein, involving a rearrangement of the conduction pathway (Lindsay et al. 1994).
Information on the dimensions of the conduction pathway of RyR and the location of sites within this region of the channel have emerged from studies involving channel block by impermeant cations (Tinker et al. 1992; Tinker and Williams 1993c, Tinker and Williams 1995). In this report we have examined the consequences of the modification of RyR channel function by a series of ryanoids on block by TEA+, a concentration- and voltage-dependent blocker of K+ current in this channel (Lindsay et al. 1991; Tinker et al. 1992).
Quantitative analysis of voltage-dependent block of K+ current in RyR indicates that the effective valence (zδ) of TEA+ is altered in the ryanoid-modified channel, which would be consistent with a relocation of the TEA+ binding site within the voltage drop across the channel. Ryanoid modification also produces alterations in the affinity of the conduction pathway for the blocking cation. These observations are consistent with the proposal that the interaction of a ryanoid with the high affinity binding site on RyR produces a conformational alteration in the channel protein resulting in modification of conduction pathway structure and a consequent change in the handling of both permeant and impermeant cations.
MATERIALS AND METHODS
Materials
Phosphatidylethanolamine was purchased from Avanti Polar Lipids, Inc., and phosphatidylcholine from Sigma-Aldrich. [3H]Ryanodine was supplied by New England Nuclear Ltd. Aqueous counting scintillant was purchased from Packard Instrument Co. Other reagents were obtained as the best available grade from BDH Ltd. or Sigma-Aldrich. The ryanoids used in these studies were synthesized as described previously (21-amino-9α-hydroxyryanodine, 21-p-nitrobenzoylamino-9α-hydroxyryanodine, 21-azido-9α-hydroxyryanodine [Welch et al. 1997]; ryanodol [Deslongchamps et al. 1990)]) and stored as stock solutions in 50% ethanol at −20°C.
As more than one modified conductance state was observed after the addition of 21-p-nitrobenzoylamino-9α-hydroxyryanodine to RyR (see results), the purity of the compound was reinvestigated to ensure that there had been no decomposition during storage. Samples used in the experiments reported here were found to be homogeneous by TLC (both charring and fluorescence detection) and by MALDI/TOF mass spectroscopy.
Molecular Modeling
The conformations of the ryanoids shown in Fig. 1 were analyzed by molecular mechanics using the Tripos force field (SYBYL; Tripos Inc.) and semiempirical quantum mechanics (MOPAC). In Fig. 1 A, ball and stick drawings of 21-amino-9α-hydroxyryanodine (bottom left), 21-azido-9α-hydroxyryanodine (bottom right), ryanodol (top right), and 21-p-nitrobenzoylamino-9α-hydroxyryanodine (top left) are shown. All are shown in the conformation corresponding to their respective global minima. Although all of these ryanoids can exist in multiple conformations, the bulk of the long floppy tail of 21-p-nitrobenzoylamino-9α-hydroxyryanodine makes the conformers of this ryanoid of experimental interest. Molecular dynamics (SYBYL) simulations indicate that the side chain exists in three conformational families arising principally from the high rotational energy barriers about the single bond proximal to carbon 9. The local minimum of each of these families is shown in Fig. 1 B, and the significance of the existence of these conformers is discussed later.
Figure 1.
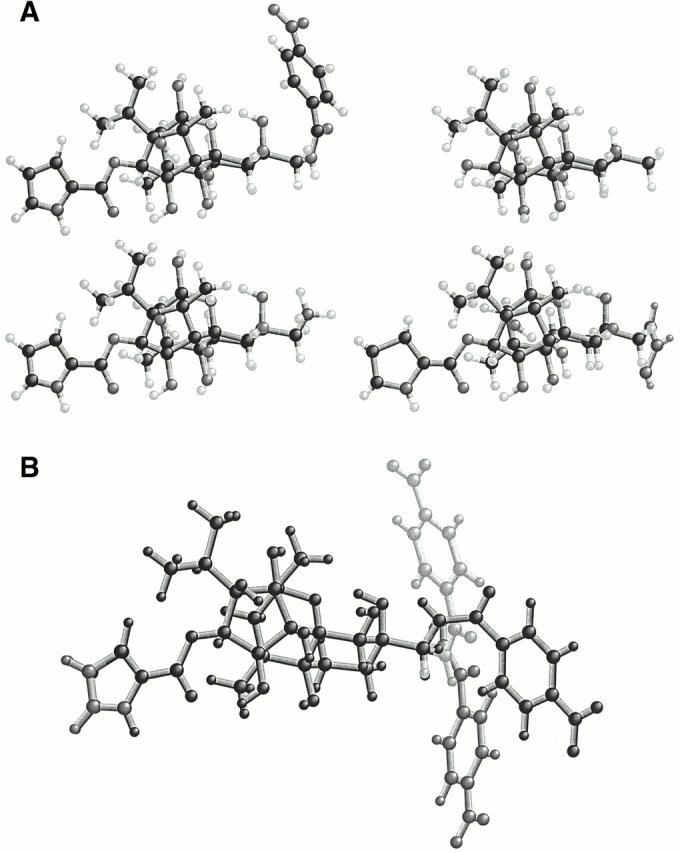
(A) Ball and stick models of 21-amino-9α-hydroxyryanodine (bottom left), 21-p-nitrobenzoylamino-9α-hydroxyryanodine (top left), ryanodol (top right), and 21-azido-9α-hydroxyryanodine (bottom right) are shown in the conformation corresponding to the respective global energy minimum. All are oriented with the 3-position to the left (note the pyrrole group at the extreme left) and the 21-position to the right (a horizontal line connects these two positions). Ryanodol (top right) is the core structure of all of the other compounds illustrated in Fig. 1. (B) The local minima (differing shades of gray) of the three most populated conformations of 21-p-nitrobenzoylamino-9α-hydroxyryanodine are superimposed by the polycyclic fused ring system. The conformational space was investigated by molecular dynamics at a simulated temperature of 25°C. Note the changes in physical shape of the molecule due to large movements of the p-nitrobenzoyl group compared with the other pendant groups.
Isolation of Sheep Cardiac Heavy Sarcoplasmic Reticulum Membrane Vesicles and Solubilization and Separation of the RyR
Sheep hearts were collected from a local abattoir in ice-cold cardioplegic solution and heavy sarcoplasmic reticulum membrane vesicles prepared using procedures described previously (Sitsapesan and Williams 1990).
Heavy sarcoplasmic reticulum membrane vesicles were solubilized with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS) and RyR isolated and reconstituted into unilamellar liposomes for incorporation into planar phospholipid bilayers, as described previously (Lindsay and Williams 1991).
Planar Phospholipid Bilayers
Phospholipid bilayers were formed from suspensions of phosphatidylethanolamine in n-decane (35 mg/ml) across a 200-μm-diam hole in a styrene copolymer partition which separated two chambers referred to as cis (volume 0.5 ml) and trans (volume 1.0 ml). The trans chamber was held at virtual ground, whereas the cis chamber could be clamped at holding potentials relative to ground. Current flow across the bilayer was monitored using an operational amplifier as a current-voltage converter (Miller 1982). Bilayers were formed with solutions containing 600 mM KCl, 20 mM HEPES, titrated to pH 7.4 with KOH, resulting in a solution containing 610 mM K+ in both chambers. An osmotic gradient was created by the addition of an aliquot (50–100 μl) of 3 M KCl to the cis chamber. Proteoliposomes were added to the cis chamber and stirred. Under these conditions, channels usually incorporated into the bilayer within 2–3 min. If channels did not incorporate, a second aliquot of 3 M KCl could be added to the cis chamber. After channel incorporation, further fusion was prevented by perfusion of the cis chamber with 610 mM K+. Channel proteins incorporate into the bilayer in a fixed orientation so that the cytosolic face of the channel is exposed to the solution in the cis chamber and the luminal face of the channel to the solution in the trans chamber. Single-channel P o was increased by the addition of 100 μM EMD 41000 to the cytosolic face of the channel (McGarry and Williams 1994; Tanna et al. 1998). Experiments were carried out at room temperature (21 ± 2°C).
Modification of RyR channel function by 21-amino-9α-hydroxyryanodine, ryanodol, 21-azido-9α-hydroxyryanodine, and 21-p-nitrobenzoylamino-9α-hydroxyryanodine was achieved by adding an appropriate concentration of the ryanoid to the solution at the cytosolic face of the bilayer. Although TEA+ is an effective blocker of the RyR channel only from the cytosolic face of the channel (Lindsay et al. 1991), it was added symmetrically to the solutions at both sides of the bilayer to avoid the possibility of asymmetric surface potentials arising from the binding of the cation to the bilayer.
Single-channel Data Acquisition and Analysis
Single-channel current fluctuations were displayed on an oscilloscope and stored on Digital Audio Tape. For analysis, data were replayed, filtered at 1 kHz with an 8-pole Bessel filter, and digitized at 4 kHz using Satori v3.2 (Intracel). Single-channel current amplitudes were measured from digitized data. The representative traces shown in the figures were obtained from digitized data acquired with Satori v3.2 and transferred as an HPGL graphics file to a graphics software package (CorelDraw; Corel Systems Corporation) for annotation and printing.
RESULTS
The high affinity association of a ryanoid with the RyR channel produces a dramatic alteration in channel function. With a ryanoid bound, rates of Ca2+ and K+ translocation through RyR are reduced and the channel enters a characteristic modified conductance state. The fractional conductance (FC) of the modified state (the amplitude of the ryanoid-modified state expressed as a proportion of the full amplitude of the channel; Lindsay et al. 1994) is determined by structural features of the ryanoid (Tinker et al. 1996; Welch et al. 1997). Dwell times in the various ryanoid-modified states differ considerably, lasting for seconds with, for example, 21-amino-9α-hydroxyryanodine, to hours with ryanodine (Tanna et al. 1998, Tanna et al. 2000).
The Influence of Voltage on TEA+ Block of Unmodified and Ryanoid-modified RyR Channels
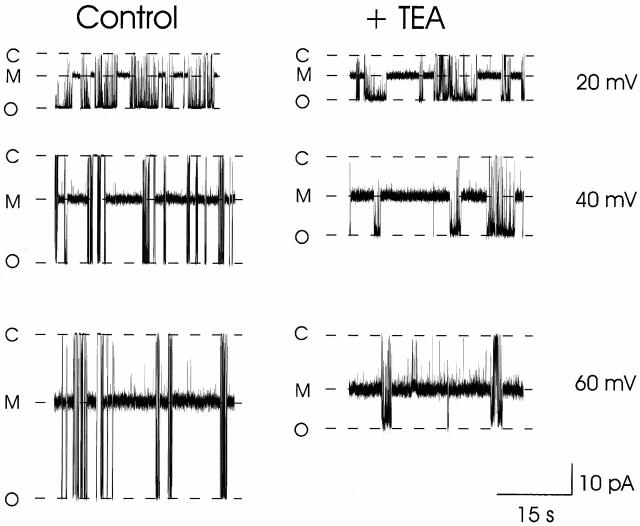
The left panel of Fig. 2 shows current fluctuations of a single RyR channel in the presence of 500 nM 21-amino-9α-hydroxyryanodine with K+ as the permeant ion. Under these conditions, this ryanoid associates with and readily dissociates from the channel. With the ryanoid bound, RyR channel P o increases markedly and single-channel conductance is reduced to a modified level (M) with an FC of 0.44. After dissociation of the ryanoid, the channel gates normally with fluctuations between the closed (C) and fully open conductance level (O). The probability of 21-amino-9α-hydroxyryanodine associating with the channel increases and the probability of the ryanoid dissociating from the channel decreases as the holding potential is made more positive (Tanna et al. 1998).
Figure 2.
The influence of transmembrane holding potential on the single-channel current amplitude of the unmodified and 21-amino-9α-hydroxyryanodine-modified conductance states of the RyR channel in the absence and presence of 20 mM TEA+. C, closed conductance level; M, modified conductance level; O, fully open conductance level.
The right panel of Fig. 2 shows current fluctuations of the same channel after the addition of 20 mM TEA+ to the solutions at both sides of the channel. In the absence of ryanoid, TEA+ is a concentration- and voltage-dependent blocker of K+ conductance in the RyR channel. Dwell times in the TEA+ blocked state are too short to be resolved and, as a consequence, RyR channel block by this cation appears as a time-averaged reduction in single-channel current amplitude (Lindsay et al. 1991; Tinker et al. 1992). Block of this form can be seen in Fig. 2 in the periods of unmodified gating when the ryanoid is not bound to the channel. In agreement with previous observations (Lindsay et al. 1991), the degree of block of this unmodified state of the channel by TEA+ varies as transmembrane potential is changed; block increases as the holding potential is made more positive. Current-voltage relationships for unmodified conductance states of several RyR channels in the absence and presence of 20 mM TEA+ are shown in Fig. 3 A. TEA+ has no effect on K+ translocation at negative holding potentials but blocks K+ current with increasing effectiveness as the holding potential is shifted to more positive values.
Figure 3.
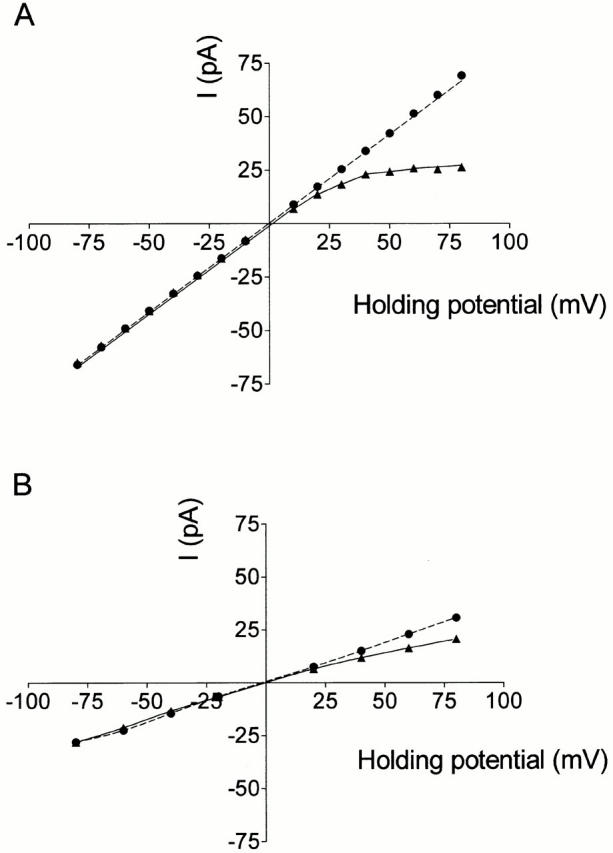
(A) Single-channel current-voltage relationships for unmodified channels in the absence (•; mean ± SEM of 22–51 experiments) and presence (▴; mean ± SEM of 4–25 experiments) of 20 mM TEA+. (B) Single-channel current-voltage relationships for 21-amino-9α-hydroxyryanodine–modified channels in the absence (•; ± SEM of 4–15 experiments) and presence (▴; ± SEM of 4–10 experiments) of 20 mM TEA+. Where no error bars are visible they are contained within the symbol.
TEA+ also blocks K+ current in the 21-amino-9α-hydroxyryanodine–modified conductance state. However, TEA+ is a less effective blocker of the ryanoid-modified conductance state of RyR than the unmodified conductance state. At all three holding potentials shown in Fig. 2, the reduction in current amplitude produced by TEA+ is greater for transitions in the unmodified state (transitions from C to O) than in the ryanoid-modified state (transitions from C to M). Fig. 3 B shows current-voltage relationships for the 21-amino-9α-hydroxyryanodine–modified states of several channels in the absence and presence of 20 mM TEA+. As is the case for the unmodified state of RyR, TEA+ produces no block of K+ current at negative holding potentials, but is an increasingly effective blocker of the ryanoid-modified state as holding potential is made more positive. However, a comparison of the data in Fig. 3 (A and B) establishes the observation made for the representative channel in Fig. 2; TEA+ is less effective as a blocker of K+ current in the ryanoid-modified conductance state of RyR. As a point of comparison, at a holding potential of +80 mV, 20 mM TEA+ reduces mean single-channel current amplitude of the 21-amino-9α-hydroxyryanodine–modified conductance state of RyR by 33%; the equivalent block of the unmodified conductance state is 62%.
What mechanisms are involved in the alteration in effectiveness of TEA+ as a blocker of RyR after the interaction of 21-amino-9α-hydroxyryanodine with the channel? In previous investigations, we have demonstrated that ryanoid-induced modification of permeant ion handling in RyR is strongly correlated to specific structural loci on the ryanoid molecule with contributions from both electrostatic and steric effects (Welch et al. 1997). To investigate the possibility that similar structural features underlie the alteration of the effectiveness of TEA+ as a blocker of K+ current in RyR, we have examined the influence of three additional members of the ryanoid group of ligands: ryanodol, 21-azido-9α-hydroxyryanodine, and 21-p-nitrobenzoylamino-9α-hydroxyryanodine. These ryanoids vary in both steric bulk and charge and, after interaction with the channel, yield values of FC ranging from 0.67 to 0.17. Single-channel current amplitude of both unmodified and ryanoid-modified conductance states were monitored at holding potentials within the range ±80 mV in the absence and presence of 20 mM TEA+. Examples of current fluctuations of representative channels at a holding potential of +40 mV are given in Fig. 4. The left panel of Fig. 4 demonstrates modification of the RyR channel that results from the interaction of the indicated ryanoid. In all cases, the interaction of the ryanoid with the RyR channel results in the occurrence of a modified conductance state with high P o. As outlined above, the amplitude of the modified conductance state is dependent on the structure of the ryanoid bound to the channel (Tinker et al. 1996; Welch et al. 1997). The values of FC induced by the ryanoids shown in Fig. 4 are 0.67 for ryanodol, 0.55 for 21-azido-9α-hydroxyryanodine, and 0.27 for 21-p-nitrobenzoylamino-9α-hydroxyryanodine. The influence of 20 mM TEA+ on the current amplitude of these various ryanoid-modified states is shown in the right panel of Fig. 4. In all cases, the addition of 20 mM TEA+ leads to a reduction in current amplitude. However, a comparison of the current amplitudes of the ryanoid-modified conductance states in the absence and presence of the blocking cation demonstrates that the effectiveness of TEA+ differs in the various ryanoid-modified conductance states. At this holding potential, the magnitude of block produced by TEA+ of the ryanoid-modified conductance state is in order
Figure 4.
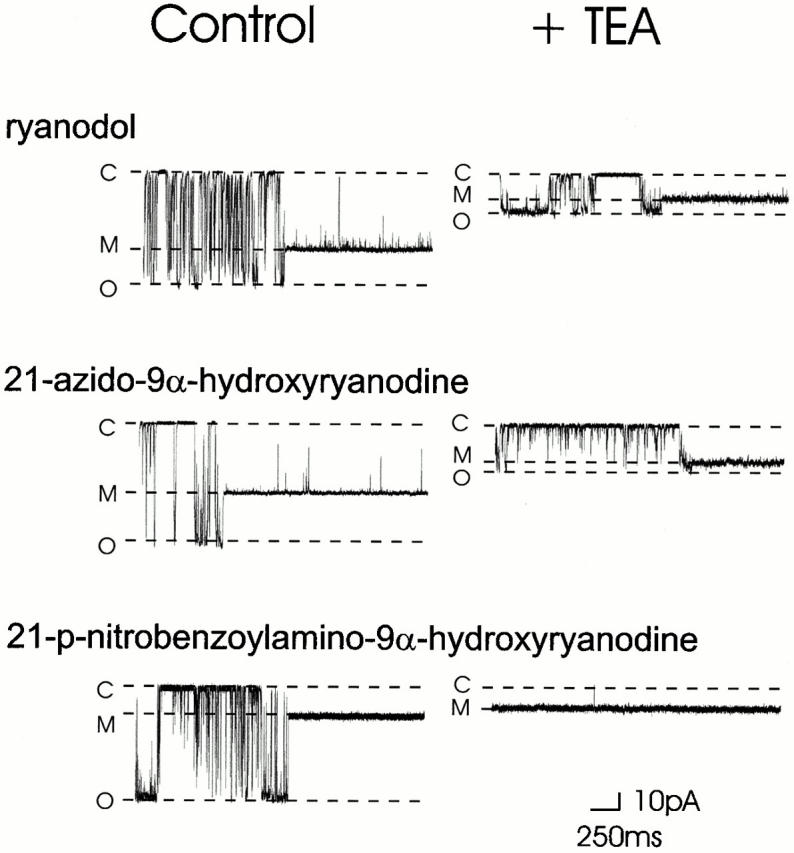
Single-channel traces showing the influence of 20 mM TEA+ on the amplitude of ryanoid-modified conductance states at +40 mV. Both ryanodol and 21-azido-9α-hydroxyryanodine interact rapidly with RyR. For this reason, both unmodified and ryanoid-modified conductance states are shown in the presence of TEA+ for these ryanoids. In contrast, 21-p-nitrobenzoylamino-9α-hydroxyryanodine is a slow-binding modifier of RyR channel function and, as a consequence, traces in the presence of TEA+ show only the ryanoid-modified conductance state. C, closed conductance level; M, modified conductance level; O, fully open conductance level.
ryanodol > 21-azido-9α-hydroxyryanodine > 21-p- nitrobenzoylamino-9α-hydroxyryanodine.
The reduction in amplitude of both the unmodified and ryanoid-modified conductance states of RyR by TEA+ with varying holding potential can be fitted by a model originally described by Woodhull 1973. This scheme envisages a single site, accessible to a blocking ion of valence z from only one side of the channel and located a fraction (δ) into the voltage drop across the channel. Under these conditions, the relationship between relative conductance (i/i0; the current in the presence of TEA+ divided by the current in the absence of the blocking cation) and holding potential is:
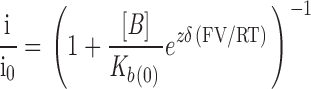 |
1 |
where [B] is the concentration of TEA+ (20 mM), K b(0) is the dissociation constant at a holding potential of 0 mV, and zδ is conventionally referred to as the effective valence. F, R, and T have their usual meanings, and RT/F is 25.2 mV at 20°C. Values of K b(0) and zδ were determined for 21-amino-9α-hydroxyryanodine, ryanodol, 21-azido-9α-hydroxyryanodine, and 21-p-nitrobenzoylamino-9α-hydroxyryanodine using nonlinear regression to establish the best fit of to mean data from four or more experiments (GraphPad Prism v2.01; Fig. 5). Blocking parameters determined from these plots are shown in Table .
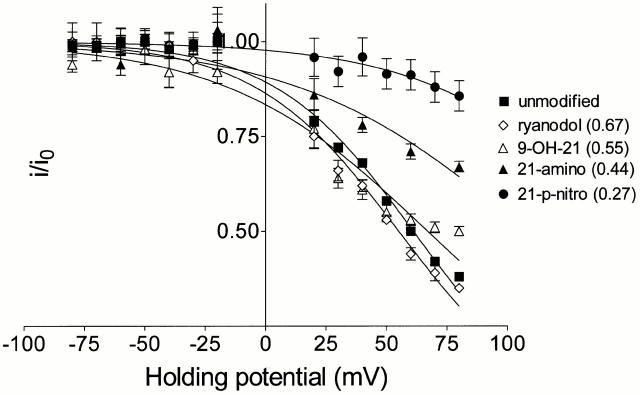
Figure 5.
Woodhull plots for the indicated unmodified and ryanoid-modified conductance states in the presence of 20 mM TEA+. Points are mean normalized conductance values (i/i0) ± SEM (unmodified, n = 4-51; ryanodol, n = 4–11; 21-azido-9α-hydroxyryanodine, n = 4–19; 21-amino-9α-hydroxyryanodine, n = 4–15; 21-p-nitrobenzoylamino-9α-hydroxyryanodine [0.27], n = 10–11). The solid lines were obtained by nonlinear regression fits to with the parameters quoted in Table . Figures in brackets indicate the FC induced by the various ryanoids. 21-p-nitro, 21-p-nitrobenzoylamino-9α-hydroxyryanodine; 21-amino, 21-amino-9α-hydroxyryanodine; 9-OH-21, 21-azido-9α-hydroxyryanodine.
Table 1.
Blocking Parameters for the Interaction of TEA+ with RyR Channels in the Absence of Ryanoid and in the Indicated Ryanoid-modified States
| Ryanoid | FC | K b(0) (mM) | zδ |
|---|---|---|---|
| None | 1.00 | 167.3 | 0.88 |
| Ryanodol | 0.67 | 126.6 | 0.84 |
| 21-azido-9α-hydroxyryanodine | 0.55 | 100.3 | 0.60 |
| 21-amino-9α-hydroxyryanodine | 0.44 | 195.1 | 0.53 |
| 21-p-nitrobenzoylamino-9α-hydroxyryanodine | 0.27 | 853.9 | 0.63 |
| 21-p-nitrobenzoylamino-9α-hydroxyryanodine | 0.17 | No block | No block |
Parameters were determined using nonlinear regression to establish the best fit of for the data displayed in Fig. 5. No block was seen in the 0.17 FC state of 21-p-nitrobenzoylamino-9α-hydroxyryanodine. Blocking parameters determined for the 0.59 FC state induced by 21-p-nitrobenzoylamino-9α-hydroxyryanodine (n = 1) are K b(0) = 177.7 mM and zδ = 0.66.
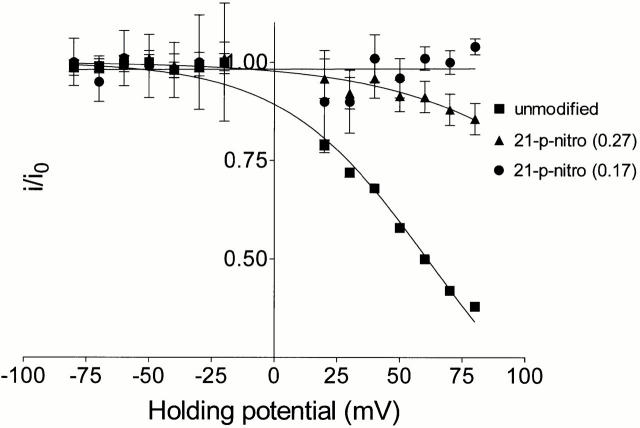
In addition to the FC of 0.27 reported above, we have observed two other modified conductance states of RyR in the presence of 21-p-nitrobenzoylamino-9α-hydroxyryanodine (Tinker et al. 1996). In these studies, out of a total of 15 experiments conducted with 21-p-nitrobenzoylamino-9α-hydroxyryanodine, we observed 11 modified conductance states with an FC of 0.27 (73% of total events), 3 with an FC of 0.17 (20% of total events), and on one occasion a modified state with an FC of 0.59 (7% of total events). This observation of the occurrence of more than one modified conductance state is entirely consistent with the existence of multiple conformations of 21-p-nitrobenzoylamino-9α-hydroxyryanodine, as determined by molecular dynamics (see materials and methods and Fig. 1 B). Furthermore, these determinations demonstrate that the possible conformations of 21-p-nitrobenzoylamino-9α-hydroxyryanodine are not isoenergetic. When added to the solution at the cytosolic face of RyR, 21-p-nitrobenzoylamino-9α-hydroxyryanodine will be present in several conformations with different probabilities of occurrence. The three most common conformers exist in a ratio of 73:24:1%, with the remaining 2% corresponding to multiple conformers rarely populated. The striking similarity between the observed probability of occurrence of the three different modified conductance states induced by 21-p-nitrobenzoylamino-9α-hydroxyryanodine and the ratio of the three common conformers of this ryanoid determined by molecular dynamics indicates that on binding to the RyR channel, the different conformers of the bulky p-nitrobenzoyl tail (Fig. 1 B) produce different FC states. We have demonstrated previously a strong correlation between structure at this locus and FC (Welch et al. 1997). The advantage of the p-nitrobenzoyl conformers is that they provide a built-in control. The different values of FC observed after the interaction of 21-p-nitrobenzoylamino-9α-hydroxyryanodine are due only to changes in conformation of the ryanoid and not to other ligand–receptor interactions that may arise when the covalent structure of the ryanoid is modified. Examples of the 21-p-nitrobenzoylamino-9α-hydroxyryanodine–induced 0.27 and 0.17 FC states are shown in Fig. 6. The figure shows the modified conductance states in the absence (Fig. 6, left) and presence (Fig. 6, right) of 20 mM TEA+. The influence of TEA+ on the 0.59 FC induced by 21-p-nitrobenzoylamino-9α-hydroxyryanodine is not included in Fig. 6, as only one such event occurred during the course of this investigation. The low probability of occurrence of a third 21-p-nitrobenzoylamino-9α-hydroxyryanodine–induced modified conductance state is not surprising in light of the ratio of possible conformers of this ryanoid determined by molecular dynamics. TEA+ blocking parameters of the 0.59 FC state are quoted in the text. The degree of block induced by TEA+ and the influence of holding potential on block differs for the different FC states. Mean single-channel current amplitude at +80 mV in the presence of symmetrical 20 mM TEA+ was reduced by 14% in the 0.27 FC state, but no reduction in current amplitude was seen under these conditions in the 0.17 FC state. Single-channel current amplitude at +80 mV was reduced by 44% in the 0.59 FC state (data not shown). Woodhull analysis of the influence of holding potential on block of K+ current by TEA+ () of the 0.27 and 0.17 21-p-nitrobenzoylamino-9α-hydroxyryanodine–induced FC states is shown in Fig. 7. Blocking parameters for the 0.17 FC state were not determined, as we observed no significant block of K+ current by TEA+ in this ryanoid-modified state at any holding potential. Blocking parameters for the 0.59 FC state are quoted in the legend of Table .
Figure 6.
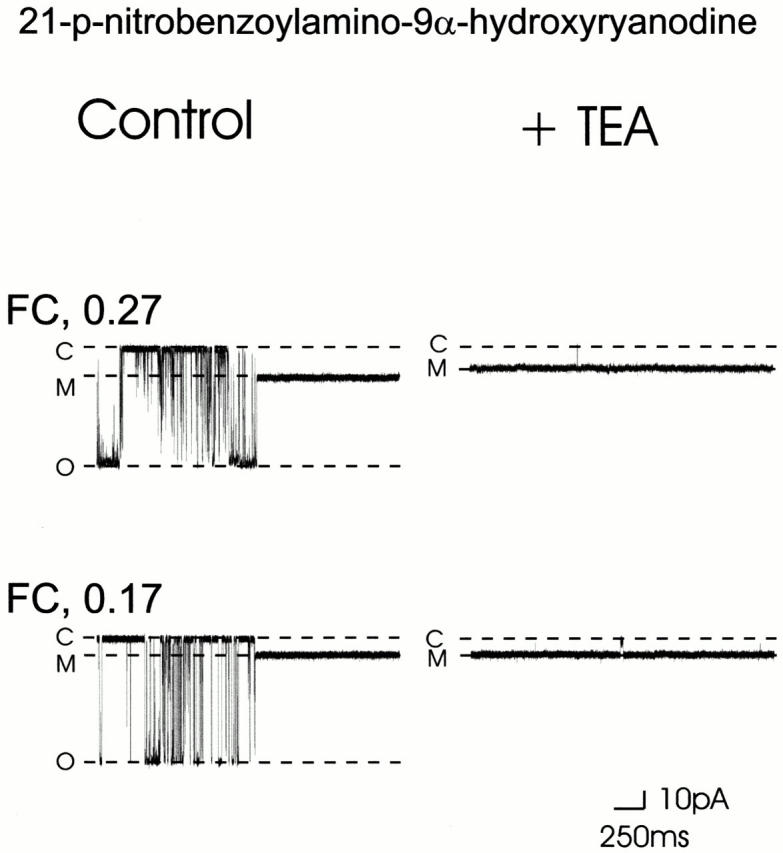
Single-channel traces showing the influence of 20 mM TEA+ on the amplitude of two 21-p-nitrobenzoylamino-9α-hydroxyryanodine–modified conductance states at +40 mV. Irrespective of the FC induced, 21-p-nitrobenzoylamino-9α-hydroxyryanodine is an irreversible modifier of RyR and, as a consequence, traces in the presence of TEA+ show only the ryanoid-modified conductance state. C, closed conductance level; M, modified conductance level; O, fully open conductance level.
Figure 7.
Woodhull plots for the indicated unmodified and 21-p-nitrobenzoylamino-9α-hydroxyryanodine–modified conductance states in the presence of 20 mM TEA+. Points are mean normalized conductance values (i/i0) ± SEM (unmodified, n = 4–51; FC 0.27, n = 10–11; FC 0.17, n = 3). The solid lines were obtained by nonlinear regression to with the parameters quoted in Table . Figures in brackets indicate the FC of the various conductance states. 21-p-nitro, 21-p-nitrobenzoylamino-9α-hydroxyryanodine.
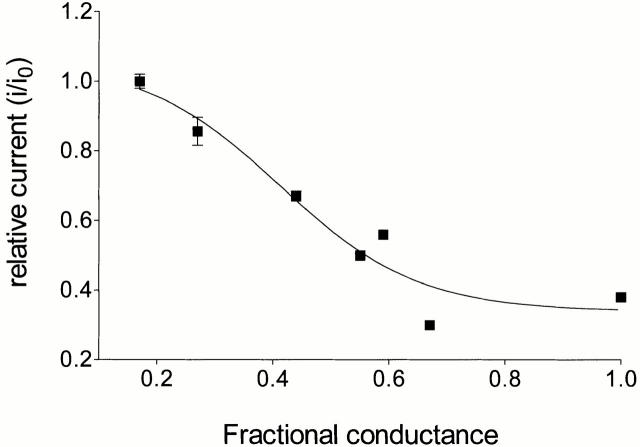
Overall, our investigations of the influence of holding potential on block by TEA+ of ryanoid-modified conductance states of RyR indicate that there are marked quantitative differences in the degree of block produced by TEA+ in the modified states induced by the various ryanoids. The alteration of the effectiveness of TEA+ as a blocker appears to be correlated with the FC of the ryanoid. A comparison of the degree of block (expressed as relative current; single-channel current in the presence of TEA+ divided by single-channel current in the absence of TEA+) produced by 20 mM TEA+ at a holding potential of +80 mV of the various ryanoid-modified conductance states monitored in this study is presented in Fig. 8. The degree of block induced by TEA+ increases as FC rises from 0.17 to 0.67; block in the ryanodol-modified state is essentially the same as that in the RyR channel in the absence of ryanoid (FC = 1.0).
Figure 8.
Relationship between relative conductance (i/io) produced by TEA+ in the ryanoid-modified state at + 80 mV and FC of the ryanoid-modified state. The line plotted through the data is of no theoretical significance.
Quantitative analysis of the influence of holding potential on block by TEA+ provides additional information. The relationships between FC in the range 0.27 to 1.0 and the blocking parameters derived from the Woodhull analysis are shown in Fig. 9 (no block was seen in the 0.17 FC state of 21-p-nitrobenzoylamino-9α-hydroxyryanodine). The values of zδ determined for TEA+ block of K+ conductance of channels in the absence of ryanoid, and channels modified with various ryanoids, show a broad correlation with relative K+ conductance (FC; Fig. 9 A). Ryanoid-modified conductance states with values of FC in the range 0.67 to 0.44 yield dissociation constants for TEA+ at 0 mV that show little or no variation from the value determined in the absence of ryanoid. Dissociation constants increased markedly at FCs below 0.44 (Fig. 9 B).
Figure 9.
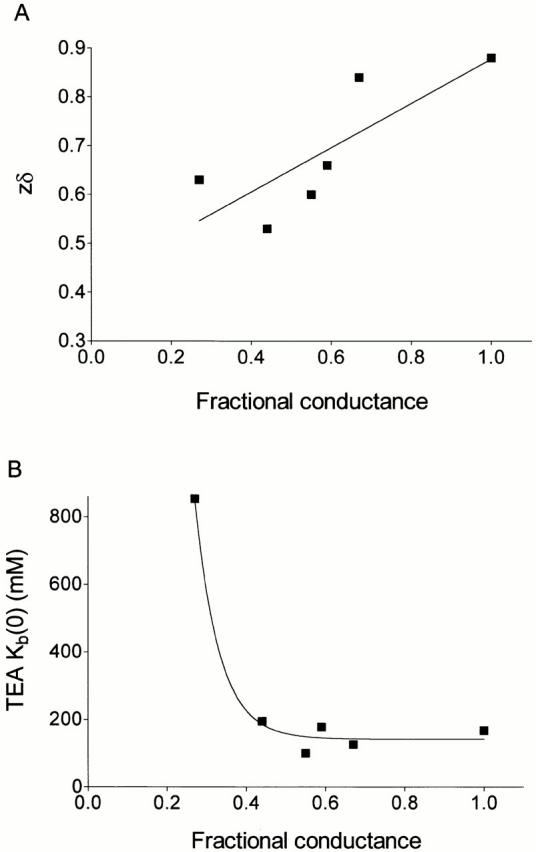
(A) Variations in zδ of TEA+ in the unmodified RyR channel and ryanoid-modified states with FCs in the range 0.27–0.67. It should be noted that no measurable block by TEA+ was seen, and therefore no value of zδ was determined, for the 0.17 FC state induced by 21-p-nitrobenzoylamino-9α-hydroxyryanodine. The solid line is the best-fit line obtained by linear regression. (B) Variations in K b(0) of TEA+ in the unmodified RyR channel and ryanoid-modified states with FCs in the range 0.27–0.67. Again, in the absence of measurable block, no value of K b(0) could be determined for the 0.17 FC state induced by 21-p-nitrobenzoylamino-9α-hydroxyryanodine. The line plotted through the data has no theoretical significance.
DISCUSSION
We have demonstrated that the interaction of a range of ryanoids with RyR alters the blocking characteristics of TEA+ in the channel. An inspection of the influence of voltage on block by TEA+ in the unmodified and ryanoid-modified conductance states reveals something of the mechanism involved in this action. The interaction of ryanoids with RyR can affect both the zδ of the blocking cation and the affinity of the conduction pathway for the blocker (K b(0)).
For a cation, such as TEA+, that blocks by entering the electric field across the RyR channel, δ is a measure of the relative depth of penetration of the blocking cation into the field (French and Shoukimas 1985). As z, the valence of TEA+, is constant, a change in zδ after channel modification by a ryanoid implies that the location of the site of interaction of TEA+ within the voltage drop across the channel is altered. This relocation of the TEA+ binding site within the voltage drop is likely to be the consequence of a reorganization of the RyR conduction pathway induced by the binding of the ryanoid.
The interaction of some of the ryanoids investigated produces a concomitant alteration of the affinity of the TEA+ site in RyR. In previous studies we have demonstrated that a reduction in the rate of association of a large organic blocking cation (tetrabutylammonium) with RyR underlies a reduction in affinity of the channel for this cation after the interaction of ryanodine (Tinker and Williams 1993b). In addition, hydrophobic interactions are likely to contribute to the strength of binding at the TEA+ site. In the absence of ryanoids, K b(0) decreases as the hydrophobicity of cations interacting with this site is increased by the substitution of methyl and methylene groups (Tinker et al. 1992). If, as we suggest, the binding of ryanoids brings about conformational changes in RyR, then alterations in K b(0) are likely to reflect the rearrangement of groups in and around the TEA+ site. Decreased access to the site, changes in electrical potential, or a lowering of the hydrophobicity of the site could all contribute to the observed reductions in affinity.
Quantitative differences in blocking parameters were monitored in the various ryanoid-modified states. This is reminiscent of the influence of ryanoids on the conductance of permeant cations such as K+. On binding to the high affinity site on RyR, all ryanoids so far examined reduce single-channel K+ current amplitude, but the relative amplitude of the ryanoid-modified conductance state (FC) varies and is dependent on the electrostatic and steric features of the bound ryanoid (Tinker et al. 1996; Welch et al. 1997). By monitoring the influence of voltage on TEA+ block of K+ conductance in RyR channels modified with ryanoids yielding different FCs, we have examined the possibility that the mechanisms underlying the alteration of TEA+ blocking parameters by ryanoids might also be responsible for the alterations in K+ conductance induced by these ligands.
The results of these experiments are consistent with the proposal that the interaction of a ryanoid with RyR can induce structural rearrangements in the channel. In effect, the position of the TEA+ binding site is relocated relative to the voltage drop across the channel. In each case, the TEA+ site is shifted towards the cytosolic limit of the voltage drop.
When considering how a binding site might move within the voltage drop of the RyR channel, it is relevant to discuss what constitutes the TEA+ binding site. Does TEA+ merely traverse a portion of the RyR voltage drop until it jams in a narrow region of the conduction pathway, or are more specific interactions involved? The evidence that has emerged from previous investigations of ion translocation and block in RyR indicates that small tetraalkylammonium cations do not block RyR by simply jamming in a constriction in the conduction pathway. Studies with these cations, trimethylammonium derivatives, and charged local anesthetics have identified two regions within the voltage drop across RyR with which blocking cations can interact. Tetramethylammonium (TMA+) blocks K+ conductance in RyR by binding at a site located ∼50% into the voltage drop from the cytosolic face of the channel (Tinker et al. 1992). Larger tetraalkylammoniums and related cations are excluded from the TMA+ site and bind further into the voltage drop (80–90%; Tinker et al. 1992; Tinker 1993; Tinker and Williams 1993a). As outlined above, the affinity of this site for blocking cations increases in proportion to the hydrophobicity of the cations, indicating that hydrophobic forces are likely to be involved in this interaction. The exclusive association of the smallest tetraalkylammonium, TMA+, with the 50% site has been interpreted as indicating that this site is likely to be a small, relatively rigid structure from which larger blocking cations are excluded by steric restrictions (Tinker et al. 1992).
Within the context of the picture of the RyR conduction pathway set out above, it is probable that the observed relocation and changes in affinity of the TEA+ blocking site within the voltage drop result from rearrangement in this region of the channel after ryanoid interaction. We can envisage movement of the relative position of the TEA+ site within the voltage drop across the RyR channel as a physical relocation of the site; alternatively, the monitored variations in zδ could arise from a change in the dimensions or position of the voltage drop across the channel. In such a scheme, the interaction of a ryanoid with RyR would induce a structural reorganization within the channel that results in the elongation of the voltage drop or a shift of the region of the channel over which transmembrane voltage drops towards the luminal face of the channel. Assuming that the physical location of the TEA+ site within the conduction pathway of the channel is unchanged, either of these maneuvers would result in a lowering of zδ.
The absence of voltage-dependent block of K+ current by TEA+ in the 21-p-nitrobenzoylamino-9α-hydroxyryanodine–modified state with an FC of 0.17 is likely to result from either, or both, a relocation of the blocking site outside the voltage drop across the channel or a dramatic decrease in the affinity of the TEA+ blocking site in the conduction pathway of RyR. The trends in the data observed in Fig. 9 indicate that alterations in affinity of the TEA+ binding site are likely to be the dominant factor.
In previous investigations, we reported modifications of permeant ion conductance in ryanoid-bound channels and suggested that these variations were likely to result from ryanoid-induced structural reorganization of the conduction pathway of RyR (Lindsay et al. 1994; Tinker et al. 1996; Welch et al. 1997). The experiments reported in this communication provide direct evidence for this postulated ryanoid-induced restructuring. Although structural rearrangements within the conduction pathway of the RyR channel are unlikely to have exactly equivalent effects on the interactions of permeant and blocking cations, the broad correlation of TEA+ blocking parameters with the FC induced by individual ryanoids with different steric and electronic properties indicates that the structural reorganizations identified here are likely to underlie ryanoid-induced alterations in permeant ion translocation in RyR.
Acknowledgments
We thank J.J. Hull (University of Nevada) for the MALDI/TOF mass spectroscopy.
This work was supported by grants from The British Heart Foundation (to A. Williams), The Biotechnology and Biological Sciences Research Council (to A. Williams), The Wellcome Trust (to A. Williams, W. Welch, and J. Sutko), National Institutes of Health (No. HL53677 to J. Sutko), and National Science Foundation (No. MCB-9817605 to W. Welch).
Footnotes
Abbreviations used in this paper: FC, fractional conductance; P o, open probability; RyR, ryanodine receptor; TMA, tetramethylammonium; zδ, effective valence.
References
- Ashley R.H., Williams A.J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J. Gen. Physiol. 1990;95:981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslongchamps P., Belanger A., Berney D.J.F., Borschberg H.J., Brousseau R., Doutheau A., Durand R., Katayama H., Lapalme R., Leturc D.M. The total synthesis of (+)-ryanodol. Can. J. Chem. 1990;68:115–192. [Google Scholar]
- French R.J., Shoukimas J.J. An ion's view of the potassium channel. The structure of the permeation pathway as sensed by a variety of blocking ions. J. Gen. Physiol. 1985;85:669–698. doi: 10.1085/jgp.85.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A.R.G., Williams A.J. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1991;1064:89–102. doi: 10.1016/0005-2736(91)90415-5. [DOI] [PubMed] [Google Scholar]
- Lindsay A.R.G, Manning S.D., Williams A.J. Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J. Physiol. 1991;439:463–480. doi: 10.1113/jphysiol.1991.sp018676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A.R.G, Tinker A., Williams A.J. How does ryanodine modify ion-handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel? J. Gen. Physiol. 1994;104:425–447. doi: 10.1085/jgp.104.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry S.J., Williams A.J. Activation of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by analogues of sulmazole. Br. J. Pharmacol. 1994;111:1212–1220. doi: 10.1111/j.1476-5381.1994.tb14874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1982;299:401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J.S., Meissner G. Ryanodine modifies conductance and gating behaviour of single Ca2+ release channel. Am. J. Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A.J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. Interactions of a reversible ryanoid (21-amino-9alpha-hydroxy-ryanodine) with single cardiac ryanodine receptor channels. J. Gen. Physiol. 1998;112:55–69. doi: 10.1085/jgp.112.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. The interaction of a neutral ryanoid with the ryanodine receptor channel provides insights into the mechanisms by which ryanoid binding is modulated by voltage. J. Gen. Physiol. 2000;116:1–9. doi: 10.1085/jgp.116.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A. Ionic conduction and block in the sheep cardiac sarcoplasmic reticulum ryanodine receptor-channel. Ph.D. thesis 1993. University of London; London, UK: pp. 1–225 [Google Scholar]
- Tinker A., Williams A.J. Charged local anesthetics block ionic conduction in the sheep cardiac sarcoplasmic reticulum calcium-release channel Biophys. J. 65 1993. 852 864a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Williams A.J. Using large organic cations to probe the nature of ryanodine modification in the sheep cardiac sarcoplasmic reticulum calcium-release channel Biophys. J. 65 1993. 1678 1683b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Williams A.J. Probing the structure of the conduction pathway of the sheep cardiac sarcoplasmic reticulum calcium-release channel with permeant and impermeant organic cations J. Gen. Physiol. 102 1993. 1107 1129c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Williams A.J. Measuring the length of the pore of the sheep cardiac sarcoplasmic reticulum calcium-release channel using trimethylammonium ions as molecular calipers. Biophys. J. 1995;68:111–120. doi: 10.1016/S0006-3495(95)80165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Lindsay A.R.G., Williams A.J. Block of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by tetraalkyl ammonium cations. J. Membr. Biol. 1992;127:149–159. doi: 10.1007/BF00233287. [DOI] [PubMed] [Google Scholar]
- Tinker A., Sutko J.L., Ruest L., Deslongchamps P., Welch W., Airey J.A., Gerzon K., Bidasee K.R., Besch H.R., Jr., Williams A.J. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys. J. 1996;70:2110–2119. doi: 10.1016/S0006-3495(96)79777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W., Williams A.J., Tinker A., Mitchell K.E., Deslongchamps P., Lamothe J., Gerzon K., Bidasee K.R., Besch H.R., Jr., Airey J.A. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry. 1997;36:2939–2950. doi: 10.1021/bi9623901. [DOI] [PubMed] [Google Scholar]
- Woodhull A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]