Abstract
Skeletal muscle dihydropyridine (DHP) receptors function both as voltage-activated Ca2+ channels and as voltage sensors for coupling membrane depolarization to release of Ca2+ from the sarcoplasmic reticulum. In skeletal muscle, the principal or α1S subunit occurs in full-length (∼10% of total) and post-transcriptionally truncated (∼90%) forms, which has raised the possibility that the two functional roles are subserved by DHP receptors comprised of different sized α1S subunits. We tested the functional properties of each form by injecting oocytes with cRNAs coding for full-length (α1S) or truncated (α1SΔC) α subunits. Both translation products were expressed in the membrane, as evidenced by increases in the gating charge (Qmax 80–150 pC). Thus, oocytes provide a robust expression system for the study of gating charge movement in α1S, unencumbered by contributions from other voltage-gated channels or the complexities of the transverse tubules. As in recordings from skeletal muscle, for heterologously expressed channels the peak inward Ba2+ currents were small relative to Qmax. The truncated α1SΔC protein, however, supported much larger ionic currents than the full-length product. These data raise the possibility that DHP receptors containing the more abundant, truncated form of the α1S subunit conduct the majority of the L-type Ca2+ current in skeletal muscle. Our data also suggest that the carboxyl terminus of the α1S subunit modulates the coupling between charge movement and channel opening.
Keywords: Ca2+ channels, skeletal muscle, Xenopus oocyte expression, cut-open oocyte voltage clamp, gating charge movement
INTRODUCTION
Skeletal muscle dihydropyridine (DHP) receptors are L-type Ca2+ channels that serve a dual role in muscle, functioning both as Ca2+-conducting pores and as the voltage sensors for excitation–contraction (E-C) coupling (Ríos and Brum 1987; Tanabe et al. 1988; Melzer et al. 1995). These channels are heteromeric complexes containing the pore-forming α1S subunit plus the β1, α2δ, and γ regulatory subunits (Hofmann et al. 1994). Two forms of the α1S subunit have been detected in skeletal muscle: a 212-kD full-length translation product (α1S), present as a minor component, and a more abundant 175-kD form (α1SΔC, 90% of α1 subunit recovered from transverse tubule membrane preparations) created by post-translational cleavage of 175 amino acids from the COOH terminus (De Jongh et al. 1989, De Jongh et al. 1991). While the COOH termini of other cloned Ca2+ channel α1 subunits have been shown to play a role in channel regulation (Wei et al. 1994; Lee et al. 1999; Zühlke et al. 1999), the functional significance of the COOH-terminal truncation of α1S is unknown.
One long-standing hypothesis holds that some DHP receptors in skeletal muscle are specialized voltage sensors for E-C coupling, while others are capable of both voltage sensing and Ca2+ conduction. Comparison of DHP binding to L-type Ca2+ current amplitude in intact muscle fibers suggested that only a small percentage of DHP receptors are functional Ca2+ channels (Schwartz et al. 1985). The discovery of two size forms of α1S occurring in a 90%:10% ratio led to the hypothesis that the rarer full-length form is capable of both sensing the voltage and passing current, while the more abundant truncated form is a dedicated voltage sensor for E-C coupling (De Jongh et al. 1989).
Injection of cDNAs encoding either α1S or a COOH-terminally truncated form of α1S (at Asn 1662) into the nuclei of dysgenic mouse myotubes lacking α1S restores contraction, gating charge movement, and L-type Ca2+ current (Tanabe et al. 1988; Adams et al. 1990; Beam et al. 1992). While the version of α1SΔC used in these experiments was artificially truncated slightly upstream of the native cleavage site, these results clearly suggest that α1SΔC can function as both an ion channel and a voltage sensor for E-C coupling. However, the role of full-length α1S remains unknown, since it is possible that an undetermined proportion of the α1S subunits were cleaved to α1SΔC in the myotubes. One recent study has address this issue. When the green fluorescent protein was fused to the COOH terminus of α1S and expressed in dysgenic myotubes, the fluorescence was localized to the t tubules, which suggests a significant amount of full-length α1S was inserted into the membrane and little cleavage occurred within the 48–72-h duration of the experiment (Flucher et al. 1999).
Heterologous expression in nonmuscle cells provides an alternative opportunity to distinguish the functional roles of the two α1S forms. Injection of cRNAs encoding α1SΔC plus the muscle-associated β1b, α2δ, and γ subunits into Xenopus oocytes was recently found to give rise to robust DHP-sensitive L-type currents, whereas injection of full-length α1S cDNA gave little or no current (Ren and Hall 1997). While these results suggest a difference in channel function for α1S and α1SΔC, they leave open the possibility that full-length α1S simply expresses poorly in oocytes, but conducts Ca2+ just as well as α1SΔC. Moreover, measurements of ionic current alone cannot address the crucial question of whether α1S and α1SΔC function differently as voltage sensors.
To study the current-carrying and voltage-sensing capabilities of the two size forms of α1S in parallel, we expressed α1S and α1SΔC with the β1, α2δ, and γ auxiliary subunits in Xenopus oocytes. We examined the β1a and β1b splice variants, both of which are expressed in muscle tissue (Hofmann et al. 1994; Ren and Hall 1997), because of a previous report that the less-abundant β1b variant was critical for forming channels capable of conducting Ba2+ current (Ren and Hall 1997). The cut-open oocyte voltage clamp technique (Stefani and Bezanilla 1998) was used to record ionic currents (in 10 mM Ba2+) and gating charge movement (in 2 mM Co2+) in parallel. We found that both size forms of the α1S subunit supported gating charge movements in 2 mM Co2+ when expressed with auxiliary subunits in oocytes, even though only the truncated form conducted appreciable L-type currents in 10 mM Ba2+. This result was independent of whether the β1a or β1b splice variant was used. We conclude that, while α1S and α1SΔC function almost identically as voltage sensors, α1SΔC is the form specialized to carry L-type current, while α1S appears to be somehow inhibited from passing substantial ionic current.
METHODS
The rabbit α1S, rat brain β1b, rabbit α2δ, and rabbit γ Ca2+ channel cDNAs were obtained in the pKCRH, pBluescript, pcDNA3, and pcD-x vectors, respectively, as a gift from Dr. Kevin Campbell (University of Iowa, Iowa City, IA). The rabbit β1a subunit was obtained in the pNKS2 vector as a gift from Dr. Bernhard Flucher (University of Innsbruck, Innsbruck, Austria). α1S and γ were subcloned into the pGEMHE oocyte expression vector (gift of Dr. Emily Liman, Harvard University) at the HindIII and BamHI polylinker sites, respectively. β1b was subcloned into pGEMHE between the SacII and HindIII sites, while α2δ and β1a were left in pCDNA3 and pNKS2, respectively. α1SΔC-pGEMHE was made from α1S-pGEMHE using the Clontech Transformer site-directed mutagenesis kit (CLONTECH Laboratories, Inc.). The mutagenic primer caused a loop-out deletion of base pairs 5320–5844 (amino acids 1698–1893) of the rabbit α1S sequence, while the selection primer mutated the MfeI site at position 2788 of the pGEMHE sequence to a unique NdeI site. For in vitro synthesis of RNA, the plasmids containing the Ca2+ channel subunits were linearized using the following restriction enzymes: Sse8387I for α1S and α1SΔC, XbaI for β1a, NotI for β1b, PvuII for α2δ, and NheI for γ. Capped cRNA was synthesized using the mMessage mMachine T7 kit (for α1S, α1SΔC, β1b, α2δ, and γ) or SP6 kit (for β1a; Ambion Corp.) and extracted using the RNAid purification kit (Bio101).
Stage V and VI oocytes were harvested from egg-bearing female Xenopus laevis frogs under anesthesia with 3-aminobenzoic acid ethyl ester (1 mg ml−1 in a cold water bath for 25 min; Sigma-Aldrich), in accordance with the guidelines of the Subcommittee on Research Animal Care at the Massachusetts General Hospital. Oocytes were removed into Ca2+-free OR-2 solution containing (mM): 82.5 NaCl, 2.5 KCl, 1 MgCl2, and 5 HEPES, pH 7.6. The egg sacs were manually torn open using forceps, and the oocytes were incubated in OR-2 containing 2 mg ml−1 collagenase (GIBCO BRL) for 2.5 h in a room temperature shaker (60 rpm) to remove the follicular membrane. The oocytes were then washed four times in OR-2 solution and transferred for storage to ND-96 solution containing (mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 2.5 pyruvate, 5 HEPES, and 50 μg ml−1 gentamicin (GIBCO BRL), pH 7.6. Oocytes were injected with 50–100 nl of a 1:1:1:1 mixture of α1S (or α1SΔC), β1a (or β1b), α2δ, and γ cRNA using injection pipettes pulled from thin-wall capillary glass (World Precision Instruments) and were stored at 18°C for 5–7 d before recording.
Oocytes were voltage clamped in the cut-open configuration, with active clamp of the upper and guard compartments, using an oocyte clamp (CA-1B; Dagan Corp.) under control of a custom stimulation/recording program written in AxoBASIC and running on an IBM-compatible computer. Voltage-sensing electrodes, fabricated from borosilicate capillary glass (1.65-mm outer diameter; VWR Scientific) using a multi-stage puller (Sutter Instrument Co.), were filled with 3 M KCl and had resistances of 0.2–1 MΩ. The leads of the amplifier headstage, attached to Ag/AgCl pellets in plastic wells containing 2 M NaCl solution, were connected to the upper, guard, and lower oocyte compartments using glass agar bridges containing 110 mM Na-methanesulfonic acid and 10 mM HEPES, in 3% agarose, pH 7.0, and threaded with a platinum wire. Current traces, evoked by depolarizing test pulses from a holding potential of −90 mV, were corrected for linear leak and capacity currents (minimized by the analog compensation of the amplifier) by addition of control currents evoked by hyperpolarizing pulses of equal amplitude from the holding potential (P/−1 protocol). Since the charge movement was not noticeably altered by changing the holding potential to −110 or −120 mV (data not shown), we judged our leak subtraction protocol to be sufficient to selectively subtract linear components. Signals were filtered at 1 kHz and sampled at 20 kHz (for gating currents) or 2 kHz (for ionic currents). Analysis and curve fitting was performed using a combination of custom AxoBASIC routines and SigmaPlot (Jandel Scientific). Student's t test was used to make pairwise comparisons between sets of data (P values are reported in the text; P = 0.05 was taken as the limit of statistical significance).
Solutions for recording Ba2+ currents and Ca2+ channel gating currents were prepared following Olcese et al. 1996 and Stefani and Bezanilla 1998 and were Cl− free to avoid contamination with oocyte Cl− currents (including Ba2+-activated Cl− currents). For gating current measurements, the external solution (applied in the external and guard compartments) contained (mM): 2 Co(OH)2, 96 NaOH, and 10 HEPES, adjusted to pH 7.0 with methanesulfonic acid. For ionic current measurements, the external solution contained 10 mM Ba(OH)2 instead of 2 mM Co(OH)2, but was otherwise identical. The lower chamber in contact with the part of the oocyte permeabilized with 0.1% saponin (Sigma-Aldrich) contained 110 mM potassium glutamate and 10 mM HEPES, adjusted to pH 7.0 with NaOH. To avoid possible contamination with nonlinear charge movement related to the oocyte Na+–K+ pump (Neely et al. 1993; Olcese et al. 1996; Stefani and Bezanilla 1998), 0.1 mM ouabain (Sigma-Aldrich) was present in all external solutions.
RESULTS
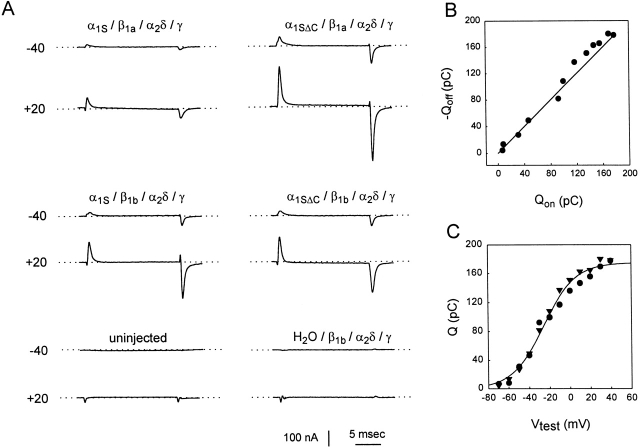
Oocytes injected with either α1S or α1SΔC plus the auxiliary subunit cRNAs showed transient currents at the beginning and end of short depolarizing pulses, while uninjected oocytes and oocytes injected with auxiliary subunit cRNAs alone lacked these currents (Fig. 1 A). These transient currents had the properties expected of gating currents arising from the movement of charged elements in the ion channel protein: (a) they depended on the presence of the pore-forming α subunit (Fig. 1 A); (b) they always followed the direction of the voltage step (A); (c) the integral of the current at the onset of a voltage step (Qon) matched the integral of the current at the offset of the step (Qoff) (B); and (d) Qon and Qoff approached limiting values at both positive and negative potentials, with a voltage dependence that followed a Boltzmann distribution (C).
Figure 1.
Gating currents from oocytes expressing the truncated and full-length forms of the rabbit skeletal muscle L-type Ca2+ channel α1 subunit (α1SΔC and α1S). (A) Voltage-clamp traces recorded in six oocytes during 20-ms depolarizing steps to the values shown on the left from a holding potential of −90 mV. The combination of cRNAs injected is shown above each set of traces. Each trace is the average of four responses filtered at 1 kHz and sampled at 20 kHz. The dotted lines represent zero current. (B) Qon vs. −Qoff plot for the α1SΔC, β1b-injected oocyte from A, middle right, shows charge conservation. A straight line with a slope of 1 is shown as a reference. Values of Qon and Qoff were obtained by integrating the gating currents during and after the pulse. 10 datum points (0.5 ms) near the end of the pulse were used to define the baseline for Qon, while 10 datum points near the end of the trace (3–5 ms after the end of the pulse) were used to define the baseline for Qoff. Qon was integrated throughout the pulse to allow slow components of charge movement to be included. Qoff did not have prominent slow components and was integrated over the first ∼3 ms after the pulse. A number of cells showed small, slow tail currents at depolarized potentials (see +20 mV traces in A, top right and middle left) that were excluded from Qoff by baseline subtraction. (C) Qon (•) and Qoff (▾) versus voltage for the same oocyte were fitted by Boltzmann distributions of the form: Q-V = Qmax/{1 + exp[−(V − V1/2Q)/k Q]}, where Qmax is the maximal charge moved at depolarized voltages, V1/2Q is the voltage at which charge movement is half maximal, and k Q describes the steepness of the distribution. The solid line shows the average of the Qon and Qoff fits: Qmax = 175 pC, V1/2Q = −25.9 mV, and k Q = 15.6 mV.
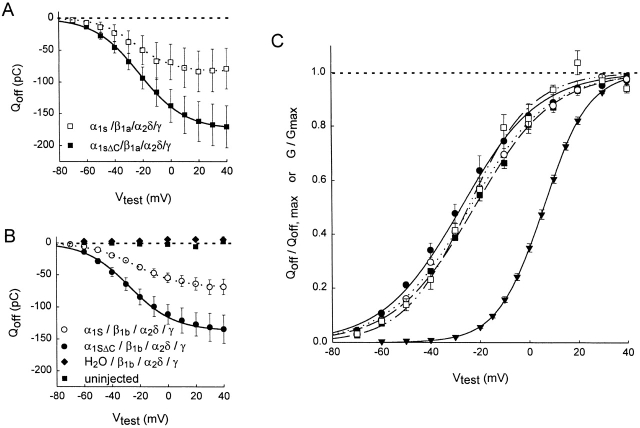
Fig. 2, which compares the magnitude and voltage dependence of the charge movement measured in oocytes expressing α1S and α1SΔC, shows that both constructs were clearly expressed abundantly at the surface membrane regardless of which β1 subunit isoform was coexpressed. Qoff was used for the comparison instead of Qon because of the faster kinetics (and hence greater single-to-noise ratio at small test depolarizations) of the OFF response. The maximum Qoff appeared larger in oocytes expressing α1SΔC than in oocytes expressing α1S (Fig. 2A and Fig. B). While this effect on Qoff, max was not statistically significant in the presence of β1a (Fig. 2 A; P = 0.13), it was statistically significant in the presence of β1b (B; P = 0.008). The normalized Qoff versus voltage (Q-V) curves for α1S and α1SΔC had roughly the same midpoints and slopes (Fig. 2 C). As has been observed for other voltage-dependent ion channels and for native L-type Ca2+ channels in skeletal muscle, the Q-V curve started at significantly more negative potentials and was less steep than the G-V curve for L-type current (Fig. 2 C, ▾). This is consistent with the idea that skeletal muscle Ca2+ channels undergo rapid voltage-dependent transitions among several closed conformations before opening, some of which are presumably involved in excitation–contraction coupling (Feldmeyer et al. 1990; Ma et al. 1996).
Figure 2.
The magnitude and voltage dependence of charge movement in oocytes expressing α1SΔC and α1S. (A) Qoff versus voltage from five oocytes expressing α1S, β1a, α2δ, and γ (□) and 12 oocytes expressing α1SΔC, β1a, α2δ, and γ (▪). The solid and dashed lines through the data points show the mean Boltzmann fits to the data from individual cells (fitted as described in Fig. 1). α1S/β1a/α2δ/γ: Qoff, max = −82.9 ± 32.8 pC, V1/2Q = −24.7 ± 2.2 mV, k Q = 13.1 ± 1.0 mV, n = 5; α1SΔC/β1a/α2δ/γ: Qoff, max = −175 ± 34.0 pC, V1/2Q = −22.2 ± 0.8 mV, k Q = 16.1 ± 0.3 mV, n = 12. The differences in Qoff, max between these two data sets are not statistically significant (P = 0.13; see text). (B) Qoff versus voltage from nine oocytes expressing α1S, β1b, α2δ, and γ (○), eight oocytes expressing α1SΔC, β1b, α2δ, and γ (•), six oocytes expressing the auxiliary subunits alone (♦), and eight uninjected oocytes (▪). Error bars correspond to the standard error of the mean and are smaller than the symbol when not visible. Gating currents were recorded in 2 mM extracellular Co2+, and Qoff was calculated as described in Fig. 1. The solid and dashed lines through the symbols are mean Boltzmann fits, as above. α1S/β1b/α2δ/γ: Qoff, max = −69.0 ± 11.0 pC, V1/2Q = −23.9 ± 1.3 mV, k Q = 16.2 ± 0.5 mV, n = 9; α1SΔC/β1b/α2δ/γ: Qoff, max = −137 ± 20.1 pC, V1/2Q = −27.5 ± 3.2 mV, k Q = 15.9 ± 1.4 mV, n = 8. The differences in Qoff, max between these two data sets are statistically significant (P = 0.008; see text). (C) Normalized Qoff versus voltage (Q-V) curves for oocytes expressing α1S (open symbols) and α1SΔC (filled symbols) with β1a, α2δ, and γ (squares) or β1b, α2δ, and γ (circles). The Q-V curves are compared with the mean G-V curve measured in 10 mM Ba2+ solution for oocytes expressing α1SΔC, β1b, α2δ, and γ (▾). To obtain the Q-V curves, data from individual oocytes were fitted by Boltzmann distributions as described above and normalized by Qoff, max. To obtain the G-V curve, inward L-type currents were evoked by 300-ms depolarizations in 10 mM extracellular Ba2+, filtered at 1 kHz, sampled at 2 kHz, and measured at the end of the pulse. For eight individual oocytes, the I-V curves so obtained were fit to the function: I-V = Gmax(V − Erev)/{1 + exp[−(V − V1/2G)/k G]}, where Gmax is the maximal conductance attained, Erev is the current reversal potential, V1/2G is the half-activation potential, and k G describes the steepness of activation. In the fitting procedure, Gmax, Erev, V1/2G, and k G were all allowed to be free parameters. Normalized G-V curves were then obtained by dividing each I-V curve by Gmax(V − Erev). The lines in C show the average normalized Boltzmann distributions fit to the Q-V and G-V relations measured in individual cells. The fitted parameters for the Q-V curves are given for A and B, above. For the G-V curve: Gmax = 3.4 ± 0.3 μS, V1/2G = 5.9 ± 0.6 mV, and k G = 9.4 ± 0.2 mV.
To compare directly the voltage-sensing and current-carrying properties of α1S and α1SΔC, we measured both ionic and gating currents in individual oocytes expressing these constructs (Fig. 3). In these experiments, ionic currents were measured in the presence of 10 mM Ba2+, the extracellular Ba2+ was then replaced with 2 mM Co2+ using a manual pipet, and gating charge movements were measured (see Fig. 3, legend). For these experiments, Qon was measured instead of Qoff, despite the lower signal-to-noise ratio of the ON response, to avoid the possibility of contamination by small Ba2+ tail currents remaining after the solution exchange. These experiments confirmed that the full-length form of the channel conducts Ba2+ current very poorly compared with the truncated form (Ren and Hall 1997), but that injecting cRNA coding for either full-length or truncated α1S resulted in large voltage-driven gating charge movements not found in uninjected eggs. Moreover, this difference was observed whether β1a (Fig. 3A and Fig. B) or β1b (C and D) cRNA was coinjected. The maximal amplitude of the gating currents measured in each egg was comparable with that of the largest ionic current, consistent with the idea that channel expression was high but the channel open probability was low. Indeed, while the ionic currents, even those carried by α1SΔC, were small compared with those measured for other cloned Ca2+ channels expressed in oocytes, the gating currents were as large as or larger than those of other Ca2+ channels (Neely et al. 1993; Olcese et al. 1996). This suggests that oocyte expression of the skeletal muscle Ca2+ channels is considerably more robust than has been previously believed. Oocytes injected with cRNA coding for the auxiliary subunits alone had DHP-insensitive inward Ba2+ currents comparable in size with the L-type current, but with rapid kinetics, presumably carried by an endogenous Ca2+ channel (Fig. 3 E, top). These oocytes did not have measurable gating currents (Fig. 3 E, bottom), suggesting that the endogenous channel had a much smaller ratio of gating to ionic current than the skeletal muscle channel. Based on its kinetic properties, we judged the small ionic current seen in oocytes injected with cRNA coding for full-length α1S to be mostly, though not completely, endogenous in origin (a conclusion confirmed by separate experiments in which 5 μM nimodipine was found to block <10% of the current expressed in these cells; data not shown). These currents were consistently smaller than the endogenous current observed in oocytes injected with the auxiliary subunit cRNAs alone, most likely because of competition between α1S subunits and endogenous Ca2+ channel subunits for the auxiliary subunits.
Figure 3.
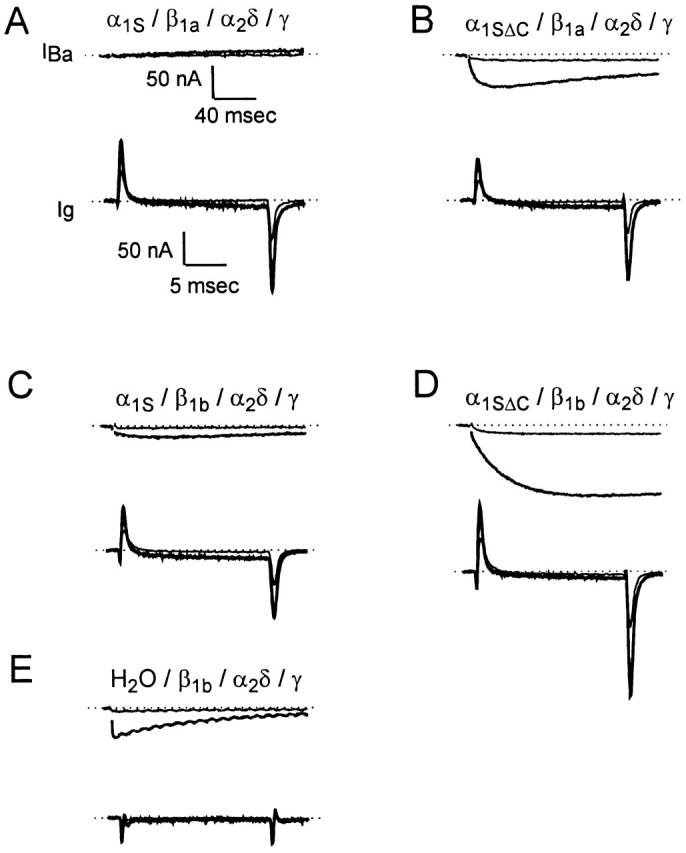
Ionic and gating currents recorded in the same cell for oocytes expressing α1S plus auxiliary subunits, α1SΔC plus auxiliary subunits, or auxiliary subunits alone. For each oocyte, inward ionic currents were recorded in 10 mM Ba2+ solution. The Ba2+ solution was replaced by 2 mM Co2+ solution (applied in the bath using a Pasteur pipet), and then gating currents were recorded after capacitance compensation. In A–E, the ionic currents evoked by test pulses to −40 and +20 mV from the holding potential of −90 mV are shown on a slow time scale (top, average of two traces in each case), and the gating currents evoked by test pulses to the same voltages are shown on a much faster time scale (bottom, average of four traces). The lighter traces show the responses at −40 mV, and the darker traces show the responses at +20 mV. Ionic currents were evoked by 300-ms depolarizations, filtered at 1 kHz and sampled at 2 kHz, and are presented with the residual capacitance transients blanked. Gating currents were evoked by 20-ms depolarizations, filtered at 1 kHz and sampled at 20 kHz.
Comparison of the mean Qon-V and I-V curves measured in oocytes expressing α1SΔC and α1S with either the β1a subunit (Fig. 4A and Fig. B) or the β1b subunit (C and D) emphasizes that while the full-length and truncated subunits are comparable voltage sensors, the truncated version is a much better conductor of ionic current. We quantified this functional distinction by calculating the ratio of maximum ionic current to maximum charge movement (Imax/Qon, max) for each oocyte (Fig. 4 E). Imax/Qon, max was sixfold greater for oocytes expressing α1SΔC than for oocytes expressing α1S when either the β1a or the β1b subunit was used; a difference that was highly statistically significant in each case (P = 4.8 × 10−6 and 6.2 × 10−5, respectively). This difference is a conservative estimate, since at least 90% of the current observed in α1S-injected oocytes was conducted by a DHP-insensitive α subunit, as mentioned above (data not shown). Imax/Qon, max was smaller for channels containing β1a than for channels containing β1b, reflecting both the somewhat smaller ionic currents and larger gating currents observed, on average, when β1a was injected. As was also seen in the separate experiments of Fig. 2, truncation of the α1S increased the size of gating currents significantly in the presence of the β1b subunit (P = 0.02), but not in the presence of the β1a subunit (P = 0.63).
Figure 4.
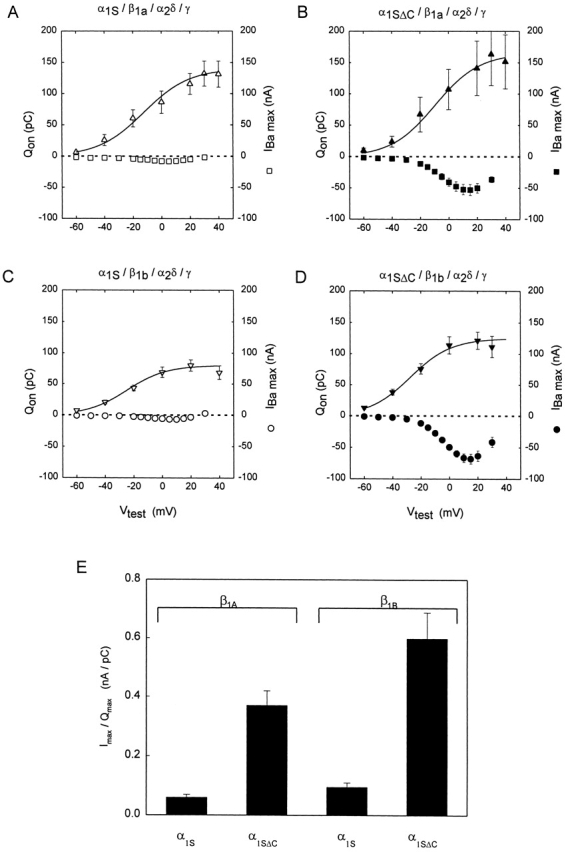
Comparison of ionic current to gating charge movement in oocytes expressing α1S or α1SΔC plus the auxiliary subunits. (A–D) Qon versus voltage (left scale) and current versus voltage (right scale) measured in the same oocytes. (A) Data from six cells expressing α1S, β1a, α2δ, and γ. (B) Data from five cells expressing α1SΔC, β1a, α2δ, and γ. (C) Data from 10 cells expressing α1S, β1b, α2δ, and γ. (D) Data from nine cells expressing α1SΔC, β1b, α2δ, and γ. Experiments were performed as in Fig. 3. Qon, measured as described in Fig. 1, was used instead of Qoff in these experiments to avoid the possibility of contamination with residual Ba2+ tail current. The solid lines are the mean Boltzmann Q-V curves determined as in Fig. 2 for each set of data. In A, Qon, max = 140 ± 21.6 pC, V1/2Q = −12.3 ± 5.5 mV, k Q = 16.0 ± 1.7 mV, Imax = −8.5 ± 1.1 nA, and n = 6. In B, Qon, max = 165 ± 47.6 pC, V1/2Q = −9.2 ± 3.3 mV, k Q = 15.6 ± 2.3 mV, Imax = −53.0 ± 8.6 nA, and n = 5. The difference in Qon, max between the data in A and in B was not statistically significant (P = 0.63). In C, Qon, max = 80.1 ± 9.6 pC, V1/2Q = −24.6 ± 1.8 mV, k Q = 13.8 ± 1.4 mV, Imax = −7.3 ± 1.1 nA, and n = 10. In D, Qon, max = 125 ± 16.0 pC, V1/2Q = −27.4 ± 2.6 mV, k Q = 14.4 ± 1.8 mV, Imax = −68.8 ± 7.2 nA, and n = 6. The difference in Qon, max between C and D was statistically significant (P = 0.02). (E) Ratio of maximum Ba2+ current (Imax) to Qon, max in cells expressing α1SΔC or α1S plus auxiliary subunits. For each cell, the maximum inward current evoked by a 300-ms depolarization to +10 or +15 mV in 10-mM Ba2+ solution was divided by the value of Qon, max determined from the Boltzmann fit to the Qon versus voltage relationship. In cells expressing the β1a subunit, Imax/Qon, max was 0.06 ± 0.01 nA/pC when α1S was expressed and 0.37 ± 0.05 nA/pC when α1SΔC was expressed, a difference which was statistically significant (P = 4.8 × 10−6). In cells expressing the β1b subunit, Imax/Qon, max was 0.10 ± 0.01 nA/pC when α1S was expressed and 0.60 ± 0.09 nA/pC when α1SΔC was expressed, a difference which was also statistically significant (P = 6.2 × 10−5).
DISCUSSION
The primary goal of this study was to determine whether the different magnitudes of L-type Ba2+ current observed previously when the full-length α1S and truncated α1SΔC subunits were expressed in Xenopus oocytes (Ren and Hall 1997) represents a function specialization of these two size forms of the skeletal muscle L-type Ca2+ channel. Using the cut-open oocyte voltage clamp method, we measured large transient currents from oocytes expressing α1S or α1SΔC with the β1 (β1a or β1b), α2δ, and γ subunits that represented gating charge movements in the α1 subunit molecule, demonstrating that both size forms of the channel are expressed in oocytes and constitute working voltage sensors. The range and steepness of the voltage dependence of the charge movement mediated by α1S and α1SΔC were essentially the same, independent of which β1 subunit was coexpressed. The maximum amount of charge moved at depolarized voltages, Qmax, tended to be larger for α1SΔC than for α1S. However, this difference in Qmax was statistically significant (both Qon, max and Qoff, max) only when the β1b subunit, rather than the β1a subunit, was coexpressed. Measurements of both charge movement and ionic current from the same oocyte showed that: (a) oocytes expressing the truncated subunit plus auxiliary subunits supported both charge movements and L-type Ba2+ current; (b) oocytes expressing the full-length subunit plus auxiliary subunits had charge movements but very little L-type Ba2+ current; and (c) oocytes expressing the auxiliary subunits alone had neither detectable charge movements nor L-type Ba2+ current. The stark differences observed between cells expressing α1S versus α1SΔC suggest there was no appreciable COOH-terminal truncation of α1S in the oocyte.
We conclude that the two naturally occurring size forms of the skeletal muscle DHP receptor α subunit are functionally specialized when expressed with the auxiliary subunits found in muscle. α1S and α1SΔC are both functional voltage sensors, but α1SΔC is a much more effective Ca2+ channel. Only tiny ionic currents were seen when the α1S subunit was coexpressed with either the β1a or β1b subunit (Fig. 4A and Fig. C), and the currents observed when the α1S/β1b/α2δ/γ combination was injected were ∼90% insensitive to 5 μM nimodipine (data not shown). This differs from the data of Ren and Hall 1997, who observed what appeared to be L-type current when α1S was expressed with β1b, α2δ, and γ (although this conclusion was based on current kinetics rather than on dihydropyridine block). We observed robust ionic currents when either β1a or β1b cRNAs were coinjected with α1SΔC cRNA, in contrast to the original report of a requirement for β1b (Ren and Hall 1997). Moreover, separate experiments in which 5 μM nimodipine was applied to oocytes expressing the α1SΔC/β1a/α2δ/γ combination confirmed that the inward current seen in this case was 80–90% L-type (data not shown). Our results therefore support the hypothesis that the predominant form of native DHP receptors in skeletal muscle, those containing α1SΔC and β1a, can function both as voltage sensors and as L-type calcium channels. Given the strong similarity of the kinetics and voltage dependence of the ionic and gating currents measured here in oocytes to those measured previously in mammalian muscle cells (Dulhunty and Gage 1983; Simon and Beam 1985; Lamb 1987; Delbono 1992; Garcia et al. 1992), we believe that the functional differences we observed for α1S and α1SΔC in oocytes are likely to occur in native DHP receptors. Nevertheless, experimental confirmation of the distinct functional roles of the α1S and α1SΔC subunits must ultimately be delineated in native cells, especially given the mounting evidence from dyspedic myotubes that both functions of the DHP receptor are strongly influenced by interacting ryanodine receptors (Nakai et al. 1996; Avila and Dirksen 2000). Definitive proof of distinct roles for the two size forms of the α1S subunit may ultimately require the identification and selective inhibition of the enzyme responsible for COOH-terminal truncation.
The mechanism by which the carboxyl terminus modulates the coupling between voltage-dependent gating and channel opening remains unknown. A similar effect has been observed for the cardiac L-type Ca2+ channel. Deletion of amino acids 307–472 from the 665-amino acid carboxyl terminus of the α1C subunit increased Ba2+ currents four- to sixfold in the oocyte expression system (Wei et al. 1994). The increase in ionic current did not appear to be accompanied by an increase in gating charge, consistent with the notion that the coupling was enhanced, rather than an increase in the number of channels. The confidence in this conclusion is somewhat limited, however, by the fact that charge movements were measured in the presence of large, unblocked inward Ba2+ currents. Several motifs important for modulation of Ca2+ currents have been identified in the carboxyl tails of α subunits. The carboxyl terminus deletion of α1S studied herein removes several consensus sites for PKC and cAMP-mediated phosphorylation, but leaves intact an EF hand and calmodulin-binding IQ consensus motifs, both of which have been implicated in Ca2+-dependent modulation of inactivation and facilitation of ionic current (Lee et al. 1999; Zühlke et al. 1999). It remains to be determined whether the enhanced coupling of gating charge movement to channel opening we observed in α1SΔC is dependent on any of these previously identified modulatory roles of the carboxyl terminus. Our observation that truncation of the α1S subunit significantly increased the magnitude of gating charge movements (an effect not seen with COOH-terminal truncation of α1C) suggests that the COOH terminus of α1S may play a role in channel expression in the membrane as well as in the coupling of gating to pore opening.
Our experiments give the unexpected result that both size forms of the α1S subunit express as well as or better than other cloned Ca2+ channel isoforms in Xenopus oocytes, as judged by gating current amplitudes (Neely et al. 1993; Olcese et al. 1996). This finding contradicts the common belief that the small ionic currents seen by us and others trying to express α1S in heterologous systems (Perez-Reyes et al. 1989; Nargeot et al. 1992; Johnson et al. 1997) reflect an inability of this channel to express stably outside the muscle environment. On the other hand, the local environment of the triadic junction does enhance the efficacy of DHP receptors to function as Ca2+ channels, as shown by a decrease in the ratio of ionic to gating current in dyspedic mice lacking the skeletal muscle isoform of the ryanodine receptor (Nakai et al. 1996). The large gating currents we observed relative to the small ionic currents underscore the idea that the coupling of charge movement to pore opening is poor in DHP receptors (Flockerzi et al. 1986; Ma et al. 1991; Dirksen and Beam 1995), even for channels containing the truncated α1S subunit. Robust expression of the skeletal muscle L-type Ca2+ channel in a heterologous system will be useful in elucidating the functional consequences of muscle-specific modulatory mechanisms, which may include modifications to the channel protein (such as phosphorylation or the COOH-terminal truncation studied here) or interactions with other proteins (such as the auxiliary Ca2+ channel subunits or other components of the triad junction). In addition, the ability to record skeletal muscle Ca2+ channel gating currents in an expression system lacking the electrically complex features of muscle cells, such as t tubules and additional voltage-gated channels, may allow for unprecedented precision in the study of the channel voltage sensor.
Acknowledgments
The authors thank B.P. Bean for lending the oocyte clamp and for comments on the manuscript.
This work was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases (AR42703 to S.C. Cannon) and the Quan Fellowship to Harvard Medical School (J.A. Morrill).
Footnotes
Abbreviations used in this paper: DHP, dihydropyridine; E-C, excitation–contraction.
References
- Adams B.A., Tanabe T., Mikami A., Numa S., Beam K.G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Avila G., Dirksen R.T. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J. Gen. Physiol. 2000;115:467–480. doi: 10.1085/jgp.115.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K.G., Adams B.A., Niidome T., Numa S., Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature. 1992;360:169–171. doi: 10.1038/360169a0. [DOI] [PubMed] [Google Scholar]
- De Jongh K.S., Merrick D.K., Catterall W.A. Subunits of purified calcium channelsa 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc. Natl. Acad. Sci. USA. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K., Warner C., Colvin A.A., Catterall W.A. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc. Natl. Acad. Sci. USA. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O. Calcium current activation and charge movement in denervated mammalian skeletal muscle fibres. J. Physiol. 1992;451:187–203. doi: 10.1113/jphysiol.1992.sp019160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen R.T., Beam K.G. Single calcium channel behavior in native skeletal muscle. J. Gen. Physiol. 1995;105:227–247. doi: 10.1085/jgp.105.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A.F., Gage P.W. Asymmetrical charge movement in slow- and fast-twitch mammalian muscle fibres in normal and paraplegic rats. J. Physiol. 1983;341:213–231. doi: 10.1113/jphysiol.1983.sp014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zollner P. Fast gating kinetics of the slow Ca2+ current in cut skeletal muscle fibres of the frog. J. Physiol. 1990;425:347–367. doi: 10.1113/jphysiol.1990.sp018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H.-J., Hofmann F., Pelzer D., Cavalie A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986;323:66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Flucher B.E., Neuhuber B., Gerster U. The full-length form of the skeletal muscle calcium channel α1S subunit is inserted into triads. Biophys. J. 1999;76:A342. [Google Scholar]
- Garcia J., McKinley K., Appel S.H., Stefani E. Ca2+ current and charge movement in adult single human skeletal muscle fibres. J. Physiol. 1992;454:83–196. doi: 10.1113/jphysiol.1992.sp019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu. Rev. Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Johnson B.D., Brousal J.P., Peterson B.Z., Gallombardo P.A., Hockerman G.H., Lai Y., Scheuer T., Catterall W.A. Modulation of the cloned skeletal muscle L-type Ca2+ channel by anchored cAMP-dependent protein kinase. J. Neurosci. 1997;17:1243–1255. doi: 10.1523/JNEUROSCI.17-04-01243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G.D. Asymmetric charge movement in polarized and depolarized muscle fibres of the rabbit. J. Physiol. 1987;383:349–367. doi: 10.1113/jphysiol.1987.sp016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Wong S.T., Gallagher D., Lin B., Storm D.R., Scheuer T., Catterall W.A. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- Ma J., Gonzalez A., Chen R. Fast activation of dihydropyridine-sensitive calcium channels of skeletal musclemultiple pathways of channel gating. J. Gen. Physiol. 1996;108:221–232. doi: 10.1085/jgp.108.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Mundina-Weilenmann C., Hosey M.M., Ríos E. Dihydropyridine-sensitive skeletal muscle Ca channels in polarized planar bilayers. 1. Kinetics and voltage dependence of gating. Biophys. J. 1991;60:890–901. doi: 10.1016/S0006-3495(91)82123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Hermann-Frank A., Lüttgau H.C. The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Nakai J., Dirksen R.T., Nguyen H.T., Pessah I.N., Beam K.G., Allen P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Nargeot J., Dascal N., Lester H.A. Heterologous expression of calcium channels. J. Membr. Biol. 1992;126:97–108. doi: 10.1007/BF00231908. [DOI] [PubMed] [Google Scholar]
- Neely A., Wei X., Olcese R., Birnbaumer L., Stefani E. Potentiation by the β subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- Olcese R., Neely A., Qin N., Wei X., Birnbaumer L., Stefani E. Coupling between charge movement and pore opening in vertebrate neuronal α1E calcium channels J . Physiol. 1996;497:675–686. doi: 10.1113/jphysiol.1996.sp021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H.S., Lacerda A.E., Horne W., Wei X., Rampe D., Campbell K.P., Brown A.M., Birnbaumer L. Induction of calcium currents by the expression of the α1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989;340:233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Ren D., Hall L.M. Functional expression and characterization of skeletal muscle dihydropyridine receptors in Xenopus oocytes. J. Biol. Chem. 1997;272:22393–22396. doi: 10.1074/jbc.272.36.22393. [DOI] [PubMed] [Google Scholar]
- Ríos E., Brum G. Involvement of dihydropyridine receptors in excitation–contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L.M., McCleskey E.W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. Nature. 1985;314:747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Simon B.J., Beam K.G. Slow charge movement in mammalian skeletal muscle. J. Gen. Physiol. 1985;85:1–19. doi: 10.1085/jgp.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Bezanilla F. Cut-open oocyte voltage clamp technique. Methods Enzymol. 1998;293:300–318. doi: 10.1016/s0076-6879(98)93020-8. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K.G., Powell J.A., Numa S. Restoration of excitation–contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Wei X., Neely A., Lacerda A.E., Olcese R., Stefani E., Perez-Reyes E., Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J. Biol. Chem. 1994;269:1635–1640. [PubMed] [Google Scholar]
- Zühlke R.D., Pitt G.S., Deisseroth K., W Tsien R., Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]