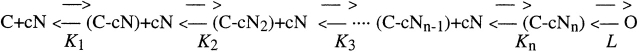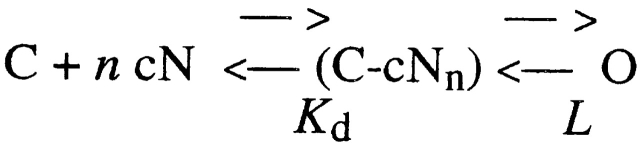Abstract
Upon stimulation by odorants, Ca2+ and Na+ enter the cilia of olfactory sensory neurons through channels directly gated by cAMP. Cyclic nucleotide–gated channels have been found in a variety of cells and extensively investigated in the past few years. Glutamate residues at position 363 of the α subunit of the bovine retinal rod channel have previously been shown to constitute a cation-binding site important for blockage by external divalent cations and to control single-channel properties. It has therefore been assumed, but not proven, that glutamate residues at the corresponding position of the other cyclic nucleotide–gated channels play a similar role. We studied the corresponding glutamate (E340) of the α subunit of the bovine olfactory channel to determine its role in channel gating and in permeation and blockage by Ca2+ and Mg2+. E340 was mutated into either an aspartate, glycine, glutamine, or asparagine residue and properties of mutant channels expressed in Xenopus laevis oocytes were measured in excised patches. By single-channel recordings, we demonstrated that the open probabilities in the presence of cGMP or cAMP were decreased by the mutations, with a larger decrease observed on gating by cAMP. Moreover, we observed that the mutant E340N presented two conductance levels. We found that both external Ca2+ and Mg2+ powerfully blocked the current in wild-type and E340D mutants, whereas their blockage efficacy was drastically reduced when the glutamate charge was neutralized. The inward current carried by external Ca2+ relative to Na+ was larger in the E340G mutant compared with wild-type channels. In conclusion, we have confirmed that the residue at position E340 of the bovine olfactory CNG channel is in the pore region, controls permeation and blockage by external Ca2+ and Mg2+, and affects channel gating by cAMP more than by cGMP.
Keywords: cyclic guanosine monophosphate, cyclic adenosine monophosphate, olfactory sensory neurons, site-directed mutagenesis
INTRODUCTION
Olfactory transduction is initiated by the binding of odorant molecules to receptor proteins located in the cilia of olfactory sensory neurons. This binding causes the activation of a receptor-coupled G protein, which in turn activates an adenylyl cyclase that synthesizes cAMP. The resultant increase in cAMP concentration leads to the opening of cyclic nucleotide–gated (CNG) ion channels in the ciliary membrane (Nakamura and Gold 1987), through which flows an odorant-induced current carried by Na+ and Ca2+ ions. Ca2+ entry in olfactory sensory neurons is physiologically important since a rise in intracellular Ca2+concentration is involved both in the excitation process, which includes the activation of a Ca2+-activated Cl− current, and in adaptation (for a recent review, see Menini 1999).
Native CNG channels from olfactory sensory neurons have been supposed to be heterotetrameric complexes with two types of subunits, named differently by different authors. However, a third type of subunit, a short splice variant of the retinal rod β subunit, has recently been cloned (Sautter et al. 1998) and CNG channels from rat olfactory sensory neurons have been shown to be comprised of three different types of subunits (Bönigk et al. 1999). Unfortunately, an agreement on the nomenclature for CNG channels has not yet been reached, creating confusion for the nonspecialist reading papers on CNG channels. We will use the nomenclature of Bönigk et al. 1999; i.e., α3 for the principal olfactory α subunit, α4 for the second cloned subunit (this subunit was originally named rOCNC2 by Bradley et al. 1994, and Liman and Buck 1994, and subsequently named β subunit by other authors) and β1b for the most recently cloned subunit (Sautter et al. 1998; Bönigk et al. 1999). The α3 subunit expressed in Xenopus laevis oocytes forms functional channels (Dhallan et al. 1990; Altenhofen et al. 1991; Goulding et al. 1992), while the other two subunits, α4 and β1b, are modulatory subunits and do not form functional CNG channels on their own. The α3 subunit has a glutamate residue (E340) that corresponds to E363 of the α subunit of the bovine rod CNG channel. The two subunits α4 and β1b have an aspartate and a glycine residue, respectively, at the corresponding location.
Each CNG channel subunit is formed by six transmembrane segments, with the pore region located between the fifth and sixth transmembrane segment (for reviews, see Kaupp 1995; Menini 1995; Zimmerman 1995; Finn et al. 1996; Zagotta and Siegelbaum 1996). Structure–function studies have identified several domains of the channel protein involved in channel gating: a cyclic nucleotide-binding domain, near the COOH terminus (Kaupp et al. 1989; Altenhofen et al. 1991; Brown et al. 1993; Scott and Tanaka 1998), a region near the NH2 terminus (Goulding et al. 1994; Gordon and Zagotta 1995; Varnum et al. 1995; Tibbs et al. 1997), the C-linker, which connects the sixth transmembrane segment and the cyclic nucleotide-binding domain (Brown et al. 1998; Zong et al. 1998; Paoletti et al. 1999), and the pore (Bucossi et al. 1997; Fodor et al. 1997). The cyclic nucleotide–binding site is physically separated from the pore region and the mechanism by which the conformational change in the cyclic nucleotide–binding domain is coupled to channel opening is likely to be very complex, with several regions of the channel being involved (Sun et al. 1996; Gordon et al. 1997; Varnum and Zagotta 1997; Becchetti et al. 1999; Sunderman and Zagotta 1999a,Sunderman and Zagotta 1999b).
A number of previous studies have reported that extracellular Ca2+ effectively blocks the current carried by monovalent ions through retinal rod, cone, and olfactory CNG channels in a voltage-dependent way (for reviews, see Kaupp 1995; Menini 1995; Finn et al. 1996). Site-directed mutagenesis on the α subunit of CNG channels from bovine retinal rods showed that blockage by external Ca2+ and Mg2+ critically depends on E363 (Root and MacKinnon 1993; Eismann et al. 1994). Retinal rod, cone, and olfactory CNG channels share many functional properties, but also differ in some respects. For example, cGMP and cAMP activate similar maximal currents in olfactory CNG channels, whereas cAMP activates only a small fraction of the current activated by cGMP in rod and cone CNG channels. Moreover, Ca2+ permeation is higher in CNG channels of olfactory sensory neurons and cones compared with rods (Perry and McNaughton 1991; Frings et al. 1995; Picones and Korenbrot 1995).
The aim of the present study was to investigate the role in permeation, gating, and block by Ca2+ and Mg2+ of the residue at position 340 in the α3 subunit of the bovine olfactory CNG channel.
MATERIALS AND METHODS
Site-directed Mutagenesis and Functional Expression
Site-directed mutagenesis was performed following the procedure of Herlitze and Koenen 1990. The cDNA of the bovine olfactory CNG channel (Altenhofen et al. 1991) was mutated by PCR reaction at the level of the putative pore region, where the sequence encoding the glutamate residue in position 340 was substituted with the sequence encoding a residue of aspartic acid or with the sequence encoding noncharged amino acids such as glutamine, asparagine, and glycine. Mutant clones were selected by their sensitivity to a nearby introduced SgrAI site and verified by sequencing (Sanger et al. 1977). mRNAs specific for the wild-type and mutant channels were then synthesized in vitro (Melton et al. 1984) from XhoI-linearized DNA using T3-RNA polymerase; synthesis was primed with m7G(5′)ppp(5′)G. Xenopus laevis oocytes (Centre National de la Recherche Scientifique, Montpellier, France) were prepared and injected with individual mRNAs as previously described (Gavazzo et al. 1997).
Current Recordings
Current measurements were performed from patches excised from the plasma membrane of oocytes in the inside-out or outside-out configuration.
Macroscopic currents were measured with the Axopatch 1D patch-clamp amplifier (Axon Instruments, Inc.), filtered at 1 kHz, and sampled at 2 or 2.5 kHz using a TL-1 DMA interface board (Axon Instruments, Inc.) with a PC-type computer and pClamp5 software (Axon Instruments, Inc.).
Single-channel recordings, performed 1–3 d after mRNA injection, allowed the determination of single-channel current and open probability of wild-type and mutant channels. Single-channel traces were recorded filtering at 2 kHz and sampled at 10 kHz. All-points amplitude histograms were constructed from 30 s of data recordings. The single-channel current and the open probability were determined by analyzing amplitude histograms normalized to a total area of 1. Histograms were fitted using the Levenberg-Marquardt least square algorithm by the sum of two or more Gaussian functions with pClamp6 software (Axon Instruments, Inc.).
Ionic Solutions
The ionic composition of the divalent cation-free solution at the extracellular and intracellular sides of the membrane patch was: 110 mM NaCl, no added divalent salts, 2 mM EDTA, and 10 mM HEPES, buffered to pH 7.4 with NaOH or with tetramethylammonium hydroxide. Low Ca2+ or Mg2+ concentrations were buffered by 2 mM nitrilotriacetic acid or by EDTA, respectively. The concentrations of CaCl2 or MgCl2, necessary to give the indicated free Ca2+ or Mg2+ concentrations were calculated using the chelator program of Fabiato 1988. Free Ca2+ or Mg2+ concentrations higher than 0.1 mM were not buffered.
Channels were activated with various concentrations of cyclic nucleotides, cGMP or cAMP, applied at the intracellular side of an excised patch. Exchange of bath solutions was achieved by positioning the excised patch in front of one of four glass pipes from which test solutions were continuously flowing, as previously described by Menini and Nunn 1990.
Current–Voltage Relations
Macroscopic current–voltage relations were measured by applying voltage steps of 150-ms duration from a holding potential of 0 mV in ±20-mV steps. For the measurement of the current blockage by divalent cations, current–voltage relations were obtained by ramping the voltage command from −80 to +80 mV in 1 s; five individual ramps were averaged in each measurement. I-V relations at the beginning of each experiment were also measured by applying voltage steps of 150-ms duration to investigate whether the patch presented ion accumulation effects, as described by Zimmerman et al. 1988. The experiments were continued only when such effect was not present and therefore the I-V relations obtained from ramps or from voltage steps gave the same values.
Outside-out patches were used to investigate the blockage by external Ca2+ and Mg2+. In these experiments, 1 mM cGMP was present in the patch pipette, unless otherwise indicated. Similar results were obtained when channels were activated by cAMP. The leak conductance was assumed not to be voltage dependent, and was usually estimated by perfusing the external side of the patch with a solution containing 73.3 mM CdCl2 or MgCl2 buffered to pH 7.4 with tetramethylammonium hydroxide. The residual conductance measured at negative potentials was taken as the leak conductance and was subtracted from each I-V relation. Further experiments were only performed when the leak current did not exceed 10% of the CNG current in divalent cation-free solution.
Data Analysis of Dose–Response Relations
Macroscopic currents as a function of cyclic nucleotide concentration were fitted by:
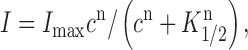 |
1 |
where I is the activated current, I max is the maximal current, c is the cyclic nucleotide concentration, K 1/2 is the cyclic nucleotide concentration activating half of the maximal current, and n is the Hill coefficient.
A similar equation was used to fit the single-channel open probability, P o, as a function of cyclic nucleotide concentration:
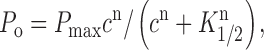 |
2 |
where P max is the maximal open probability and K 1/2 is the cyclic nucleotide concentration giving the half-maximal open probability.
A model for channel activation involving the cooperative binding of cyclic nucleotides to n binding sites, followed by an allosteric closed to open transition, similar to that originally proposed by Liu et al. 1994, was also used (Fig. 1):
Scheme S1.
where C is the channel in the closed state, cN is the cyclic nucleotide, O is the channel in the open state, K i are the dissociation constants for the binding sites, and L is the equilibrium “gating” constant, defined as the ratio between open and closed channels. In the limiting case of infinite cooperativity between the binding sites, the above kinetic scheme reduces to (Fig. 2):
Scheme S2.
with K d representing the “effective” dissociation constant, defined as K d = (K 1 K 2·····Kn−1 Kn)1/ n. In this model, the channel open probability, P o, is given by:
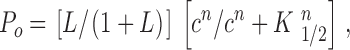 |
3 |
where c is the cyclic nucleotide concentration and K 1/2 = K d/(1 + L)1/ n. Therefore, K 1/2 values are determined by both the apparent ligand affinity for cyclic nucleotides and the probability of the allosteric transition. The maximal open probability, P max, is given by L/(1 + L).
The parameters obtained by fitting the single-channel dose–response data to the Hill equation (P max, K 1/2, and n) () were used to calculate starting points for the parameters L, K d, and n for the best fit of . Curve fittings were done with IgorPro3.1 (WaveMetrics).
Data Analysis of Current Blockage
Current values in the presence of various concentrations of Ca2+ or Mg2+ were normalized to the current in the absence of divalent cations at a given voltage and plotted versus divalent cation concentration. Data were fitted with the following equation:
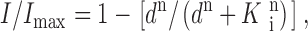 |
4 |
where I is the current measured in the presence of the divalent cation concentration d, I max is the current measured in the absence of divalent cations, K i is the divalent cation concentration blocking half of I max, and n is the Hill coefficient.
was fitted to the data for each patch separately and mean values for K i and n were calculated. All data are given as mean ± SD.
RESULTS
Macroscopic Currents
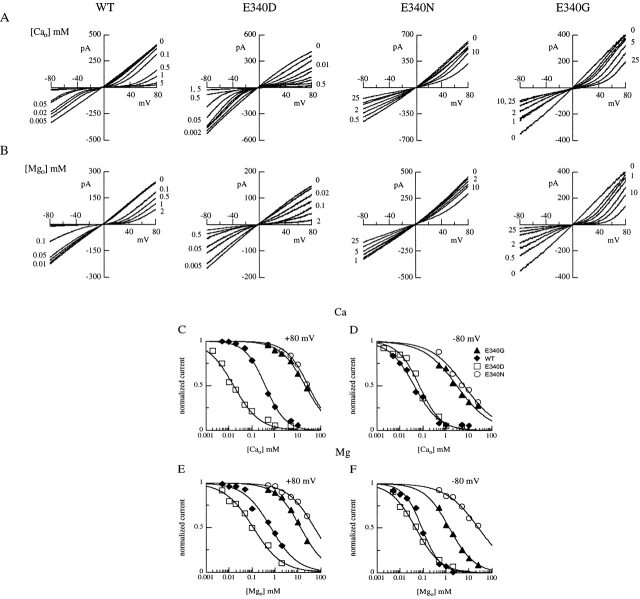
Currents activated by cGMP or cAMP were studied in excised inside-out patches from oocytes expressing wild-type or individual mutant channels. Fig. 1A and Fig. B, shows macroscopic currents measured at voltage pulses between −80 and +80 mV and activated by cGMP (A) or by cAMP (B) concentrations producing maximal currents.
Figure 1.
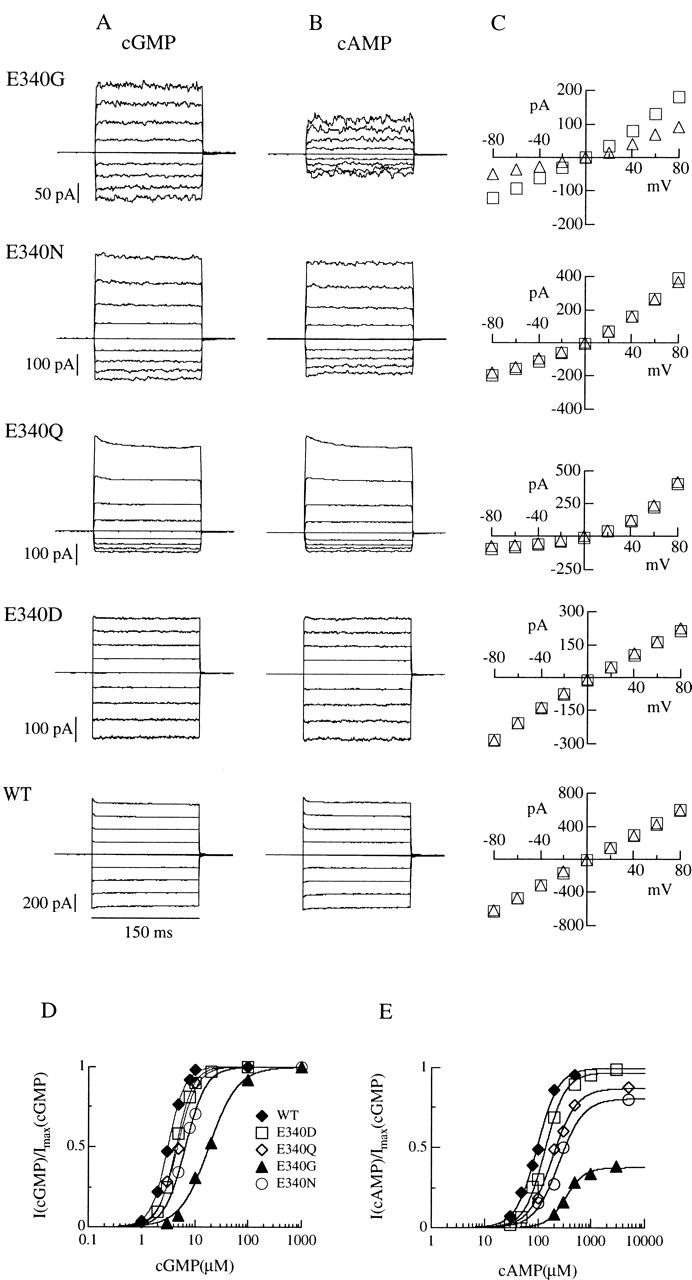
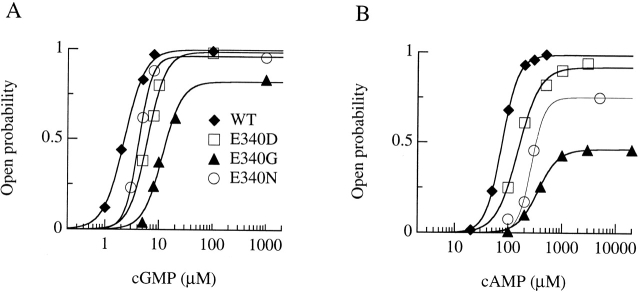
Maximal currents activated by cGMP or cAMP for wild-type and mutant olfactory CNG channels. Currents in each row were from the same excised inside-out patch. Voltage steps of 150-ms duration from a holding potential of 0 mV were given from −80 to +80 mV in 20-mV steps. Currents were activated by cyclic nucleotide concentrations eliciting maximal currents (see dose–response relations in D–E): (A) cGMP concentration was 1 mM, (B) cAMP concentration was 3 mM for E340G and E340D, 5 mM for E340N and E340Q, and 500 μM for wild-type channels. (C) Current–voltage relations from recordings shown in A and B for cGMP (□) and cAMP (▵). (D–E) Dose–response relations. Currents activated by cGMP or by cAMP at −80 mV were measured in the same patch, normalized to the maximal current activated by cGMP at −80 mV, and plotted versus cGMP (D) or cAMP (E) concentrations. Continuous lines represent the best fit of the Hill equation () to the data with the following values. Wild-type: I max,cA/I max,cG = 0.99, K 1/2,cG = 3.2 μM, n cG = 2.7, K 1/2,cA = 97 μM, n cA = 2.2; E340D: I max,cA/I max,cG = 0.97, K 1/2,cG = 4.5 μM, n cG = 2.7, K 1/2,cA = 137 μM, n cA = 2.3; E340Q: I max,cA/I max,cG = 0.87, K 1/2,cG = 4.8 μM, n cG = 2.4, K 1/2,cA = 189 μM, n cA = 2.0; E340N: I max,cA/I max,cG = 0.81, K 1/2,cG = 6.6 μM, n cG = 2.3, K 1/2,cA = 251 μM, n cA = 1.9; E340G: I max,cA/I max,cG = 0.37, K 1/2,cG = 18.5 μM, n cG = 1.6, K 1/2,cA = 335 μM, n cA = 2.5.
In wild-type channels, maximal currents activated by cGMP or cAMP have very similar values; whereas, quite surprisingly, we found that in the E340G mutant, the current activated by cAMP was reduced to ∼50% of the current activated by cGMP. Mutation of E340 to aspartate, asparagine, or glutamine had much smaller effects on maximal currents activated by cAMP (see Table ).
Table 1.
Parameters of Dose–Response Relations from Macroscopic Currents from Mutant and Wild-Type CNG Channels
| K 1/2, μM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I(cAMP)/I(cGMP) | I(−80 mV)/I(+80 mV) | cAMP | cGMP | n(−80 mV) | ||||||
| −80 mV | +80 mV | cAMP | cGMP | −80 mV | +80 mV | −80 mV | +80 mV | cAMP | cGMP | |
| Wild Type | 0.98 ± 0.02 | 0.97 ± 0.20 | 1.12 ± 0.03 | 1.11 ± 0.03 | 73 ± 19 | 78 ± 21 | 2.5 ± 0.5 | 2.6 ± 0.6 | 2.3 ± 0.4 | 2.7 ± 0.2 |
| (7) | (7) | (7) | (7) | (7) | (7) | (7) | (7) | (7) | (7) | |
| E340D | 0.91 ± 0.09 | 0.93 ± 0.06 | 1.30 ± 0.08 | 1.30 ± 0.10 | 152 ± 20 | 155 ± 20 | 4.9 ± 1.1 | 4.8 ± 1.1 | 1.9 ± 0.2 | 2.6 ± 0.4 |
| (8) | (8) | (8) | (8) | (6) | (6) | (6) | (6) | (6) | (6) | |
| E340G | 0.44 ± 0.05 | 0.50 ± 0.06 | 0.69 ± 0.12 | 0.79 ± 0.09 | 430 ± 66 | 453 ± 37 | 15.0 ± 1.6 | 13.0 ± 1.5 | 2.0 ± 0.4 | 1.8 ± 0.4 |
| (8) | (8) | (8) | (8) | (5) | (5) | (3) | (3) | (5) | (3) | |
| E340N | 0.83 ± 0.07 | 0.91 ± 0.06 | 0.51 ± 0.10 | 0.55 ± 0.10 | 299 ± 31 | 206 ± 35 | 7.6 ± 4.0 | 5.6 ± 1.8 | 2.0 ± 0.3 | 2.1 ± 0.3 |
| (6) | (6) | (6) | (6) | (7) | (7) | (7) | (7) | (7) | (7) | |
| E340Q | 0.89 ± 0.05 | 0.96 ± 0.06 | 0.18 ± 0.05 | 0.19 ± 0.06 | 194 ± 83 | 141 ± 31 | 5.6 ± 1.7 | 4.0 ± 1.2 | 1.8 ± 0.4 | 2.7 ± 0.5 |
| (5) | (5) | (5) | (5) | (4) | (4) | (4) | (4) | (4) | (4) | |
Data are obtained by fitting the experimental values with the Hill equation (). All values are means ± SD, with the number of experiments in parenthesis.
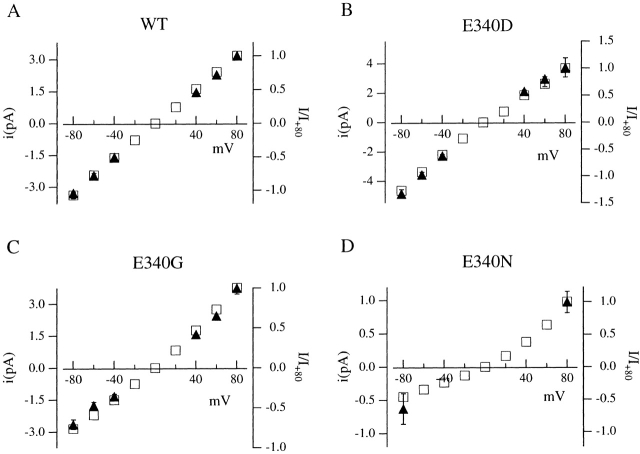
In Fig. 1 C, the modification of the current–voltage relations induced by mutations of E340 is shown. The mean ratio between the cAMP-gated current flowing at −80 and +80 mV, I(−80)/I(+80), was 1.12 ± 0.03 (n = 7) in wild-type channels, while it was slightly more inwardly rectifying in the mutated channel E340D, with a ratio of 1.30 ± 0.08 (n = 8) and became progressively outwardly rectifying with ratios of 0.69 ± 0.12 (n = 8) in the E340G, 0.51 ± 0.10 (n = 6) in the E340N, and 0.18 ± 0.05 (n = 5) in the E340Q mutants. Rectification ratios were similar for channels activated by cAMP or by cGMP (Table ). Whether changes of the current–voltage dependence were due to a modification of the channel permeation or gating properties caused by the mutations will be investigated later (see Fig. 4).
Figure 4.
Comparison of normalized I-V relations from macroscopic and single-channel currents for wild-type and mutant channels. Macroscopic currents were measured at saturating cGMP in a voltage range from −80 to + 80 mV and normalized to unity at +80 mV. Average values of normalized macroscopic currents (□) and of single-channel amplitudes (▴) were plotted versus applied voltage. Each point is the average from at least four patches. Bars indicate standard deviation. Error bars smaller than the size of the symbols were not plotted.
Normalized dose–response relations at −80 mV are shown in Fig. 1D and Fig. E. Currents for each channel type were measured from the same patch, plotted versus the concentration of cGMP or cAMP, fitted by the Hill equation (), and normalized to the maximal current measured in cGMP at −80 mV. For all mutants, the dose–response relations were shifted towards higher cyclic nucleotide concentrations with respect to the wild-type channel (Table ). At −80 mV, K 1/2 for cGMP increased from an average value of 2.5 ± 0.5 μM (n = 7) for the wild-type to 15.5 ± 1.6 μM (n = 3) for the E340G mutant, and K 1/2 for cAMP increased from an average value of 73 ± 19 μM (n = 7) for the wild-type to 430 ± 66 μM (n = 5) for the E340G mutant. Moreover, the maximal current of the E340G mutant activated by cAMP was 0.44 ± 0.05 (n = 8) of that activated by cGMP. Other mutations affected the current ratio less (Table ).
Therefore, mutations of glutamate 340 changed the voltage dependence of currents and modified the channel activation by increasing K 1/2 and decreasing the ratio between maximal currents activated by cAMP and cGMP.
Single-Channel Properties
To interpret properties of the macroscopic currents in terms of permeation and gating behavior, currents from patches containing only one single olfactory CNG channel were recorded.
Fig. 2A and Fig. B, shows recordings at −80 mV from a patch containing a single E340G channel. The open probability was plotted in Fig. 2 C as a function of cyclic nucleotide concentrations and fitted by the Hill equation (). At −80 mV, the average K 1/2 was 14 ± 5 μM (n = 4) for cGMP and 400 ± 214 μM (n = 4) for cAMP. These values are very similar to those obtained from macroscopic dose–response relations (see Table ).
Figure 2.
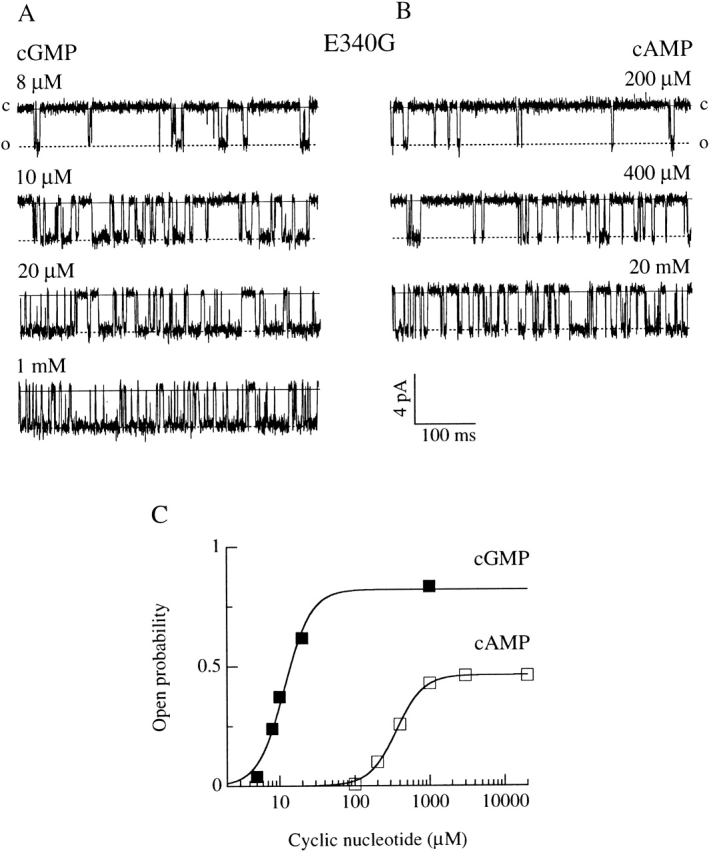
Single-channel dose–response relation for the E340G mutant. Current recordings from a membrane patch containing one E340G channel activated at −80 mV by various concentrations of cGMP (A) or cAMP (B). The continuous lines indicate the closed channel level and the dotted lines indicate the open level. (C) Dose–response relations were plotted as the open probability versus cyclic nucleotide concentration from the patch shown in A and B. Continuous curves were the best fit of the Hill equation () to the data with the following values: P max,cG = 0.82, K 1/2,cG = 11.6 μM, n cG = 2.4, P max,cA = 0.46, K 1/2,cA = 360 μM, n cA = 2.4.
In the presence of cGMP, the average maximal open probability, P max, for E340G channels was 0.79 ± 0.06 (n = 9), whereas, in the presence of cAMP, P max was only 0.27 ± 0.12 (n = 9). These values were much lower than P max for the wild-type channel, 0.996 ± 0.001 (n = 3) with cGMP and 0.985 ± 0.004 (n = 5) with cAMP (data not shown). The open probability was largely independent of voltage (see Table ).
Table 2.
Single-Channel Properties of Wild-Type and Mutant CNG Channels
| P max (−80 mV) | P max (+80 mV) | i, pA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP | cGMP | cAMP | cGMP | −80 mV | +80 mV | |||||||
| Wild Type | 0.985 ± 0.004 | 5 | 0.996 ± 0.001 | 3 | 0.984 ± 0.002 | 2 | 0.993 ± 0.004 | 2 | −3.2 ± 0.2 | 17 | 3.0 ± 0.1 | 11 |
| E340D | 0.958 ± 0.029 | 4 | 0.987 ± 0.007 | 4 | 0.978 ± 0.009 | 4 | 0.985 ± 0.007 | 2 | −4.8 ± 0.2 | 4 | 3.8 ± 0.6 | 4 |
| E340G | 0.27 ± 0.12 | 9 | 0.79 ± 0.06 | 9 | 0.23 ± 0.08 | 5 | 0.77 ± 0.08 | 5 | −2.6 ± 0.2 | 11 | 3.6 ± 0.3 | 13 |
| E340N* | 0.88 ± 0.02 | 2 | 0.96 ± 0.01 | 3 | ND | 0.87 ± 0.02 | 2 | −0.5 ± 0.1 | 5 | 1.0 ± 0.2 | 6 | |
| E340N‡ | 0.03 ± 0.01 | 2 | 0.04 ± 0.01 | 3 | ND | 0.10 ± 0.07 | 2 | −1.8 ± 0.1 | 4 | 1.6 ± 0.1 | 4 | |
P max values are obtained by fitting the experimental data with . All values are means ± SD, with the number of experiments given. *Low level of current; ‡high level of current determined with respect to low level.
Therefore, the maximal open probability measured in the E340G mutant was significantly lower than that of the wild-type channel and its value depended on which cyclic nucleotide activated the channel.
Single-channel properties were also investigated in other mutants. Single-channel currents from the mutant E340Q could not be measured, probably because of their small amplitude. Assuming the existence of only one open state, an estimate of the current amplitude was obtained by the relation between current variance and mean amplitude of macroscopic currents at various cGMP concentrations (data not shown). The estimated single-channel current was 0.1 pA at +80 mV and 0.03 pA at −80 mV, with a maximal open probability of 0.9.
Fig. 3 A shows recordings from a patch containing one single E340N mutant channel obtained at two cGMP concentrations at +80 and −80 mV.
Figure 3.
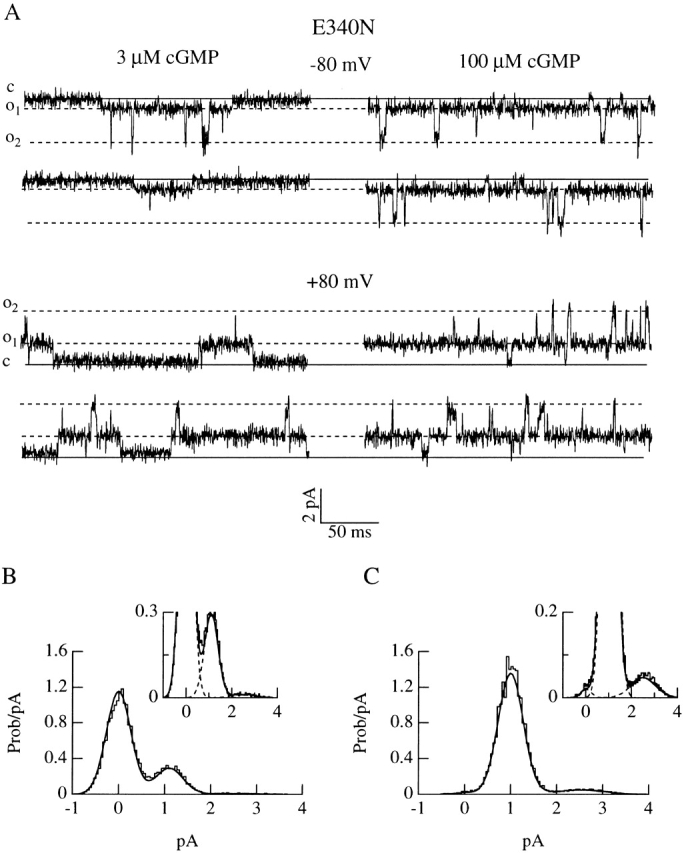
Single-channel voltage and concentration dependence of E340N mutant. (A) Current recordings from a membrane patch containing one E340N channel activated by 3 or 100 μM cGMP at −80 or +80 mV. The continuous lines indicate the closed channel level and the dotted lines indicate two conductance open levels. Amplitude histograms at +80 mV for 3 μM (B) or 100 μM (C) cGMP were fitted as the sum of three Gaussians and shown enlarged in the insets. Histograms were fitted with the following parameters. For 3 μM cGMP: P closed = 0.765, P o,low = 0.22, ilow = 1.1 pA, P o,high = 0.015, ihigh = 2.5 pA; for 100 μM cGMP: P closed = 0.01, P o,low = 0.925, ilow = 1 pA, P o,high = 0.065, ihigh = 2.5 pA.
Two conductance levels were present. The low conductance state had a current amplitude of ∼1 pA at +80 mV. From the low conductance state, the channel made brief transitions to a higher conductance state of ∼2.5 pA. Occasionally, the current trace returned to baseline from the low conductance state. Transitions to the high conductance state were never observed from the baseline, but only from the low conductance state. At a low cGMP concentration (3 μM), the channel spent most of the time in the closed state, from which it opened to the low conductance state, and very rarely to the high conductance state, as shown in the amplitude histogram of Fig. 3 B. At a high cGMP concentration (100 μM), the channel spent most of the time in the low conductance state from which it made more frequent but brief transitions to the high conductance state (Fig. 3 C).
The low conductance state was outwardly rectifying [i = −0.5 ± 0.1 pA (n = 5) at −80 mV, and 1.0 ± 0.2 pA (n = 6) at +80 mV], whereas the high conductance state had similar values at both voltages (∼2.5 pA). Similar results were obtained when the E340N channel was activated by cAMP. However, from single-channel dose–response curves (data not shown, see Table ), it was found that the maximal open probability of the low conductance state for cAMP at −80 mV was 0.88 ± 0.02 (n = 2), lower than that for cGMP, which was 0.96 ± 0.01 (n = 3).
Therefore, the E340N mutation modifies the gating properties of the channel by introducing a new conductance state and by reducing the efficacy of activation by cyclic nucleotides more so for cAMP than cGMP.
Voltage Dependence of Permeation Properties
To investigate whether changes in the voltage dependence of the macroscopic currents recorded from various mutants (Fig. 1 A) were due to effects of voltage on the permeation or on the gating process, the I-V relations of macroscopic and single-channel currents for wild-type and mutant channels were compared in Fig. 4. Macroscopic currents were normalized to their respective values measured at +80 mV, and the values of single-channel and normalized macroscopic currents were averaged from several patches and plotted as a function of voltage. For the mutant E340N, only the single-channel current value of the low conductance state, in which the channel spent most of its time, is shown. Among the different channel types, the amplitude and rectification properties of single-channel current varied; however, they did not depend on which cyclic nucleotide, cAMP or cGMP, activated the channel (data not shown). At −80 mV, the average single-channel current was −3.2 pA in the wild-type, −4.8 pA in the mutated channel E340D, −2.6 pA in the E340G, and −0.5 pA for the low-conductance state in the E340N (see Table ). The mean ratio between the single-channel current at −80 and +80 mV was 1.07 in the wild type, 1.26 in the mutated channel E340D, 0.72 in the E340G, and 0.5 for the low conductance state in the E340N.
The superimposition of the single-channel I-V curve with the macroscopic I-V curve for the various channel types indicates that the changes in the rectification properties in different mutants are mainly caused by changes in the voltage dependence of the permeation process, rather than by changes in gating (see also Table for values of P max at positive and negative voltages).
Gating Model for Channel Activation
The single-channel dose responses of wild-type and mutant channels were plotted in Fig. 5, along with the best fit obtained by . In the presence of cGMP, K d had similar values: 29 ± 10 μM (n = 3) wild-type and 31 ± 15 μM (n = 3) for E340D, and 24 ± 12 μM (n = 4) for E340G. On the other hand, L varied from 334 ± 82 for the wild-type to 66 ± 15 for E340D and 5.2 ± 1.3 for E340G mutation. In the presence of cAMP, K d also had similar values: 333 ± 96 μM (n = 3) for wild-type and 558 ± 120 μM (n = 3) for E340D and 479 ± 241 μM (n = 4) for E340G, though these values differ from those obtained for cGMP. L for cAMP varied from 70 ± 22 for the wild type to 12 ± 7 for E340D and 0.6 ± 0.3 for E340G mutant channels.
Figure 5.
Single-channel dose–response relations for wild-type, E340D, E340N, and E340G channels. Continuous curves show fits of to the dose–response relations using the following parameters. Wild-type: L cG = 334, K d,cG = 29 μM, n cG = 2.3, L cA = 70, K d,cA = 333 μM, n cA = 2.9; E340D: L cG = 66, K d,cG = 31 μM, n cG = 2.6, L cA = 12, K d,cA = 558 μM, n cA = 3; E340G: L cG = 4.6, K d,cG = 24 μM, n cG = 2.4, L cA = 0.9, K d,cA = 472 μM, n cA = 2.3; E340N: L cG = 10, K d,cG = 24 μM, n cG = 3.5, L cA = 3, K d,cA = 400 μM, n cA = 3.5.
Therefore, mutant channels have K d values similar to those estimated for the wild-type channel, but are characterized by very different values of the gating constant L, which determines the value for the maximal open probability. Two major conclusions can be drawn from these results. First, mutations at position 340 do not affect the “effective” dissociation constant (K d) for cAMP and cGMP. Second, mutations at position 340 modify the opening efficiency of the fully liganded channel. Thus, changes in the value of K 1/2 in the various mutants originate from changes of L.
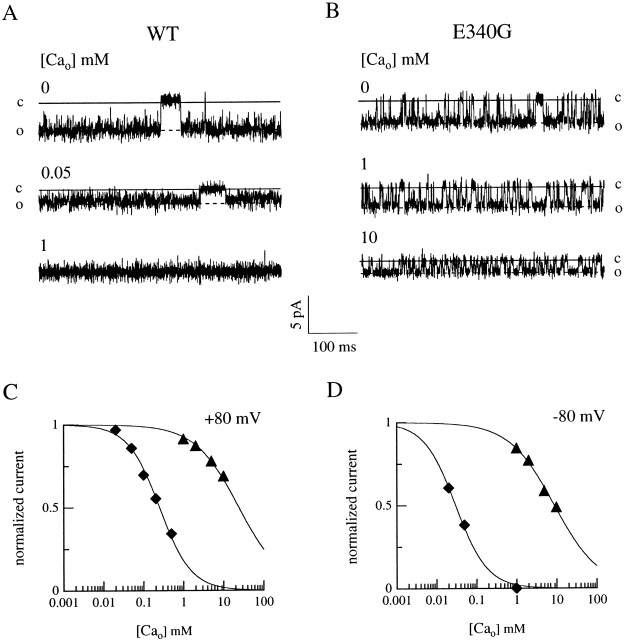
Divalent Cation Blockage
It is well known from previous work on the α subunit of the bovine retinal rod CNG channel that E363 controls channel blockage by extracellular divalent cations. We investigated here the role on such blockage of the residue E340 at the corresponding position in the olfactory CNG channel.
First, we measured the macroscopic dose–response relations for currents activated by cAMP or cGMP in inside-out patches from wild-type, E340G, and E340N channels in the presence of 1 mM Ca2+ in the patch pipette. The presence of 1 mM external Ca2+ did not significantly affect K 1/2 for either cyclic nucleotide (data not shown), indicating that external Ca2+ does not modify the gating properties of these channels (see also Fig. 7).
Figure 7.
External Ca2+ blockage on single-channel current for wild-type and E340G mutant channels. (A and B) Single-channel currents at −80 mV activated by cGMP in outside-out patches in the presence of the indicated concentrations of external Ca2+. The cGMP concentration was 5 μM for wild-type (A) and 1mM for E340G (B) mutant channels. (C and D) Currents from each patch were determined from amplitude histograms in the presence of various Ca2+ concentrations at +80 or −80 mV, normalized to the current measured at the same voltage in the absence of divalent cations, and plotted as a function of external Ca2+ concentrations (C and D). Continuous lines were the best fit of to the data with the following values. At +80 mV, (C) wild type (♦): K i,Ca = 250 μM, n = 1; E340G (▴): K i,Ca = 32 mM, n = 0.7. At −80 mV, (D) wild type (♦): K i,Ca = 31 μM, n = 1.1; E340G (▴): K i,Ca = 8.8 mM, n = 0.8.
The blocking effect of external Ca2+ and Mg2+ was therefore investigated by measuring the I-V relations of wild-type and mutant CNG channels in the presence of increasing Ca2+ or Mg2+ concentrations at the extracellular side of outside-out patches.
Fig. 6A and Fig. B, (left) shows the I-V relations for wild-type channels in which increasing concentrations of external Ca2+ (A) or Mg2+ (B) progressively reduced both the inward and outward currents in a voltage-dependent manner. When glutamate 340 was replaced by a neutral asparagine or glycine residue, blockage by external divalent cations was greatly reduced, and even high concentrations of external Ca2+ or Mg2+, 5–10 mM, only partially blocked the current (Fig. 6).
Figure 6.
External Ca2+ and Mg2+ blockage in wild-type and mutant channels. cGMP-gated currents were measured in outside-out patches in the presence of the indicated concentrations of Ca2+ (A) or Mg2+ (B) in the extracellular solution. Currents were activated by 100 μM cGMP in the patch pipette. Voltage ramps were from −80 to +80 mV. Currents from the experiments shown in A and B in the presence of various Ca2+ or Mg2+ concentrations were measured at +80 or −80 mV, normalized to the current measured in the absence of divalent cations at the same voltage and plotted as a function of external Ca2+ (C–D) or Mg2+ (E–F) concentrations. Continuous lines were the best fit of to the data with the following values. (C) At +80 mV, wild type (♦): K i,Ca = 380 μM, n = 1; E340D (□): K i,Ca = 16 μM, n = 1; E340G (▴): K i,Ca = 21 mM, n = 0.8; E340N (○): K i,Ca = 28 mM, n = 0.9. (D) At −80 mV, wild type (♦): K i,Ca = 42 μM, n = 0.8; E340D (□): K i,Ca = 70 μM, n = 0.9; E340G (▴): K i,Ca = 3 mM, n = 0.6; E340N (○): K i,Ca = 6.3 mM, n = 0.6. (E) At +80 mV, wild type (♦): K i,Mg = 650 μM, n = 0.7; E340D (□): K i,Mg = 112 μM, n = 0.7; E340G (▴): K i,Mg = 13 mM, n = 0.8; E340N (○): K i,Mg = 51 mM, n = 0.7. (F) At −80 mV, wild type (♦): K i,Mg = 90 μM, n = 1.1; E340D (□): K i,Mg = 48 μM, n = 0.8; E340G (▴): K i,Mg = 1.4 mM, n = 0.8; E340N (○): K i,Mg = 26 mM, n = 0.6.
To investigate whether an acidic residue at position 340 was crucial for Ca2+ and Mg2+ blockage, the charge-conserving substitution E340D was analyzed. Fig. 6 shows that external divalent cations powerfully blocked E340D channels, and that the voltage dependence of the blockage by external Ca2+ differed from that observed in wild-type channels. Indeed (see also Table ), the blocking effect at positive voltages was more pronounced in the E340D mutant than in wild-type channels (see discussion).
Table 3.
Inhibition Constants from Macroscopic Currents from Wild-Type and Mutant CNG Channels
| Ca2+ | Mg2+ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K i, μM | n | K i, μM | n | |||||||||||||
| −80 mV | +80 mV | −80 mV | +80 mV | −80 mV | +80 mV | −80 mV | +80 mV | |||||||||
| Wild Type | 47 ± 21 | 6 | 383 ± 145 | 6 | 1.3 ± 0.4 | 6 | 0.9 ± 0.1 | 6 | 70 ± 34 | 11 | 920 ± 640 | 11 | 1.2 ± 0.2 | 11 | 1.0 ± 0.1 | 11 |
| E340D | 47 ± 7 | 6 | 18 ± 10 | 6 | 1.2 ± 0.5 | 6 | 1.3 ± 0.5 | 6 | 53 ± 17 | 4 | 155 ± 56 | 4 | 0.7 ± 0.2 | 4 | 0.9 ± 0.2 | 4 |
| E340G | 6300 ± 2500 | 6 | 26000 ± 9800 | 6 | 0.4 ± 0.1 | 6 | 0.6 ± 0.2 | 6 | 1100 ± 300 | 7 | 9500 ± 3900 | 7 | 0.7 ± 0.1 | 7 | 0.8 ± 0.2 | 7 |
| E340N* | 6500 ± 4000 | 4 | 29000 ± 6000 | 4 | 0.6 ± 0.1 | 4 | 0.6 ± 0.2 | 4 | 17000 ± 7200 | 4 | 50000 ± 3600 | 4 | 0.6 ± 0.1 | 7 | 0.6 ± 0.1 | 7 |
| E340N‡ | 2100 ± 600 | 4 | 19000 ± 12000 | 4 | 0.4 ± 0.2 | 4 | 0.5 ± 0.2 | 4 | 3400 | 1 | 140000 | 1 | 0.5 | 1 | 0.3 | 1 |
Inhibition constants, K i, and Hill coefficients, n, for the blockage by external Ca2+ or Mg2+ were obtained from the best fit of to the data. All values are means ± SD, with the number of experiments given.
The concentration dependence of extracellular Ca2+ and Mg2+ blockage is better illustrated in Fig. 6C–F. Current values from the experiments shown in Fig. 6A and Fig. B, measured at +80 or −80 mV in the presence of various concentrations of Ca2+ or Mg2+ were normalized to the current in the absence of divalent cations and plotted versus divalent cation concentration. For external divalent cation blockage at various voltages, K i was calculated from the best fit of to the data (see Table ). At −80 mV for Ca2+, K i was found to increase from a similar average value of 47 ± 21 μM (n = 6) for wild-type and 47 ± 7 μM (n = 6) for E340D, to 6.3 ± 2.5 mM (n = 6) for E340G and 6.5 ± 4.0 mM (n = 4) for E340N mutant channels. At −80 mV for Mg2+, K i increased from an average value of 70 ± 34 μM (n = 11) for wild-type and 53 ± 17 μM (n = 4) for E340D, to 1.1 ± 0.3 mM (n = 7) for E340G and 17.0 ± 7.2 mM (n = 4) for E340N mutant channels.
The blockage by external divalent cations was also found to be relieved in the E340Q mutant channels (data not shown), with average values reported in Table . Hill coefficients for wild type and E340D were always close to one, indicating that one divalent cation is likely sufficient to block the pore in these channels. A 1,000-fold higher external divalent concentration was necessary to block 50% of the current in mutant channels with a neutral residue in position 340, and Hill coefficients were below unity.
The blockage by internal Ca2+ of wild-type and mutant channels was also investigated. Macroscopic currents in inside-out patches were activated by 1 mM cGMP in the presence of various Ca2+ concentrations at the cytoplasmic side of the patch (data not shown), and the average K i for internal Ca2+ blockage at +80 mV was found to be 1.0 ± 0.7 mM (n = 5) for wild-type and 1.5 ± 0.4 mM (n = 4) for E340G mutant channels. Similar results were also found for the E340N mutation. Therefore, charge neutralization of E340 does not significantly modify the blockage by intracellular Ca2+ of the olfactory CNG channel.
These results show that the presence of an acidic residue at position 340 of the bovine olfactory channel is an important determinant of blockage by external divalent cations.
Single-Channel Analysis of External Ca2+ Blockage
To investigate whether external divalent cation blockage on macroscopic current is due to a change in permeation and/or gating properties of the olfactory CNG channel, single-channel properties were examined. Fig. 7 A shows recordings from a patch in the outside-out configuration containing wild-type channels activated by 5 μM cGMP at −80 mV. At saturating cGMP concentrations, wild-type channels are open most of the time, making it difficult to estimate the single-channel amplitude reliably, so a lower concentration of cGMP was used for estimating the single-channel amplitude. The addition of 50 μM external Ca2+ caused a partial blockage of the single-channel current and in the presence of 1 mM Ca2+, no current was detected at −80 mV.
When the same experiments were repeated with a patch containing a single E340G mutant channel (Fig. 7 B), activated by 1 mM cGMP, the single-channel amplitude was only slightly reduced in the presence of 1 or even 10 mM external Ca2+.
Single-channel currents from the patches shown in Fig. 7A and Fig. B, in the presence of increasing concentrations of extracellular Ca2+, were estimated from amplitude histograms. The obtained values were normalized to the single-channel current measured in the absence of divalent cations and plotted as a function of Ca2+ concentration at +80 and −80 mV (see Fig. 7C and Fig. D). The best fit of to the data (continuous lines) gave a value of K i for Ca2+ at +80 mV of 250 μM for wild-type and 32 mM for E340G, while at −80 mV, K i for Ca2+ was 31 μM for wild-type and 8.8 mM for E340G. Similar results were obtained in two other patches for each channel type.
A comparison between these results on single-channel current and those on macroscopic currents (Table ) shows that K i and n values are in good agreement, indicating that external Ca2+ blockage diminishes the single-channel amplitude, affecting permeation rather than gating.
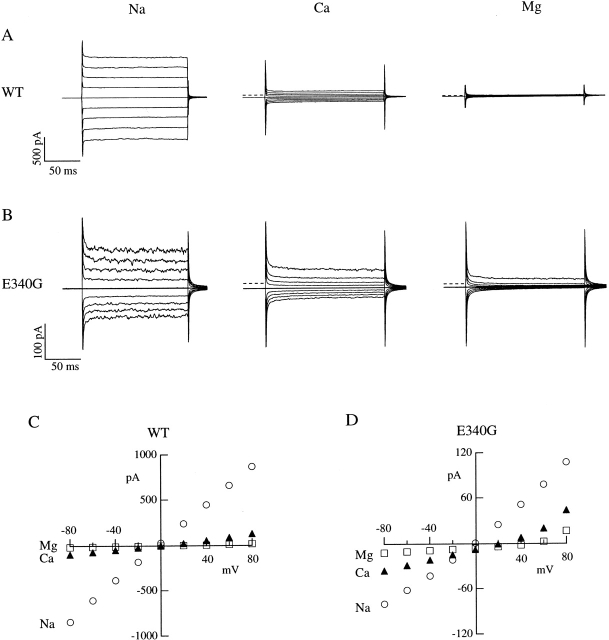
Divalent Cation Permeation
The permeation of divalent cations was investigated in outside-out patches by substituting external NaCl (110 mM) with equiosmolar concentrations (73.3 mM) of CaCl2 or MgCl2. Channels were activated by 1 mM cGMP in the patch pipette and voltage steps of 150-ms duration from a holding potential of 0 mV were given from −80 to +80 mV in 20-mV steps. Fig. 8A and Fig. B, shows recordings from wild-type and E340G mutants, respectively. I-V relations were plotted in Fig. 8C and Fig. D.
Figure 8.
Permeation of external Ca2+ and Mg2+ in wild-type and E340G mutant channels. Current recordings from individual outside-out patches in bi-ionic conditions. The patch pipette contained 1 mM cGMP and 110 mM NaCl, while the external solution contained either 110 mM NaCl, 73.3 mM CaCl2, or 73.3 mM MgCl2, as indicated in the figure. Voltage was changed in 20-mV steps from −80 to +80 mV from a holding potential of 0 mV. The dashed lines indicate the zero-current level. Raw data are illustrated without leak subtraction. Capacitance artifacts were not subtracted. Current–voltage relations are plotted in C and D for wild-type and E340G, respectively. Symbols indicate the bi-ionic conditions: symmetrical NaCl (○), internal NaCl and external CaCl2 (▴), and internal NaCl and external MgCl2 (□).
From these experiments, the ratio between the current carried by external Ca2+ and Na+ ions, I Ca/I Na, at −80 mV was calculated. For wild-type channels, the average value of I Ca/I Na at −80 mV was 0.049 ± 0.004 (n = 4) and increased to 0.16 ± 0.03 (n = 6) for E340G mutant channels. The average reversal potential, V rev, under bi-ionic conditions (internal Na and external divalent cations) was 25 ± 6 mV (n = 8) for Ca2+ and 45 ± 7 mV (n = 5) for Mg2+ for wild-type channels. For E340G mutant channels, V rev was 24 ± 5 mV (n = 5) for Ca2+ and 45 ± 15 mV (n = 4) for Mg2+.
These results show that reversal potentials and therefore permeability ratios, were not affected by the E340G mutation. However, the E340G mutation caused an increase of the inward Ca2+ current relatively to the Na+ current.
DISCUSSION
The results presented in this paper demonstrate that the residue at position 340 of the bovine olfactory CNG channel affects permeation and gating properties in the absence of divalent cations and controls the efficacy of blockage by external Ca2+ and Mg2+ ions. Moreover, our findings highlight similarities and differences with the role played by the corresponding residue 363 of the bovine rod CNG channel.
Gating by cAMP and cGMP
It is well established that cGMP and cAMP elicit similar maximal responses in wild-type olfactory channels, whereas K 1/2 for cGMP is several-fold lower than that for cAMP. All mutant channels presented in this study had higher K 1/2 values compared with wild-type channels, and showed a decrease of the maximal current activated by cAMP with respect to cGMP, especially in the E340G mutant. The explanation of these results is not obvious: the decrease in cAMP maximal current could derive either from a reduced single-channel amplitude or from different gating properties of CNG channels when activated by cAMP or cGMP. Moreover, the shift in K 1/2 could originate from a modification in the binding reactions for cyclic nucleotides (i.e., K d) or the subsequent allosteric rearrangement leading to channel opening (i.e., L) or from both effects.
Single-channel analysis allowed us to distinguish between these possible explanations. In all tested mutants, the single-channel conductance activated by cAMP and cGMP was the same, but open probabilities were different: in the E340G mutant P max did not exceed 50% of that observed with cGMP. Moreover, analysis of the data by using an allosteric model similar to that used by Liu et al. 1994 revealed that the substitutions at position 340 leave the effective affinity of the binding steps almost unaffected for a given cyclic nucleotide, but lower the equilibrium gating constant L. Therefore, differences in activation can be explained by assuming that mutants open less easily than wild-type channels without significant changes in the binding of cyclic nucleotides. These results show that the pore is involved in the allosteric conformational change after ligand binding.
Permeation
We found that mutation of E340 caused a modification in the I-V relations. The analysis of single-channel activity (Table ) showed that the rectification of the I-V curves is primarily determined by the voltage dependence of ion permeation rather than of the gating process. This result differs from what was previously measured in retinal rod. Indeed, Root and MacKinnon 1993 showed that the rectification of single-channel currents induced by mutations of E363 is smaller than that of macroscopic currents, implying that the difference must be accounted for by an effect of the mutations on voltage dependence of channel gating.
Two conductance levels were observed in the E340N mutant. Although it was outside the scope of our study to develop a kinetic model explaining these multiple conductance levels, this result further shows that the bovine olfactory CNG channel has single-channel properties different from those of the catfish olfactory CNG channel. Indeed, the CNG channel from catfish has multiple conductance states (Goulding et al. 1992) that have been ascribed to protonation of the glutamate residue (Root and MacKinnon 1994). One of the key experiments aimed at demonstrating that these subconductance states arise from proton binding in the pore was to show that they are not present when glutamate was replaced with the neutral glycine (Root and MacKinnon 1994). We found here that, although subconductance states are not evident in the wild-type bovine olfactory CNG channel, multiple conductance states are present when glutamate was replaced with the neutral asparagine, E340N, suggesting that proton block of the 340 residue is not responsible for subconductance states.
These results show that channels from different species could have some specific properties that are not necessarily shared by the majority of CNG channels, despite belonging to the same class.
Divalent Cation Blockage
Ca2+ and Mg2+ block the current carried by Na+ ions through olfactory CNG channels from the extracellular side and to a lesser extent from the intracellular side. The external divalent cation blockage was drastically reduced in mutant channels where E340 was replaced by a neutral amino acid (Table ), while internal divalent blockage was largely unaffected. These findings are in agreement with similar results obtained with mutations at position E363 of the α subunit from bovine retinal rods (Root and MacKinnon 1993; Eismann et al. 1994; Park and MacKinnon 1995).
A special case is the E340D mutant. The charge-conserving mutation E340D preserved the external divalent cation blockage, but altered its voltage dependence with a more pronounced effect for Ca2+ than for Mg2+ (Table ). Indeed, we unexpectedly found that external Ca2+ blockage was more effective at positive (K i = 18 μM at +80 mV) than negative (K i = 47 μM at −80 mV) voltages. In the E363D mutant from retinal rods (Root and MacKinnon 1993; Park and MacKinnon 1995; Seifert et al. 1999), the external divalent cation blockage was also preserved, although the voltage dependence was not dramatically altered as in the olfactory channel.
How can the different voltage dependence between Ca2+ and Mg2+ blockage in the olfactory E340D channel be explained? A similar result has also been previously observed in the blockage by internal divalent cations in native CNG channels from retinal rods (Colamartino et al. 1991; Zimmerman and Baylor 1992). The voltage dependence of block by internal Ca2+ was opposite that of block by internal Mg2+ (see Figure 10, B and C, from Zimmerman and Baylor 1992): Mg2+ in the internal solution caused a strong inward rectification, while Ca2+ caused a very pronounced outward rectification. Zimmerman and Baylor 1992 proposed a simple model, based on Eyring rate theory, in which cations permeate and block the channel by combining with a single binding site located in the transmembrane electrical field. They showed that the opposite voltage dependence of blockage by Ca2+ and Mg2+ arises from their different energy profiles for permeation: for Mg2+, the external barrier is higher than the internal barrier and depolarization favors occupancy of the well by internal Mg2+; for Ca2+, the internal barrier is higher and depolarization disfavors the occupancy of the well by internal Ca2+ (see Figure 1 in Zimmerman and Baylor 1992). A similar model can be used to explain the different voltage dependence of blockage by external Ca2+ and Mg2+ between the wild-type and E340D mutant channel; i.e., the mutation could differentially alter the external barrier for Ca2+ and Mg2+.
From a molecular point of view, these differences could have several origins. Although glutamate and aspartate have the same charge, they have a different length of the amino acid side chains and the protonation of aspartate could be different from that of glutamate. Alternatively, the binding site may change its location within the transmembrane electrical field, and/or the stereochemical coordination of divalent cations may be altered.
Comparison with Native Channels
The voltage dependence of blockage in α3 homomers and native channels is largely similar in that blockage was more pronounced at negative than at positive potentials. However, the values for K i were different. The K i for external Ca2+ blockage in native olfactory CNG channels from the frog Rana esculenta was found to be 265 μM at −70 mV and ∼2 mM at +80 mV (see Figure 6 B of Dzeja et al. 1999; see also Kleene 1995), while in α3 homomers we found 47 μM at −80 mV and 383 μM at +80 mV. These different K i values could originate from the different subunit composition of native channels: indeed, native olfactory CNG channels from the olfactory sensory neurons of the rat are composed by the coassembly of the three different types of subunits α3, α4, and β1b (Bönigk et al. 1999) having a glutamate, an aspartate, and a glycine residue, respectively, at the positions corresponding to E340 of the α3 subunit of bovine olfactory CNG channels. These residues are likely to play the key role in the blockage by external divalent cations, since we have shown that mutation of glutamate at position 340 of the α3 subunit into aspartate or glycine changes the sensitivity to external Ca2+ and Mg2+ by more than two orders of magnitude.
Recent experiments by Kleene 1999 show that external Ca2+ reduces the sensitivity to cAMP of the native olfactory channel with a mechanism different from the effect of cytoplasmic Ca2+. In the presence of 3 mM external Ca2+, K 1/2 for cAMP increased eightfold. We did not observe this effect in our experiments, using expressed channels composed only of the principal α3 subunit. Hackos and Korenbrot 1999 found that divalent cation selectivity depends on the cGMP concentration in retinal rod recombinant channels composed of α and β subunits, and not on channels formed from α subunits alone. Whether the additional olfactory subunits are responsible for the reduced sensitivity to cAMP induced by external Ca2+ in native olfactory channels remains to be determined.
Acknowledgments
We thank V. Torre, F. Gambale and A. Miri for comments on the manuscript and F. Pittaluga, G. Boido, G. Gaggero, P.G. Gagna, D. Magliozzi, and P. Guastavino for technical assistance. L. Giovanelli checked the English. Special thanks to B. Norcio for many helpful discussions.
This work was supported by European Community contract BIO4-CT96-0593 (U.B. Kaupp and A. Menini) and a grant from the Ministerium für Wissenschaft und Forschung des Landes Nordrhein-Westfalen IVA-10201095 (U.B. Kaupp).
Footnotes
Abbreviation used in this paper: CNG channel, cyclic nucleotide–gated channel.
References
- Altenhofen W., Ludwig J., Eismann E., Kraus W., Bönigk W., Kaupp U.B. Control of ligand specificity in cyclic nucleotide-gated channels from rod photoreceptors and olfactory epithelium. Proc. Natl. Acad. Sci. USA. 1991;88:9868–9872. doi: 10.1073/pnas.88.21.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti A., Gamel K., Torre V. Cyclic nucleotide–gated channels. Pore topology studied through the accessibility of reporter cysteines. J. Gen. Physiol. 1999;114:377–392. doi: 10.1085/jgp.114.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönigk W., Bradley J., Müller F., Sesti F., Boekhoff I., Ronnett G.V., Kaupp U.B., Frings S. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 1999;13:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J., Li J., Davidson N., Lester H.A., Zinn K. Heteromeric olfactory cyclic nucleotide-gated channelsa subunit that confers increased sensitivity to cAMP. Proc. Natl. Acad. Sci. USA. 1994;91:8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.L., Geber W.V., Karpen J.W. Specific labeling and permanent activation of the retinal rod cGMP-activated channel by the photoaffinity analog 8-p-azidophenacylthio-cGMP. Proc. Natl. Acad. Sci. USA. 1993;90:5369–5373. doi: 10.1073/pnas.90.11.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.L., Snow S.D., Haley T.L. Movement of gating machinery during the activation of rod cyclic nucleotide-gated channels. Biophys. J. 1998;75:825–833. doi: 10.1016/s0006-3495(98)77571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucossi G., Nizzari M., Torre V. Single-channel properties of ionic channels gated by cyclic nucleotides. Biophys. J. 1997;72:1165–1181. doi: 10.1016/S0006-3495(97)78765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamartino G., Menini A., Torre V. Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J. Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan R.S., Yau K.-W., Schrader K.A., Reed R.R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Dzeja C., Hagen V., Kaupp U.B., Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann E., Muller F., Heinemann S.H., Kaupp U.B. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc. Natl. Acad. Sci. USA. 1994;91:1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Finn J.T., Grunwald M.E., Yau K.W. Cyclic nucleotide-gated ion channelsan extended family with diverse functions. Annu. Rev. Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- Fodor A.A., Black K.D., Zagotta W.N. Tetracaine reports a conformational change in the pore of cyclic nucleotide–gated channels. J. Gen. Physiol. 1997;110:591–600. doi: 10.1085/jgp.110.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S., Seifert R., Godde M., Kaupp U.B. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Gavazzo P., Picco C., Menini A. Mechanisms of modulation by internal protons of cyclic nucleotide-gated channels cloned from sensory receptor cells. Proc. R. Soc. Lond. B Biol. Sci. 1997;264:1157–1165. doi: 10.1098/rspb.1997.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.E., Zagotta W.N. Localization of regions affecting an allosteric transition in cyclic nucleotide-activated channels. Neuron. 1995;14:857–864. doi: 10.1016/0896-6273(95)90229-5. [DOI] [PubMed] [Google Scholar]
- Gordon S.E., Varnum M.D., Zagotta W.N. Direct interaction between amino- and carboxyl-terminal domains of cyclic nucleotide-gated channels. Neuron. 1997;19:431–441. doi: 10.1016/s0896-6273(00)80951-4. [DOI] [PubMed] [Google Scholar]
- Goulding E.H., Ngai J., Kramer R.H., Colicos S., Axel R., Siegelbaum S.A., Chess A. Molecular cloning and single channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 1992;8:45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- Goulding E.H., Tibbs G.R., Siegelbaum S.A. Molecular mechanism of cyclic-nucleotide-gated channel activation. Nature. 1994;372:369–374. doi: 10.1038/372369a0. [DOI] [PubMed] [Google Scholar]
- Hackos D.H., Korenbrot J.I. Divalent cation selectivity is a function of gating in native and recombinant cyclic nucleotide–gated ion channels from retinal photoreceptors. J. Gen. Physiol. 1999;113:799–817. doi: 10.1085/jgp.113.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S., Koenen M. A general and rapid mutagenesis method using polymerase chain reaction. Gene. 1990;91:143–147. doi: 10.1016/0378-1119(90)90177-s. [DOI] [PubMed] [Google Scholar]
- Kaupp U.B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N.J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U.B. Family of cyclic nucleotide gated ion channels. Curr. Opin. Neurobiol. 1995;5:434–442. doi: 10.1016/0959-4388(95)80002-6. [DOI] [PubMed] [Google Scholar]
- Kleene S.J. Block by external calcium and magnesium of the cyclic nucleotide-activated current in olfactory cilia. Neuroscience. 1995;66:1001–1008. doi: 10.1016/0306-4522(94)00634-h. [DOI] [PubMed] [Google Scholar]
- Kleene S.J. Both external and internal calcium reduce the sensitivity of the olfactory cyclic nucleotide-gated channel to cAMP. J. Neurophysiol. 1999;81:2675–2682. doi: 10.1152/jn.1999.81.6.2675. [DOI] [PubMed] [Google Scholar]
- Liman E.R., Buck L.B. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Liu M., Chen T.Y., Ahamed B., Li J., Yau K.W. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- Melton, D.A., P.A. Krieg, M.R. Rebagliati, Maniatis, T.K. Zinn, and M.R. Green. 1984. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acid Res. 12:7035–7056. [DOI] [PMC free article] [PubMed]
- Menini A., Nunn B.J. The effect of pH on the cyclic GMP-activated conductance in retinal rods. In: Borsellino A., Cervetto L., Torre V., editors. Sensory Transduction. Plenum Publishing Corp; New York, NY: 1990. pp. 175–181. [Google Scholar]
- Menini A. Cyclic nucleotide-gated channels in visual and olfactory transduction. Biophys. Chem. 1995;55:185–196. doi: 10.1016/0301-4622(94)00153-b. [DOI] [PubMed] [Google Scholar]
- Menini A. Calcium signalling and regulation in olfactory neurons. Curr. Opin. Neurobiol. 1999;9:419–426. doi: 10.1016/S0959-4388(99)80063-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G.H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Paoletti P., Young E.C., Siegelbaum S.A. C-linker of cyclic nucleotide-gated channels controls coupling of ligand binding to channel gating. J. Gen. Physiol. 1999;113:17–33. doi: 10.1085/jgp.113.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.S., MacKinnon R. Divalent cation selectivity in a cyclic nucleotide-gated ion channel. Biochemistry. 1995;34:13328–13333. doi: 10.1021/bi00041a008. [DOI] [PubMed] [Google Scholar]
- Perry R.J., McNaughton P.A. Response properties of cones from the retina of the tiger salamander. J. Physiol. 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J.I. Permeability and interaction of Ca with cGMP-gated ion channels differ in retinal rod and cone photoreceptors. Biophys. J. 1995;69:120–127. doi: 10.1016/S0006-3495(95)79881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M.J., MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993;11:459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- Root M.J., MacKinnon R. Two identical noninteracting sites in an ion channel revealed by proton transfer. Science. 1994;265:1852–1856. doi: 10.1126/science.7522344. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter A., Zong X., Hofmann F., Biel M. An isoform of the rod photoreceptor cyclic nucleotide-gated channel beta subunit expressed in olfactory neurons. Proc. Natl. Acad. Sci. USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S.P., Tanaka J.C. Three residues predicted by molecular modeling to interact with the purine moiety alter ligand binding and channel gating in cyclic nucleotide-gated channels. Biochemistry. 1998;37:17239–17252. doi: 10.1021/bi981185d. [DOI] [PubMed] [Google Scholar]
- Seifert R., Eismann E., Ludwig J., Baumann A., Kaupp U.B. Molecular determinants of a Ca2+-binding site in the pore of cyclic nucleotide-gated channelsS5/S6 segments control affinity of intrapore glutamates. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:119–130. doi: 10.1093/emboj/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.-P., Myles H.A., Goulding E.H., Karlin A., Siegelbaum S.A. Exposure of residues in cyclic nucleotide-gated channel poreP region structure and function in gating. Neuron. 1996;16:141–149. doi: 10.1016/s0896-6273(00)80031-8. [DOI] [PubMed] [Google Scholar]
- Sunderman E.R., Zagotta W.N. Mechanism of allosteric modulation of rod cyclic nucleotide-gated channels J. Gen. Physiol. 113 1999. 601 619a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderman E.R., Zagotta W.N. Sequence of events underlying the allosteric transition of rod cyclic nucleotide–gated channels J. Gen. Physiol. 113 1999. 621 640b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbs G.R., Goulding E.H., Siegelbaum S.A. Allosteric activation and tuning of ligand efficacy in cyclic nucleotide-gated channels. Nature. 1997;386:612–615. doi: 10.1038/386612a0. [DOI] [PubMed] [Google Scholar]
- Varnum M.D., Black K.D., Zagotta W.N. Molecular mechanism for ligand discrimination of cyclic nucleotide-gated channels. Neuron. 1995;15:619–625. doi: 10.1016/0896-6273(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Varnum M.D., Zagotta W.N. Interdomain interactions underlying activation of cyclic nucleotide gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- Zagotta W.N., Siegelbaum S.A. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- Zimmerman A.L. Cyclic nucleotide-gated channels. Curr. Opin. Neurobiol. 1995;5:296–303. doi: 10.1016/0959-4388(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman A., Baylor D.A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J. Physiol. 1992;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A., Karpen J.W., Baylor D.A. Hindered diffusion in excised membrane patches from retinal rods outer segments. Biophys. J. 1988;54:351–355. doi: 10.1016/S0006-3495(88)82966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Zucker H., Hofmann F., Biel M. Three amino acids in the C-linker are major determinants of gating in cyclic nucleotide-gated channel. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:353–362. doi: 10.1093/emboj/17.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]