Abstract
Selective permeability in voltage-gated Ca2+ channels is dependent upon a quartet of pore-localized glutamate residues (EEEE locus). The EEEE locus is widely believed to comprise the sole high-affinity Ca2+ binding site in the pore, which represents an overturning of earlier models that had postulated two high-affinity Ca2+ binding sites. The current view is based on site-directed mutagenesis work in which Ca2+ binding affinity was attenuated by single and double substitutions in the EEEE locus, and eliminated by quadruple alanine (AAAA), glutamine (QQQQ), or aspartate (DDDD) substitutions. However, interpretation of the mutagenesis work can be criticized on the grounds that EEEE locus mutations may have additionally disrupted the integrity of a second, non-EEEE locus high-affinity site, and that such a second site may have remained undetected because the mutated pore was probed only from the extracellular pore entrance. Here, we describe the results of experiments designed to test the strength of these criticisms of the single high-affinity locus model of selective permeability in Ca2+ channels. First, substituted-cysteine accessibility experiments indicate that pore structure in the vicinity of the EEEE locus is not extensively disrupted as a consequence of the quadruple AAAA mutations, suggesting in turn that the quadruple mutations do not distort pore structure to such an extent that a second high affinity site would likely be destroyed. Second, the postulated second high-affinity site was not detected by probing from the intracellularly oriented pore entrance of AAAA and QQQQ mutants. Using inside-out patches, we found that, whereas micromolar Ca2+ produced substantial block of outward Li+ current in wild-type channels, internal Ca2+ concentrations up to 1 mM did not produce detectable block of outward Li+ current in the AAAA or QQQQ mutants. These results indicate that the EEEE locus is indeed the sole high-affinity Ca2+ binding locus in the pore of voltage-gated Ca2+ channels.
Keywords: ion selectivity, selectivity filter, L-type Ca2+ channel, patch clamp, cysteine mutagenesis
INTRODUCTION
For voltage-gated Ca2+ channels, selective permeability is believed to involve interaction between two or more permeant ions. However, the number of pore-localized ion binding sites that may mediate these interactions has been contentious, with assertions of two or more binding sites (Almers and McCleskey 1984; Hess and Tsien 1984; Hess et al. 1986; Lansman et al. 1986; Friel and Tsien 1989; Yue and Marban 1990; Rosenberg and Chen 1991; Kuo and Hess 1993a,Kuo and Hess 1993b,Kuo and Hess 1993c), one binding site (Kostyuk et al. 1983; Kostyuk and Mironov 1986; Lux et al. 1990; Armstrong and Neyton 1991), or no discrete binding sites (Nonner and Eisenberg 1998) made by various researchers. Two-site models have been the most widely considered (McCleskey 1999; Miller 1999), and they are similar in nature to the multi-ion, multi-site models that have been used to describe block and flux in voltage-gated K+ channels (Hodgkin and Keynes 1955; Hille and Schwarz 1978; Neyton and Miller 1988a,Neyton and Miller 1988b). The general validity of these kinds of multi-ion, multi-site models has been strongly supported by the recently obtained crystal structure of a bacterial K+ channel (Doyle et al. 1998). This structure reveals a narrow, single-file selectivity filter containing a pair of discrete sites of dehydrated metal ion localization (binding) separated by a short distance of ∼7.5 Å. This small separation very likely allows significant ion–ion interaction to occur within the K+ channel's pore, as has also been postulated for Ca2+ channels. In the case of Ca2+ channels, tight binding of one Ca2+ ion within the single-file region of the pore obstructs permeation by foreign ions (selectivity), whereas Ca2+–Ca2+ interaction inside the pore overcomes tight Ca2+ binding to generate high Ca2+ throughput (unitary Ca2+ current). These two processes can be observed in an experimental setting, where the probability of pore occupancy by Ca2+ can be manipulated: with approximately micromolar Ca2+ in the bath, the probability of single occupancy by Ca2+ is high, so that Ca2+ effectively blocks monovalent metal cation (e.g., Na+) flux; with approximately millimolar Ca2+ in the bath, the probability of double occupancy by Ca2+ is high, so that Ca2+–Ca2+ interactions within the pore are frequent and high unitary Ca2+ flux is achieved (Almers and McCleskey 1984; Hess and Tsien 1984; Fukushima and Hagiwara 1985; Matsuda 1986). In a physiological setting, Na+ competes with Ca2+ for entry into the pore of a Ca2+ channel so that the pore fluctuates between single and double occupancy by Ca2+, resulting in a Na+-interrupted Ca2+ flux (Polo-Parada and Korn 1997). This basic description of the selective permeability of voltage-gated Ca2+ channels has remained largely intact up to the present, but its structural underpinnings have been revised in recent years.
Site-directed mutagenesis studies have identified a set of four conserved glutamate residues that contribute to Ca2+ binding, with the glutamates referred to in ensemble as the EEEE locus (Heinemann et al. 1992; Kim et al. 1993; Mikala et al. 1993; Tang et al. 1993; Yang et al. 1993; Yatani et al. 1994; Parent and Gopalakrishnan 1995). The roles of the EEEE locus glutamates in permeant ion binding have been investigated using a systematic set of EEEE locus mutants (Ellinor et al. 1995). In these experiments, block by a high-affinity cation of current carried by a low-affinity cation was used to estimate the effects of the mutations upon binding of the high-affinity cation. Because single amino-acid substitutions for any one of the glutamates affected block, and because none of the double substitution mutants retained high-affinity binding, it was concluded that the EEEE locus forms a single high-affinity binding site for Ca2+. Replacement of the entire quartet of EEEE locus glutamates with alanine or glutamine residues (AAAA or QQQQ mutants) eliminated all high-affinity binding, leading to the conclusion that the EEEE locus forms the only high-affinity binding site in the pore of Ca2+ channels. Indeed, theoretical studies have indicated that models incorporating a single high-affinity Ca2+ binding site are capable of accounting for nearly all of the selective permeability properties of Ca2+ channels, although low-affinity flanking sites may be required as well (Armstrong and Neyton 1991; Dang and McCleskey 1998).
Yet the existence of a second high-affinity, pore-localized Ca2+ binding site has remained a lurking possibility, according to either of the following two lines of reasoning. One criticism holds that the single and double mutations introduced into the EEEE locus not only reduced ion binding affinity in that locus, but that, in addition, the mutations disrupted function of the second high-affinity binding site. Such an effect could be viewed as arising from a more extensive disruption of pore structure than is hoped for with site-directed mutagenesis, or more elaborately, as a consequence of disruption by the mutations of a hypothesized allosteric interaction between the two high-affinity sites. A second criticism focuses on the fact that the conclusions of Ellinor et al. 1995 were based upon study of block by external divalent cations, but divalent cations were also found in that work to be essentially impermeant in the AAAA or QQQQ mutants. Thus, neither Ca2+ nor Ba2+ currents are detectable in the AAAA or QQQQ mutant channels. In principle, therefore, the pore might possess a second high-affinity binding site internal to the EEEE locus, but in the AAAA and QQQQ mutants, this site might be inaccessible to external divalent cations. Because the EEEE locus is thought to be situated near the extracellular entrance to the pore, a putative second high-affinity binding site might be located anywhere along most of the length of the pore.
Irrespective of the specific formulations of these two criticisms, their salience lies in the exposure of a weakness in the case for a single high-affinity locus model of selective permeability in Ca2+ channels. In this paper, we present two tests of the validity of these arguments: we have used the substituted-cysteine accessibility method (Akabas et al. 1992) to compare side-chain orientation in the AAAA mutant with that of the wild-type channel, and we have probed from the intracellular side of the channel for high-affinity pore binding of Ca2+ in channels lacking an EEEE locus (AAAA and QQQQ mutants).
MATERIALS AND METHODS
Molecular Biology and Expression of Ca2+ Channels
To enhance channel expression in Xenopus oocytes, cDNAs for the pore-forming α1C (Mikami et al. 1989) and the auxiliary Ca2+ channel subunits α2δ (Mikami et al. 1989) and β2b (Hullin et al. 1992) were subcloned into a modified version of the pGEMHE vector (modified polylinker), which contains the 5′ and 3′ untranslated regions of the Xenopus β-globin gene (Liman et al. 1992). The α1C cDNA was also reconstructed to include a consensus Kozak sequence for initiation of translation. Quadruple alanine (AAAA) and quadruple glutamine (QQQQ) mutants were made by substituting either alanine (A) or glutamine (Q) for the EEEE locus glutamate (E) in each of the four motifs of wild-type (WT) α1C. All mutants were constructed using megaprimer PCR-based mutagenesis (Barik 1995) within cassettes centered on the EEEE locus glutamate codons. Each cassette was delimited by a pair of unique restriction sites (motif I: SstI–BamHI, 308 bp; motif II: StuI–EcoRI, 380 bp; motif III: SalI–SnaBI, 401 bp; motif IV: SacII–BclI, 371 bp). The four single A or four single Q mutations were subsequently combined to make the AAAA or QQQQ constructs. For experiments in which amino acid side-chain accessibility was probed with methanethiosulfonate reagents, single cysteine (C) substitutions were introduced at various positions in the AAAA mutant using a similar PCR-based mutagenesis strategy. Mutations and cassette sequence fidelity were confirmed by cDNA sequencing.
Ovarian tissue was removed from anesthetized female Xenopus laevis (Xenopus One) and agitated in Ca2+-free OR-2 solution (mM: 82.5 NaCl, 2.5 KCl, 1 MgCl2, 5 HEPES, pH 7.6 with NaOH) containing 2 mg/ml collagenase A or B (Boehringer) for 60–120 min. After a 30-min rinse in fresh OR-2 solution, stage V and VI oocytes were selected by hand. cRNAs encoding α1C (WT or mutant), α2δ, and β2b subunits were transcribed in vitro using the mMESSAGE mMACHINE kit (Ambion) and injected into oocytes in an equimolar ratio. Injected oocytes were stored at 18°C in ND-96 solution (mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, pH 7.6 with NaOH) containing 2.5 mM sodium pyruvate (Sigma-Aldrich), 100 U/ml penicillin (Sigma-Aldrich), 0.1 mg/ml streptomycin (Sigma-Aldrich), and 0.1 mg/ml gentamicin (Boehringer) and studied 3–14 d after injection.
For expression in HEK293 cells, WT and mutant cDNAs were subcloned into the pBK-CMV N/B-200 vector, a version of pBK-CMV (Stratagene) in which the lac promoter and the initiation codon for β-galactosidase have been removed. HEK293 cells (CRL-1573; American Type Culture Collection) were grown at 37°C and 5% CO2 in Minimal Essential Medium (GIBCO BRL) supplemented with 10% fetal bovine serum (GIBCO BRL), 100 U/ml penicillin (Sigma-Aldrich), and 0.1 mg/ml streptomycin (Sigma-Aldrich). An equimolar ratio of α1C, α2δ, β2b, and green fluorescent protein (GFP; GIBCO BRL) cDNAs were transiently transfected using the Effectene transfection kit (QIAGEN). Cells expressing GFP (as determined by their fluorescence) were studied 48–72 h after transfection. Approximately 10–20% of cells were transfected (expressed GFP) under these conditions.
Electrophysiological Recording from Xenopus Oocytes
Single-channel currents were recorded from cell-attached patches on oocytes that had been stripped of their vitelline membrane. The oocytes were bathed in a high K+ solution designed to zero the membrane potential (mM: 100 KCl, 10 HEPES, 10 EGTA, pH 7.4 with TEA-OH). The Ca2+ channel agonist FPL 64176 (RBI) was included in the bath solution (2 μM) to prolong channel open time, which facilitated the study of pore block. Patch pipets were made of borosilicate glass (Warner Instruments Co.), coated with either Sylgard (Dow Corning Corp.) or wax (Kerr Corp.) and had resistances of ∼20–30 MΩ when filled with solution for recording inward Li+ currents (mM: 100 LiCl, 10 HEPES, 10 HEDTA, 14 TEA-Cl, plus various concentrations of CaCl2, pH 7.4 with TEA-OH). “Chelator” software (Theo J.M. Schoenmakers, University of Nijmegen, Nijmegen, The Netherlands) was used to determine the [CaCl2] to add to the recording solution for a desired final free [Ca2+]. Currents were recorded with an Axopatch 200B amplifier (Axon Instruments, Inc.). The amplifier's internal filter was set to 10 kHz and an external filter (eight-pole Bessel filter; Frequency Devices, Inc.) was set to 2 kHz, yielding a final corner frequency of 1.96 kHz. Data were sampled at 10 kHz using Pulse software (HEKA, distributed by Instrutech Corp.).
Two-electrode voltage clamp recording from oocytes was performed as previously described (Sather et al. 1993). Briefly, pipets were made from borosilicate glass (Frederick Haer and Co.), filled with 3 M KCl, and had resistances of 0.3–1 MΩ. The bath was continuously perfused (∼1 ml/min) with either Li+ solution (same as above for single-channel recording) or Ba2+ solution [mM: 40 Ba(OH)2, 52 TEA-OH, 5 HEPES, pH 7.4 with methane sulfonic acid]. In experiments where amino acid side-chain accessibility was studied in cysteine mutants, the sulfhydryl-modifying reagent 2-aminoethyl methanethiosulfonate hydrobromide (MTSEA·Br; Toronto Research Chemicals) was dissolved in Li+ solution immediately before application to the oocyte via the bath perfusion system. Currents were recorded with an amplifier (OC-725C; Warner Instruments Co.), filtered at 500 Hz (four-pole Bessel filter; Warner Instruments Co.) and sampled at 1 kHz. Computer programs to control the data acquisition and analyze the data were custom written in Axobasic (Axon Instruments, Inc.). Leak currents were subtracted using a modified P/4 protocol: 10 pulses to −P/4 were averaged.
Electrophysiological Recording from HEK293 Cells
Whole-cell currents were recorded from HEK293 cells with a 100 mM Li+ solution (as above for oocytes) in the bath and a Cs+ solution in the pipet (mM: 135 CsCl, 10 EGTA, 10 HEPES, 4 Mg-ATP, pH 7.5 with TEA-OH). Patch pipets were made from thin-walled borosilicate glass (Warner Instruments Co.) and had resistances of ∼1–3 MΩ. Currents were recorded and filtered with the same equipment, settings, and software as described above for single-channel current recording from oocytes, and they were sampled at 4 kHz. Leak currents were subtracted using the average of eight pulses to −P/10. Uncompensated series resistance was <9 MΩ, and this was compensated by 70–80% during experiments.
Following previous work by Kuo and Hess 1993a, outward Li+ currents through single channels in inside-out, excised patches from HEK293 cells were recorded with 300 mM Li+ in the bath (mM: 300 LiCl, 10 HEPES, 10 HEDTA, plus various [CaCl2], pH 7.4 with CsOH) and 55 mM Li+ in the patch pipet (mM: 55 LiCl, 55 CsCl, 10 HEPES, 10 HEDTA, 150 glucose, pH 7.4 with CsOH). CaCl2 was added to the bath solution to achieve the desired free [Ca2+], as calculated using the Chelator program. The Ca2+ channel agonist BayK 8644 (RBI) was included in the bath solution at 5 μM to prolong channel openings; BayK 8644 was more effective than FPL 64176 at the positive potentials required to study outward currents. Pipets, amplifier, data filtering, and sampling were the same as described above for unitary current recording from oocytes. The kinetics of pore block were analyzed using Tac and TacFit software (Bruxton). Based on the corner frequency of 1.96 kHz, the rise time of our recording system was 0.169 ms. We therefore excluded events of duration <0.2 ms from the analysis. The data were not corrected for missed events.
All chemicals for which a source is not specifically mentioned were obtained from either Sigma-Aldrich or Fluka. All mean values are reported as mean ± SEM.
RESULTS
Accessibility of Substituted Cysteines Suggests that AAAA Pore Structure Is Not Globally Disrupted
The fact that divalent cations do not permeate the AAAA channel raises the possibility that quadruple mutations in the EEEE locus produce a nonspecific disruption of pore structure, so that divalent cations do not permeate, although monovalent cations do. It has been suggested that this kind of disruption of structure might also destroy the postulated second high-affinity Ca2+ binding site. As a means of assessing the structural integrity of the AAAA channel, we have compared the orientations of amino acid side-chains of pore-lining residues in WT channels with those of the corresponding residues in AAAA channels. Side-chain orientation, that is, whether a side-chain projects into the pore or not, was investigated by measuring whether a bath-applied sulfhydryl-modifying reagent could irreversibly block current through cysteine-substituted EEEE or AAAA channels. We analyzed side-chain orientation at two positions in each motif, the position occupied in WT channels by an EEEE locus glutamate (position 0), and the immediate amino-terminal neighbor (position −1), which is located farther from the channel's external entrance than is the EEEE locus glutamate. For EEEE channels, four 0-position cysteine substitution mutants were studied (CEEE, ECEE, EECE, and EEEC), and, for AAAA channels, four additional 0-position mutants were studied (CAAA, ACAA, AACA, and AAAC). Cysteine substitution at the −1 position yielded four mutants in the EEEE channel (M392C, G735C, F1144C, and G1445C), and four mutants in the AAAA channel (M392C/AAAA, G735C/AAAA, F1144C/AAAA, and G1445C/AAAA).
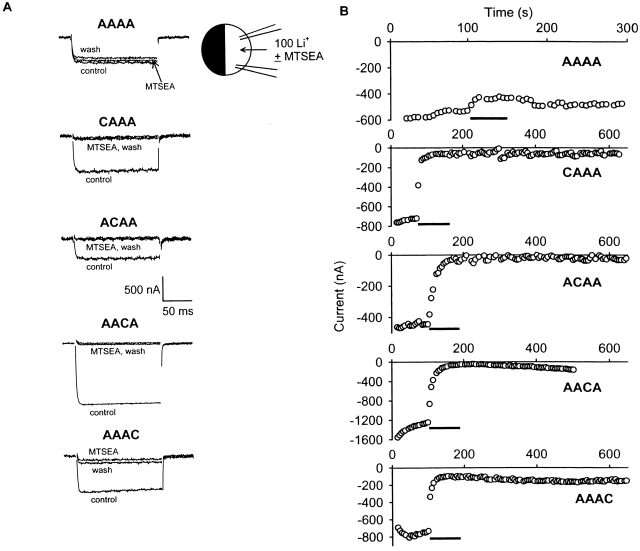
The effects of the methanethiosulfonate reagent MTSEA on inward, whole-cell Li+ currents carried by non- and cysteine-substituted AAAA channels (0 position only) are illustrated in Fig. 1 (A and B). For the non–cysteine-substituted AAAA channel, MTSEA block was small and rapidly reversible. The average steady state block of AAAA channels by MTSEA was 18 ± 1% (Fig. 2 A), and the time constant for recovery from MTSEA block was 21 ± 6 s (n = 6). This unblock rate is similar to that measured for Cd2+ unblock in oocytes, and most likely reflects the rate at which rapidly reversible blocking agents can be washed out of the oocyte recording chamber. In contrast, the cysteine-substituted AAAA channels (CAAA, ACAA, AACA, and AAAC) were all more strongly and persistently blocked by MTSEA. Average values of MTSEA block for the 0 position cysteine mutants were 63% for CAAA, 83% for ACAA, 90% for AACA, and 90% for AAAC (Fig. 2 A). All of the testable −1 position cysteine substitutions were also persistently blocked by MTSEA, with average values of block being 32% for M392C/AAAA, 52% for F1144C/AAAA, and 67% for G1445C/AAAA. The G735C/AAAA either did not express or did not produce functional channels, since oocytes injected with this cRNA exhibited little or no inward Li+ current.
Figure 1.
Determination of side-chain orientation of pore-lining amino acids in the AAAA channel. (A) Superimposed two-electrode voltage clamp records illustrating the effects of MTSEA upon whole-cell Ca2+ channel currents. Currents (100 mM Li+) were elicited by step depolarization (150 ms) from the holding potential of −80 to −20 mV every 5 s. In each recording, after a stable baseline was established, MTSEA·Br (final concentration of MTSEA: 2 mM) was dissolved in the 100 mM Li+ solution and applied immediately to the oocyte via bath perfusion. (B) Time course of MTSEA action on peak inward Li+ currents.
Figure 2.
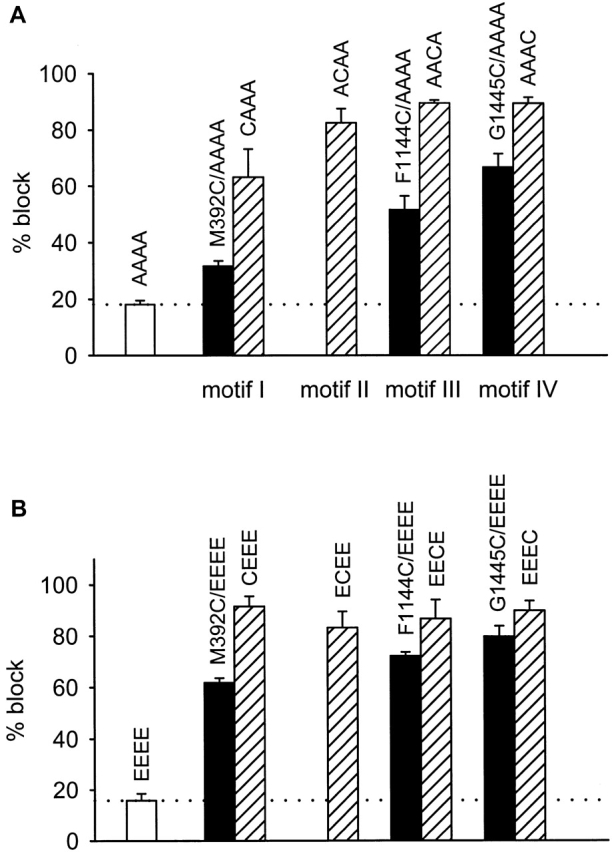
Summary of MTSEA block of substituted-cysteine mutants. (A) Percent block (±SEM) for the quadruple alanine mutant (AAAA) and cysteine substitutions in the AAAA channel. Block was calculated as the average for n = 4–9 oocytes, except for F1144C/AAAA, for which n = 3. Cysteine-substituted mutants differed from the AAAA channel in that block of the mutants was not reversible with wash, and their block was larger (P < 0.00005). Current was carried by Li+, and data were collected as described for Fig. 1. (B) Percent block (±SEM) for the EEEE channel and cysteine substitutions in the EEEE channel. Block was calculated as the average for n = 4–6 oocytes. Block of the cysteine-substituted mutant versions of the EEEE channel was not reversible with wash, unlike the rapidly reversible block of the EEEE channel, and block of the mutants was much larger than for the EEEE channel (P < 0.00002). Current was carried by Ba2+ (40 mM Ba2+ solution), holding potential was −80 mV, test potential was +20 mV, and 150-ms step depolarizations were applied every 15 s. Open bars indicate reversible block (AAAA and EEEE channels only), filled bars indicate −1 position cysteine substitution mutants, and hatched bars indicate 0-position cysteine substitutions. For reference, EEEE locus glutamates are located at positions 393, 736, 1145, and 1446 in motifs I–IV, respectively.
The results of MTSEA block of WT EEEE channels and cysteine-substituted EEEE channels are summarized in Fig. 2 B. WT EEEE channels were blocked by only 16 ± 3%, and this small block was reversed with wash. In contrast, all −1- and 0-position cysteine substitution mutants of the EEEE channel were persistently blocked by MTSEA. For these mutants, percent block values ranged from 83 to 92% for 0-position mutants, and from 62 to 80% for −1-position mutants. These results indicate that all eight −1- and 0-position side-chains project into the pore of the EEEE channel, as has been described previously (Chen et al. 1996; Klockner et al. 1996; Chen and Tsien 1997; Wu, X., H.D. Edwards, and W.A. Sather, manuscript submitted for publication).
Comparison of the MTSEA block patterns for EEEE and AAAA channels shows that all −1- and 0-position mutants were blocked by MTSEA in EEEE and AAAA channels, indicating that all of the tested positions are accessible to MTSEA in both channel types. Furthermore, within each motif, MTSEA block of the 0-position mutant was larger than that of the −1-position mutant for both EEEE and AAAA channels. These points of similarity in the MTSEA block patterns indicate that the orientations of amino acid side-chains at the 0 and −1 positions were not drastically altered by introduction of four alanines at the EEEE locus. In other details, the block patterns differed so that, for some cysteine substitution positions, fractional block by MTSEA differed in degree between the EEEE and AAAA channels. In comparing cysteine-substituted EEEE and AAAA channels at a given position, the differences in degree of fractional block by MTSEA are not surprising: with four small alanine side chains (-CH3) projecting into the pore in place of four larger, and charged, glutamate side chains (-CH2CH2COO-), substituted cysteine thiols may be somewhat differently accessible, or these thiols may be modified to a somewhat different degree, or thiol modification may not result in an identical degree of physical obstruction of the pore. The key finding, however, is that the EEEE and AAAA block patterns exhibited a degree of similarity that suggests that structural differences between the AAAA channel and WT are largely limited to the side-chain makeup of the EEEE locus.
High-affinity Block by External Ca2+ Occurs at the EEEE Locus
EEEE locus mutations have previously been shown to attenuate block by divalent cations entering the pore via its extracellularly oriented, or external, entrance (Kim et al. 1993; Tang et al. 1993; Yang et al. 1993; Yatani et al. 1994; Ellinor et al. 1995; Parent and Gopalakrishnan 1995). The IC50 for external Ca2+ block of whole-cell, inward Li+ currents in WT α1C (∼1 μM) was shifted ∼10–50-fold by single alanine substitutions and ∼1,000-fold by substitution of alanine for all four EEEE locus glutamates (AAAA; Ellinor et al. 1995). Here we have studied effects of the EEEE locus quadruple mutations upon various configurations of Ca2+ block of single-channel Li+ currents, starting with block by external Ca2+ of inward Li+ current. In cell-attached patches on oocytes expressing WT α1C channels, step depolarizations in the presence of 2 μM FPL 64176 produced inward Li+ currents of long duration (Fig. 3 A, top). Including 3 μM Ca2+ in the 100-mM Li+ solution bathing the extracellular surface of the patch (pipet solution) introduced frequent interruptions in the unitary inward currents, which represent individual Ca2+ block/unblock transitions. By contrast, inward Li+ currents through AAAA mutant channels were not detectably affected by 3 μM, or even 100 μM, external Ca2+ (Fig. 3 A, bottom). When 1 mM Ca2+ was included in the patch pipet solution, a small degree of flicker block was observed. This presumably is the manifestation of Ca2+ binding to a low affinity site in the pore. These results coincide with those obtained at the whole-cell level (Yang et al. 1993; Ellinor et al. 1995), demonstrating that high-affinity binding of divalent cations that enter the pore from the external solution occurs at the EEEE locus; no high-affinity binding is detectable in the AAAA mutant channel when probed by ions entering from the external solution.
Figure 3.
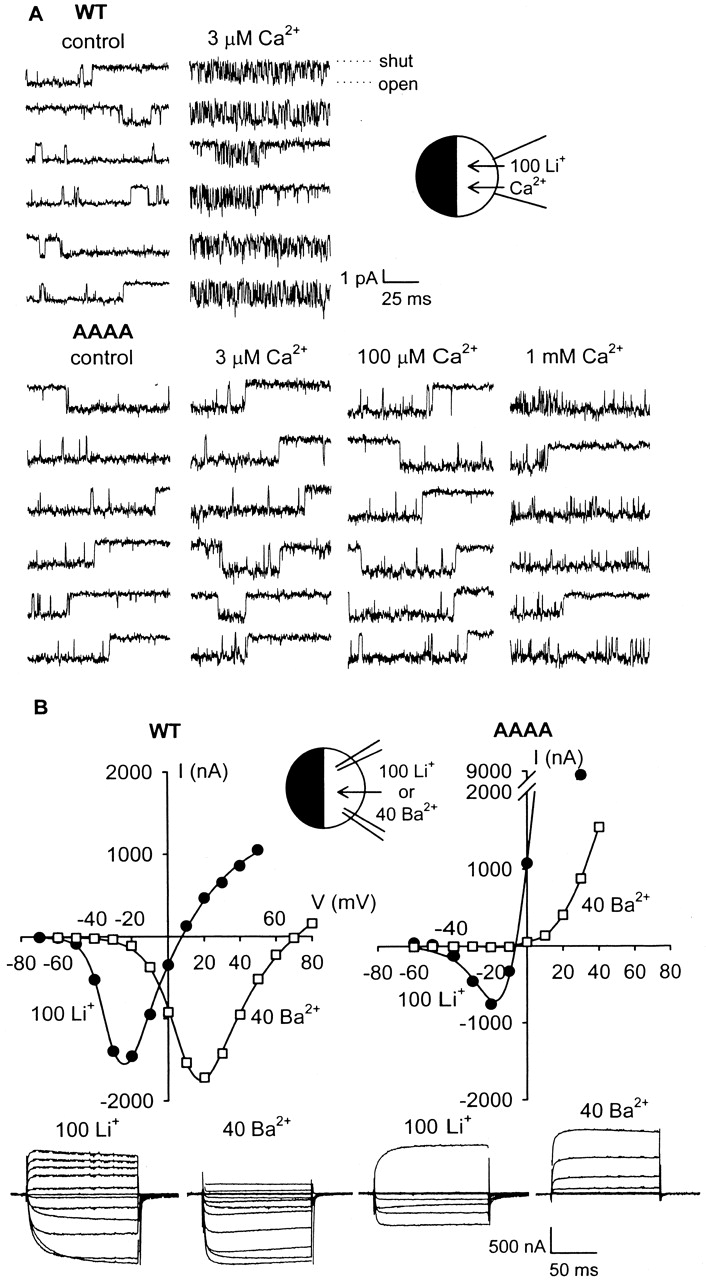
Absence of divalent cation block or flux in the AAAA mutant Ca2+ channel. (A) High-affinity block of single-channel inward Li+ currents by external Ca2+ was abolished by replacing all four EEEE locus residues with alanine. Inward Li+ currents were studied in cell-attached patches on oocytes expressing either WT or AAAA channels. The patch pipet contained 100 mM Li+ plus various [Ca2+]. Control solution contained 3 nM Ca2+. Holding potential was −100 mV, channels were activated by 25–50-ms prepulses to +40 mV, and the illustrated single-channel currents were recorded at −40 mV. (B) Li+, but not Ba2+, permeates AAAA channels. Example current–voltage relationships and current records from two-electrode voltage-clamp recordings from individual oocytes expressing either WT or AAAA are illustrated. The bath solution contained either 100 mM Li+ (•) or 40 mM Ba2+ (□). Currents were recorded during step depolarizations from a holding potential of −100 mV (Li+) or −80 mV (Ba2+) every 15 s. Reversal potentials in Li+ were 3.7 ± 1.2 mV (n = 6) for WT and −8.0 ± 1.7 (n = 6) for AAAA. Smooth curves drawn through the data were best-fits calculated according to: I = (1/{1 + exp[(V0.5 − VM)/b]}) · ([A 1 · exp(zFδVM/RT)] − {A 2 · exp[−2zF(1 − δ)V M/RT]}), where I = membrane current, VM = membrane potential, V0.5 = half-activation voltage, b = Boltzmann slope factor, A 1 and A 2 are amplitude factors related to the concentrations and permeabilities of the internal and external ions, z = ion valence, δ = electrical distance, and F, R, and T are Faraday's constant, ideal gas law constant, and temperature (K).
Examples of the high fluxes of both divalent (Ba2+) and monovalent (Li+) cations through WT α1C channels are illustrated in Fig. 3 B (left). Fig. 3 B (right) shows that, although Li+ is highly permeant in the AAAA channel, Ba2+ is not. In fact, lack of divalent cation permeability is characteristic of quadruple EEEE locus mutants, so that little or no inward current was observed with external Ba2+ or Ca2+ in the AAAA or QQQQ mutants (Ellinor et al. 1995). The immeasurably low permeability of quadruple EEEE locus mutants to divalent cations raises a key question for the one-site model of Ca2+ channel pore function: is it possible that another high-affinity site exists, distinct from the EEEE locus and deeper in the pore, and that this deeper site is not accessible to external divalent cations in the quadruple EEEE locus mutants?
Outward Ca2+ Channel Currents in Excised Patches
If there is a second high-affinity binding site for divalent cations located further from the external pore entrance than is the EEEE locus, then this deeper site could reasonably be expected to be accessible from the intracellular side of WT, AAAA, and QQQQ channels. We addressed this possibility by studying, in Ca2+ channel-bearing inside-out patches, block of outward Li+ currents by Ca2+ entering the pore from the internal (cytosolically oriented) pore entrance. For this set of experiments, we had greater success studying Ca2+ channels expressed in HEK293 cells than in oocytes. In inside-out patches from oocytes, with 100 mM Li+ in the bath (internal solution), outward single-channel Li+ currents were not readily observed. L-type Ca2+ channels are known to exhibit rapid rundown or loss of activity after patch excision, perhaps due to the loss of positive regulation by intracellular components (Armstrong and Eckert 1987; Lambert and Feltz 1995; Hao et al. 1998). In addition, single-channel outward currents through Ca2+ channels in either cell-attached or excised patches have been notoriously difficult to study, possibly due to block by intracellular Mg2+ (Kuo and Hess 1993c) and/or polyamines (Sjöholm et al. 1993; Gomez and Hellstrand 1995). Xenopus oocytes in particular have high concentrations of internal polyamines that do not easily wash out even from excised patches and that hamper the observation of unitary outward currents carried by K+ channels (Lu et al. 1999). Use of HEK293 cells, addition of HEDTA to the bath for chelation of divalent cations including Mg2+, and a higher concentration (300 mM) of Li+ in the bath facilitated the observation of unitary outward Li+ currents in inside-out excised patches. Nevertheless, channel activity was observed in only ∼12–15% of excised patches. Rundown, as well as residual block by patch-associated polyamines and Mg2+, was likely responsible for this low probability of observing unitary outward currents.
Five lines of evidence indicate that the single-channel currents that we recorded from inside-out patches were produced by transfected Ca2+ channels and not by ion channels endogenous to HEK293 cells. This is a particularly significant issue for the AAAA and QQQQ mutant channels, since their unitary conductance and block properties have not been previously described. First, consider the endogenous channels of HEK293 cells. Although Ca2+ channels native to HEK293 cells display some of the characteristics of L-type Ca2+ channels, these native channels are insensitive to the dihydropyridine agonist BayK 8644, and they were therefore not responsible for the long-duration openings that we studied (Berjukow et al. 1996). The activity of K+ channels native to HEK293 cells is sparse, their conductances are inconsistent with those we have measured (Yu and Kerchner 1998; Zhu et al. 1998), and K+ channels are typically only poorly permeated by Li+, with PLi/PK permeability ratios generally <0.1 (Hille 1992). The unitary conductances of the three Cl− channels identified in HEK293 cells are (in symmetrical 150 mM Cl−) 55, 240, and 350 pS (Zhu et al. 1998). These large conductances are expected to be even larger with the 300 mM internal and 260 mM external Cl− used in our experiments, and such large conductances differ greatly from those we have investigated. Cl− channels in HEK293 cells are also relatively rare, occurring in only 4–5% of patches (Zhu et al. 1998).
Second, reversal potentials determined from whole-cell current–voltage relationships were consistent with the overwhelming majority of current in transfected cells being attributable to exogenous Ca2+ channels (Fig. 4 A). Average reversal potential for α1C-transfected cells was +31.2 ± 2.2 mV (n = 5), reflecting a significant preference in the WT α1C channel for Li+ over Cs+. AAAA-transfected cells had a reduced preference for Li+ over Cs+ (average reversal potential = +10.7 ± 1.3 mV, n = 6), which is consistent with the loss of selectivity caused by the quadruple EEEE locus mutations.
Figure 4.

Whole-cell current densities in HEK293 cells. (A) Examples of current–voltage relationships from individual cells expressing WT (○), AAAA (▴), or GFP (control, □). The bath solution contained 100 mM Li+ and the pipet contained 135 mM Cs+. Currents were elicited every 5 s by step depolarizations from a holding potential of −100 mV. (B) Average current densities in WT-, AAAA-, and GFP-transfected (control) HEK293 cells. Inward current density was measured at the inward peak of the I-V, and outward current density was measured at +80 mV. Data plotted as mean ± SEM (n = 10–13).
Third, measurement of whole-cell currents in HEK293 cells demonstrated that the Li+ currents through single channels in excised patches were dominantly due to permeation of heterologously expressed Ca2+ channels, rather than endogenous channels. Fig. 4 B shows that inward Li+ current density was ∼50-fold higher for cells expressing WT α1C compared with control cells (GFP-transfected only) and ∼8-fold higher for cells expressing AAAA compared with control. Likewise, outward Cs+ current densities were significantly greater in Ca2+ channel-transfected than in control cells (∼17-fold for WT and ∼7-fold for AAAA; Fig. 4 B).
Fourth, a dihydropyridine antagonist of L-type Ca2+ channels, nimodipine (10 μM), blocked inward Li+ currents through AAAA-transfected cells by 67 ± 8% (n = 3; −20 mV), which is similar to the degree of block of WT α1C channels expressed in oocytes (Furukawa et al. 1999). Fifth, and finally, in patches from α1C-transfected cells, when micromolar Ca2+ was present in the bath, WT α1C channels were always blocked by Ca2+; there was no contamination with nonblocked channels. In the case of the AAAA-transfected cells, the absence of internal Ca2+ block of outward Li+ currents eliminates the possibility that currents recorded from these cells might have been carried by endogenous Ca2+ channels.
Internal Ca2+ Blocks Wild-Type Ca2+ Channels with High Affinity
Recordings of outward Li+ currents through single α1C channels in inside-out patches from HEK293 cells are shown in Fig. 5 A. These channels exhibited multiple conductance states, a well-documented behavior of voltage-gated Ca2+ channels (e.g., Cloues and Sather 2000). The amplitude to which they opened most frequently was the largest one, and this amplitude was analyzed for kinetics of pore block. WT α1C channels were blocked when at least micromolar Ca2+ was present in the solution bathing the cytosolic surface of the patch. Representative WT single-channel records illustrate the flicker block that was introduced by internal Ca2+, with the number of flicker block events increasing with [Ca2+] (Fig. 5 A). Quantitation of open and shut times yielded Ca2+ on rates (1/τopen) that increased with [Ca2+] and off rates (1/τshut) that were concentration independent, findings that are indicative of a bimolecular reaction. At a membrane potential of +20 mV, the average off rate was 2,965 s−1 and the on-rate coefficient, determined as the slope of a linear regression fit, was 2.3 × 108 M−1 s−1. The WT channel exhibited high-affinity binding of Ca2+ in this configuration, as the apparent K d is calculated to be ∼13 μM (k off/k on). The concentration-dependent on rate is similar to that of Kuo and Hess 1993a, who studied Ca2+ block of Li+ current through endogenous Ca2+ channels in PC12 cells. Likewise, the magnitude and concentration independence of the off rate agree with that measured by Kuo and Hess 1993a. The voltage dependence of block by Ca2+ was determined over the voltage range 0 to +20 mV. The on rate was voltage independent, whereas the off rate increased with depolarization, e-fold/19 mV (Fig. 5 B). These characteristics match those of on and off rates obtained by Kuo and Hess 1993a, as expected if the channels we have studied are WT α1C channels.
Figure 5.

Concentration and voltage dependence of Ca2+ block of outward Li+ current carried through WT α1C channels in inside-out patches excised from HEK293 cells. (A) Internal Ca2+ blocked outward Li+ currents through single WT channels with high affinity. The bath solution contained 300 mM Li+ plus various [Ca2+], and the patch pipet contained 55 mM Li+. Control Li+ solution contained 3 nM Ca2+. Single-channel currents were recorded during a test depolarization to +20 mV from a holding potential of −100 mV. In some cases, a 25- or 50-ms prepulse to +100 mV was given to facilitate channel activation. Ca2+ on (○) and off (▪) rates are plotted as mean ± SEM versus [Ca2+] for n = 3–5. The linear regression fit through the on-rate data points has a slope of 2.3 × 108 M−1 s−1. The horizontal line fit to the off-rate data indicates the average off rate was 2,965 s−1. (B) Voltage dependence of internal Ca2+ block of outward Li+ currents through single WT channels. The bath solution contained 300 mM Li+ and 3 μM Ca2+, while the pipet solution contained 55 mM Li+. Single-channel currents were recorded during a step depolarization from the holding potential of −100 mV. In some experiments, a 25- or 50-ms prepulse to +100 mV was given to facilitate channel activation. Ca2+ on (○) and off (▪) rates plotted as mean ± SEM versus test potential (n = 4–5). The horizontal line fit to the on-rate data indicates the average on rate was 846 s−1. The exponential curve fit to the off-rate data indicates that off rate increased e-fold per 19 mV.
AAAA and QQQQ Channels Are Not Effectively Blocked by Internal Ca2+
In contrast to the case for WT channels, unitary outward Li+ currents through AAAA channels did not exhibit detectable flicker block with internal (bath) [Ca2+] as high as 1 mM (Fig. 6), nor even 3 mM (data not shown). If Ca2+ block and unblock of AAAA channels occurred on a time scale shorter than the dead time of our recording system, then discrete Ca2+ block events would not be fully resolved and Ca2+ block would reduce unitary current amplitude in a manner graded with [Ca2+]. A potentially complicating factor is that, like WT, the AAAA mutant exhibited four clear conductance levels. However, no graded reductions in AAAA channel unitary current amplitudes were detected as internal Ca2+ was increased, so that AAAA channels exhibited the same unitary current amplitudes in the presence of 1 mM internal Ca2+ as in control internal solution. Thus, measurements of unitary current amplitudes also did not reveal block by internal Ca2+ of outward Li+ currents in AAAA channels.
Figure 6.
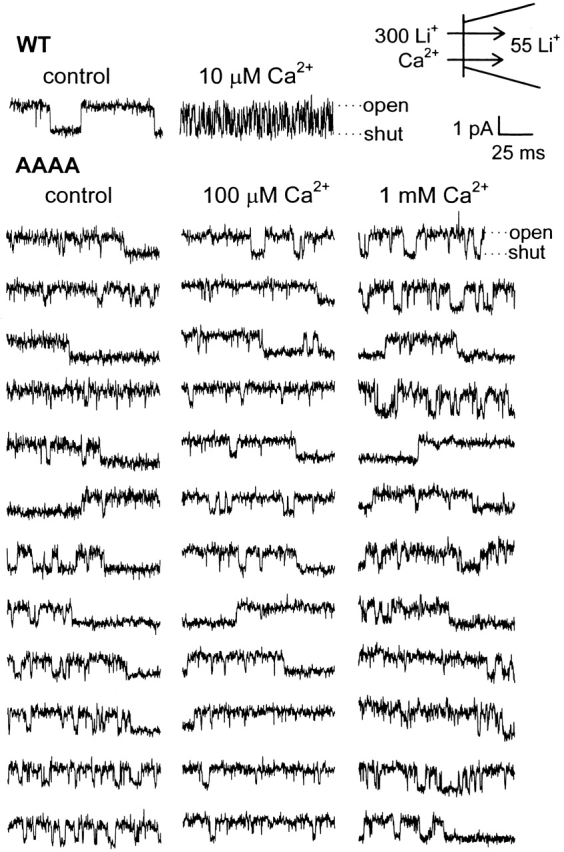
Internal Ca2+ does not block outward single-channel Li+ currents through AAAA channels. Currents were studied in inside-out patches excised from HEK293 cells expressing AAAA channels (WT records shown for comparison). The bath solution contained 300 mM Li+ plus various [Ca2+], and the patch pipet contained 55 mM Li+. [Ca2+] was 3 nM in the control solution. Single-channel currents were recorded during a test depolarization to +20 mV, following a 25- or 50-ms prepulse to +100 mV. Holding potential was −100 mV.
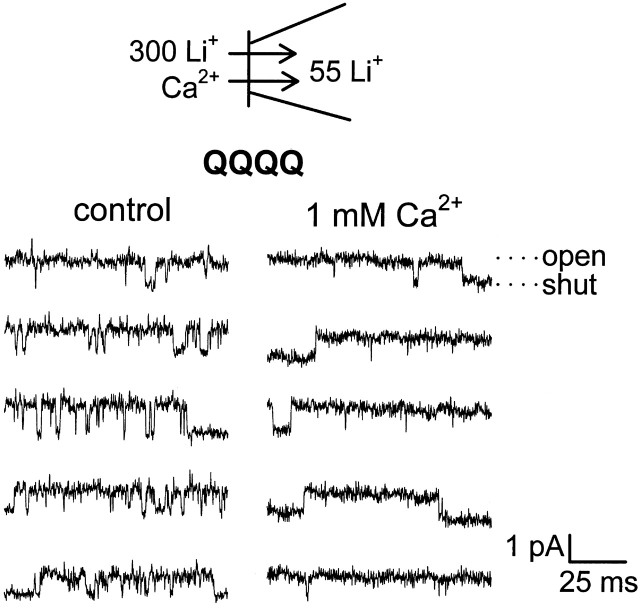
QQQQ is another EEEE locus quadruple mutant that lacks high-affinity binding of Ca2+ when probed via the pore's external entrance (Ellinor et al. 1995). QQQQ channels behaved like AAAA channels in the present study: with 1 mM Ca2+ in the internal solution (bath), there was no detectable block of outward Li+ currents (Fig. 7). As found for the AAAA mutant, reduced unitary conductance as a consequence of fast, unresolved flicker block by Ca2+ was not detected for the QQQQ mutant either. The combined results from WT, AAAA, and QQQQ channels argue that Ca2+ entering the channel from the intracellular entrance can bind with high affinity, but only in channels with an intact EEEE locus, and that in the absence of an intact EEEE locus, there is no high-affinity binding and therefore no second high-affinity binding site in the pore.
Figure 7.
Outward Li+ currents through QQQQ channels are not blocked by internal Ca2+. Currents were studied in inside-out patches excised from HEK293 cells expressing QQQQ channels. The bath contained 300 mM Li+ plus either 3 nM Ca2+ (control) or 1 mM Ca2+, and the patch pipet contained 55 mM Li+. Single-channel currents were recorded during a test depolarization to +20 mV, following a 25- or 50-ms prepulse to +100 mV. Holding potential was −100 mV.
DISCUSSION
Side-Chain Orientation in the AAAA Mutant
Because high-resolution structures are not available for the pores of mutant or WT Ca2+ channels, the effects of mutations upon pore structure can only be inferred from indirect information such as altered pore block. This is one reason why the idea that Ca2+ channels possess only a single locus of high-affinity binding in their pore-lining regions has remained tantalizingly short of conclusive.
We have tried to determine whether amino acid substitutions in the EEEE locus have little effect on structure other than to replace one side chain with another while preserving general spatial organization, or whether substitutions may have in addition the undesired effect of significantly altering side-chain orientation: in one extreme example, side-chains that project into the lumen of the WT pore to interact with permeating ions might, upon amino acid substitution, project away from the lumen of the mutant pore. Even more problematic, it is possible that EEEE locus substitutions could conceivably cause significant reorientation of side chains outside the EEEE locus. To address this kind of problem, we investigated the accessibility of substituted cysteines to determine side-chain orientation in the pore, but one view of this approach is that relying on additional mutagenesis work in the absence of “real” structural information only compounds the problems inherent in the earlier mutagenesis work. This may be true. Yet atomic-scale pore structures for both WT and mutant Ca2+ channels are not likely to be in hand any time soon, and further mutagenesis work allows comparison of the effects of various mutations, thereby providing tests for the consistency of conclusions regarding pore structure. Following this logic, we have examined whether side chains that have been found in previous mutagenesis work to project into the pore of WT channels also do so in the relatively severe AAAA mutant.
Based on substituted-cysteine accessibility analysis of WT channels (Wu, X., H.D. Edwards, and W.A. Sather, manuscript submitted for publication) and on evidence that the EEEE locus side-chains together form a single protonatable site in the pore (Chen et al. 1996; Klockner et al. 1996; Chen and Tsien 1997), in WT EEEE channels the side chains at the 0 and −1 positions project into the pore. Cysteines substituted at three of the four −2 positions in the EEEE channel did not support MTSEA block, leaving the side-chain orientation at these positions unknown for now (Wu, X., H.D. Edwards, and W.A. Sather, manuscript submitted for publication). Here, we have found that cysteines substituted into the AAAA mutant at all 0 and all testable −1 positions are susceptible to long-lasting modification by MTSEA. By this very simple index, side-chain orientation thus appears to be at least crudely preserved despite the introduction of four or more mutations into the selectivity filter region.
Because we were specifically concerned about the possible existence of Ca2+ binding sites located internally to the EEEE locus, we did not examine side-chain orientation at more external positions (+1, +2) in the AAAA channel. Examination of substituted-cysteine accessibility at deeper pore positions was not attempted, based on suspected large-scale structural similarity with the KcsA pore structure (Doyle et al. 1998). Only a short segment of the P-loop in the KcsA K+ channel lines the pore and analysis of protein secondary structure for Ca2+ channels strongly suggests that the P-loops of these channels have a roughly similar architecture to that of the KcsA channel. Specifically, a pore helix, which does not line the pore in KcsA channels, is predicted to occupy positions −4 through −15 in all Ca2+ channel P-loops. Thus, at this point, residues lining the deeper regions of the Ca2+ channel pore remain largely unknown. Furthermore, the deeper pore of Ca2+ channels is very likely to comprise an aqueous cavity like that found in the KcsA channel, because this aqueous cavity is thought to be essential in lowering the dielectric barrier to ion permeation through cell membranes. Though Ca2+ occupancy of this region of the pore may be important in supporting high Ca2+ flux, it is difficult to envision how a high-affinity Ca2+ binding site in such a wide-diameter section of the pore could be involved in the selectivity-by-binding process.
Considering all of the above, the most probable conclusion that can be drawn from the substituted-cysteine accessibility work is this: despite the limited nature of the information provided, the concern that fourfold alanine substitution at the EEEE locus might result in substantial rearrangement of pore structure seems unlikely owing to the absence of a drastic affect on structure near and at the EEEE locus. Even so, a viable alternative view is that the small structural differences detected between EEEE and AAAA channels hint at larger structural differences deeper in the pore. Identification of sequences lining the deeper pore will allow the more extensive comparison of pore structure between EEEE and AAAA channels needed to address this lingering concern.
In contrast to the situation with cysteine-substituted mutants, the modest block by MTSEA of the non–cysteine-substituted AAAA channel was reversible, and occurred at a rate similar to that of Cd2+ unblock of Ca2+ channels in oocytes. One reasonable interpretation of these observations is that the cationic MTSEA is attracted into the pore, where it obstructs permeant ion flow. Whatever the mechanism of this kind of block by MTSEA, however, block did not result from covalent attachment of -SCH2CH2NH3 + to the non–cysteine-substituted AAAA channel, and so this kind of block was separable from that measured for the cysteine-substituted mutants.
The Number of High-Affinity Binding Sites Involved in Selective Ion Permeability
Our examination of AAAA and QQQQ channels for block of outward Li+ current by Ca2+ entering the pore via its intracellularly oriented entrance showed that Ca2+ at up to 3 mM failed to effectively block these mutants. Specifically, we were unable to detect the kind of discrete Ca2+ block and unblock events that are the kinetic signature of high-affinity Ca2+ binding. Thus, Ca2+ channels lacking an intact EEEE locus are unable to bind Ca2+ with approximately micromolar affinity, whether Ca2+ enters the pore via the external entrance, as shown in previous work, or via the internal entrance, as shown here. Combining these results with substituted-cysteine accessibility results suggesting that pore structure was not extensively disrupted in the AAAA mutant solidifies the view that the EEEE locus comprises the only high-affinity Ca2+ binding site in the pore of voltage-gated Ca2+ channels. Our results are consonant with those obtained in studies of double alanine substitution mutants, which are permeated by at least one divalent cation, Ba2+, but that exhibit greatly weakened Ca2+ block and binding (Ellinor et al. 1995). As it is likely that double alanine substitutions would disrupt structure even less than would quadruple substitutions, and because the Ba2+ permeability of the double mutants suggests that Ca2+ would be able to reach and bind to any nondisrupted second site if it existed in these mutants, the lack of high-affinity Ca2+ binding observed in the double alanine mutants represents strong evidence against the existence of a second pore-localized, high-affinity Ca2+ binding site.
In the AAAA and QQQQ mutants, 1 mM internal Ca2+ not only failed to produce resolvable, discrete block/unblock events, but this very high level of Ca2+ also failed to produce a detectable change in unitary Li+ current amplitude. Two-electrode voltage-clamp measurements in oocytes have shown that currents carried by 100 mM Li+ through AAAA and QQQQ channels are half-blocked by ∼1.2 mM Ca2+ (Ellinor et al. 1995), so it might have been anticipated that AAAA or QQQQ unitary current amplitudes would be reduced by ∼50% at 1 mM Ca2+, despite the absence of any high-affinity Ca2+ binding site. However, the recording conditions and solutions differ between two-electrode voltage-clamp and inside-out patch work, shifting the half-block value for WT from the 1-μM value measured by two-electrode voltage clamp to 13 μM, measured here with inside-out patches. Scaling the mutant channel half-block values by this factor of 13 predicts that mutant channels would only be blocked by 6% at 1 mM Ca2+, a reduction in unitary current amplitude so small that we were unable to detect it.
An External Low-Affinity Ca2+ Binding Site?
We were unable to detect discrete block events in AAAA channels when Ca2+ entered the pore from the internal entrance, but we did detect what appeared to be discrete block events when Ca2+ entered the AAAA pore via the external entrance. Although it was difficult to separate the external block events from channel gating events, there appeared to be more resolved open/shut transitions in high Ca2+ than in low Ca2+ (Fig. 3 A). Assuming that the brief open/shut transitions produced in high external Ca2+ represent block of Li+ flux by Ca2+ binding in the permeation pathway, where could such binding occur in AAAA channels that lack an EEEE locus? A speculative possibility is that Ca2+ binds to a low-affinity site just external to the EEEE locus. Such a site would necessarily possess low Ca2+ binding affinity because the AAAA channel is only weakly blocked by Ca2+; an external localization is plausible based on the fact that Ca2+, which is impermeant in the AAAA channel, produced discrete block only when present on the external side of the membrane. Detection of discrete block events is possible for a low-affinity site assuming that a large majority of block events are too brief to be resolved, and that only the rare, very longest events in the distribution of blocked-state lifetimes are detected.
The low-affinity block by Ca2+ of monovalent cation flux through AAAA channels (IC50 ∼ 1 mM) has been thought, since it was first observed, to be mediated by a superficial, externally localized site (Ellinor et al. 1995). WT Ca2+ channels also possess a low-affinity, superficial metal ion binding site (Polo-Parada and Korn 1997). Perhaps these previously considered external, low-affinity Ca2+ binding sites are one and the same with the low-affinity site we have speculated upon here, a site that may represent the physical correlate of the external flanking potential energy well in the Dang and McCleskey 1998 step model of selective permeability in Ca2+ channels.
Acknowledgments
We thank Tsutomu Tanabe, Veit Flockerzi, and Franz Hofmann for gifts of Ca2+ channel cDNAs, and Emily Liman for the gift of the pGEMHE vector.
This work was supported by grant NS35245 (W.A. Sather) and fellowship MH11717 (S.M. Cibulsky) from the National Institutes of Health.
Footnotes
Abbreviations used in this paper: GFP, green fluorescent protein; MTSEA, 2-aminoethyl methanethiosulfonate; WT, wild type.
References
- Akabas M.H., Stauffer D.A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Almers W., McCleskey E.W. Non-selective conductance in calcium channels of frog musclecalcium selectivity in a single-file pore. J. Physiol. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M., Neyton J. Ion permeation through calcium channelsa one-site model. Ann. NY Acad. Sci. 1991;635:18–25. doi: 10.1111/j.1749-6632.1991.tb36477.x. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc. Natl. Acad. Sci. USA. 1987;84:2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S. Site-directed mutagenesis by PCRsubstitution, insertion, deletion, and gene fusion. In: Sarkar G., editor. Methods in Neuroscience. Vol. 26. Academic Press; San Diego, CA: 1995. pp. 309–323. [Google Scholar]
- Berjukow S., Döring F., Froschmayr M., Grabner M., Glossmann H., Hering S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br. J. Pharmacol. 1996;118:748–754. doi: 10.1111/j.1476-5381.1996.tb15463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.H., Bezprozvanny I., Tsien R.W. Molecular basis of proton block of L-type Ca2+ channels. J. Gen. Physiol. 1996;108:363–374. doi: 10.1085/jgp.108.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.H., Tsien R.W. Aspartate substitutions establish the concerted action of P-region glutamates in repeats I and III in forming the protonation site of L-type Ca2+ channels. J. Biol. Chem. 1997;272:30002–30008. doi: 10.1074/jbc.272.48.30002. [DOI] [PubMed] [Google Scholar]
- Cloues R.K., Sather W.A. Permeant ion binding affinity in subconductance states of an L-type Ca2+ channel expressed in Xenopus laevis oocytes. J. Physiol. 2000;524:19–36. doi: 10.1111/j.1469-7793.2000.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang T.X., McCleskey E.W. Ion channel selectivity through stepwise changes in binding affinity. J. Gen. Physiol. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D.A., Cabral J.M., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channelmolecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Ellinor P.T., Yang J., Sather W.A., Zhang J.-F., Tsien R.W. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Friel D.D, Tsien R.W. Voltage-gated calcium channelsdirect observation of the anomalous mole fraction effect at the single-channel level. Proc. Natl. Acad. Sci. USA. 1989;86:5207–5211. doi: 10.1073/pnas.86.13.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J. Physiol. 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Yamakawa T., Midera T., Sagawa T., Mori Y., Nukada T. Selectivities of dihydropyridine derivatives in blocking Ca2+ channel subtypes expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1999;291:464–473. [PubMed] [Google Scholar]
- Gomez M., Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflügers Arch. 1995;430:501–507. doi: 10.1007/BF00373886. [DOI] [PubMed] [Google Scholar]
- Hao L.-Y., Kameyama A., Kuroki S., Nishimura S., Kameyama M. Run-down of L-type Ca2+ channels occurs on the α1 subunit. Biochem. Biophys. Res. Commun. 1998;247:844–850. doi: 10.1006/bbrc.1998.8886. [DOI] [PubMed] [Google Scholar]
- Heinemann S.H., Terlau H., Stühmer W., Imoto K., Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R.W. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J.B., Tsien R.W. Calcium channel selectivity for divalent and monovalent cationsvoltage and concentration dependence of single channel current in ventricular heart cells. J. Gen. Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 1978;72:409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes 2nd ed 1992. Sinauer Associates, Inc; Sunderland, MA: pp. 607 [Google Scholar]
- Hodgkin A.L., Keynes R.D. The potassium permeability of a giant nerve fibre. J. Physiol. 1955;128:61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. Calcium channel β subunit heterogeneityfunctional expression of cloned cDNA from heart, aorta and brain. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-S., Morii T., Sun L.-X., Imoto K., Mori Y. Structural determinants of ion selectivity in brain calcium channel. FEBS Lett. 1993;318:145–148. doi: 10.1016/0014-5793(93)80009-j. [DOI] [PubMed] [Google Scholar]
- Klockner U., Mikala G., Schwartz A., Varadi G. Molecular studies of the asymmetric pore structure of the human cardiac voltage-dependent Ca2+ channel. Conserved residue, Glu-1086, regulates proton-dependent ion permeation. J. Biol. Chem. 1996;271:22293–22296. doi: 10.1074/jbc.271.37.22293. [DOI] [PubMed] [Google Scholar]
- Kostyuk P.G., Mironov S.L. Some predictions concerning the calcium channel model with different conformational states. Gen. Physiol. Biophys. 1986;6:649–659. [PubMed] [Google Scholar]
- Kostyuk P.G., Mironov S.L., Shuba Y. M. Two ion-selecting filters in the calcium channel of the somatic membrane of mollusc neurons. J. Membr. Biol. 1983;76:83–93. [Google Scholar]
- Kuo C.-C., Hess P. Ion permeation through the L-type Ca2+ channel in rat phaeochromocytoma cellstwo sets of ion binding sites in the pore J. Physiol. 466 1993. 629 655a [PMC free article] [PubMed] [Google Scholar]
- Kuo C.-C., Hess P. Characterization of the high-affinity Ca2+ binding sites in the L-type Ca2+ channel pore in rat phaeochromocytoma cells J. Physiol. 466 1993. 657 682b [PMC free article] [PubMed] [Google Scholar]
- Kuo C.-C., Hess P. Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaeochromocytoma cells J. Physiol. 466 1993. 683 706c [PMC free article] [PubMed] [Google Scholar]
- Lambert R.C., Feltz A. Maintained L-type Ca2+ channel activity in excised patches of PTX-treated granule cells of the cerebellum. J. Neurosci. 1995;15:6014–6022. doi: 10.1523/JNEUROSCI.15-09-06014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J.B., Hess P., Tsien R.W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E.R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lu T., Nguyen B., Zhang X., Yang J. Architecture of a K+ channel inner pore revealed by stoichiometric covalent modification. Neuron. 1999;22:571–580. doi: 10.1016/s0896-6273(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Lux H.D., Carbone E., Zucker H. Na currents through low-voltage–activated Ca channels of chick sensory neuronsblock by external Ca and Mg. J. Physiol. 1990;430:159–188. doi: 10.1113/jphysiol.1990.sp018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflügers Arch. 1986;407:465–475. doi: 10.1007/BF00657502. [DOI] [PubMed] [Google Scholar]
- McCleskey E.W. Calcium channel permeationa field in flux. J. Gen. Physiol. 1999;113:765–772. doi: 10.1085/jgp.113.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikala G., Bahinski A., Yatani A., Tang S., Schwartz A. Differential contribution by conserved glutamate residues to an ion-selectivity site in the L-type Ca2+ channel pore. FEBS Lett. 1993;335:265–269. doi: 10.1016/0014-5793(93)80743-e. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Miller C. Ionic hopping defended. J. Gen. Physiol. 1999;113:783–787. doi: 10.1085/jgp.113.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel J. Gen. Physiol. 92 1988. 549 567a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel J. Gen. Physiol. 92 1988. 569 586b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W., Eisenberg B. Ion permeation and glutamate rersidues linked by Poisson-Nernst-Plank theory in L-type calcium channels. Biophys. J. 1998;75:1287–1305. doi: 10.1016/S0006-3495(98)74048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent L., Gopalakrishnan M. Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+ channel. Biophys. J. 1995;69:1801–1813. doi: 10.1016/S0006-3495(95)80050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L., Korn S.J. Block of N-type calcium channels in chick sensory neurons by external sodium. J. Gen. Physiol. 1997;109:693–702. doi: 10.1085/jgp.109.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R.L., Chen X.-H. Characterization and localization of two ion-binding sites within the pore of cardiac L-type calcium channels. J. Gen. Physiol. 1991;97:1207–1225. doi: 10.1085/jgp.97.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W.A., Tanabe T., Zhang J.-F., Mori Y., Adams M.E., Tsien R.W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel α1 subunits. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Sjöholm Å., Arkhammar P., Welsh N., Bokvist K., Rorsman P., Hallberg A., Nilsson T., Welsh M., Berggren P.-O. Enhanced stimulus-secretion coupling in polyamine-depleted rat insulinoma cells. J. Clin. Invest. 1993;92:1910–1917. doi: 10.1172/JCI116784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Mikala G., Bahinski A., Yatani A., Varadi G., Schwartz A. Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel. J. Biol. Chem. 1993;268:13026–13029. [PubMed] [Google Scholar]
- Yang J., Ellinor P.T., Sather W.A., Zhang J.-F., Tsien R.W. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Bahinski A., Mikala G., Yamamoto S., Schwartz A. Single amino acid substitutions within the ion permeation pathway alter single-channel conductance of the human L-type cardiac Ca2+ channel. Circ. Res. 1994;75:315–323. doi: 10.1161/01.res.75.2.315. [DOI] [PubMed] [Google Scholar]
- Yu S.P., Kerchner G.A. Endogenous voltage-gated potassium channels in human embryonic kidney (HEK293) cells. J. Neurosci. Res. 1998;52:612–617. doi: 10.1002/(SICI)1097-4547(19980601)52:5<612::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yue D.T., Marban E. Permeation in the dihydropyridine-sensitive calcium channelmulti-ion occupancy but no anomalous mole-fraction effect between Ba2+ and Ca2+ . J. Gen. Physiol. 1990;95:911–939. doi: 10.1085/jgp.95.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Zhang Y., Xu H., Jiang C. Identification of endogenous outward currents in the human embryonic kidney (HEK 293) cell line. J. Neurosci. Methods. 1998;81:73–83. doi: 10.1016/s0165-0270(98)00019-3. [DOI] [PubMed] [Google Scholar]