Abstract
The effects of the muscle relaxant dantrolene on steps of excitation-contraction coupling were studied on fast twitch muscles of rodents. To identify the site of action of the drug, single fibers for voltage-clamp measurements, heavy SR vesicles for calcium efflux studies and solubilized SR calcium release channels/RYRs for lipid bilayer studies were isolated. Using the double Vaseline-gap or the silicone-clamp technique, dantrolene was found to suppress the depolarization-induced elevation in intracellular calcium concentration ([Ca2+]i) by inhibiting the release of calcium from the SR. The suppression of [Ca2+]i was dose-dependent, with no effect at or below 1 μM and a 53 ± 8% (mean ± SEM, n = 9, cut fibers) attenuation at 0 mV with 25 μM of extracellularly applied dantrolene. The drug was not found to be more effective if injected than if applied extracellularly. Calculating the SR calcium release revealed an equal suppression of the steady (53 ± 8%) and of the early peak component (46 ± 6%). The drug did not interfere with the activation of the voltage sensor in as much as the voltage dependence of both intramembrane charge movements and the L-type calcium currents (ICa) were left, essentially, unaltered. However, the inactivation of ICa was slowed fourfold, and the conductance was reduced from 200 ± 16 to 143 ± 8 SF−1 (n = 10). Dantrolene was found to inhibit thymol-stimulated calcium efflux from heavy SR vesicles by 44 ± 10% (n = 3) at 12 μM. On the other hand, dantrolene failed to affect the isolated RYR incorporated into lipid bilayers. The channel displayed a constant open probability for as long as 30–50 min after the application of the drug. These data locate the binding site for dantrolene to be on the SR membrane, but be distinct from the purified RYR itself.
Keywords: calcium current, intramembrane charge, calcium release, ryanodine receptor, single channel
INTRODUCTION
In susceptible individuals, the inhalation of certain volatile anesthetics triggers a sudden increase in body temperature, which, if left untreated, is usually lethal. The underlying reason for this increase in body temperature (malignant hyperthermia [MH]) is the abnormal activation of skeletal muscle fibers and the consequent increase in metabolic rate throughout the body. In MH, apparently, the presence of the anesthetic is sufficient to trigger the release of calcium from its internal store, the SR in resting, unstimulated skeletal muscle fibers. To prevent this increase in muscle activity, dantrolene is administered. Dantrolene itself is a hydantoid derivative muscle relaxant that has been shown to decrease contractile force (Flewellen et al. 1983) and shift the excitation threshold to more positive voltages (Hainaut and Desmedt 1974). In spite of the fact that dantrolene plays a pivotal role in MH treatment and its effects on most major steps of excitation-contraction coupling have been investigated, the actual site of dantrolene action is still controversial.
During normal excitation of a skeletal muscle fiber the change in transverse (t)-tubular voltage governs the conformational changes of the t-tubular dihydropyridine receptors (DHPRs) acting as voltage sensors. The interaction of the DHPRs with the calcium release channel of the SR, the RYRs, leads to the release of calcium from the SR into the myoplasmic space. In MH, this chain of events is disrupted, and calcium release can occur even in the absence of voltage sensor activation. The effect of dantrolene is, by a yet unknown mechanism, to suppress SR calcium release.
It is currently accepted that dantrolene must, either directly or indirectly, affect the RYR itself (Parness and Palnitkar 1995; Nelson et al. 1996; Pessah et al. 1996; Palnitkar et al. 1997). Several lines of evidence favor this interpretation. Measurements have shown that intramembrane charge movement is slightly reduced, and its voltage dependence is shifted to more positive voltages (Hui 1983; Jong et al. 1997). The effect of the drug was preferentially on the Qγ component (Hui 1983). Considering the proposed connection between Qγ and calcium release (Pizarro et al. 1991; Jong et al. 1995), these observations link the effects of dantrolene to SR calcium release.
Dantrolene or its water-soluble analogue azumolene has been reported to inhibit calcium efflux from heavy SR vesicles (Francis 1978; Fruen et al. 1997). Not only were these drugs shown to reduce doxorubicin (Tian et al. 1991) or calcium- and calmodulin-induced (Fruen et al. 1997) calcium release, but dantrolene was also shown to inhibit [3H]ryanodine binding to SR membrane fractions. Furthermore, [3H]dantrolene and [3H]ryanodine binding sites were localized to closely associated SR membrane fractions from skeletal muscle (Parness and Palnitkar 1995; Palnitkar et al. 1997). Single-channel measurements have revealed a high affinity activatory and a lower affinity inhibitory binding site on the SR membrane (Nelson et al. 1996).
Despite this close association, the calcium release channel and the dantrolene-binding site in skeletal muscle have not been equated. On the one hand, Fruen and co-workers (Fruen et al. 1997) have shown that isolated RYR reconstituted into liposomes display a reduced [3H]ryanodine binding in the presence of dantrolene, which is indicative of a dantrolene-binding site on the channel molecule itself. On the other hand, Palnitkar and co-workers (Palnitkar et al. 1999) identified a 160-kD protein in the SR membrane with a high affinity against [3H]azidodantrolene as the putative binding site. However, recently, the same authors (Paul-Pletzer et al. 2001) have provided evidence for the 160-kD protein to be identical with the NH2 terminus of the RYR. Single-channel data that could resolve the controversy were obtained from vesicles incorporated into planar lipid bilayers (Suarez-Isla et al. 1986; Nelson et al. 1996), leaving the possibility of an SR membrane–bound molecule to be the dantrolene-binding site open.
To date, three isoforms of the calcium release channel have been identified in mammals. In spite of the homology between isoforms found, predominantly, in skeletal (RYR1) and cardiac (RYR2) muscles, dantrolene is known to preferentially inhibit calcium release in skeletal, but not in cardiac muscle (Chamberlain et al. 1984). Although the inhibitory effect of dantrolene on calcium release from internal stores in neurons has been demonstrated (Nelson et al. 1999), the involvement of RYR3 in this effect remains to be elucidated, since neurons are known to express all three RYR isoforms. A possible action of the drug on RYR3 would be interesting from the therapeutic point of view, since the expression of RYR3, although to a limited extent, has been demonstrated in skeletal muscle as well (Flucher et al. 1999).
Although dantrolene was shown to suppress the calcium transients in frog skeletal muscle (Desmedt and Hainaut 1977; Kovács et al. 1983), similar effects in mammalian skeletal muscle haven't been documented so far. Furthermore, a detailed description of the alteration of SR calcium release as well as a functional comparison of the purified channel with the channel in native interaction with other proteins is also missing. Here we show, for the first time, that dantrolene inhibits calcium release in mammalian skeletal muscle by suppressing both steady and peak components of SR calcium release flux. This suppression was achieved without major alterations in the voltage dependence of intramembrane charge movement and calcium current. We also demonstrate that the isolation and reconstitution of RYR into planar lipid bilayers results in the loss of dantrolene sensitivity, which was clearly present when the release channel was in the SR membrane. These data argue against a direct effect of dantrolene on the purified RYR, but place the dantrolene-binding site on an SR membrane–bound molecule.
MATERIALS AND METHODS
Enzymatic Isolation of Single Fibers
Single skeletal muscle fibers were isolated enzymatically from the extensor digitorum communis or flexor digitorum brevis muscles of rats or mice and mounted into a double Vaseline gap chamber or silicone clamp as described earlier (Szentesi et al. 1997; Jacquemond 1997, respectively). In brief, rats or mice of either sex were anesthetized and killed by cervical dislocation in accordance with the guidelines of the European Community (86/609/EEC). The muscles were removed and were treated with collagenase (Type I; Sigma-Aldrich) for 1–1.5 h at 37°C. After the dissociation, fibers were allowed to rest for at least 20 min, and only those that showed no signs of membrane damage were used.
For the Vaseline-gap experiments, the selected fiber was transferred into a recording chamber (Kovács, et al. 1983) filled with relaxing solution ([in mM] 150 potassium glutamate, 2 MgCl2, 10 HEPES, and 1 EGTA). The fiber segments in the open-end pools were permeabilized using 0.01% saponin. After completing the permeabilization, the solutions were exchanged to internal solution in the open-end pools ([in mM] 120 cesium glutamate, 5.5 MgCl2, 5 Na2-ATP, 10 sodium phosphocreatine, 10 glucose, 10 HEPES, and 5 EGTA) and to external solution in the middle pool ([in mM] 140 TEA-CH3SO3, 2 CaCl2, 2 MgCl2, 10 HEPES, 0.0003 tetrodotoxin, and 1 3,4-diamino-pyridine). All solutions were adjusted to pH 7.2 and 300 mosmol/liter. The internal solution also contained 1 mM antipyrylazo III (APIII) and 100 μM Fura-2 for the detection of intracellular calcium concentration. For calcium current measurements, the external solution was modified to have 5 mM CaCl2 and appropriately reduced TEA-CH3SO3, whereas the EGTA concentration in the internal solution was increased to 20 mM with reducing cesium glutamate. The length of the fiber segment in the middle pool was set to 500 μm.
For silicone-clamp experiments, the major part of an isolated mouse skeletal muscle fiber was electrically insulated with silicone grease so as to achieve whole-cell voltage clamp on a short portion (50–100 μm long) of the fiber extremity (Jacquemond 1997). Intracellular indo-1 loading was then performed through local pressure microinjection, using a micropipette containing 0.5 mM indo-1 (pentapotassium salt) dissolved in an “intracellular-like” solution containing (in mM): 120 potassium glutamate, 5 Na2-ATP, 5 Na2-phosphocreatine, 5.5 MgCl2, and 10 HEPES, adjusted to pH 7.2 with KOH. The microinjection was performed with the micropipette tip inserted through the silicone within the insulated part of the fiber. Fibers were then left for a 30–45-min period of time before voltage clamping to allow for dye equilibration throughout the entire fiber volume. In some experiments, dantrolene was present within the injected solution at a concentration of 25 μM (see Chemicals). The composition of the external solution was identical to that used for the Vaseline-gap experiments, except that it contained 2.5 mM CaCl2, 2 μM tetrodotoxin, and no 3,4 diamino-pyridine. For testing the effect of extracellularly applied dantrolene, a thin polyethylene capillary perfusion system operating by gravity was used.
Optical Set-up and Voltage Clamp
The experimental set-up and the data acquisition have been described in detail in our earlier reports (Jacquemond 1997; Csernoch et al. 1999b). In the Vaseline gap, the fiber was trans-illuminated using a tungsten halogen light source (λ > 600 nm) and was also epi-illuminated at 380 nm or at the isosbestic wavelength of Fura-2 using a 75 W Xenon arc lamp (model 60000; Oriel). Using appropriate interference filters and dichroic mirrors, light intensities were simultaneous recorded at 510, 720, and 850 nm for the detection of APIII absorbance and Fura-2 fluorescence. Fibers were voltage-clamped and the holding potential was set to −100 mV. In silicone-clamp experiments, the light from a high pressure mercury bulb (model USH102DH;, USHIO INC.) was used for fiber illumination. Excitation was set at 335 nm and fluorescence was detected simultaneously at 405 and 470 nm by two photo multipliers, using an appropriate set of filters and dichroic mirror. Fibers were voltage-clamped at a holding potential of −80 mV using a patch-clamp amplifier (model RK-400; Bio-Logic) in whole-cell configuration, connected to a microelectrode filled with the “intracellular-like” solution (see above). All experiments were performed at room temperature (20–22°C).
Preparation of SR Vesicles and RYR
Heavy and light SR (HSR and LSR, respectively) vesicles and the RYR calcium release channel were isolated from rat skeletal muscle as described earlier (Csernoch et al. 1999b). In brief, skeletal muscles were removed from the front and hind legs of those rats that were used in the Vaseline-gap experiments. The muscles were cut into small pieces, quickly frozen using liquid nitrogen, and stored at −70°C until further use. Vesicles were obtained by first homogenizing the tissue, and then removing the unsolubilized particles in a clinical centrifuge. Crude membrane fraction was collected from the supernatant by centrifugation at 40,000 g (30 min). The actomyosin content of the pellet was then dissolved in 600 mM KCl, and the crude microsome fraction was collected by centrifugation at 109,000 g (30 min). The microsome was loaded onto a 20–45% linear sucrose gradient and spun for 16 h at 90,000 g (4°C) in a swing-out (SW-27) Beckman rotor. The protein ring corresponding to the HSR fraction was extracted from the 36–38%, whereas that for LSR was extracted from the 32–34% region. Vesicles were collected at 124,000 g for 60 min, and then resuspended in 92.5 mM KCl − 18.5 mM K-MOPS, pH 7.0, for vesicular measurements or in 0.4 M sucrose − 10 mM K-PIPES, pH 7.2, for RYR preparation. Samples were stored at −70°C until further use.
For preparation of RYR, the HSR vesicles (3 mg/ml) were solubilized for 2 h at 4°C with 1% CHAPS (3[(3-chloramidopropyl)dimethyl-amino]-1-propanesulfonate) in a solution containing 1 M NaCl, 100 μM EGTA, 150 μM CaCl2, 5 mM AMP, 0.45% phosphatidylcholine, 20 mM Na-PIPES, pH 7.2, and protease inhibitors (Csernoch et al. 1999b). Unsolubilized proteins were removed by centrifugation at 59,000 g, and subsequently the supernatant was layered on top of a 10–28% sucrose gradient also containing 1% CHAPS, 0.7 M NaCl, 10 mM AMP, 0.5% phosphatidylcholine, 70 μM EGTA, 100 μM CaCl2, 1 mM DTT, 13 mM Na-PIPES, pH 7.2. Solubilized SR membranes were centrifuged through the sucrose gradient for 16 h at 90,000 g (4°C) in swing-out Beckman rotor. Fractions containing RYR were collected in small aliquots. They were rapidly frozen in liquid nitrogen and stored at −70°C.
Calculation of [Ca2+]i and Rrel
In Vaseline-gap experiments, the changes in myoplasmic free calcium concentration ([Ca2+]i) were calculated from APIII absorbance as described by Kovács et al. 1983 with the correction for intrinsic fiber absorbance at 850 nm (Melzer et al. 1986) and from Fura-2 fluorescence using the kinetic correction (Klein et al. 1988). Under silicone-clamp conditions, [Ca2+]i was calculated from the ratio of indo-1 fluorescence measured at 405 and 470 nm, as described previously (Jacquemond 1997; Collet et al. 1999).
The rate of calcium release from the SR (Rrel) was calculated from the calcium transients measured in Vaseline-gap experiments using the procedure described in Szentesi et al. 1997. Four parameters of the removal model were fitted simultaneously, koff,M–P (Mg2+ off rate from parvalbumin), PVmax (maximal transport rate of SR calcium pump), and koff,Ca-E and kon,Ca-E (the rate constants of calcium binding to EGTA). The calculated Rrel records were corrected for the depletion of calcium in the SR and expressed as a percentage of SR content (Csernoch et al. 1993). The voltage (Vm) dependence of either component of calcium release (Rrel,i(Vm), where i is the peak or steady level) was assessed by fitting the two state Boltzmann function:
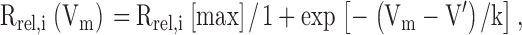 |
1 |
where Rrel,i[max] is the maximal release rate, V′ is the voltage at half-maximal release rate, and k is the slope factor to the calculated data.
Intramembrane Charge Movement and Calcium Current
Intramembrane charge transfer and calcium current through L-type calcium channels were calculated by measuring membrane currents in response to depolarizing and hyperpolarizing pulses as described in detail previously (Szentesi et al. 1997). Briefly, the linear capacitive current was determined from hyperpolarizing pulses of −20 mV in amplitude. This was then scaled and subtracted from the current measured during depolarizing steps. The nonlinear capacitative current, representing intramembrane charge transfer, was integrated to give the amount of charge moved during the pulse (Q). To assess the membrane potential dependence of charge transfer (Q(Vm)) the measured charge was fitted with the Boltzmann function:
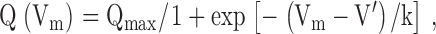 |
2 |
where Qmax is the maximal available charge, and V′ and k have their usual meaning.
Calcium currents (ICa) were measured using 800-ms-long depolarizing pulses exploring the −50 to +60 mV voltage range. To enable the subtraction of the linear capacitive component the −20-mV hyperpolarizing pulses were also extended to 800 ms in duration. The external calcium concentration was elevated to 5 mM as detailed above. The peak ICa versus voltage relationship was fitted with:
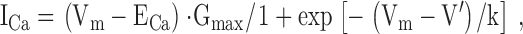 |
3 |
where ECa is the calcium equilibrium potential, Gmax is the maximal conductance, and all other parameters have their usual meaning. All currents, the amount of charge moved, and the maximal conductance were normalized to fiber capacitance to take the size of the individual fibers into account.
Calcium Efflux from HSR Vesicles
HSR vesicles were actively loaded with calcium, resulting in a 1.20 ± 0.11 μmol/mg protein average calcium content of the vesicles. Calcium efflux was determined by measuring the extravesicular calcium concentration using a Fluoromax (SPEX Inc.) spectrofluorometer modified for absorption measurements by monitoring the transmittance at 710 and 790 nm, and calculating the corrected absorbance change (A710 − A790) as described earlier (Sárközi et al. 1996). In brief, vesicles were suspended in the cuvette (final volume, 2 ml) in a medium containing the following (in mM): 92.5 KCl, 1 MgCl2, 0.180 APIII, 0.054 CaCl2, 18.5 K-MOPS, and 1 ATP up to a final protein concentration of 260 μg/ml. Vesicles were actively loaded with calcium in the cuvette by the addition of CaCl2. After completion of the ATP to ADP conversion, the release of calcium was initiated by the addition of 300 μM thymol. The samples were continuously stirred by special magnetic stirrers having a mixing time of 0.2–0.5 s. Initial rate of release was determined using the time segments of 10–50 s after the thymol addition, since thymol exerted its full effect in a few seconds. The effect of dantrolene was tested by the preincubation of the samples with the appropriate concentration of dantrolene for 30 min before initiating the thymol-induced calcium release. The effect was assessed as a decrease of the thymol-induced release.
Planar Lipid Bilayer Measurements
CHAPS solubilized RYR molecules were incorporated into planar lipid bilayers (Tripathy and Meissner 1996) formed across a 200-μm aperture of a nolrene cup using a lipid mixture composed of phosphatidylethanolamine, phosphatidylserine and phosphatidylcholine in the weight ratio of 5:4:1. Lipids were dissolved in n-decane up to a final concentration of 20 mg/ml, as described in our previous reports (Szegedi et al. 1999). Reconstitution was initiated in symmetric buffer solution (250 mM KCl, 100 μM EGTA, 150 μM CaCl2, and 20 mM PIPES, pH 7.2), by adding a small aliquot of the solubilized receptor to one side of the bilayer chamber defined as the cytoplasmic (cis) side. K+ was used as a charge carrier in the voltage-clamp measurements, and the current signal was filtered at 1 kHz through an 8-pole lowpass Bessel filter and digitized at 3 kHz using Axopatch 200 amplifier and pClamp 6.03 software (Axon Instruments, Inc.). Channels with conductance >400 pS were accepted as RYR. Open probability values (Po) were calculated from 1-min segments of the current traces. After each change of the measuring condition, at least 5 min was allowed to reach the new equilibrium, and then at least 5-min-long records were taken for each of the experimental condition tested. In the case of dantrolene, 30-min incubation was used before the current recordings. Measurements were performed at 22–25°C, dantrolene was applied in the cis chamber in concentrations ranging from 1 nM to 50 μM.
Chemicals
Fura-2 and indo-1 were purchased from Molecular Probes Inc., APIII was purchased from ICN Biomedicals. Protease inhibitors were purchased from Boehringer, Merck, and from Sigma-Aldrich. Lipids were obtained from Avanti Polar Lipids, [3H]ryanodine was purchased from DUPONT, and all other chemicals were from Sigma-Aldrich.
For calcium efflux and bilayer experiments, a dantrolene stock of 18 mM was prepared in DMSO, and the required aliquot was added directly into the measuring chamber. This stock was diluted in the external solution to determine the molar extinction coefficient of the drug (ɛ = 14,700 cm−1M−1 at 390 nm). In Vaseline-gap or silicone-clamp experiments, dantrolene was dissolved in the applied aqueous solution to an attempted final concentration of 100 μM. Unsolubilized particles were removed by filtering the solution through 0.22-μm filters (Millipore Corp.). Using the molar extinction coefficient, this fully saturated solution was found to be 24.8 μM and will be referred to as 25 μM in results. Lower concentrations were obtained by diluting this fully saturated stock.
In statistical analyses, all values were expressed as mean ± SEM, statistical significance was calculated with the t test assuming significance for P < 0.05.
RESULTS
Dantrolene Suppresses Calcium Transients
Fig. 1 illustrates the effect of extracellular application of 25 μM dantrolene on the calcium transients elicited by short depolarizing pulses on intact mouse skeletal muscle fibers using the silicone-clamp technique. Fig. 1 A shows a series of indo-1 calcium transients elicited by 20-ms-long depolarizing pulses to 0 mV applied every 30 s. Application of dantrolene produced an ∼50% decrease in the maximal amplitude of the calcium transient from 0.88 ± 0.24 μM (n = 4), the effect being complete within <2 min. Fig. 1 B shows the time-dependent evolution of the mean maximum change in [Ca2+]i reached in response to a 20-ms duration depolarizing pulse to 0 mV, in control conditions, and upon dantrolene application and washout. Data correspond to the mean values obtained from four fibers. Values of peak change in [Ca2+]i from each fiber were normalized to the peak value measured in response to the first depolarization. In average, dantrolene produced a 58% suppression of the peak calcium transient, the effect being partly and slowly reversible upon dantrolene washout. This effect of dantrolene was not associated to any significant change in resting [Ca2+]i, which, within this series of measurements, exhibited a mean value of 64 ± 13 nM in control and 62 ± 12 nM at the time when dantrolene produced its full effect on the peak [Ca2+]i.
Figure 1.
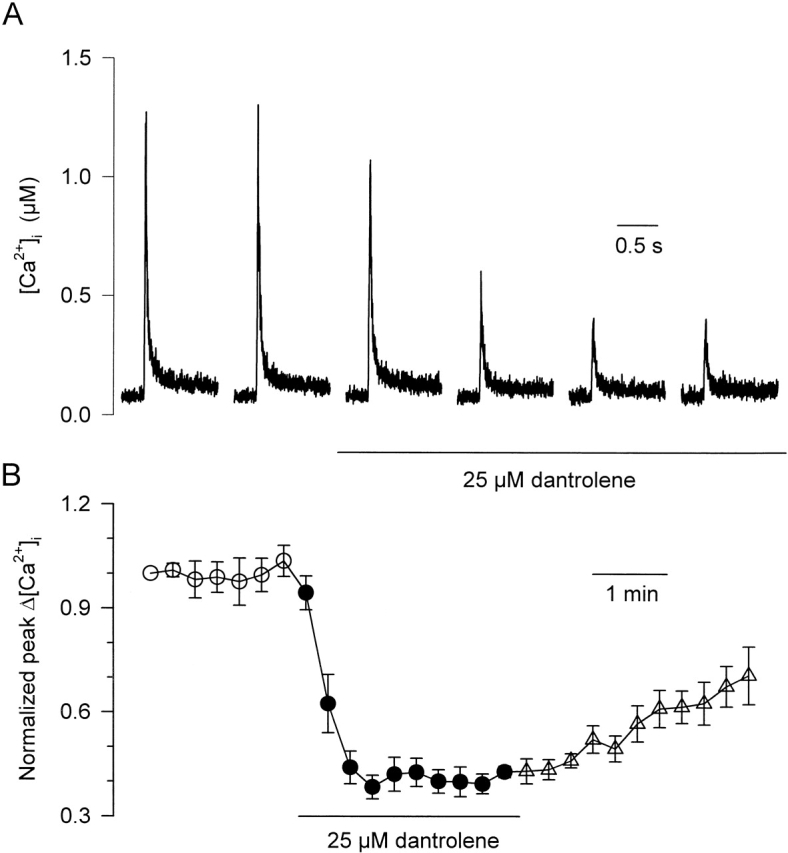
Extracellular application of dantrolene depresses calcium transients in intact mouse skeletal muscle fibers. (A) Effect of dantrolene (25 μM) on the indo-1 calcium transient elicited by a depolarization of 20 ms to 0 mV applied every 30 s. (B) Time-dependent evolution of the peak change in [Ca2+]i elicited by a 20-ms depolarization to 0 mV upon dantrolene application and after washout. Data points correspond to the mean value obtained from four fibers. For each fiber, data points were normalized to the peak value measured in response to the first depolarization. Here, and in subsequent figures, open circles (○), closed circles (•), and open triangles (Δ) denote measurement taken before, during, and after the extracellular application of dantrolene, respectively.
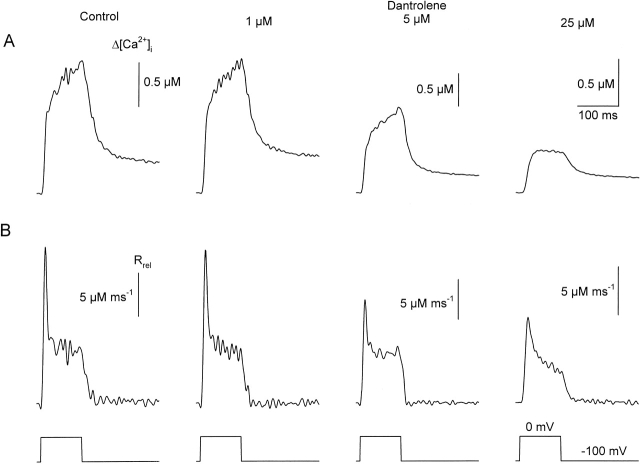
These measurements were repeated using the double Vaseline-gap system to enable the calculation of SR calcium release. Thus, Fig. 2 presents calcium transients and the calculated rate of calcium release from the SR (Rrel; see materials and methods) at different dantrolene concentrations. Measurements in dantrolene were taken 5–10 min after the solution exchange. This was, based on Fig. 1, believed to be long enough for the drug to reach its binding site and exert its full effects. As will be demonstrated in more detail later (see Fig. 4, Fig. 6, and Table ), we were unable to reach substantial recoveries from the suppression caused by dantrolene if the drug was present for several minutes in the bathing solution. Therefore, Fig. 2 presents data from three different fibers for the three different dantrolene concentrations (1, 5, and 25 μM) tested. To ease the comparison among different fibers, traces are presented on different scales that were selected to give equal maxima for the transients in control.
Figure 2.
Changes in (A) intracellular calcium concentration (Δ[Ca2+]i) and (B) the rates of calcium release from the SR (Rrel) at various dantrolene concentration. Calcium transients were evoked with 100-ms-long depolarizations to 0 mV in fibers mounted into the Vaseline gap. The first and second columns originated from the same fiber, whereas the traces in the third and fourth columns are from two other fibers. In these fibers, the peaks of the calcium transients and those of Rrel in control were close (±10%) to the values from the fiber represented in the first column. Nevertheless, to ease the comparison between the different fibers, different scales were used for the last two columns. With these, the control transients (not shown for 5 and 25 μM dantrolene) would be equal in size in the figure to the traces presented in the first column. Antipyrylazo concentrations ([APIII]) were 650, 613, and 823 μM; Fura-2 concentrations ([Fura-2]) were 48, 35, and 111 μM for the fibers with 1, 5, and 25 μM dantrolene, respectively. The parameters for calculating Rrel were koff,M-P = 9.4, 6.1, and 3.5 s−1; PVmax = 1.32, 1.88, and 2.41 mMs−1; kon,Ca-E = 0.9, 1.5, and 1.7 μM−1s−1; and koff,Ca-E = 4.9, 2.2, and 4.4 s−1, respectively. In this and subsequent figures, where the Vaseline-gap technique was applied, Fura-2 was used to measure changes in [Ca2+]i at, and close to, the resting value, whereas APIII was used for large changes in [Ca2+]i.
Figure 4.
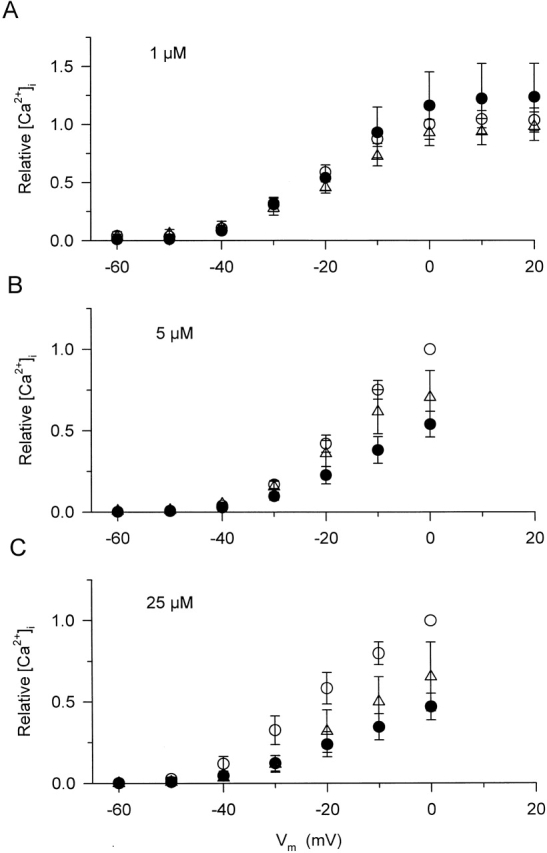
Voltage dependence of the peak of the calcium transients at different dantrolene concentrations. Measurements in each fiber and at each dantrolene concentration were taken as described in Fig. 3, first in control conditions (○), and then in the presence of the drug (•). Additionally, dantrolene was removed from the external solution before obtaining a final set of calcium transients (washout, Δ). To ease the comparison between different fibers and dantrolene concentrations, the peak [Ca2+]i for every transient in a given fiber was normalized to the value measured under control conditions at 0 mV in the given fiber. The average values for the peak of [Ca2+]i at 0 mV were 1.5 ± 0.3 (n = 13), 1.8 ± 0.3 (n = 7), and 1.6 ± 0.3 μM (n = 9) for A–C, respectively. Depolarizations to or below −70 mV did not elicit any measurable increase in [Ca2+]i and were not included in the graph. Note that the scale in A is different from that in B and C.
Figure 6.
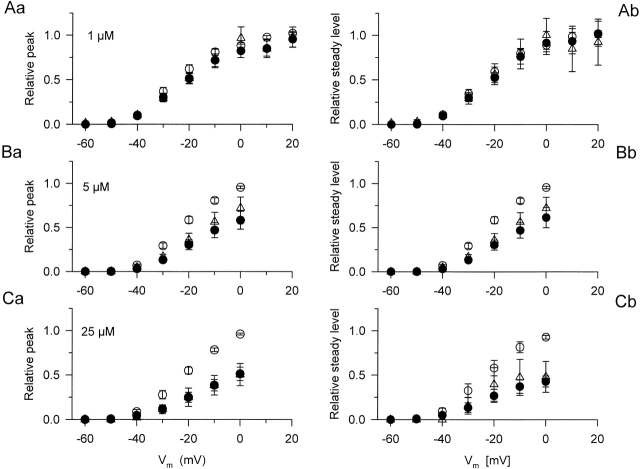
Voltage-dependent effects of dantrolene on the peak (left column) and on the steady level (right column) of SR calcium permeability. The steady level was measured as the average of the values during the last 20 ms of the test pulse. The voltage dependence of either component was fitted with in every fiber. The obtained maximum (, Rrel,i[max]) in control on the given fiber was used to normalize the values before averaging and plotting them. The parameters obtained by fitting the voltage dependence with and averaged over the fibers are presented in Table . The same fibers and symbols as in Fig. 4 are used.
Table 1.
Voltage Dependence of SR Calcium Permeability
| Peak | Steady level | |||||
|---|---|---|---|---|---|---|
| Max | V′ | k | Max | V′ | k | |
| ms−1 | mV | mV | ms−1 | mV | mV | |
| Control (n = 13) | 1.16 ± 0.27 | −23.4 ± 2.0 | 8.2 ± 0.5 | 0.39 ± 0.11 | −23.1 ± 2.1 | 8.1 ± 0.6 |
| 1 μM dantrolene | 1.09 ± 0.31 | −20.1 ± 2.0 | 9.3 ± 0.4 | 0.37 ± 0.11 | −18.1 ± 2.7 | 9.1 ± 0.7 |
| Washout | 0.91 ± 0.33 | −15.0 ± 5.9 | 10.9 ± 1.5 | 0.30 ± 0.13 | −19.5 ± 4.4 | 8.3 ± 1.1 |
| Control (n = 7) | 1.18 ± 0.05 | −22.4 ± 1.3 | 7.5 ± 0.3 | 0.49 ± 0.04 | −16.6 ± 1.9 | 8.8 ± 0.4 |
| 5 μM dantrolene | 0.75 ± 0.13 | −17.9 ± 2.6 | 7.7 ± 0.6 | 0.29 ± 0.05 | −16.1 ± 2.2 | 7.6 ± 0.4 |
| Washout | 1.04 ± 0.14 | −15.6 ± 2.5 | 9.3 ± 1.0 | 0.33 ± 0.05 | −20.3 ± 0.6 | 6.9 ± 0.7 |
| Control (n = 9) | 1.53 ± 0.14 | −21.8 ± 1.4 | 7.7 ± 0.4 | 0.57 ± 0.07 | −22.5 ± 2.8 | 6.7 ± 0.5 |
| 25 μM dantrolene | 0.94 ± 0.20 | −16.4 ± 1.8 | 8.4 ± 0.4 | 0.25 ± 0.04 | −22.0 ± 2.6 | 5.7 ± 0.8 |
| Washout | 0.81 ± 0.23 | −16.8 ± 2.9 | 7.8 ± 0.1 | 0.21 ± 0.08 | −23.2 ± 4.1 | 4.5 ± 0.1 |
Parameter values obtained by fitting to the voltage dependence of SR permeability. Values represent mean ± SEM.
Dantrolene caused a dose-dependent suppression of [Ca2+]i, reaching ∼65% at the highest concentration. The attenuation was present both in the rate of rise and in the amplitude of the calcium signals. The transients showed a continuous increase in control and after the addition of 1 and 5 μM dantrolene, whereas a definite maximum, followed by a gradual decline was measured if 25 μM dantrolene was present in the external solution. All of these characteristic changes were reflected in the calculated efflux rate of calcium from the SR. Although 1 μM dantrolene did not cause any measurable alteration in Rrel, higher concentrations gradually suppressed both the early peak and the quasi-steady component of SR calcium release.
As demonstrated in Fig. 3 for 5 μM dantrolene, the above mentioned effects on [Ca2+]i were present at every membrane potential tested. Dantrolene attenuated the calcium transients by suppressing the rate of increase of both the early, fast elevation and the more gradual increase observed after 30 ms into the depolarizing pulse. On average, the maximal increase in 5 μM dantrolene was found to be 0.9 ± 0.2 μM (n = 7) for a 100-ms-long depolarization to 0 mV as compared with 1.8 ± 0.3 μM measured on the same fibers under control conditions. Although 1 μM dantrolene did not attenuate the maximal [Ca2+]i significantly (from 1.5 ± 0.3 to 1.4 ± 0.4 μM, n = 13), 25 μM of the drug caused a 53 ± 8% suppression (from 1.6 ± 0.3 to 0.7 ± 0.1 μM, n = 9). These data were consistent with those obtained on intact fibers (Fig. 1), confirming that fibers retain their full dantrolene sensitivity after mounting them into the double Vaseline-gap system.
Figure 3.
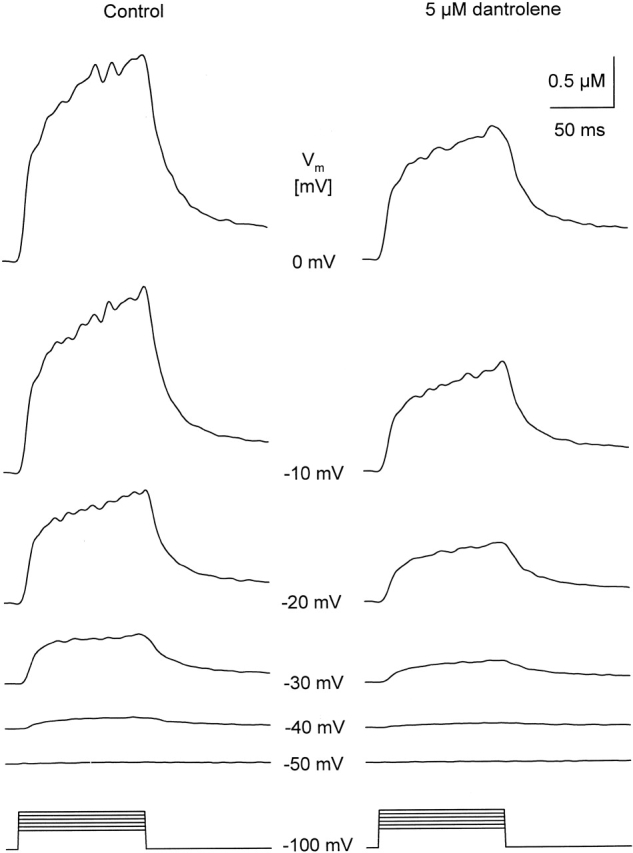
Calcium transients measured in the absence and in the presence of 5 μM dantrolene. The fiber was depolarized from the holding potential to the voltages (Vm) indicated between the traces for 100 ms. Measurements were taken first in the control, and then starting 10 min after the exchange to the dantrolene-containing solution. Note that the drug decreased the amplitude of the calcium signals at every potential tested, but it did not shift the threshold for the appearance of detectable increase in intracellular calcium concentration change. [APIII] = 605–643 μM; [Fura-2] = 28–35 μM.
Fig. 4 goes on to plot the maximal increase in [Ca2+]i in the whole voltage range examined to demonstrate that dantrolene had similar effects at every membrane potential. To ease the comparison between different fibers and drug concentrations, all data from a given fiber were normalized before averaging. For normalization, the maximum of the calcium transient measured under control conditions with a depolarization to 0 mV on the given fiber was used. Furthermore, Fig. 4 displays the results, again normalized, from measurements where dantrolene was removed from the extracellular medium as the last intervention during the experiment. In these fibers, the full [Ca2+]i versus voltage excursion was completed three times, and, as is shown in Fig. 4 A, the fibers remained fairly stable during such interventions. This observation allowed us to conclude that much of the suppression that remained after the washout of dantrolene was due to the fact that the drug was not completely removed from its binding site within the period of 15–20 min necessary to complete the measurements at all voltages. Fig. 4 A also demonstrates that increasing the amplitude of the depolarizing pulse beyond 100 mV, i.e., depolarizing the fibers to +10 or +20 mV, did not significantly increase the magnitude of the calcium transients in control. To restrict the number of depolarizing pulses to a minimum necessary to assess the voltage dependence, pulses beyond 0 mV were not included into the experimental protocol for the other two dantrolene concentrations.
Comparing the traces in Fig. 3 at −40 mV and the data presented for control conditions and in the presence of dantrolene in Fig. 4 (B and C) reveals that the drug did not, significantly, alter the membrane potential at which detectable increase in [Ca2+]i was observed (−51.1 ± 2.5 and −53.3 ± 3.0 mV in control and in the presence of 25 μM dantrolene, respectively, n = 9). These data, taken together, confirm that dantrolene suppresses the depolarization-induced elevation of [Ca2+]i in a generally voltage-independent manner in mammalian skeletal muscle fibers.
SR Permeability Is Attenuated by Dantrolene
To describe the effects of dantrolene on the SR calcium release process, the rate of calcium release was corrected for depletion of calcium in the SR and the obtained transients were normalized to SR contents. This content was found to be fairly stable over the fibers included into the present study (1.8 ± 0.1, 2.0 ± 0.1, and 1.9 ± 0.2 mM for the fibers subjected to 1, 5, and 25 μM dantrolene; n = 13, 7, and 9) and to be unaltered by the treatment with dantrolene. Similarly, dantrolene left the resting [Ca2+]i ([Ca2+]rest) as well as the parameters of the removal model unaltered. As an example, the values of [Ca2+]rest were found to be 109 ± 17 nM in control and 107 ± 12 nM after the addition of 25 μM dantrolene. The corresponding values of the parameters of the removal model, if fitted independently in control and in the presence of the drug, were as follows: koff,M-P = 8.5 ± 2.1 and 5.2 ± 1.6 s−1, PVmax = 3.1 ± 0.5 and 2.5 ± 0.3 μMs−1, koff,C-E = 3.3 ± 0.8 and 3.6 ± 0.8 s−1, and kon,C-E = 2.1 ± 0.4 and 2.0 ± 0.4 μMs−1. Therefore, in the calculations, we used the same values for the removal parameters for control, in the presence of the drug and after washout in most fibers, unless they gave unrealistic Rrel records (e.g., large negative phases after repolarization). In the latter case, separate sets of parameters were used.
Fig. 5 presents SR calcium permeabilities calculated from the calcium transients in Fig. 3. In this case, the same set of removal parameters was used before and after the addition of the drug. Dantrolene caused a marked suppression of both the early and the sustained steady components of SR permeability at all membrane potentials tested. This observation is presented in Fig. 6, where pooled data of the two kinetic components of SR permeability are shown as a function of membrane potential in control in the presence of the drug and after washout. The organization of Fig. 6 follows that of Fig. 4, individual data points were normalized before averaging. To this end, was fitted to the data (peak or steady level versus voltage) in control for each and every fiber and the obtained maximum (, Rrel,i[max]) was used for normalization. Fig. 6 reveals a dose-dependent suppression of both the early peak and the steady component of SR permeability reaching >50% in case of 25 μM dantrolene. It also shows that, as expected from the data on the calcium transients, no significant recovery was achieved after the removal of the drug.
Figure 5.
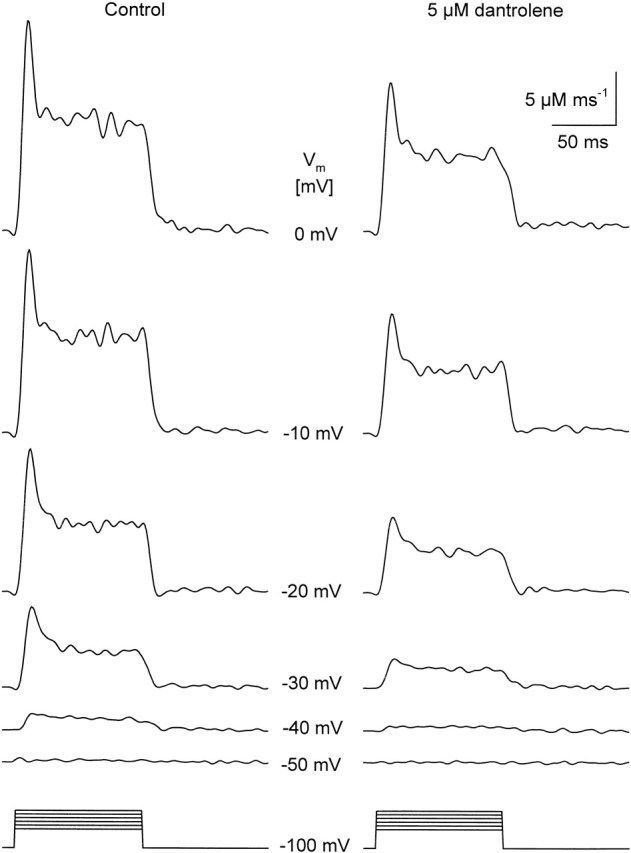
The effects of 5 μM dantrolene on SR calcium permeability. Traces were calculated from the calcium transients presented in Fig. 3 as detailed in materials and methods. Note that dantrolene suppressed both the early peak and the maintained steady level at each and every membrane potential (Vm) tested. The parameters of the removal model were koff,M-P = 8.2 s−1, PVmax = 2.24 mMs−1, kon,Ca-E = 1.4 μM−1s−1, and koff,Ca-E = 1.7 s−1. The estimated SR content for this fiber was 2.5 mM.
To quantify the suppression caused by the different concentrations, was fitted to the data (peak or steady level versus voltage) in the presence and absence of dantrolene and after washout in every fiber. The obtained parameters, Pmax or SLmax (, Rrel,i[max] with i = peak or steady level, respectively), the midpoint voltage, and the slope factor, are presented in Table after averaging. The data reveal that 1 μM dantrolene did not have any measurable effects on the depolarization-induced increase in SR permeability. On the other hand, 5 and 25 μM of the drug significantly (P < 0.02) attenuated both the early peak and the steady level. Although the effect on the steady level was somewhat larger than on the peak, 53 ± 8 vs. 46 ± 6% for 25 μM, this difference did not prove to be statistically significant.
Comparing the voltage dependence of the different components as well as the effect of dantrolene on the voltage dependence did not reveal any systematic difference or trend. The only statistically significant change occurred at 25 μM, where a shift toward more positive membrane potentials was detected in the voltage dependence of the peak. The fact that dantrolene had very little effects on the voltage dependence of either component suggested a voltage-independent block of SR permeability by the drug. This was confirmed by directly calculating the suppression in every fiber in the −40 to 0 mV voltage range and averaging these relative data. The largest and the smallest values were, respectively, 0.63 ± 0.14 (at −40 mV) and 0.45 ± 0.09 (at −30 mV) for the peak and 0.65 ± 0.13 (at 0 mV) and 0.55 ± 0.1 (at −30 mV) for the steady level with 5 μM dantrolene. Corresponding values in 25 μM were 0.59 ± 0.06 (at −40 mV) and 0.43 ± 0.08 (at −20 mV) for the peak and 0.51 ± 0.02 (at −40 mV) and 0.39 ± 0.05 (at 0 mV) for the steady level, none of which was found to be significantly different. These data establish that dantrolene attenuates SR permeability in a voltage-independent manner. The extent of suppression is equal for the two kinetic components, peak and steady level, of SR permeability.
Intracellular Application Does Not Alter the Effective Concentration for Dantrolene
The following series of measurements were designed to test whether or not extracellular application of dantrolene could somehow limit the access of the drug to its putative intracellular binding site, since submicromolar dissociation constants have been reported (150 nM; Fruen et al. 1997) in vesicles. To this end calcium transients were measured in fibers that had been pressure-injected with an “internal-like” solution containing 25 μM dantrolene. Due to the low solubility of the drug in water, this involved the injection of a large volume relative to that of the cell. To ensure that the injection had minimal effects on fiber conditions, the silicone-clamp technique was used, where injections of large volumes are known to cause little, if any, damage.
Under our standard conditions of microinjection, the concentration of the fluorescent dye present in the injected solution is estimated to be diluted by a factor of 5–10, once fully equilibrated within the entire volume of the cell (Csernoch et al. 1998). In these experiments, as dantrolene was present at 25 μM in the injected solution, its final concentration within the fibers was believed to reach 2.5–5 μM. This, of course, is a rough estimation. It assumes that dantrolene was diffusing freely within the fibers, and, furthermore, that intracellular binding (within the insulated part) did not alter its final concentration in the silicone-free portion of the fibers.
Fig. 7 shows the results from a fiber where dantrolene was injected a few minutes before voltage clamping and giving depolarizing pulses. In this fiber, a 20-ms-long pulse to 0 mV was applied every minute. Fig. 7 A shows calcium transients measured ∼13 (trace a), 53 (trace b) and 103 min (trace c) after dantrolene had been injected into the fiber. Each trace is the average of five consecutive calcium transients. Fig. 7 B follows the evolution of the change in the peak of [Ca2+]i over the course of the experiment. No significant suppression of the peak [Ca2+]i occurred under these conditions, only toward the end of the experiment. However, note that the mean value for the maximum of the first calcium transients obtained in these injected fibers was 0.76 ± 0.1 μM (n = 8) as compared with 0.88 ± 0.24 μM in control cells (Fig. 1).
Figure 7.
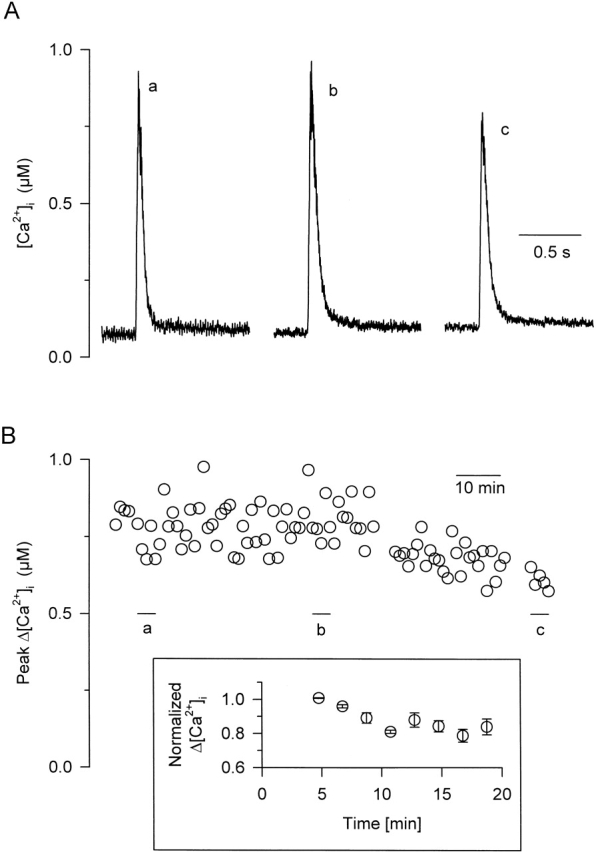
Effect of intracellularly applied dantrolene on calcium transients measured in intact mouse and rat fibers. (A) Indo-1 calcium transients measured in response to a 20-ms-long depolarization to 0 mV at various times after a microinjection of dantrolene into a mouse fiber. Each trace corresponds to the average of five successive transients. (B) Time-dependent evolution of the depolarization-induced peak change in [Ca2+]i after the microinjection of dantrolene into the same fiber. Horizontal bars (labeled a–c) indicate the period used to calculate the transients presented in A. The inset shows pooled data (n = 3) from experiments repeated on fibers isolated from rats to increase the diffusion length. The evolution of the amplitude of the calcium transients is displayed after normalizing to the first calcium transient measured on the corresponding fiber.
To test the possibility that the relatively little, if any, effect of injected dantrolene was due to its fast diffusion, the above experiments were repeated on fibers isolated from rats. In these fibers, the length of diffusion was, on average, twice that in mouse fibers. The inset in Fig. 7 displays the evolution of the relative amplitude of the calcium transients from these experiments. As in Fig. 1 B, the first calcium transient was used for normalization and the normalized values were averaged over the fibers. The injection of dantrolene caused an ∼20% decrease in the amplitude of the calcium transients that was complete within 15 min.
Assuming that the first calcium transient (taken ∼5 min after injection) represents the control situation, these data suggest that intracellularly applied dantrolene has relatively little effect on the micromolar concentration. The magnitude of this effect is comparable to that seen with extracellular application. If, on the other hand, the injected dantrolene exerted the majority of its effects before the first calcium transient was taken, then additional application of external dantrolene should have little, or no, further effect.
Fig. 8 tests this hypothesis by showing results from two other fibers that were injected with dantrolene. In Fig. 8 (A and B), measurements were taken 30 min after dantrolene had been injected. The fiber was repeatedly depolarized using 20-ms-long pulses to 0 mV, and the effect of a brief, transient extracellular application of 25 μM dantrolene was tested. Results show that, under these conditions, extracellular dantrolene still maintained its potency to partially depress the depolarization-induced increase in [Ca2+]i. This conclusion is further strengthened by the results shown in Fig. 8 (C and D), which were obtained from another fiber ∼1 h after dantrolene had been injected. A 30-ms-long pulse to 0 mV was applied every minute. Traces shown in Fig. 8 C correspond to the average of three successive calcium transients measured in control (a), and in the presence of 0.5 (b), 12.5 (c), and 25 μM (d) extracellular dantrolene. In this experiment, the presence of each dantrolene concentration was maintained for 5 min and followed by a 5-min washout period. Under these conditions, dantrolene at 12.5 and 25 μM had an effective, dose-dependent depressing effect on the peak calcium transients. Similar results were obtained on four other fibers with 40 ± 2% and 67 ± 11% suppression at 12.5 and 25 μM dantrolene, respectively. Overall, these results strongly argue against the possibility that dantrolene, when applied intracellularly would suppress calcium transients at a much lower concentration than when applied in the extracellular medium. This implies that the surface membrane does not limit the ability of dantrolene to access its binding site.
Figure 8.
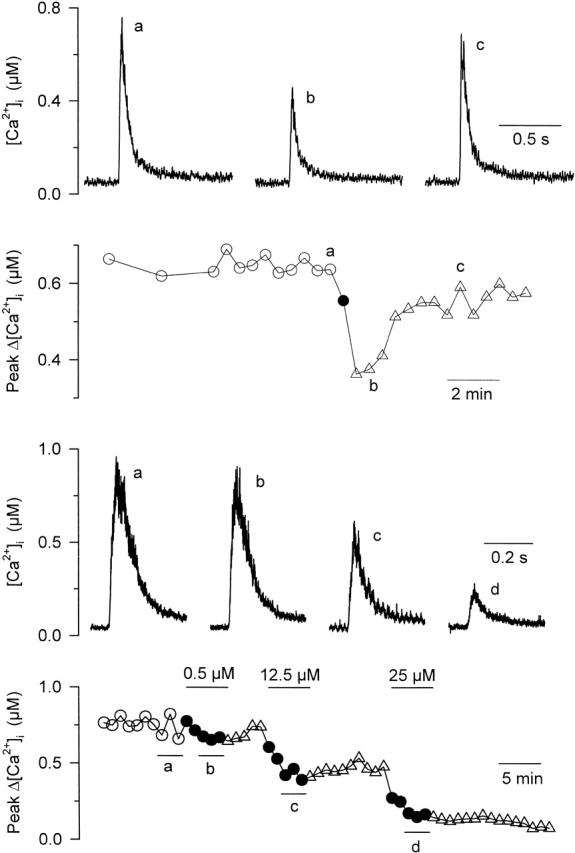
Dantrolene-injected fibers are still susceptible to extracellular dantrolene application. Results shown in A–D were obtained from two distinct fibers that had been injected with dantrolene. (A) Indo-1 calcium transients were elicited by 20-ms-long depolarizations to 0 mV in control (a), and 2 min (b) and 6 min (c) after a brief extracellular application of 25 μM dantrolene. (B) Time-dependent evolution of the peak change in [Ca2+]i before and after the application of dantrolene. (C) Indo-1 calcium transients elicited by a 30-ms duration pulse to 0 mV in control (a), and upon extracellular application of 0.5 (b), 12 (c), and 25 μM (d) dantrolene. (D) Corresponding time-dependent evolution of the peak change in [Ca2+]i during the course of the experiment.
Effects of Dantrolene on the Dihydropyridine Receptor
Results so far suggest an interaction of the drug with a step of excitation-contraction coupling subsequent to the voltage-sensing event. To directly exclude the involvement of the voltage sensor as the putative dantrolene-binding site, its two functional manifestations, intramembrane charge movements and L-type calcium currents, were measured in separate sets of experiments. These experiments also tested the possibility of any “backward” flow of information in the coupling process, i.e., did the suppression of SR calcium release affect the functioning of the DHPRs.
Fig. 9 A presents nonlinear capacitive currents representing intramembrane charge movement in the absence and presence of 25 μM dantrolene. The transients were recorded in response to 100-ms-long depolarizing pulses exploring the −80 to 0–mV voltage range. The actual currents were normalized to linear capacitance to take into account the differences in fiber size, when comparing different fibers, or any possible change in passive electric properties during the experiments. However, the linear capacitance, on average, did not change significantly during the course of these experiments since it was found to be 13.7 ± 0.7 nF (n = 12) under control conditions and 13.7 ± 0.9 nF on the same cells after the addition of dantrolene. Thus, the fibers were stable not only from the aspect of calcium release, but also electrically.
Figure 9.
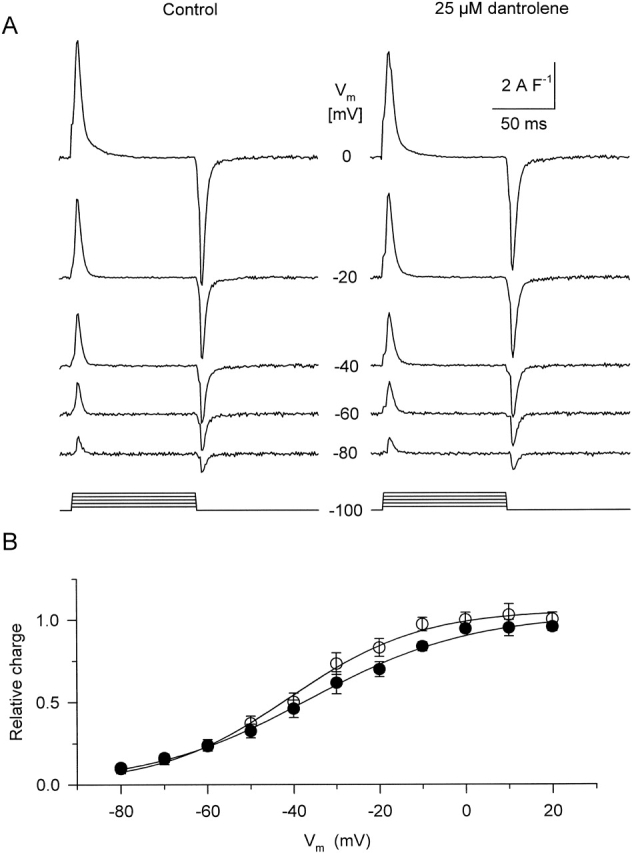
Intramembrane charge movement in the absence and presence of 25 μM dantrolene. (A) Nonlinear capacitive currents representing the movement of intramembrane charge. The currents were obtained in response to 100-ms-long depolarizations to various membrane potentials (Vm). Currents are presented after normalization to fiber capacitance (14.4 and 16.0 nF, control and dantrolene). (B) Voltage dependence of charge transfer. The amount of charge moved was fitted with , and the data are presented as normalized to the fitted maximum and averaged over the fibers (n = 9). Superimposed are the theoretical two-state Boltzmann functions fitted to the averaged data. The parameters of the curves are as follows: V′ = −40.8 and −36.4 mV; and k = 15.3 and 18.9 mV for control and dantrolene, respectively.
To assess the voltage dependence of charge transfer the currents were integrated and was fitted to the charge versus voltage data both before and after the addition of dantrolene. Average values of the parameters from these fits are presented in Table . To visualize the effects of dantrolene on the voltage dependence of charge transfer, Fig. 9 B plots the normalized charge, amount of charge at a given voltage divided by Qmax determined under the given condition in that fiber, as a function of membrane potential during the test pulse. Although there seemed to be a slight shift of the voltage dependence to the right along the voltage axis, by 5 mV, together with a slight decrease in steepness (an increase in parameter k) these changes did not prove to be statistically significant. Similarly, the elevation of the total amount of available charge, from 23.8 ± 2.4 to 27.6 ± 3.9 nCμF−1 on average (n = 10), remained within the limits of statistical variation.
Table 2.
Effect of 25 μM Dantrolene on Intramembrane Charge Movement and on the L-Type Calcium Current
| Charge movement | ICa | ||||||
|---|---|---|---|---|---|---|---|
| Qmax | V′ | k | Gmax | V′ | k | ECa | |
| nCμF−1 | mV | mV | SF−1 | mV | mV | mV | |
| Control | 23.8 ± 2.4 | −38.9 ± 2.3 | 13.1 ± 1.1 | 200 ± 16 | −10.8 ± 1.5 | 7.1 ± 0.5 | 73.2 ± 2.6 |
| Dantrolene | 27.6 ± 3.9 | −33.5 ± 3.3 | 15.8 ± 1.0 | 143 ± 8 | −8.2 ± 1.5 | 6.5 ± 0.7 | 80.1 ± 4.3 |
Parameter values obtained by fitting or to the voltage dependence of intramembrane charge movement or L-type calcium current, respectively.
Another manifestation of DHPR function, the L-type calcium current was measured in a separate set of experiments using 800-ms-long depolarizing pulses and elevated calcium concentration in the external solution while exploring a wider, from −50 to +60 mV, voltage range. Calcium currents were readily observed under these conditions, and they displayed all the usual characteristics in control (Fig. 10 Aa) as described earlier (McDonald et al. 1994). Currents showed a relatively slow activation at negative membrane potentials that became faster with increasing depolarization. After reaching a peak, ∼160 ms into the pulse, currents slowly inactivated with a time constant on the order of 0.2–0.5 s (0.26 ± 0.02 s at 0 mV; Fig. 10 E). After the addition of 25 μM dantrolene, currents became smaller and their inactivation was drastically slowed down (Fig. 10 Ab). To describe the voltage dependence of current activation, the maximal current, averaged over the fibers, was plotted as a function of membrane potential in Fig. 10 C and fitted with . Although the fit revealed a substantial decrease in maximal conductance, from 184 to 111 SF−1, the other parameters describing the voltage dependence remained, essentially, unaltered.
Figure 10.
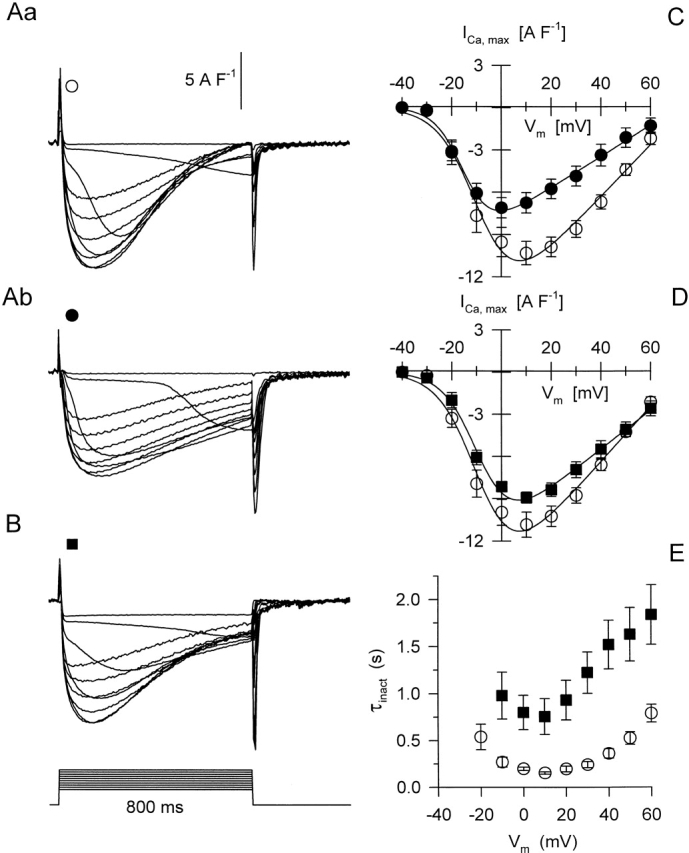
L-type calcium currents in the absence and presence of 25 μM dantrolene. (A) Calcium currents were elicited by 800-ms-long depolarizations exploring the −40 to +60 mV voltage range in 10-mV increments in control (Aa) and after the addition of the drug (Ab). (B) Calcium currents from a fiber where dantrolene was already present in the external solution when the experiment started. All current traces were normalized to the linear capacitance of the given fiber (7.3, 6.2, and 17.6 nF for Aa, Ab, and B, respectively). (C) Current-voltage relationship for the calcium current measured in control and, on the same fibers, after the addition of the drug (n = 8). Superimposed are the theoretical voltage dependencies obtained by fitting to the averaged data points with Gmax = 184 and 111 SF−1; V′ = −8.3 and −14.6 mV; k = 7.7 and 5.5 mV; and ECa = 73.4 and 71.2 mV in control and in the presence of dantrolene. (D) Current-voltage relationship in control conditions and in the presence of dantrolene. In this case, all of those fibers (n = 10) were included with the control where the experiment started with normal external solution, whether or not dantrolene was afterwards applied. Furthermore, only those fibers were averaged under dantrolene (n = 10) where the drug was present from the beginning of the experiment, i.e., no control series were measured beforehand (as in B). Superimposed curves represent the least squares fit of with Gmax = 196 and 138 SF−1; V′ = −8.3 and −8.7 mV; k = 7.7 and 6.9 mV; and ECa = 72.4 and 79.6 mV in control and in the presence of dantrolene. (E) Time constant of current inactivation as a function of membrane voltage. Same fibers as in D are used.
Since the alteration in calcium current seen in the presence of dantrolene resembled that of current run-down (Markwardt and Nilius 1990), in another set of experiments calcium currents were measured on fibers where dantrolene was present from the beginning of the experiment. Fig. 10 B displays such currents, confirming that much of the alteration seen going from Fig. 10 Aa to 10 Ab was due to rundown rather than to the presence of dantrolene itself. Fig. 10 D goes on to plot the current voltage relationship for all fibers, provided that the measurements in the given condition were the first series of depolarizations. Fitting to the data points revealed a slight suppression of maximal conductance and no significant change in any other parameter. This observation was confirmed by fitting the voltage dependence in each fiber and then averaging the obtained parameters (Table ). Although the maximal conductance was reduced from 200 ± 16 to 143 ± 8 SF−1, the change in the other parameters, a slight shift to more positive voltages in V′ and in the equilibrium potential with a small increase in steepness, was found not to be significant.
Since the maximal conductance was indeed suppressed by dantrolene, it was of interest to see whether some of the alteration in inactivation was also due to the presence of the drug. Thus, Fig. 10 E plots the time constants of inactivation, obtained by fitting a single exponential function to the decaying part of the current traces, as a function of the membrane potential. As for Fig. 10 D, only those data were included in the figure where the measurements in the given condition, control or dantrolene, comprised the first series of records. As demonstrated in Fig. 10 E, dantrolene caused a three- to fourfold increase (from 0.26 ± 0.02 to 0.80 ± 0.18 s at 0 mV) in the time constant of inactivation at every membrane potential tested. It is not clear at present, whether the decrease in the conductance L-type calcium channels and in the rate of inactivation is a direct or an indirect effect of dantrolene on the DHPRs. Nevertheless, the data establish that the drug does not affect the voltage sensor of EC coupling in a way that could explain its effects on SR permeability. Therefore, the putative binding site must lay further downstream in the steps of events leading to the opening SR calcium channels.
Dantrolene Inhibits Calcium Efflux from HSR Vesicles
Previous sections have established that 25 μM dantrolene suppresses SR permeability by ∼50% without altering the voltage sensor function of the DHPRs. However, they do not exclude a putative binding site to be located in the surface membrane that might influence the interaction of DHPRs and the RYRs. To exclude this possibility, HSR vesicles were prepared, and the effect of dantrolene was tested on the efflux of calcium.
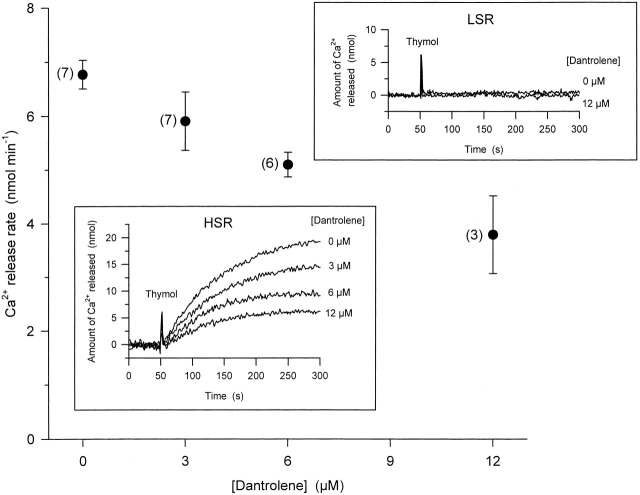
HSR vesicles were actively loaded with calcium in the presence of ATP and, after the ATP was essentially used up, to prevent reuptake during release, calcium efflux was initiated by the addition of 300 μM thymol, a potent activator of RYRs (Palade 1987). As demonstrated in the inset (marked HSR) in Fig. 11, the addition of thymol caused a massive release of calcium, which eventually led to the complete emptying of the vesicles. This effect of thymol was specific to RYR, since the addition of the drug to LSR vesicles did not initiate any calcium release (Fig. 11, inset marked LSR).
Figure 11.
Concentration-dependent effects of dantrolene on thymol-induced calcium release from heavy SR vesicles. Vesicles were actively loaded with calcium and preincubated with dantrolene for 30 min. Calcium release was initiated by the addition of 300 μM thymol. Calcium release was calculated as the maximal rate of rise after the addition of thymol. Data present averages from individual experiments (number of experiments given in parentheses) where the protein content and the amount of calcium loaded into the vesicles were the same. Insets show actual records of calcium release from HSR (bottom) and LSR vesicles (top), monitored as the amount of extravesicular calcium with APIII, from representative experiments at each concentration tested. Transient upward deflections mark the addition of thymol (Thymol). Note that neither thymol nor dantrolene had any effect on LSR vesicles, whereas dantrolene inhibited the thymol-induced calcium release in HSR vesicles.
Since thymol could not be removed from the solution during the course of an experiment, Fig. 11 compares different experiments for the different dantrolene concentrations. However, it should be noted that the amount of protein and the amount of calcium loaded into the HSR vesicles were identical for the different experimental conditions. The latter was ensured by the addition of known and set amounts of calcium into the extravesicular space, and by continuously monitoring its uptake into the vesicles. The amounts of calcium released, therefore, can be compared directly.
The presence of dantrolene in the extravesicular medium substantially reduced the rate of calcium efflux from HSR vesicles. The attenuation of the maximal release rate reached 44 ± 11% at 12 μM, a value that is comparable with that obtained from intact fibers for the suppression of SR permeability (Table ), suggesting that the putative dantrolene-binding site was fully preserved during the isolation of the HSR vesicles. These data clearly place the putative binding site to be on a SR-associated molecule.
Dantrolene Does Not Influence the Isolated RYR
Previous experiments (Suarez-Isla et al. 1986; Nelson et al. 1996) used SR vesicles incorporated into planar lipid bilayers to explore the effects of dantrolene on RYR function. However, those measurements do not directly confirm whether the binding site for dantrolene is on the RYR itself, or it is on a closely associated protein. Therefore, we purified the calcium release channel and used the purified protein for bilayer studies.
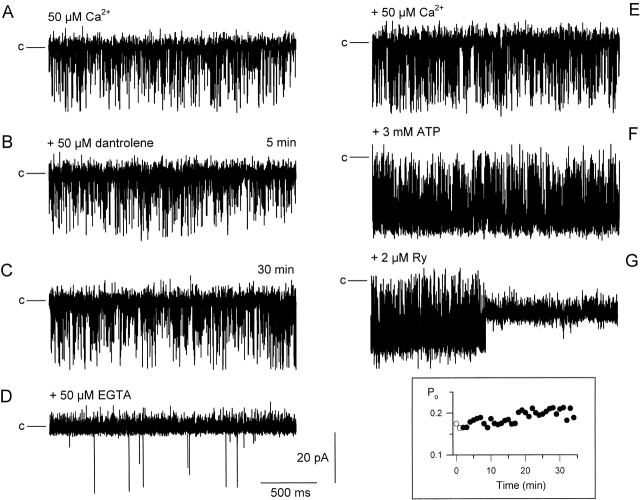
Fig. 12 demonstrates the effects of high (50 μM) dantrolene concentration on the gating of the RYR. The traces are representative (2.2-s long) segments of current records from an experiment that lasted for more than an hour. The incorporation was initiated under control conditions with 50 μM [Ca2+] on either side of the bilayer. Under these conditions (Fig. 12 A), the channel displayed an open probability (Po) of 0.175 and a conductance of 555 pS, as expected from earlier studies. However, in contrast to previous results using SR vesicles, the addition of 50 μM dantrolene into the cis chamber influenced neither the open probability nor the conductance of the purified channel. This is shown in Fig. 12B and Fig. C, at 5 and 30 min after the addition of the drug, respectively. The inset of Fig. 12 follows the evolution of the open probability of the channel during the 30-min after the addition of the drug, demonstrating that dantrolene did not have any transient or long-term effects on channel gating.
Figure 12.
The effect of 50 μM dantrolene on the gating properties and pharmacology of the purified RYR. Incorporation was initiated in symmetric 250 mM KCl with 50 μM calcium concentration in the cis chamber. Holding potential was −40.3 mV, channel openings are downward deflections. Horizontal lines before each current record mark the closed state. (A) Single-channel recording in control conditions. (B and C) Representative segments of single-channel behavior taken 5 and 30 min after the additions of dantrolene. (D–G) Single-channel currents measured after the successive additions of EGTA (D), calcium (E), 3 mM ATP (F) and, finally, RYR (G) into the cis chamber. Inset shows the evolution of the Po starting 2 min before the addition of dantrolene (open symbols) ending with the record shown in C. All recordings are from a single experiment.
Fig. 12 goes on to demonstrate that the drug did not interfere with the ability of well-known regulators to modulate channel function. First, the concentration of calcium was reduced in the cis chamber (Fig. 12 D), which resulted in a drastic reduction in Po (to 0.001). This was followed by the readministration of calcium (Fig. 12 E), and then the addition of 3 mM ATP (Fig. 12 F) into the cis chamber. This first restored the Po to its starting value and then resulted, as expected, in an activation of the channel (Po = 0.874). Finally, the addition of ryanodine caused the characteristic transition to a half-conducting state.
Since previous experiments suggested a high affinity, activatory binding site for dantrolene (Nelson et al. 1996), again using SR vesicles for incorporation, another set of experiments were used to test if the purified RYR would be influenced by 1 nM dantrolene. The drug in this low concentration neither altered the gating nor the pharmacology (activation by calcium, ATP, and caffeine, and inhibition by ruthenium red) of the purified channel (unpublished data).
These data establish that dantrolene has no effect, either transiently or permanently, on the gating behavior of the purified calcium release channel incorporated into planar lipid bilayers. The fact that the pharmacology of the channel is retained renders the possibility of major modification during the purification, unlikely. Taken together, these data strongly argue against the possibility of the binding site for dantrolene being on the purified ryanodine receptor itself.
DISCUSSION
For the first time, these experiments give a complete account of the effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. They explored the voltage sensing function of the DHPRs by measuring both intramembrane charge movements and L-type calcium currents. They also assessed the functioning of the RYRs by comparing calcium release flux from the SR in intact fibers to calcium efflux from HSR vesicles and to alterations in single-channel properties of the calcium release channel. Dantrolene was found not to interfere with the voltage-sensing event, but was found to suppress SR calcium permeability and calcium efflux to a similar extent. On the other hand, the drug failed to alter the single-channel properties and the basic pharmacology of the isolated RYR. These data place the putative binding site to be on a molecule associated with the SR membrane, but exclude the purified calcium release channel itself.
Calcium Release from the SR
Dantrolene has been in use for treating MH for more than three decades (Hainaut and Desmedt 1974). Very early it has been described that the drug suppresses twitch force (Flewellen et al. 1983) without affecting the contractile proteins or the SR calcium pump (Brocklehurst 1975). The latter were also confirmed in these experiments since the drug did not influence the uptake of the SR vesicles (unpublished data) and it did not have any nonspecific membrane effects (Fig. 11). In the late 70s and early 80s, several groups (Desmedt and Hainaut 1977; Kovács et al. 1983) demonstrated the suppression of calcium signals by dantrolene. These measurements used invertebrate striated muscle fibers (barnacle and frog, respectively) and the observation, although hypothesized, has not been confirmed on mammalian preparations. Due to the limitations of techniques present at that time, a quantitative analysis of the suppression of the depolarization-induced increase in [Ca2+]i as a function of drug concentration could not be performed. Here, we demonstrate that the calcium transients are attenuated by 53 ± 8% in the presence of 25 μM dantrolene, whereas 1 μM is without any effect. However, it should be stressed that the suppression at 25 μM was most likely far below the possible saturation of the effect of the drug, but its insolubility in the aqueous environment prevented the application of higher concentrations. Nevertheless, the attenuation presented in these experiments should provide a useful reference to the actual effect seen with in vivo applications.
Due to the lack of data on calcium transients from mammalian skeletal muscle fibers in the presence of dantrolene, no account on the effects of the drug on SR calcium release has been published so far. Not only is this true for mammalian skeletal muscle but, to our knowledge, also for striated muscle cells from other classes of vertebrates or invertebrates. These experiments would be of special interest since lower vertebrates have been shown to express two isoforms of RYR in their skeletal muscle (Olivares et al. 1991), and dantrolene has been reported to affect cardiac cells less than striated muscle fibers (Van Winkle 1976; Chamberlain et al. 1984; Fruen et al. 1997). Furthermore, cardiac glycosides, specific for cardiac over the skeletal isoform of RYR, have been reported to influence the two kinetic components of SR calcium release differently in frog skeletal muscle (Sárközi et al. 1996).
In mammalian fibers predominantly expressing the skeletal isoform of RYR, we haven't found any significant difference between the effects on the two kinetic components, peak and steady level, of SR permeability (Table ). This observation is similar to that reported for low (10 μM) concentrations of tetracaine on the same preparation (Csernoch et al. 1999b). These findings suggest a close and tight coupling between these two components, and favor the hypothesis that they both originate from the skeletal isoform of RYR. Considering that caffeine antagonizes the effects of dantrolene (Ohta et al. 1990), the augmentation of both the peak and the steady level of SR permeability by caffeine (Csernoch et al. 1999a) also strengthens the conclusion above.
Location of the Putative Dantrolene-binding Site
There is a detailed description in the literature of reduced [3H]ryanodine binding to and reduced calcium efflux from SR vesicles in the presence of dantrolene or its water-soluble analogue azumolene (Francis 1978; Tian et al. 1991; Fruen et al. 1997). On the other hand, some reports failed to demonstrate any modification in [3H]ryanodine binding to HSR vesicles by dantrolene or azumolene (Palnitkar et al. 1997). The extent of the suppression of calcium efflux reported here, 44% for 12 μM dantrolene at room temperature (Fig. 11), is consistent with, or slightly smaller than, earlier data that showed a 70% reduction of [3H]ryanodine binding at 36°C (Fruen et al. 1997). The fact that it is similar to the value reported for the suppression of SR permeability (40–50%, Table ), is of greater importance. This clearly suggests that all of the suppression seen on intact cells—under the conditions and the microscopic arrangement of EC-coupling proteins close to, or most likely identical with, that in vivo—was due to an effect mediated through an SR-bound protein. This is substantiated by the finding that dantrolene, if applied intracellularly, did not have significantly higher affinity for its putative binding site.
Whether the putative dantrolene-binding site could be equated with the RYR itself, or not, has been a long controversy in the literature. Recent biochemical data link the binding site of [3H]azidodantrolene to a 160- or a 172-kD protein (Palnitkar et al. 1999) that is likely to be the NH2-terminal portion of the skeletal type RYR isoform (Paul-Pletzer et al. 2001). Single-channel data from purified calcium release channels that could resolve the controversy, were not available, to our knowledge, in the literature. Previous studies used either SR membrane suspensions (Suarez-Isla et al. 1986) or HSR vesicles incorporated into lipid bilayers (Nelson et al. 1996) for studying the effect of dantrolene. In either case, associated SR membrane proteins were present in the preparation, and both groups described a suppression of channel activity in the presence of micromolar dantrolene. Here, we incorporated the purified receptor into planar lipid bilayers and were unable to detect any change in channel activity or pharmacology in the presence of 50 μM dantrolene (Fig. 12). In contrast to the measurement of Nelson and co-workers (Nelson et al. 1996), 1 nM dantrolene failed to enhance the activity or pharmacology of the purified RYR (unpublished data). These observations either exclude the possibility of the RYR itself being the molecular target for dantrolene or establish that, although the channel retains other regulatory sites during isolation, it loses the binding site for dantrolene.
Effects of Dantrolene of DHPR Function
Previous studies on the effect of dantrolene on intramembrane charge movements in frog skeletal muscle have revealed a slight reduction in total charge (Hui 1983) together with a shift of the voltage dependence toward more positive membrane potentials (Hui 1983; Jong et al. 1997). The reduction in Qmax (20%) was attributed to the loss of Qγ, the delayed component of intramembrane charge (Hui 1983; Jong et al. 1997). Under our experimental conditions, the total amount of charge was not altered significantly, similarly to other estimations on frogs (Morgan and Bryant 1977). On the other hand, since delayed components are not pronounced on the nonlinear capacitive currents recorded from mammalian skeletal muscle fibers, the lack of any reduction in Qmax might simply reflect the difference in species. The changes introduced by dantrolene in the voltage dependence in frog muscle fibers, as a trend, were observed in these experiments too (Table ). Although the changes did not prove to be significant, the voltage dependence was slightly shifted to the right along the voltage axis while its steepness was reduced. Since the suppression of calcium release influences intramembrane charge movement (Pizarro et al. 1991; Jong et al. 1995), the data presented here are consistent with a model where the action of dantrolene on intramembrane charge was the consequence of its effect on SR calcium release.
It has been reported that dantrolene does not influence L-type calcium currents in frog twitch muscle fibers (Cota and Stefani 1984). Similar studies, to our knowledge, are not available for mammalian fibers. Here, we found no alteration in the voltage dependence of current activation, which is in line with the data of Cota and Stefani 1984. However, both the maximal conductance and the rate of inactivation were reduced. In addition, the time constant for inactivation followed a “U-shaped” voltage dependence, both in control and after the addition of the drug. There is no clear explanation, at present, for these discrepancies, although species differences could underlie the different results.
To explain the observed voltage dependence and dantrolene action on current inactivation three possibilities should be considered: (1) the effect of reduced calcium current or SR calcium release; (2) direct action of the drug on the DHPR; and (3) retrograde effect from the putative intracellular dantrolene-binding site. The “U-shaped” voltage dependence of the time constant strongly argues in favor of a current and/or calcium-dependent inactivation, which could be due to either of the following: (1) the depletion of calcium in the restricted space of the t-tubular system; (2) a direct effect of the calcium ions passing through the L-type calcium channel itself; or (3) calcium ions released from the SR. Plotting the time constant as a function of the peak current in the control (unpublished data) gave a linear function, further strengthening the above idea. However, a similar plot in the presence of dantrolene gave a straight line with a different slope, indicating that the shift seen in the presence of the drug could not be due, simply, to a reduction in current.
SR calcium release, known to be important in the heart, seems unlikely to be the cause of current inactivation under our experimental conditions. The presence of 20 mM EGTA in the internal solution should have reduced the change in [Ca2+]i even in the relatively restricted compartment of the triadic space (see calculations in Pape et al. 1998). Furthermore, to test for SR calcium release as a major contributor to inactivation under these experimental conditions, we measured calcium currents in the presence of thymol and found no significant decrease in the time constant in spite of the increased release (unpublished data). Similarly, the slight, although significant, decrease in calcium permeability (Table ) can hardly underlie such dramatic change in t-tubular calcium concentration that could explain the action of dantrolene.
It should be stressed that the properties mentioned above do not, inevitably, rule out the current and/or calcium dependence of inactivation. Nevertheless, they suggest that other possibilities, such as voltage dependence or an interaction between dantrolene or its binding site with the DHPR, should be considered. In this framework, the drug does not influence the normal voltage sensing machinery for activation (compare intramembrane charge movements in the absence and presence of the drug in Fig. 9), but interferes with (slows the transition of) those responsible for inactivation.
This brings up the possibility that a retrograde effect from the putative intracellular dantrolene-binding site or from the altered gating of RYR would be responsible for the observed effects. The latter is supported by data obtained on dyspedic myotubes from mice, where L-type calcium currents were reduced and the gating kinetics of the calcium channels was altered (Avila and Dirksen 2000). Thus, dantrolene could, through affecting the RYRs, alter the properties of L-type calcium currents in mammalian skeletal muscle. Taken together, although the data in control would suggest a strong current and/or calcium dependence of inactivation, the measurements in the presence of dantrolene argue in favor of a retrograde effect from the SR.
Acknowledgments
The authors wish to thank Ms. R. Öri, T. Kiss-Tóth, and B. Lukács for skilful technical assistance.
This work was supported by research grants from Hungary (OTKA 030246, 034894, ETT 49/2000, FKFP 0193/2001, and AKP 98-75 3,2/44) and the European Community (CT96-0032). P. Szentesi holds a Bolyai fellowship.
Footnotes
Abbreviations used in this paper: APIII, antipyrylazo III; DHPR, dihydropyridine receptor; MH, malignant hyperthermia; Po, open probability; Rrel, rate of calcium release from the SR; t-tubular, transverse tubular.
References
- Avila G., Dirksen R.T. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J. Gen. Physiol. 2000;115:467–480. doi: 10.1085/jgp.115.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst L. Dantrolene sodium and “skinned” muscle fibers Nature. 254 1975. 364A(Abstr.) [DOI] [PubMed] [Google Scholar]
- Chamberlain B.K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J. Biol. Chem. 1984;259:7547–7553. [PubMed] [Google Scholar]
- Collet C., Allard B., Tourneur Y., Jacquemond V. Intracellular calcium signals measured with indo-1 in isolated skeletal muscle fibers from control and mdx mice. J. Physiol. 1999;520:417–429. doi: 10.1111/j.1469-7793.1999.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G., Stefani E. Saturation of calcium channels and surface charge effects in skeletal muscle fibers of the frog. J. Physiol. 1984;351:135–154. doi: 10.1113/jphysiol.1984.sp015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Jacquemond V., Schneider M.F. Microinjection of strong calcium buffers suppresses the peak of calcium release in frog skeletal muscle fibers. J. Gen. Physiol. 1993;101:297–333. doi: 10.1085/jgp.101.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Bernengo J.C., Szentesi P., Jacquemond V. Measurements of intracellular Mg2+ concentration in mouse skeletal muscle fibers with the fluorescent indicator mag-indo-1. Biophys. J. 1998;75:957–967. doi: 10.1016/S0006-3495(98)77584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Szentesi P., Kovács L. Differential effects of caffeine and perchlorate on excitation-contraction coupling in mammalian skeletal muscle J. Physiol. 520 1999. 217 230a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Szentesi P., Sárközi S., Szegedi C., Jona I. Effects of tetracaine on sarcoplasmic calcium release in mammalian skeletal muscle fibers J. Physiol. 515 1999. 843 857b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J.E., Hainaut K. Inhibition of the intracellular release of calcium by dantrolene in barnacle giant muscle fibers. J. Physiol. 1977;265:565–585. doi: 10.1113/jphysiol.1977.sp011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewellen E.H., Nelson P.E., Jones W.P., Arens J.F., Wagner D.L. Dantrolene dose response in awake manimplications for management of malignant hyperthermia. Anesthesiology. 1983;59:275–280. doi: 10.1097/00000542-198310000-00002. [DOI] [PubMed] [Google Scholar]
- Flucher B.E., Conti A., Takeshima H., Sorrentino V. Type 3 and type 1 ryanodine receptors are localized in triads of the same mammalian skeletal muscle fibers. J. Cell Biol. 1999;146:621–630. doi: 10.1083/jcb.146.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis K.T. The effect of dantrolene sodium on the efflux of Ca45 from rat heavy sarcoplasmic reticulum. Res. Commun. Chem. Pathol. Pharmacol. 1978;21:573–576. [PubMed] [Google Scholar]
- Fruen B.R., Mickelson J.R., Louis C.F. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J. Biol. Chem. 1997;272:26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- Hainaut K., Desmedt J.E. Effect of dantrolene sodium on calcium movements in single muscle fibers. Nature. 1974;252:728–730. doi: 10.1038/252728a0. [DOI] [PubMed] [Google Scholar]
- Hui C.S. Pharmacological studies of charge movement in frog skeletal muscle. J. Physiol. 1983;337:509–529. doi: 10.1113/jphysiol.1983.sp014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V. Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibers. Biophys. J. 1997;73:920–928. doi: 10.1016/S0006-3495(97)78124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong D., Pape P.C., Chandler W.K. Effects of sarcoplasmic reticulum depletion on intramembranous charge movement in frog cut muscle fibers. J. Gen. Physiol. 1995;106:659–704. doi: 10.1085/jgp.106.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong D., Stroffekova K., Heiny J.A. A surface potential change in the membranes of frog skeletal muscle is associated with excitation-contraction coupling. J. Physiol. 1997;499:787–808. doi: 10.1113/jphysiol.1997.sp021969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M.G., Simon B.J., Szücs G., Schneider M.F. Simultaneous recording of calcium transients in skeletal muscle using high and low affinity calcium indicators. Biophys. J. 1988;55:971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M.F. Measurement and modification of free calcium transients in frog skeletal muscle fibers by a metallochromic indicator dye. J. Physiol. 1983;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt F., Nilius B. Changes of calcium channel inactivation during run-down. Gen. Physiol. Biophys. 1990;9:209–218. [PubMed] [Google Scholar]
- McDonald T.F., Pelzer S., Trautwein W., Pelzer D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Melzer W., Ríos E., Schneider M.F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J. Physiol. 1986;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K.G., Bryant S.H. The mechanism of action of dantrolene sodium. J. Pharmacol. Exp. Ther. 1977;201:138–147. [PubMed] [Google Scholar]
- Nelson T.E., Lin M., Zapata-Sudo G., Sudo R.T. Dantrolene sodium can increase or attenuate activity of skeletal muscle ryanodine receptor calcium release channel. Anesthesiology. 1996;84:1368–1379. doi: 10.1097/00000542-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Nelson T.J., Zhao W.Q., Yuan S., Favit A., Pozzo-Miller L., Alkon D.L. Calcexcitin interaction with neuronal ryanodine-receptors. Biochem. J. 1999;341:423–433. doi: 10.1042/0264-6021:3410423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Ito S., Ohga A. Inhibitory action of dantrolene on Ca-induced Ca2+ release from sarcoplasmic reticulum in guinea pig skeletal muscle. Eur. J. Pharmacol. 1990;178:11–19. doi: 10.1016/0014-2999(90)94788-y. [DOI] [PubMed] [Google Scholar]
- Olivares E.B., Tanksley S.J., Airey J.A., Beck C., Ouyang Y., Deerinck T.J., Ellisman M.H., Sutko J.L. Nonmammalian vertebrate skeletal muscles express two triad junctional foot protein isoforms. Biophys. J. 1991;59:1153–1163. doi: 10.1016/S0006-3495(91)82331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. I. Use of pyrophosphate to study caffeine-induced Ca2+ release. J. Biol. Chem. 1987;262:6135–6141. [PubMed] [Google Scholar]
- Palnitkar S.S., Mickelson J.R., Louis C.F., Parness J. Pharmacological distinction between dantrolene and ryanodine binding sitesevidence from normal and malignant hyperthermia-susceptible porcine skeletal muscle. Biochem. J. 1997;326:847–852. doi: 10.1042/bj3260847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palnitkar S.S., Bin B., Jimenez L.S., Morimoto H., Williams P.G., Paul-Pletzer K., Parness J. [3H]Azidodantrolenesynthesis and use in identification of a putative skeletal muscle dantrolene binding site in sarcoplasmic reticulum. J. Med. Chem. 1999;42:1872–1880. doi: 10.1021/jm9805079. [DOI] [PubMed] [Google Scholar]
- Pape P.C., Jong D.S., Chandler W.K. Effects of partial sarcoplasmic reticulum calcium depletion on calcium release in frog cut muscle fibers equilibrated with 20 mM EGTA. J. Gen. Physiol. 1998;112:259–262. doi: 10.1085/jgp.112.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parness J., Palnitkar S.S. Identification of dantrolene binding sites in porcine skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1995;270:18465–18472. doi: 10.1074/jbc.270.31.18465. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K., Palnitkar S.S., Jimenez L.S., Morimoto H., Parness J. The skeletal muscle ryanodine receptor identified as a molecular target of [3H]azidodantrolene by photoaffinity labeling. Biochemistry. 2001;40:531–542. doi: 10.1021/bi001502s. [DOI] [PubMed] [Google Scholar]
- Pessah I.N., Lynch C., Gronert G.A. Complex pharmacology of malignant hyperthermia. Anesthesiology. 1996;84:1275–1279. doi: 10.1097/00000542-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Pizarro G., Csernoch L., Uribe I., Rodriguez M., Ríos E. The relationship between Qγ and Ca release from the sarcoplasmic reticulum in skeletal muscle. J. Gen. Physiol. 1991;97:913–947. doi: 10.1085/jgp.97.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sárközi S., Szentesi P., Jona I., Csernoch L. Effects of cardiac glycosides on excitation-contraction coupling in frog skeletal muscle fibers. J. Physiol. 1996;495:611–626. doi: 10.1113/jphysiol.1996.sp021620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Isla B.A., Orozco C., Heller P.F., Froehlich J.P. Single calcium channels in native sarcoplasmic reticulum membranes from skeletal muscle. Proc. Natl. Acad. Sci. 1986;83:7741–7745. doi: 10.1073/pnas.83.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi C., Sárközi S., Herzog A., Jona I., Varsanyi M. Calsequestrinmore than “only” a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 1999;337:19–22. [PMC free article] [PubMed] [Google Scholar]
- Szentesi P., Jacquemond V., Kovács L., Csernoch L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibers. J. Physiol. 1997;505:371–384. doi: 10.1111/j.1469-7793.1997.371bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Katz A.M., Kim D.H. Effects of azumolene on doxorubicin-induced Ca2+ release from skeletal and cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1991;1094:27–34. doi: 10.1016/0167-4889(91)90022-p. [DOI] [PubMed] [Google Scholar]
- Tripathy A., Meissner G. Sarcoplasmic reticulum luminal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle W.B. Calcium release from skeletal muscle sarcoplasmic reticulumsite of action of dantrolene sodium. Science. 1976;193:1130–1131. doi: 10.1126/science.959824. [DOI] [PubMed] [Google Scholar]