Abstract
In the Kv2.1 potassium channel, binding of K+ to a high-affinity site associated with the selectivity filter modulates channel sensitivity to external TEA. In channels carrying Na+ current, K+ interacts with the TEA modulation site at concentrations ≤30 μM. In this paper, we further characterized the TEA modulation site and examined how varying K+ occupancy of the pore influenced the interaction of K+ with this site. In the presence of high internal and external [K+], TEA blocked 100% of current with an IC50 of 1.9 ± 0.2 mM. In the absence of a substitute permeating ion, such as Na+, reducing access of K+ to the pore resulted in a reduction of TEA efficacy, but produced little or no change in TEA potency (under conditions in which maximal block by TEA was just 32%, the IC50 for block was 2.0 ± 0.6 mM). The all-or-none nature of TEA block (channels were either completely sensitive or completely insensitive), indicated that one selectivity filter binding site must be occupied for TEA sensitivity, and that one selectivity filter binding site is not involved in modulating TEA sensitivity. At three different levels of K+ occupancy, achieved by manipulating access of internal K+ to the pore, elevation of external [K+] shifted channels from a TEA-insensitive to -sensitive state with an EC50 of ∼10 mM. Combined with previous results, these data demonstrate that the TEA modulation site has a high affinity for K+ when only one K+ is in the pore and a low affinity for K+ when the pore is already occupied by K+. These results indicate that ion–ion interactions occur at the selectivity filter. These results also suggest that the selectivity filter is the site of at least one low affinity modulatory effect of external K+, and that the selectivity filter K+ binding sites are not functionally interchangeable.
Keywords: permeation, outer vestibule, conformation, repulsion
INTRODUCTION
Voltage-gated K+ channels are multi-ion pores, and, while conducting, can be occupied by three to four K+ (Hodgkin and Keynes 1955; Neyton and Miller 1988). The recently resolved crystal structure of the K+ channel from Streptomyces lividans (KcsA) indicated that this bacterial channel contained two high affinity cation binding sites associated with the selectivity filter and another, lower affinity cation binding site in a water-filled cavity internal to the selectivity filter (Doyle et al. 1998; Roux and MacKinnon 1999). It is widely presumed that the pore structure of KcsA represents that of the larger voltage-gated K+ channels. The KcsA structure is remarkably consistent with a repulsion model of multi-ion permeation proposed for both K+ and Ca2+ channels (Neyton and Miller 1988; Kuo and Hess 1993). In this model, either of the two selectivity filter sites can bind K+ with high affinity if just a single cation is interacting with the selectivity filter. This high affinity binding confers ionic selectivity on Ca2+ channels (Almers and McCleskey 1984; Hess and Tsien 1984) and some K+ channels (Korn and Ikeda 1995). This model proposes that binding of a second K+ to the singly occupied selectivity filter would result in a low affinity interaction of both ions with the selectivity filter. This lowering of affinity in multiply occupied pores allows for high ionic throughput. An alternative model of multi-ion permeation, which includes just a single high affinity site flanked by lower affinity sites, can also account for several fundamental permeation characteristics of both Ca2+ and K+ channels (Dang and McCleskey 1998; Kiss et al. 1998). Whereas this model did not exclude the possibility of ion–ion interactions, it suggested that high affinity, high throughput multi-ion permeation could be achieved in a pore that contained only one truly high affinity cation binding site. An array of functional data demonstrate a low affinity interaction of external K+ with K+-conducting pores (Lopez-Barneo et al. 1993; Levy and Deutsch 1996; Baukrowitz and Yellen 1996; Kiss et al. 1998; Kiss and Korn 1998). These data are equally consistent with both of these permeation models and also with the possible existence of a low affinity binding site external to the selectivity filter binding sites. Understanding the nature of this low affinity K+ binding site requires the ability to examine the interaction of K+ with this functionally identified site under different conditions of K+ occupancy of the selectivity filter.
In the cloned potassium channel, Kv2.1, block by tetraethylammonium is cation dependent (Ikeda and Korn 1995; Immke et al. 1999). In the absence of K+ (with Na+ as the current carrier), TEA does not block Kv2.1. TEA sensitivity was reinstated at [K+] as low as 30–100 μM, and increased over the same [K+] range as that required to block Na+ current through the channel. This suggested that occupancy of a high affinity, selectivity filter binding site by K+ was involved in modulation of TEA sensitivity (Immke et al. 1999). This hypothesis was supported by independent experiments that suggested that the cation binding site associated with modulation of TEA sensitivity was located between the internal and external TEA binding sites (Immke et al. 1999), which is the location of the selectivity filter (Heginbotham et al. 1994; Doyle et al. 1998). TEA sensitivity also varied as a function of [K+] in channels conducting K+ in the outward direction (Immke et al. 1999). However, in channels carrying outward K+ current, the site responsible for modulating TEA sensitivity appeared to have a relatively low affinity. These results presented us with an opportunity to examine the effects of K+ occupancy on cation binding to a functionally identified binding site (the TEA modulation site) associated with the selectivity filter.
In Kv2.1, reduction of TEA sensitivity upon reduction of [K+] results primarily from a conformational change in the outer vestibule of the conduction pathway (Immke et al. 1999). The reduced sensitivity associated with this conformational change does not appear to reflect primarily an alteration of the TEA binding site. Rather, it appears that a lysine residue located at the outer edge of the external vestibule (K356) moves within the permeation pathway and interferes with the ability of TEA to bind to the channel. Under the conditions of previous experiments (Immke et al. 1999), a mechanistic distinction between a change in TEA potency and efficacy could not be made in K+-conducting channels. This distinction is critical for studying the relevance of different intrapore binding sites. Graded changes in TEA potency could result from complicated combinations of occupancy states of the channel. In contrast, a change in efficacy would require that occupancy of a given site would either confer TEA sensitivity or not.
In this paper, we demonstrate that changes in K+ occupancy of the pore modulate TEA efficacy in Kv2.1 channels carrying K+ current. This observation allowed us to address the following questions: (a) what is the nature of the K+ binding site responsible for conferring TEA sensitivity? and (b) how does occupancy of the selectivity filter by different cations influence the affinity of the TEA modulation site for K+? Our data indicate that TEA sensitivity is modulated by occupancy of just one of the two K+ binding sites associated with the selectivity filter. Whereas the TEA modulation site binds K+ with relatively high affinity when only one K+ occupies the selectivity filter, it binds K+ with much lower affinity when a K+ ion also occupies the other selectivity filter site. Consequently, our data indicate that ion–ion interactions occur at the selectivity filter, and that the selectivity filter is the site of at least one low affinity interaction of external K+ with the pore.
METHODS
Molecular Biology and Channel Expression
Experiments were done on two channels: wild-type Kv2.1 and a mutant channel derived from Kv2.1, Kv2.1 K356G, K382V. Mutagenesis and expression details are described in Immke et al. 1999. In brief, K+ channel cDNA was subcloned into the pcDNA3 expression vector and channels expressed in the human embryonic kidney cell line, HEK293 (American Type Culture Collection). Cells were maintained in DMEM plus 10% fetal bovine serum (GIBCO BRL) with 1% penicillin/streptomycin (maintenance media). Cells (2 − 106 cells / ml) were cotransfected by electroporation (71 μF, 375 V, Electroporator II; Invitrogen Corp.) with K+ channel expression plasmid (0.5–15 μg/0.2 ml) and CD8 antigen (1 μg/0.2 ml). After electroporation, cells were plated on protamine (1 mg/ml; Sigma Chemical Co.)-coated glass cover slips submerged in maintenance media. Electrophysiological recordings were made 18–48 h later. On the day of recording, cells were washed with fresh media and incubated with Dynabeads M450 conjugated with antibody to CD8 (1 μl/ml; Dynal). Cells that expressed CD8 became coated with beads, which allowed visualization of transfected cells (Jurman et al. 1994).
Electrophysiology
Currents were recorded at room temperature in the whole cell patch clamp configuration. Patch pipets were fabricated from N51A glass (Garner Glass Co.), coated with Sylgard and firepolished. Currents were collected with an Axopatch 1D amplifier, pClamp 6 software, and a Digidata 1200 A/D board (Axon Instruments). Currents were filtered at 2 kHz and sampled at 200–1,000 μs/pt. Series resistance ranged from 0.5 to 2.5 MΩ and was compensated 80–90%. The holding potential was −80 mV, and depolarizing stimuli were presented once every 5–15 s, depending on the experiment. In all experiments except Fig. 4 B, currents were recorded at 0 mV. Fig. 4 B experiments recorded currents at +20 mV. TEA inhibited currents identically at 0 and +20 mV (data not shown). Concentration–response curves were fit to ,
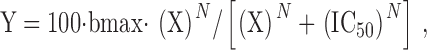 |
1 |
where X is the drug concentration, IC50 is the drug concentration that produced half maximal inhibition, bmax is the maximal level of block at saturation, and N is the slope of the curve. Data were analyzed with Clampfit (Axon Instruments); curve fitting and significance testing (unpaired Student's t test) were done with SigmaPlot 2.0. All plotted data are represented as mean ± SEM, with the number of data points denoted by n. For IC50 values, a range of values is given for n. This range represents the number of cells used for each data point in the complete concentration–response curve from which the IC50 was calculated.
Figure 4.
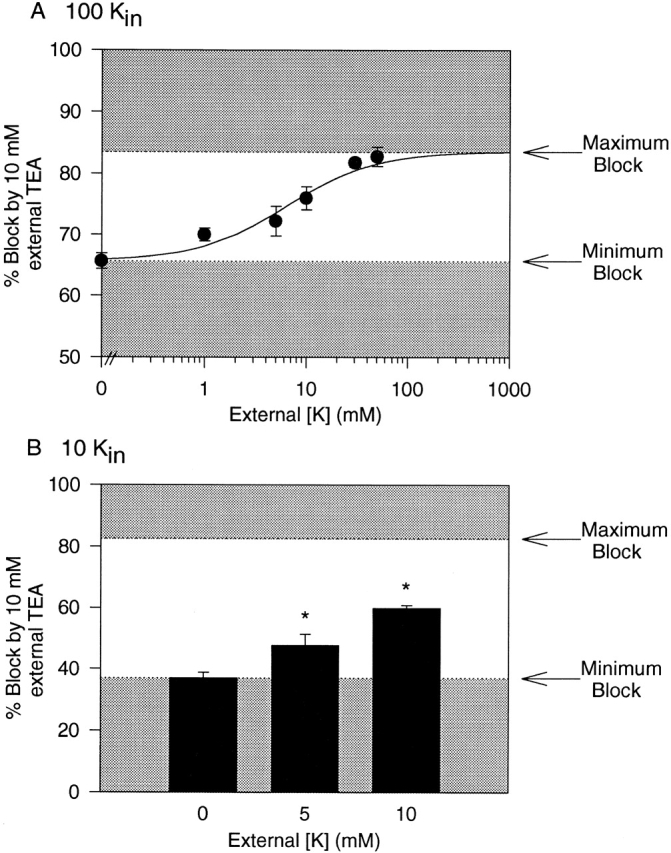
External [K+] dependence of external TEA block in the absence of internal TEA. (A) External TEA (10 mM) was applied to outward currents carried by 100 mM internal K+. As in Fig. 3 B, the shaded areas represent the minimum block (with 0 external K+) and the maximum possible block (under conditions of saturating [K+]) by 10 mM TEA under these conditions. The apparent affinity of [K+] for the site responsible for increasing external TEA block from minimum to maximum was between 8 and 10 mM (data points represent mean ± SEM for 3–16 cells). (B) External TEA (10 mM) was applied to outward currents carried by 10 mM internal K+. Currents were evoked by depolarization to +20 mV; at 0 and 5 mM external K+, TEA blocked currents identically at 0 and +20 mV (not shown). As in A and Fig. 3 B, the shaded areas represent the minimum and maximum block by 10 mM TEA under these conditions. *Statistical significance (P < 0.05 or better) for comparison between any two means (3–10 cells for each [K+]).
Electrophysiological Solutions
Currents were recorded in a constantly flowing, gravity fed bath. Solutions were placed in one of six reservoirs, each of which fed via plastic tubing into a single quartz tip (∼100 μm diameter; ALA Scientific Instruments). The tip was placed within 20 μm of the cell being recorded before the start of the experiment. One solution was always flowing, and solutions were changed by manual switching (solution exchange was complete within 5–10 s). Control internal solutions contained (mM): 140 XCl (X = a combination of K+ and NMG+), 20 HEPES, 10 EGTA, 1 CaCl2, 4 MgCl2, pH 7.3, osmolality 285. Block by external TEA was identical when Mg2+ was omitted from the internal solution (data not shown). Control external solutions contained (mM): 165 XCl, 20 HEPES, 10 glucose, 2 CaCl2, and 1 MgCl2, pH 7.3, osmolality 325. In all experiments, internal and external [K+] are reported; osmotic balance was maintained with NMG+. Other additions and substitutions are listed in the figure legends.
RESULTS
Inhibition by External TEA at Different [K+]
Fig. 1A and Fig. B, illustrates outward currents recorded through Kv2.1 with different internal and external [K+] (in this and all experiments, recordings were made in the absence of Na+). We previously demonstrated that in Kv2.1, inactivation rate was dependent on K+ occupancy of the pore (Immke et al. 1999); as occupancy of the pore by K+ was increased, inactivation rate increased. In Fig. 1A and Fig. B, comparison of control currents under these three [K+] conditions (left, middle, and right) indicates that, based on inactivation rate, exposing channels to these three different [K+] resulted in different K+ occupancy of the pore.
Figure 1.
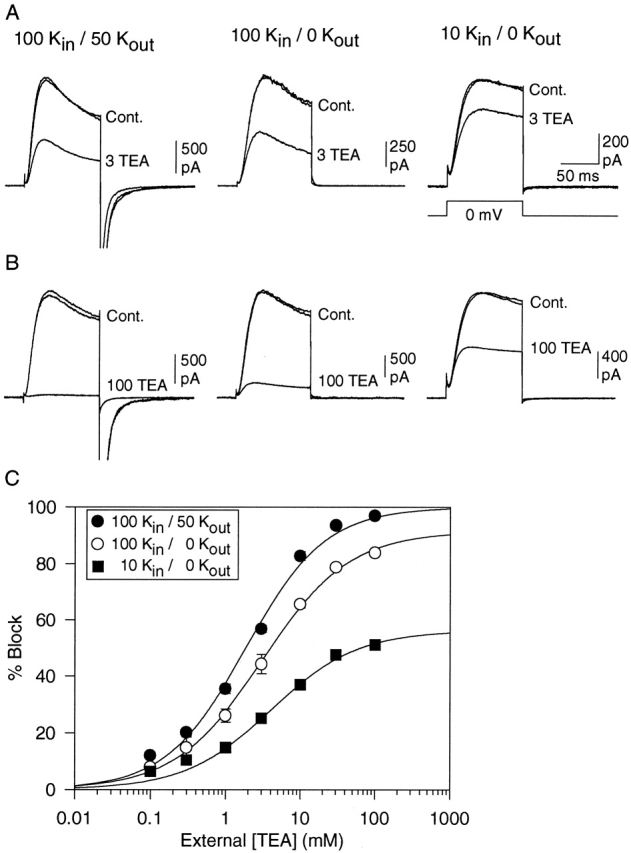
Dependence of external TEA sensitivity on [K+]. A and B each illustrate three currents, obtained before application of TEA (Cont.), during application of external TEA, and after removal of TEA. Currents were evoked by 100-ms depolarization to 0 mV for each of the three different [K+] conditions described in A (top). A illustrates block by 3 mM TEA under each of the three conditions, B illustrates block by 100 mM TEA. (C) Concentration–response curves for block of K+ current by external TEA under the three [K+] conditions. Each data point represents the mean ± SEM of data obtained from between 3 and 16 cells. Solid lines illustrate the best fit of the data by . The calculated IC50, bmax, and slope values from these fits were 1.9 ± 0.2 mM, 100 ± 1%, and 0.82 (•), 3.1 ± 0.1 mM, 92 ± 3%, and 0.75 (○), and 4.1 ± 0.9 mM, 56 ± 3%, and 0.75 (▪).
Fig. 1 A illustrates block by 3 mM TEA under these three conditions. Fig. 1 B illustrates block of K+ currents by 100 mM TEA under the same conditions. As the [K+] that the channels were exposed to was decreased, block by TEA decreased. Fig. 1 C illustrates complete [TEA]–response curves under these three conditions. With 100 mM internal and 50 mM external K+, TEA blocked K+ currents with an IC50 of 1.9 ± 0.2 mM (n = 4–5; Fig. 1, •). Importantly, 100% of the current through Kv2.1 could be blocked by TEA, which indicates that TEA interacted with 100% of the conducting channels. These data further indicate that if TEA block requires the binding of K+ to a site in the pore (see below), then the TEA modulation site is saturated with these internal and external [K+]. Under conditions of lower K+ occupancy, TEA potency was essentially unchanged, but not all channels could be blocked by external TEA. In recordings with 100 mM internal but 0 external K+, TEA blocked K+ currents with an IC50 of 3.1 ± 0.4 mM (n = 3–16; Fig. 1, ○). However, 100 mM TEA blocked just 83.9 ± 0.8% (n = 12) of the current and, according to the best fit of the experimental data, maximal possible block of total current by TEA would be just 91.6 ± 1.1%. When currents were carried by just 10 mM internal K+ (in the presence of 0 mM external K+), the change in TEA efficacy was clear. Under these conditions, there was little or no change in TEA potency (IC50 = 4.1 ± 0.9 mM, n = 3–14), but the maximum block was markedly reduced (Fig. 1, ▪). At a concentration of 100 mM, TEA blocked just 51.1 ± 1.1% (n = 14) of the current, and the best fit of the data indicated that just 56.2 ± 3.0% of the total current could be blocked. In all cases, full recovery was achieved upon removal of TEA (Fig. 1A and Fig. B).
The reduction in TEA efficacy suggests that current-carrying channels could be in one of two states. Some channels could be blocked by TEA with an IC50 of 2–4 mM; other channels were completely insensitive to TEA at concentrations of up to 100 mM. Furthermore, these data suggest that interconversion between these two states depended on the occupancy of the pore by K+, such that at higher K+ occupancy, channels were in the TEA-sensitive state and at lower K+ occupancy, channels were insensitive to TEA.
Change in External TEA Efficacy with an Internal Channel Blocker
In the experiments in Fig. 1, we altered K+ occupancy of the pore by reducing the [K+] to which the channels were exposed. An alternative approach to influencing K+ occupancy of the channel is to block the pore from the intracellular side of the channel (Baukrowitz and Yellen 1996; Khodakhah et al. 1997; Immke et al. 1999). When current is carried in the outward direction, intracellular open channel pore blockers interfere with the ability K+ to enter the pore, but K+ is still able to leave the pore and go into the external solution.
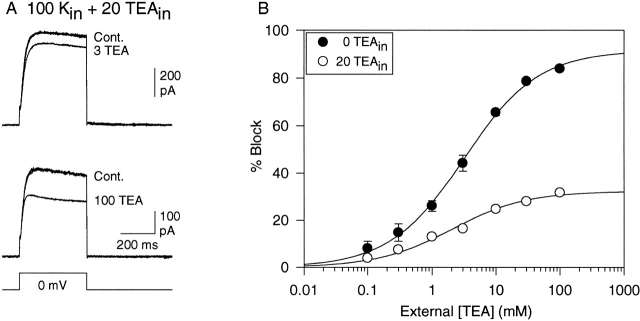
Fig. 2 A illustrates the effect of two external [TEA] on currents recorded with 100 mM intracellular K+ plus 20 mM intracellular TEA (0 external K+). Intracellular TEA at this concentration blocks inward current through Kv2.1 by 85–90% in the presence of 30 mM external K+ (Immke et al. 1999). Fig. 2 B illustrates complete concentration–response curves for external TEA in the presence (○) and absence (•) of internal TEA. Internal TEA had no effect on the IC50 for external TEA (IC50 = 2.0 ± 0.6; n = 3–6). However, in the presence of internal TEA, 100 mM external TEA blocked just 31.8 ± 0.7% (n = 6) of the current. According to the best fit of the data, the maximum possible block by external TEA was 32.4 ± 0.5%.
Figure 2.
K+ current block by external TEA in the presence of 20 mM internal TEA. Currents were recorded in the presence of 100 mM internal K+ and 0 external K+. (A) Three current traces are shown in each panel, illustrating control, block by the indicated concentration of external TEA, and recovery. Currents were evoked by 100-ms depolarization to 0 mV. (B) Concentration–response curve for block by external TEA in the absence (•) and presence (○) of internal TEA. Data points represent mean ± SEM for three to six cells. Solid lines illustrate the best fit of the data to . The calculated IC50, bmax, and slope values for the curve in the presence of internal TEA (○) were 2.0 ± 0.6, 32 ± 0.5, and 0.75. (The data for • are identical to those in Fig. 1).
[K+] Dependence of External TEA Sensitivity in the Presence of Internal TEA
In studies with Kv1.x channels, it had been suggested that internal TEA reduced external TEA potency by a direct interaction between the internal and external TEA (Newland et al. 1992). Previous studies suggested that, in Kv2.1, this is not the case. The reduction in external TEA sensitivity in the presence of internal TEA could be fully explained as resulting from the reduction in K+ occupancy of the pore (Immke et al. 1999). The observation that internal TEA did not change external TEA potency (Fig. 2 B) further supports this conclusion.
In Fig. 3, we examined the [K+] dependence of external TEA sensitivity on outward K+ currents, in the presence of 100 mM internal K+ plus 20 mM internal TEA. In all experiments, external TEA was applied to cells exposed to at least three different external [K+]. In different cells, the order of [K+] exposure was varied to control for the possibility of long term consequences of exposure (or lack thereof) to external K+. Fig. 3 A illustrates the results of one experiment. For this cell, external TEA was applied under three conditions in the following order: in the presence of 3, 5, and 1 mM external [K+]. Once current magnitude stabilized at a given [K+], TEA was applied briefly (•), and then removed. After recovery to control current amplitude, external [K+] was changed and external TEA reapplied. TEA block increased as a function of [K+]. In the presence of 1, 3, and 5 mM external K+, TEA blocked currents by 26.9 ± 1.0%, 35.0 ± 1.6%, and 38.2 ± 1.1% (n = 3), respectively.
Figure 3.
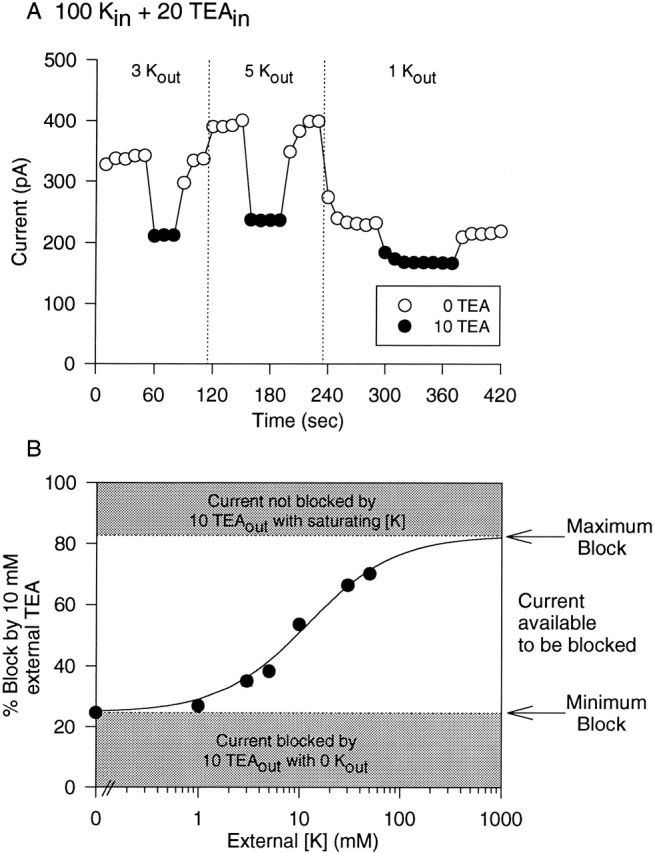
External [K+] dependence of external TEA block in the presence of internal TEA. Outward currents were recorded as in Fig. 2, with 100 mM internal K+ plus 20 mM internal TEA. Block by 10 mM external TEA was examined in the presence of different external [K+]. (A) Data obtained from a single cell, where block by 10 mM TEA was examined sequentially in the presence of 3, 5, and 1 mM external K+. Currents were evoked every 10 s; • denote current magnitude in the presence of TEA. External [K+] was changed (vertical dotted line) after washout of external TEA and return to steady state current magnitude. (B) Block by 10 mM external TEA as a function of external [K+]. The shaded area at the bottom of the graph represents the minimum block by 10 mM TEA in the presence of 100 mM internal K+ plus 20 mM internal TEA, which occurs in the absence of external K+ (Fig. 2). This percent block represents the fraction of TEA modulation sites already occupied in the absence of external K+. The shaded area at the top represents the maximum possible block by 10 mM TEA, obtained when the channel is saturated by K+ (Fig. 1, 100 K+ in, 50 K+ out). When all TEA modulation sites are occupied, 83% of current will be blocked by 10 mM TEA. The remaining, unshaded area of the graph (between 25 and 83% block) represents the percentage of current that is available to be blocked by 10 mM TEA when current is carried by 100 mM K+ in in the presence of 20 mM internal TEA. This represents current through channels with an unoccupied TEA modulation site. The best fit of the data points (three to seven cells for each point) indicates that, under these conditions, the apparent affinity of external K+ for the site responsible for increasing external TEA block from minimum to maximum is 10.8 ± 1.1 mM (slope = 1.0).
In the absence of external TEA (○), current magnitude changed with changes in external [K+]. In Kv2.1, as in other voltage-gated K+ channels, the magnitude of outward K+ currents increases as external [K+] is elevated from 0 to 10 mM (Lopez-Barneo et al. 1993; Wood and Korn 2000). Current magnitudes in the presence of 3 and 5 mM K+ were, respectively, 32.4 ± 4.7% and 40.5 ± 4.2% (n = 3; P < 0.01 for all comparisons) greater than current magnitude at 1 mM K+. This observation provides independent evidence that with these changes in external [K+], K+ occupancy of the pore increased.
In the presence of 100 mM internal K+ plus 20 mM internal TEA, changes in external [K+] were expected to modulate block by 10 mM TEA between 25 and 83% for the following reasons. In the absence of external K+, 10 mM TEA blocked channels by 24.7 ± 0.9% (n = 3; Fig. 2). Under conditions that saturated the TEA modulation site with [K+] (100 mM internal K+, 50 mM external K+, no internal TEA), 10 mM external TEA blocked 82.7 ± 1.5% (n = 4) of the total current (Fig. 1). Consequently, in this experiment, the maximum block possible upon elevation of external [K+] was expected to be 83%, which would occur if the pore became fully saturated by K+. Fig. 3 B illustrates that elevation of external [K+] between 0 and 50 mM produced a concentration-dependent increase in block, with an EC50 of 10.8 ± 1.1 mM (n = 3–7).
[K+] Dependence of External TEA Sensitivity in the Absence of Internal TEA
Our working hypothesis was that the [K+] dependence observed in Fig. 3 B simply reflected the association of external K+ with a binding site in a K+-conducting pore. This interpretation depends on the assumption that internal TEA did not influence the binding of external K+ to this site, and that the [K+] dependence was not influenced by a direct interaction between external K+ and internal TEA. It was critical, therefore, to demonstrate that the concentration dependence observed was not related to the presence of internal TEA. To do this, we examined the [K+] dependence of external TEA block under conditions that did not include internal TEA.
With 100 mM K+ in the pipet and 0 external K+, TEA efficacy was submaximal (Fig. 1). In the absence of external K+, 10 mM TEA blocked current carried by 100 mM K+ by 65.6 ± 1.2% (n = 16). As previously described, 10 mM TEA blocked current in K+-saturated channels by 83% (Fig. 1). Fig. 4 A illustrates the [K+]-dependent enhancement of block by 10 mM TEA from 66 to 83%. Even over this limited range of enhancement, the [K+] dependence is reasonably well fit by a sigmoidal function, with an EC50 that fell between 8 and 10 mM.
Fig. 4 B illustrates the [K+]-dependent increase of TEA block when currents were carried by 10 mM internal K+. As described previously, the maximum possible block by 10 mM TEA in a K+-saturated channel was 83%. In the absence of external K+, 10 mM TEA blocked 37.0 ± 1.8% (n = 10) of the total current carried by 10 mM internal K+ (Fig. 1). Current magnitude was significantly enhanced in a concentration-dependent manner by addition of both 5 and 10 mM external K+. Indeed, in the presence of 10 mM external K+, TEA blocked total current by 59.9 ± 1.0% (n = 3), which is the halfway point between 37 and 83%. Thus, under three different experimental conditions (10 Kin, 100 Kin, and 100 Kin plus 20 TEAin), which produced different K+ occupancies of the pore and different efficacies of external TEA, enhancement of TEA block by external K+ from the minimum (measured in the absence of external K+) to the maximum possible (measured in a K+-saturated pore) occurred with an EC50 of ∼10 mM. In other words, in all conducting channels with an unoccupied TEA modulation site in the pore, this site was bound by K+ with an apparent K d of ∼10 mM.
Lysines in the External Vestibule Associated with Loss of TEA Sensitivity
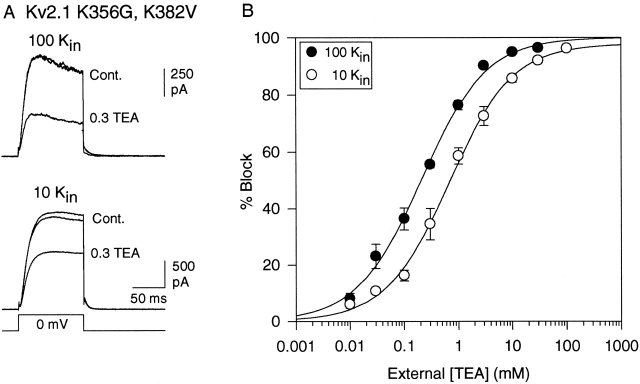
Previous work demonstrated that in Kv2.1, the loss of TEA sensitivity in the presence of Na+ (and absence of K+) was associated with a conformational rearrangement in the outer vestibule of the channel (Immke et al. 1999). In particular, two lysines in the outer vestibule appeared to influence TEA sensitivity. A lysine at position 382 did not prevent TEA block of Na+ currents, but did reduce TEA potency. More dramatically, in the absence of K+, a lysine at position 356 appeared to change its position in the permeation pathway and thus reduce TEA potency by >300-fold when K+ was replaced with Na+. Upon mutation of these lysines to smaller uncharged amino acids (Kv2.1 K356G, K382V), the conformational change and potency shift associated with removal of K+ still occurred, but TEA was able to block currents in the absence of K+ with an IC50 of 11 mM (Immke et al. 1999). To test whether the conformational rearrangement of these lysines was also associated with the loss of TEA efficacy when [K+] was reduced in the absence of Na+, we examined TEA sensitivity in the Kv2.1 K356G, K382V channel (Fig. 5).
Figure 5.
Influence of [K+] on TEA sensitivity in the mutant channel, Kv2.1 K356G, K382V. (A) Block by 0.3 mM external TEA of currents carried by 100 (top) and 10 (bottom) mM internal K+. Traces labeled “Cont.” were obtained before and after TEA application. (B) [TEA]-response curves for block at each internal [K+]. Data points represent mean ± SEM for 3–10 cells. IC50 and slope values, determined from the best fit of the data (solid lines) were, respectively, 0.21 ± 0.02 mM and 0.73 for 100 Kin, and 0.67 ± 0.07 mM and 0.74 for 10 Kin. bmax was 100% for each curve.
Fig. 5 A illustrates the block by 0.3 mM TEA on currents carried through the mutant channel by 100 (top) and 10 (bottom) mM K+. With 100 mM internal K+ (and 0 external K+), TEA blocked currents through the channel with an IC50 of 0.21 ± 0.02 mM (n = 3–12; Fig. 5 B, •). With 10 mM internal K+, TEA blocked currents with an IC50 of 0.67 ± 0.07 mM (n = 3–8; Fig. 5 B, ○). This shift in IC50 is consistent with the previously described K+-dependent change in TEA potency in this mutant channel (Immke et al. 1999), and indicates that the outer vestibule underwent a conformational change upon reduction of K+ from 100 to 10 mM. However, in the absence of the two outer vestibule lysines, TEA was able to block 100% of the current through the channel even at low [K+]. This suggests that entry of channels into the TEA-insensitive state at low [K+] was associated with the presence of these lysines in the outer vestibule.
DISCUSSION
The interaction of K+ with the selectivity filter in Kv2.1 modulates a conformational change in the outer vestibule of the pore (Immke et al. 1999). The primary findings in this paper are that: (a) this K+-dependent conformational change can completely prevent external TEA from blocking the channel, (b) TEA efficacy is regulated by just one of the two selectivity filter cation binding sites, (c) the apparent affinity of K+ for the selectivity filter site that modulates TEA sensitivity differs depending on whether the other selectivity filter site is occupied by K+, and (d) the apparent affinity of the outermost selectivity filter site, when the innermost site is occupied by K+, is ∼10 mM. The data also indicate that at physiological internal and external [K+], the selectivity filter cation binding sites in Kv2.1 are not fully saturated.
One Cation Binding Site Is Responsible for Modulation of TEA Efficacy
The crystal structure of KcsA suggests that the K+ channel pore contains three cation binding sites, two associated with the selectivity filter and one in a water-filled compartment internal to the selectivity filter (Doyle et al. 1998). Previously, independent experiments indicated that the cation binding site involved in modulation of TEA sensitivity was a high affinity site associated with ionic selectivity and that the TEA modulation site was located between the internal and external TEA binding sites (Immke et al. 1999). The observation that TEA sensitivity was all or none in Kv2.1 channels carrying K+ current allowed us to further identify and characterize the cation binding site involved in modulation of TEA sensitivity.
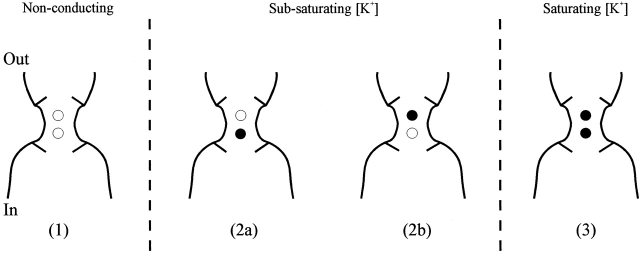
Fig. 6 illustrates a cartoon of the K+ channel pore that contains two cation binding sites associated with the selectivity filter, located between the internal and external TEA binding sites. In Fig. 6 (1), the two selectivity filter binding sites are unoccupied. These channels are not carrying current and are invisible to the experiment. (3) represents a channel with a fully saturated selectivity filter. These latter channels can all be blocked by TEA, as demonstrated by the observation that TEA blocks 100% of channels at very high internal and external [K+] (Fig. 1). (2a) and (2b)illustrate possible channel configurations, each with just one selectivity filter binding site occupied. Channels would exist in one or both of these configurations at [K+] that do not saturate the channel. There are two possible explanations for the observation that, at lower [K+], some channels cannot be blocked by TEA. First, it is possible that only fully saturated channels (3) can be blocked by TEA, and that the channel configurations represented in (2a) and (2b) are TEA-insensitive. Alternatively, it is possible that only one selectivity filter binding site must be occupied by K+ for block by TEA. Previous data indicate that, indeed, channels can be blocked by TEA when only a single selectivity filter site is occupied by K+. In Na+-conducting K+ channels, only 30 μM K+ is required for measurable inhibition of current by TEA (Immke et al. 1999). This is also the minimum [K+] that can be observed to interact with the selectivity filter, as judged by block of Na+ current. Elevation of external [K+] from 30 to 100 μM increased Na+ current block and TEA sensitivity. In the absence of Na+, [K+] up to 100 μM do not generate measurable currents. These data suggest that, at [K+] ≤ 100 μM, K+ occupies the selectivity filter, but an insignificant number of channels will have selectivity filters that are doubly occupied by K+. Thus, TEA block requires K+ occupancy of just a single selectivity filter binding site.
Figure 6.
Cartoon depicting potential occupancy states of the selectivity filter. (•) Selectivity filter cation binding sites occupied by K+, (○) unoccupied selectivity filter sites. Pairs of lines protruding into the pore, located external and internal to the cation binding sites, represent the external and internal TEA binding sites. (1) represents a channel with an unoccupied selectivity filter, (2a) and (2b) represent channels with just the inner or outer, respectively, selectivity filter sites occupied, and (3) represents a channel with a fully occupied selectivity filter.
Because occupancy of the TEA modulation site by K+ influences TEA efficacy (as opposed to potency), there must also be a current-carrying channel that cannot be blocked by TEA. Since all channels in Fig. 6 (3) can be blocked, and one of the channels in (2) can be blocked, the only other current carrying channel configuration available as a candidate for a TEA-insensitive channel is the other channel depicted in (2). Thus, only one of the two selectivity filter binding sites serves as the TEA modulation site.
The Affinity of the Second Selectivity Filter Cation Binding Site, When One Is Occupied, Is ∼10 mM: Implications for Permeation Theory
Both functional and structural studies have provided convincing evidence that there are several discrete cation binding sites in the K+ channel conduction pathway (Neyton and Miller 1988; Hurst et al. 1995; Doyle et al. 1998). The selectivity filter itself can apparently be occupied by up to two K+ ions (Doyle et al. 1998). The results of this paper, combined with previous experiments (Immke et al. 1999), indicate that a single, functionally defined site associated with the selectivity filter can bind K+ with two markedly different affinities, depending on whether the pore is already occupied by K+. In Na+-filled pores, K+ interacted with the TEA modulation site at concentrations ≤30 μM (Immke et al. 1999). In contrast, the interaction of K+ with the TEA modulation site in K+-occupied pores occurred with low affinity. With up to 100 mM internal K+ carrying outward current, the TEA modulation site remained unsaturated. Under three different experimental conditions (10 and 100 mM internal K+, and 100 mM internal K+ plus internal TEA), which produced different K+ occupancy of the pore in the absence of external K+, external K+ increased occupancy of the TEA modulation site with an apparent affinity of ∼10 mM. Consequently, these results strongly support a model of permeation that includes interactions between ions in multiply occupied pores. Our data do not address whether the occupancy-dependent change in affinity of K+ for the TEA modulation site results from electrostatic repulsion or from a nonelectrostatic mechanism (see Spassova and Lu 1999).
Is There a Low Affinity Cation Binding Site External to the Selectivity Filter Binding Sites in Voltage-gated K+ Channels?
Our results indicate that in K+ conducting channels, the low affinity modulation of TEA sensitivity by K+ occurs at a selectivity-filter binding site. The apparent affinity of ∼10 mM for the interaction of K+ with the Kv2.1 pore already occupied by one K+ ion is similar to the apparent affinity of other low affinity interactions in K+-conducting channels. For example, external K+ slows inactivation rate in Shaker with an apparent K d of 2 mM (Baukrowitz and Yellen 1996), external K+ potentiates outward K+ currents in Shaker T449 mutants with apparent K ds between 0.8 and 8 mM (Lopez-Barneo et al. 1993), and the rate of recovery from inactivation of K+ currents in Kv1.3 is modulated by external K+ at millimolar concentrations (Levy and Deutsch 1996). Whereas our data do not unequivocally rule out the existence of a low- affinity K+ binding site external to the selectivity filter, our results do indicate that the low affinity interaction that modulates TEA sensitivity occurs at a selectivity filter binding site. Interestingly, the cation binding site responsible for pseudo-irreversible “death” of some channels upon removal of external K+, which is occupied with an apparent K d of 2.2 mM in K+-conducting Shaker-like channels (Pardo et al. 1992), may also be associated with a high-affinity K+ binding site (Vergara et al. 1999).
Additional Implications of Changes in TEA Efficacy Versus Potency
In Kv2.1, reduction of K+ occupancy of the pore in the absence of a substitute permeating ion produced little or no change in TEA potency, but had a dramatic impact on TEA efficacy (Fig. 1 and Fig. 2). The reduced efficacy indicates that occupancy of a site in the pore by a cation (K+ in this case) is an absolute requirement for TEA block of the wild-type Kv2.1 channel. When Na+ was substituted for K+, TEA was completely without effect (Ikeda and Korn 1995). However, when either lys356 or lys382 was mutated to a smaller uncharged residue, TEA could block Na+ currents through the channel (Immke et al. 1999). In both mutants, TEA block was characterized by a large decrease in potency when K+ was replaced by Na+ (Immke et al. 1999). Both the potency shift observed upon substitution of Na+ for K+, and the efficacy change observed upon reduction of K+ in the absence of substitute permeating ion, were associated with a conformational change in the outer vestibule of the channel (Fig. 5; Immke et al. 1999). These data are consistent with two possibilities that are not mutually exclusive. One possibility is that permeating Na+ interacts with the TEA modulation site in the pore normally occupied by K+, and that occupancy of this site by different cations influences external TEA potency. Alternatively, the nature of specific residues in the outer vestibule may determine whether lowering K+ occupancy alters external TEA potency. The mechanistic explanation for the potency shift upon switching from K+ to Na+ must await a better understanding of the cation-dependent conformational change in the outer vestibule.
Finally, our interpretation that the change in TEA efficacy reflects all-or-none channel block depends on the assumption that TEA-bound channels do not conduct under low [K+] conditions. There is no direct evidence that addresses this assumption. However, this assumption is strongly supported by the observations that TEA binds to all four channel subunits (Heginbotham and MacKinnon 1992; Kavanaugh et al. 1992) just external to the narrowest region of the channel. One might argue that under low [K+] conditions, the channel conformation changes such that TEA binds to fewer than four subunits and that, under these conditions, TEA-bound channels can carry current at a lower conductance. However, this possibility is inconsistent with the observation that TEA potency is unchanged at lower [K+].
Acknowledgments
We thank one of the anonymous reviewers and Dr. Olaf Andersen, whose insight helped clarify our interpretation and the manuscript.
Funding was provided by the National Science Foundation and the American Heart Association, Connecticut Affiliate.
References
- Almers W., McCleskey E.W. Non-selective conductance in calcium channels of frog musclecalcium selectivity in a single file pore. J. Physiol. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 1996;271:653–656. doi: 10.1126/science.271.5249.653. [DOI] [PubMed] [Google Scholar]
- Dang T.X., McCleskey E.W. Ion channel selectivity through stepwise changes in binding affinity. J. Gen. Physiol. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D.A., Cabral J.M., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channelmolecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992;8:483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. Mutations in the K+ channel signature sequence. Biophys. J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R.W. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., Keynes R.D. The potassium permeability of a giant nerve fibre. J. Physiol. 1955;128:61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R.S., Latorre R., Toro L., Stefani E. External barium block of Shaker potassium channelsevidence for two binding sites. J. Gen. Physiol. 1995;106:1069–1087. doi: 10.1085/jgp.106.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S.R., Korn S.J. Influence of permeating ions on potassium channel block by external tetraethylammonium. J. Physiol. 1995;486:267–272. doi: 10.1113/jphysiol.1995.sp020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke D., Wood M., Kiss L., Korn S.J. Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J. Gen. Physiol. 1999;113:819–836. doi: 10.1085/jgp.113.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurman M.E., Boland L.M., Liu Y., Yellen G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- Kavanaugh M.P., Hurst R.S., Yakel J., Varnum M.D., Adelman J.P., North R.A. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992;8:493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Khodakhah K.A., Meleshchuck A., Armstrong C.M. Killing K+ channels with TEA+ . Proc. Natl. Acad. Sci. USA. 1997;94:13335–13338. doi: 10.1073/pnas.94.24.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L., Korn S.J. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys. J. 1998;74:1840–1849. doi: 10.1016/S0006-3495(98)77894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L., Immke D., LoTurco J., Korn S.J. The interaction of Na+ and K+ in voltage-gated potassium channelsevidence for cation binding sites of different affinity. J. Gen. Physiol. 1998;111:195–206. doi: 10.1085/jgp.111.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S.J., Ikeda S.R. Permeation selectivity by competition in a delayed rectifier potassium channel. Science. 1995;269:410–412. doi: 10.1126/science.7618108. [DOI] [PubMed] [Google Scholar]
- Kuo C.-C., Hess P. Characterization of the high-affinity Ca2+ binding sites in the L-type Ca2+ channel pore in rat phaeochromocytoma cells. J. Physiol. 1993;466:657–682. [PMC free article] [PubMed] [Google Scholar]
- Levy D.I., Deutsch C. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys. J. 1996;70:798–805. doi: 10.1016/S0006-3495(96)79619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J., Hoshi T., Heinemann S.H., Aldrich R.W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- Newland C.F., Adelman J.P., Tempel B.L., Almers W. Repulsion between tetraethylammonium ions in cloned voltage-gated potassium channels. Neuron. 1992;8:975–982. doi: 10.1016/0896-6273(92)90212-v. [DOI] [PubMed] [Google Scholar]
- Neyton J., Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high conductance Ca2+-activated K+ channel. J. Gen. Physiol. 1988;92:569–586. doi: 10.1085/jgp.92.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L.A., Heinemann S.H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. Extracellular K+ specifically modulates rat brain K+ channel. Proc. Natl. Acad. Sci. USA. 1992;89:2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., MacKinnon R. The cavity and pore helices in the KcsA K+ channelelectrostatic stabilization of monovalent cations. Science. 1999;285:100–102. doi: 10.1126/science.285.5424.100. [DOI] [PubMed] [Google Scholar]
- Spassova M., Lu Z. Tuning the voltage dependence of tetraethylammonium block with permeant ions in an inward rectifier K+ channel. J. Gen. Physiol. 1999;114:415–426. doi: 10.1085/jgp.114.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C., Alvarez O., Latorre R. Localization of the K+ lock-in and Ba2+ binding sites in a voltage-gated calcium-modulated channel. Implications for survival of K+ permeability. J. Gen. Physiol. 1999;114:365–376. doi: 10.1085/jgp.114.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M., Korn S.J. Potassium-dependent potentiation of K+ channel currents Biophys. J. 78 2000. 95A(Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]