Abstract
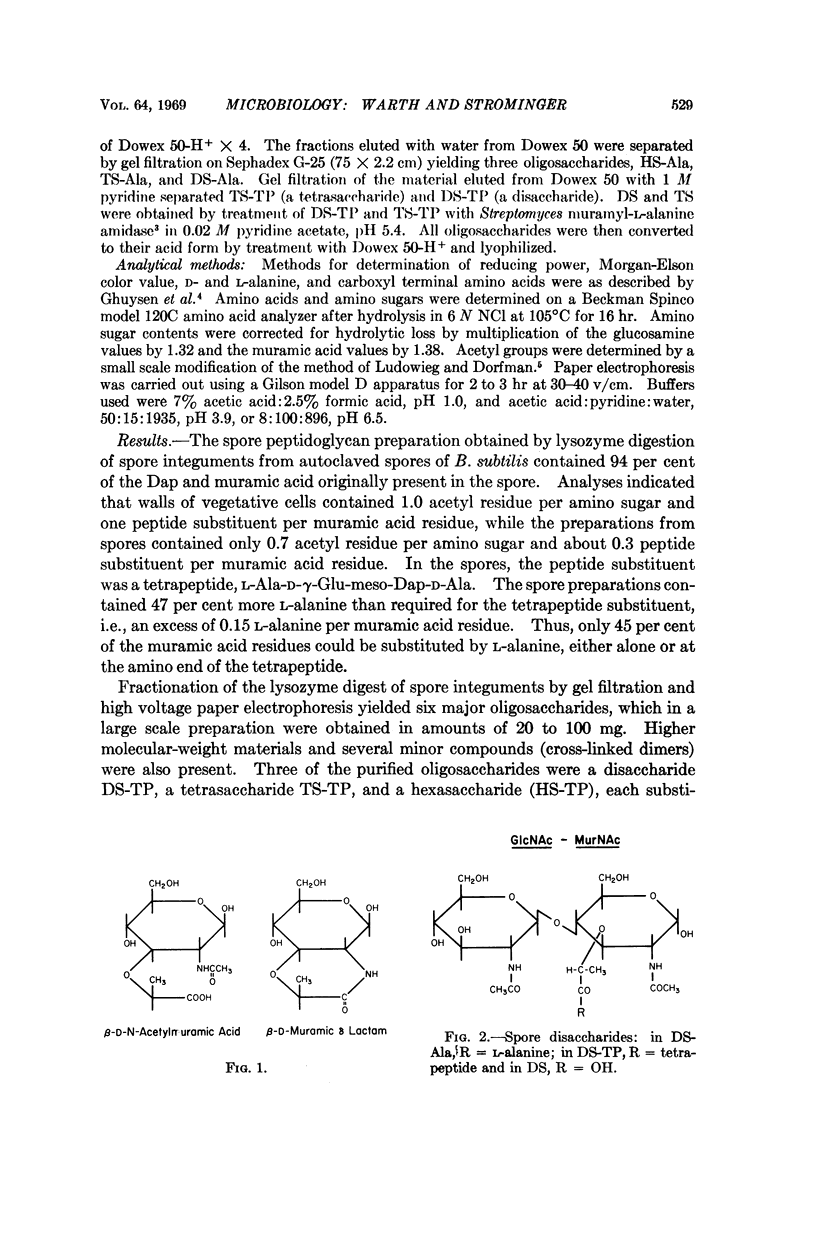
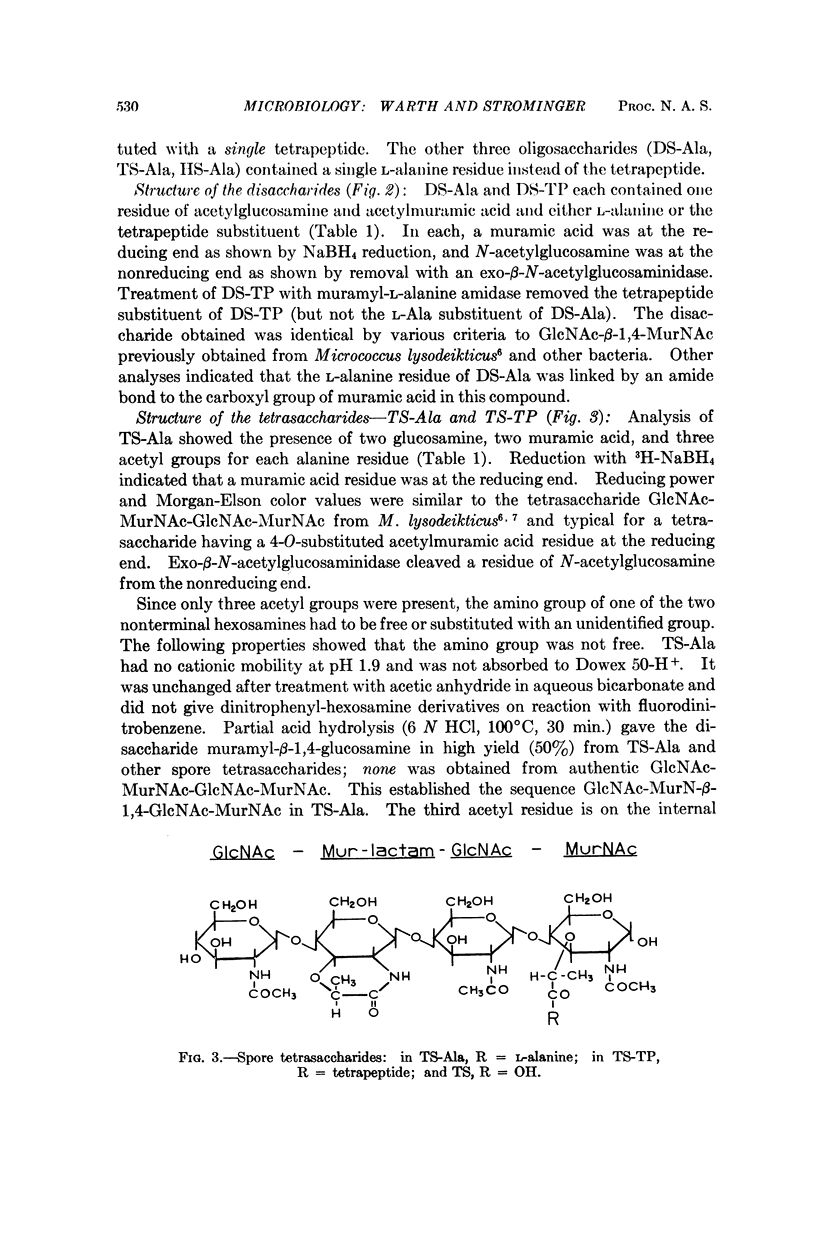
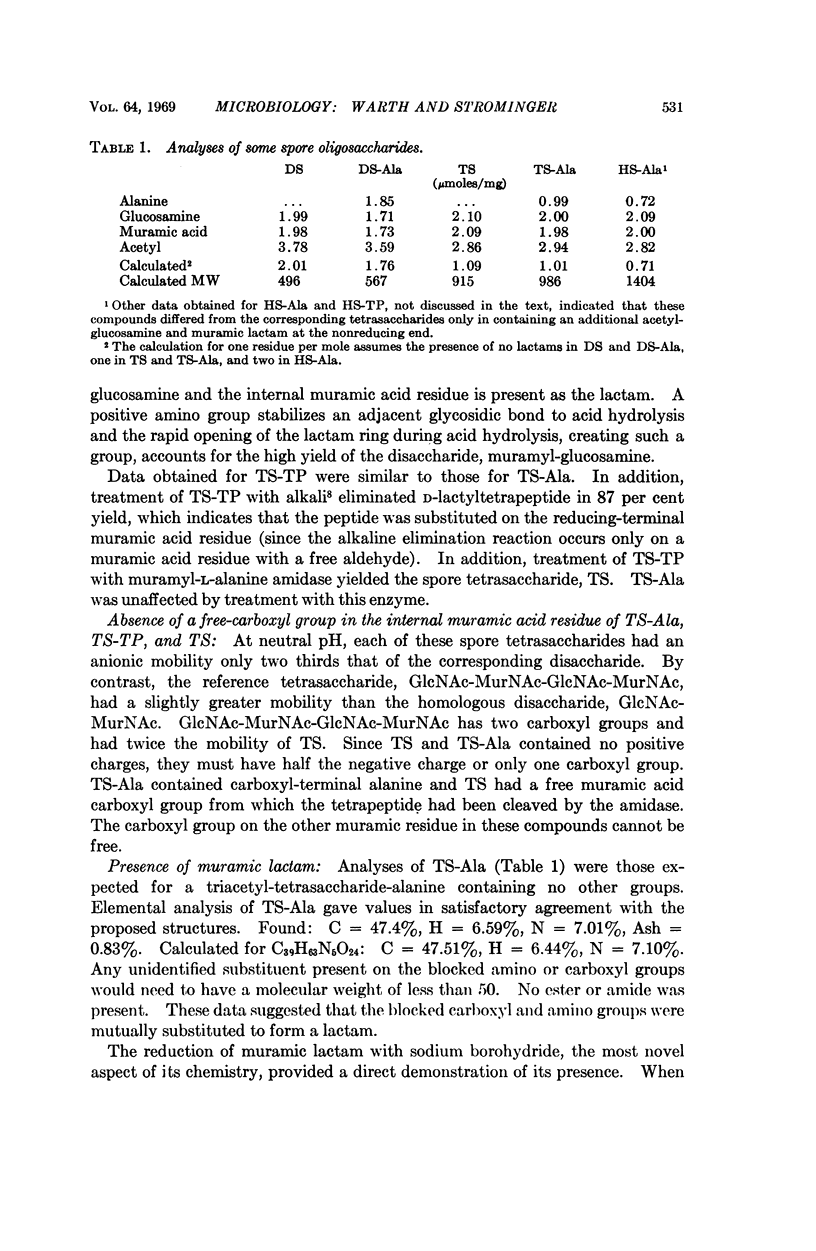
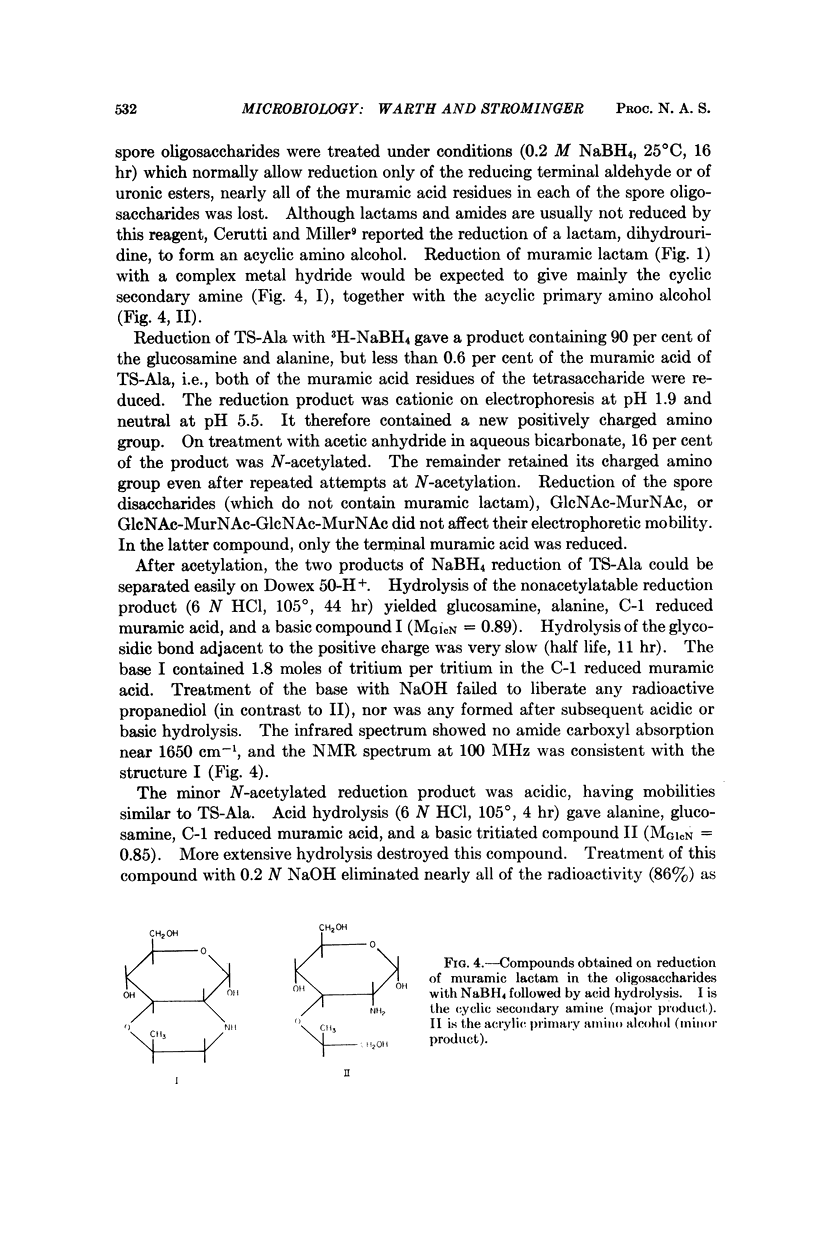
Six major oligosaccharides were released from the peptidoglycan of spores of Bacillus subtilis by lysozyme treatment. They were isolated and characterized as a disaccharide, tetrasaccharide, and hexasaccharide composed of equal amounts of muramic acid and glucosamine and containing two, three, and four acetyl groups, respectively. Three of the compounds were substituted by a single L-alanine residue, and the other three by a single tetrapeptide substituent on the acetylmuramic acid residue at the reducing end of each compound. The other muramic acid residue in the tetrasaccharides (and two of the three in the hexasaccharides) were shown to be present as muramic lactams, a sugar not previously found in nature and, hence, a unique spore constituent. Other features of the structure of spore peptidoglycan are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Cerutti P., Miller N. Selective reduction of yeast transfer ribonucleic acid with sodium borohydride. J Mol Biol. 1967 May 28;26(1):55–66. doi: 10.1016/0022-2836(67)90260-4. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Snell N. S., Burr H. K. Water Permeability of Bacterial Spores and the Concept of a Contractile Cortex. Science. 1960 Aug 26;132(3426):544–545. doi: 10.1126/science.132.3426.544. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Ghuysen J. M., Tipper D. J., Stominger J. L. Structure of the cell wall of Micrococcus lysodeikticus. I. Study of the structure of the glycan. Biochemistry. 1966 Oct;5(10):3079–3090. doi: 10.1021/bi00874a001. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., ALDERTON G. Behavior of bacterial spores in aqueous polymer two-phase systems. J Bacteriol. 1961 Sep;82:331–341. doi: 10.1128/jb.82.3.331-341.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARON N., SEIFTER S. A TRANSGLYCOSYLATION REACTION CATALYZED BY LYSOZYME. J Biol Chem. 1964 Jul;239:PC2398–PC2399. [PubMed] [Google Scholar]
- Speck J. C., Jr, Rynbrandt D. J. A convenient method for isolating the disaccharide and the tetrasaccharide in muramidase digests of Micrococcus lysodeikticus cell walls. Anal Biochem. 1967 Jun;19(3):426–433. doi: 10.1016/0003-2697(67)90232-1. [DOI] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]



