Abstract
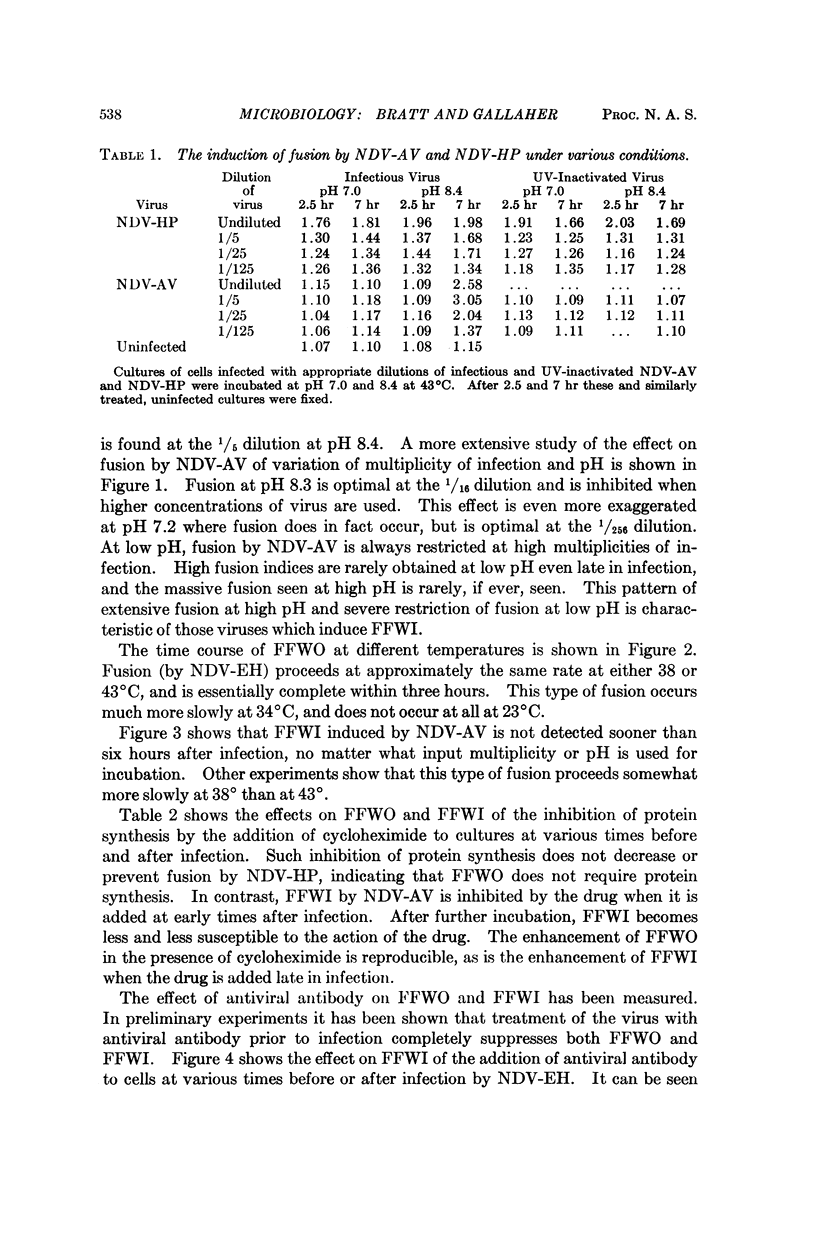
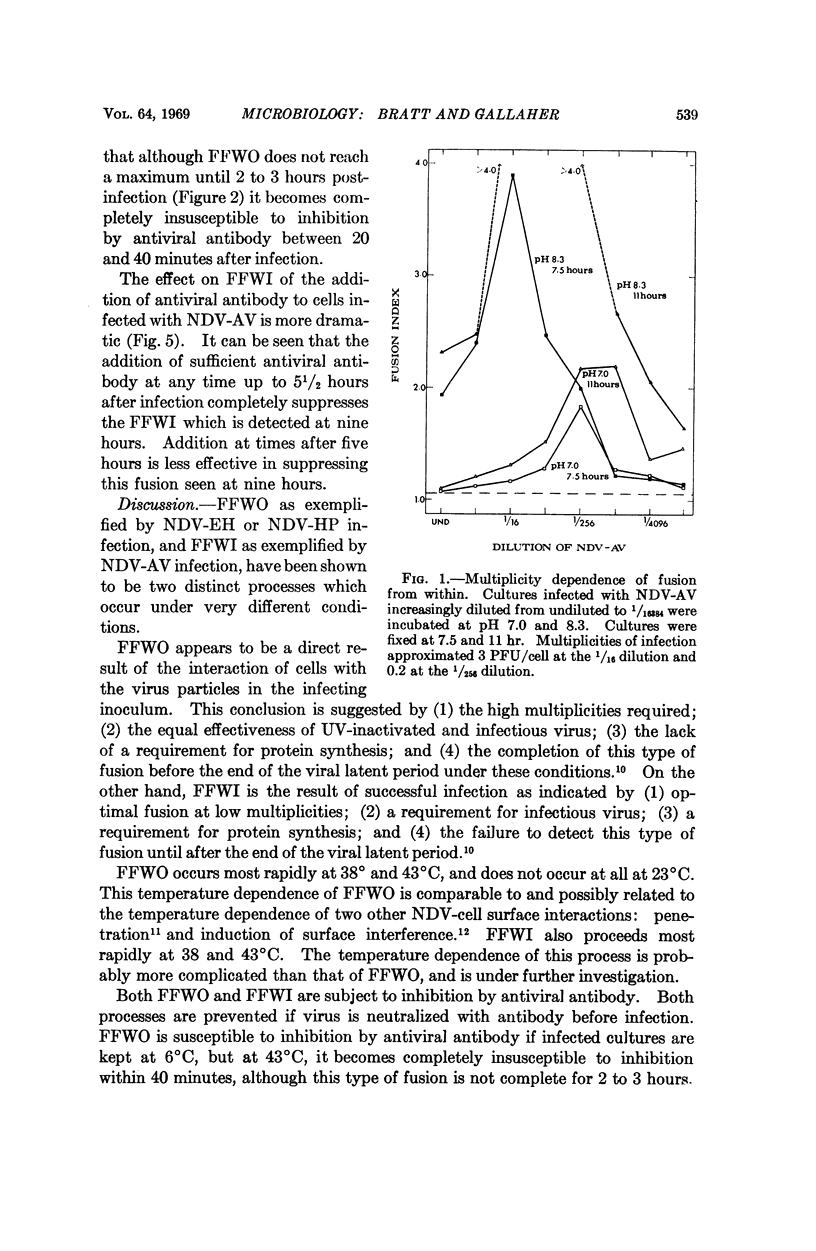
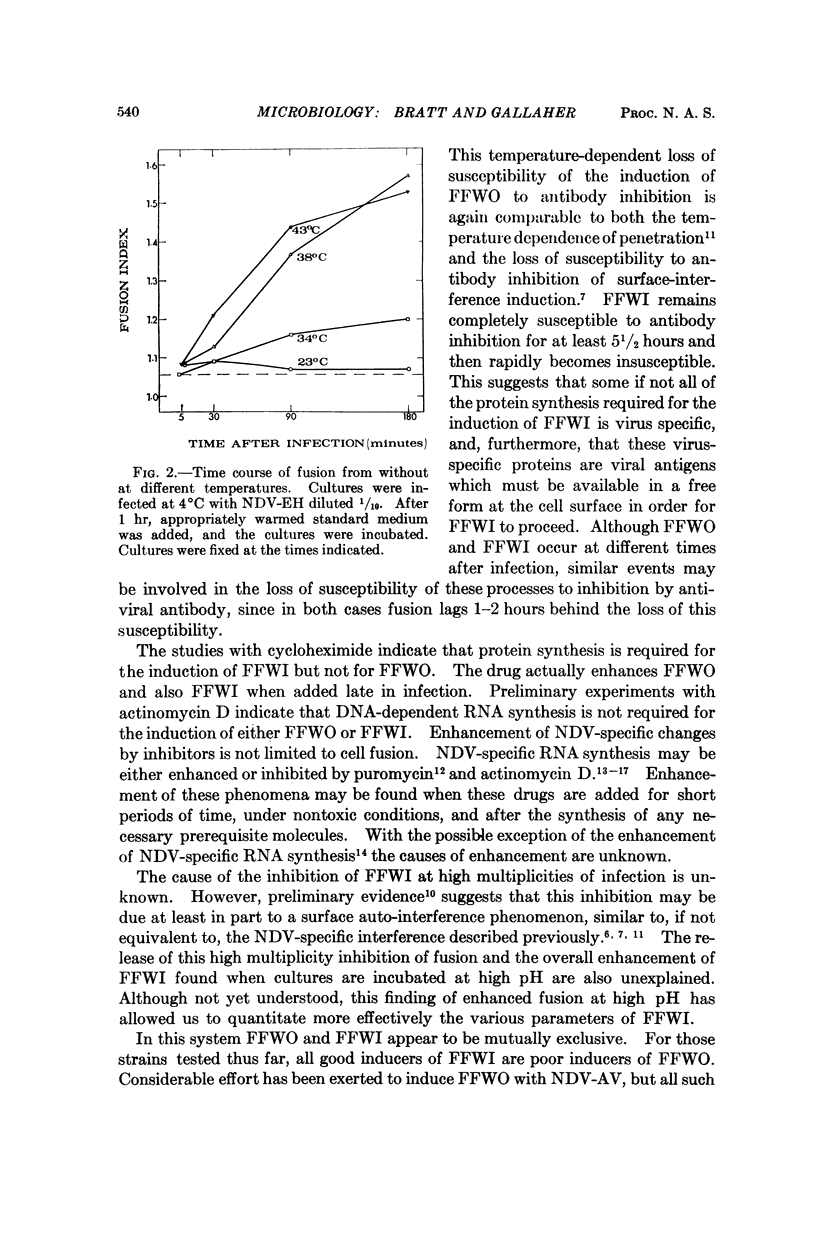
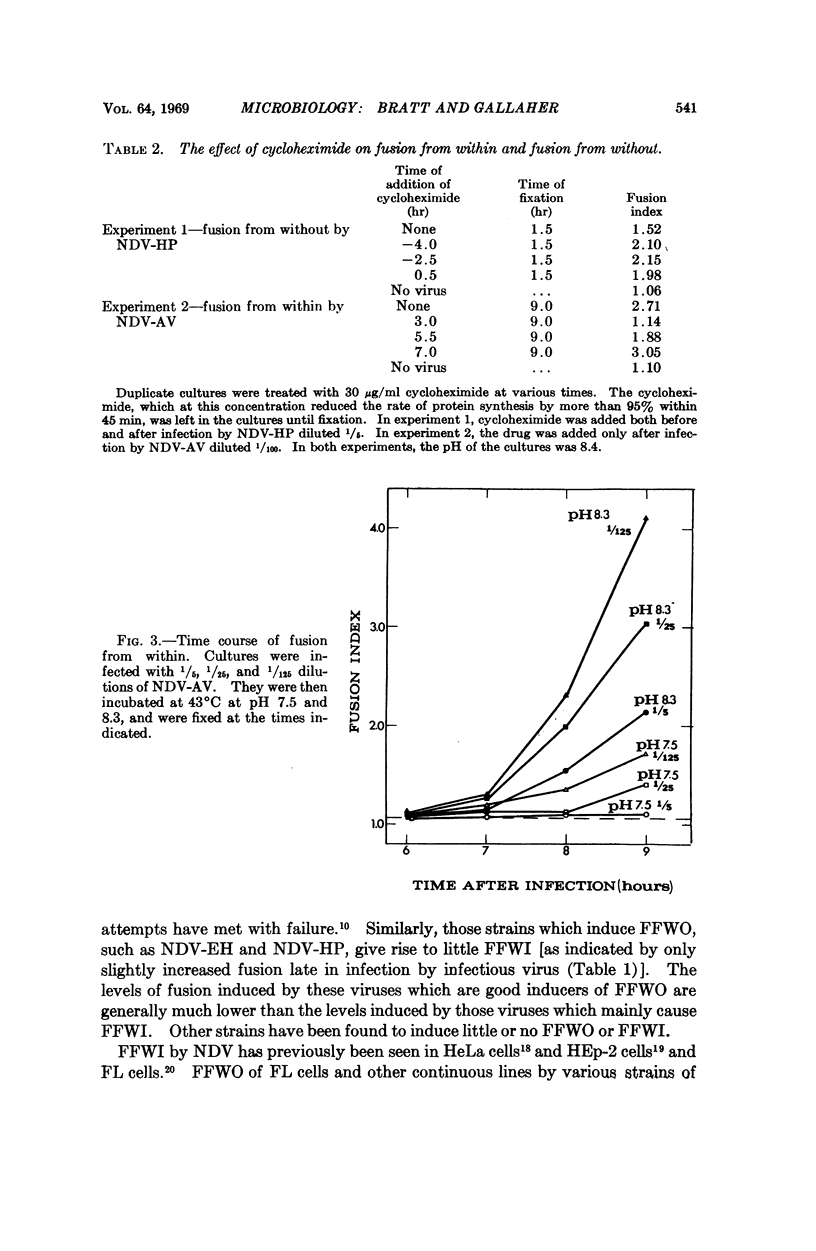
Different strains of Newcastle disease virus vary in their ability to induce cell fusion. When administered to cells at high multiplicities of infection some strains induce cell fusion within three hours. This type of fusion is apparently caused by the virus particles in the inoculum, since it can be induced by noninfectious virus and does not require protein synthesis for induction. It has been designated fusion from without (FFWO). Other strains induce fusion mainly at low multiplicities of infection. This fusion is induced only by infectious virus, and requires protein synthesis for induction. Probably included among these required proteins is a viral antigen which must be available at the cell surface in order for fusion to occur. This type of fusion has been designated fusion from within (FFWI).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY R. D., IVES D. R., CRUICKSHANK J. G. Participation of deoxyribonucleic acid in the multiplication of influenza virus. Nature. 1962 Jun 23;194:1139–1140. doi: 10.1038/1941139a0. [DOI] [PubMed] [Google Scholar]
- Bratt M. A. RNA synthesis in NDV-infected chick embryo cells treated with different concentrations of actinomycin D. Virology. 1969 Sep;39(1):141–145. doi: 10.1016/0042-6822(69)90357-2. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Rubin H. Specific interference among strains of Newcastle disease virus. 3. Mechanisms of interference. Virology. 1968 Jul;35(3):395–407. doi: 10.1016/0042-6822(68)90218-3. [DOI] [PubMed] [Google Scholar]
- CASCARDO M. R., KARZON D. T. MEASLES VIRUS GIANT CELL INDUCING FACTOR (FUSION FACTOR). Virology. 1965 Jun;26:311–325. doi: 10.1016/0042-6822(65)90279-5. [DOI] [PubMed] [Google Scholar]
- DAS M. S., GOLDBERG H. S. Inclusion bodies from Newcastle disease virus in HeLa cells. J Bacteriol. 1961 Jul;82:151–152. doi: 10.1002/path.1700820118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders J. F., Holloway A., Grogan E. A. Replication of poliovirus I in chick embryo and hamster cells exposed to sendai virus. Proc Natl Acad Sci U S A. 1967 Mar;57(3):637–644. doi: 10.1073/pnas.57.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- HARRIS H., WATKINS J. F. HYBRID CELLS DERIVED FROM MOUSE AND MAN: ARTIFICIAL HETEROKARYONS OF MAMMALIAN CELLS FROM DIFFERENT SPECIES. Nature. 1965 Feb 13;205:640–646. doi: 10.1038/205640a0. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON C. F., SCOTT A. D. CYTOLOGICAL STUDIES OF NEWCASTLE DISEASE VIRUS (NDV) IN HEP-2 CELLS. Proc Soc Exp Biol Med. 1964 Feb;115:281–286. doi: 10.3181/00379727-115-28891. [DOI] [PubMed] [Google Scholar]
- KINGSBURY D. W. Use of actinomycin D to unmask RNA synthesis induced by Newcastle disease virus. Biochem Biophys Res Commun. 1962 Sep 25;9:156–161. doi: 10.1016/0006-291x(62)90106-7. [DOI] [PubMed] [Google Scholar]
- KOHN A. POLYKARYOCYTOSIS INDUCED BY NEWCASTLE DISEASE VIRUS IN MONOLAYERS OF ANIMAL CELLS. Virology. 1965 Jun;26:228–245. doi: 10.1016/0042-6822(65)90050-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus complementary RNA: its relationship to the viral genome and its accumulation in the presence or absence of actinomycin D. Virology. 1967 Oct;33(2):227–233. doi: 10.1016/0042-6822(67)90141-9. [DOI] [PubMed] [Google Scholar]
- Kohn A., Fuchs P. Cell fusion by various strains of Newcastle disease virus and their virulence. J Virol. 1969 May;3(5):539–540. doi: 10.1128/jvi.3.5.539-540.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A., Klibansky C. Studies on the inactivation of cell-fusing property of Newcastle disease virus by phospholipase A. Virology. 1967 Feb;31(2):385–388. doi: 10.1016/0042-6822(67)90183-3. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. III. Relationship between cell condition and fusion reaction or cell degeneration reaction. Exp Cell Res. 1962 Feb;26:119–128. doi: 10.1016/0014-4827(62)90207-0. [DOI] [PubMed] [Google Scholar]
- OKADA Y., TADOKORO J. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. II. Quantitative analysis of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:108–118. doi: 10.1016/0014-4827(62)90206-9. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Fatty acid composition of three strains of Newcastle disease virus. Virology. 1969 Mar;37(3):492–494. doi: 10.1016/0042-6822(69)90237-2. [DOI] [PubMed] [Google Scholar]



