Abstract
Cognitive dysfunctions are commonly seen in many stress-related disorders, including anxiety and depression—the world's most common neuropsychiatric illnesses. Various genetic, pharmacological, and behavioral animal models have long been used to establish animal anxiety-like and depression-like phenotypes, as well as to assess their memory, learning, and other cognitive functions. Mounting clinical and animal evidences strongly supports the notion that disturbed cognitions represent an important pathogenetic factor in anxiety and depression, and may also play a role in integrating the two disorders within a common stress-precipitated developmental pathway. This paper evaluates why and how the assessment of cognitive and emotional domains may improve our understanding of animal behaviors via different high-throughput tests and enable a better translation of animal phenotypes into human brain disorders.
1. INTRODUCTION
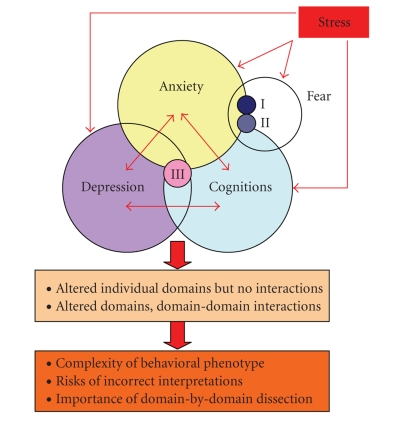
Cognitive processes play a key role in stress-related neuropsychiatric disorders, including emotional disorders such as anxiety and depression [1–5] (Figure 1). Abundant clinical and animal evidences strongly support this notion, suggesting that disturbed cognitions per se are an important part of affective illnesses, helping integrate the two disorders within a common stress-precipitated pathogenesis [6–10]. Indeed, strong negative memories play a key role not only in different subtypes of anxiety (especially in post-traumatic stress disorder or specific phobias) [6, 11–14], but also in depression and suicidality [15–20]. These findings are further supported by recent data from psychiatric genetics [2, 21–25] and brain imaging [26–29], showing how altered cognitions, associated with genetic contributions and inherited brain anatomy and physiology traits, modify emotional regulation of stress, anxiety, and depression.
Figure 1.
Interplay between fear, anxiety (including posttraumatic stress (I), and phobic disorders (II)), depression (including recurrent depression associated with negative memories (III) and cognitive domains in experimental models of neuropsychiatric disorders.
Animal experimental models of brain disorders are an indispensable tool in today's biomedical research [5, 30–32]. Animal memory-anxiety and memory-depression interplays, as well as the genetics, pharmacology, and neurophysiology of this interplay, have been comprehensively evaluated in several reviews [33–36], further strengthening the importance of memory assessment in behavioral phenotyping [37–41].
Do we routinely do this? Clearly not, as there exist several objective and subjective reasons. First, there is a traditional dichotomy between “emotional” domains (such as anxiety and depression) and “cognitive” domains (such as memory and learning) in behavioral neuroscience. Albeit relatively artificial, these boundaries somehow seem to preprogram researchers, who often enter (and remain loyal until the retirement party) the field as either “stress scientists” or “memory researchers.” While some inquisitive scholars may subsequently move from one “cast” to another during their careers, in many cases it is the initial professional choice, triggered by personal preferences and reinforced by age-dependent conservatism, that dictates the whole line of subsequent behavioral research of a scientist. Sadly, such heterogeneity often further divides behavioral neuroscientists, who sometimes tend to attend only specialized meetings within their “own” domains, concepts and paradigms.
Another reality is that “anxiety” or “depression” laboratories rather rarely study memory and learning phenotypes in depth (and vice versa), and do so mostly when a gross cognitive deficit is apparent and seems to influence all outgoing animal behaviors. In many such cases, memory testing becomes rather formal, is limited to selected “reference” memory tests, and does not focus on complex interactions between memory, anxiety, and depression domains (see, however, several encouraging exceptions discussed further).
Likewise, despite a growing recognition of the deleterious consequences of restricted behavioral battery usage [42, 43], current routine problems of an average behavioral laboratory include limits in testing and animal holding space, the lack of proper behavioral training, personnel, limited research budgets, or all of them together. Collectively, this leads to an extensive use and reuse of animals in high-throughput batteries [44–46]. In reality, this means that emotionality (e.g., anxiety and depression) tests are routinely run in the same cohorts of animals with relatively little attention to possible cognitive mechanisms or alterations that are triggered by such batteries, and that may, in fact, influence dramatically the subsequent behavioral scores of “anxiety” and “depression” [44]. Furthermore, learning and memory per se may also be affected by such batteries [44], further complicating behavioural phenotyping, and most likely exerting secondary effects on anxiety and depression.
Is this of concern? Can our routine laboratory practice lead to confounded findings and, even worse, potential misinterpretations of data? The aim of this paper is to analyze why and how an in-depth assessment of cognitive and emotional domains may improve our understanding of animal behaviors in different high-throughput tests, and their translation into human behavioral disorders.
2. TARGETING MEMORY-ANXIETY INTERPLAY IN ANIMAL BEHAVIORAL MODELS
Learning, memory, and anxiety have long been known as interactive dimensions in both animal and clinical studies [47, 48]. The importance of in-depth assessment of memory and anxiety together is further illustrated in Table 1. The interplay of these two domains in this table may hypothetically lead to multiple alternative states, whose misinterpretations in different behavioral tests (as well as psychopharmacological data obtained in such models) would generally be unavoidable if only single domains were assessed (also see: [31, 32] for discussion). In a similar vein, a recent review [41] has evaluated anxiety and memory/learning phenotypes in various genetically modified mouse models, including mutant mice lacking various receptors or other brain proteins. A common (but not mandatory) situation noted in this study, when the same mutation leads to simultaneously altered anxiety and memory phenotypes, illustrates the overlap between these two key domains, and demonstrates the extent to which their interplay may affect other animal outgoing behaviors.
Table 1.
Examples of possible interplay between memory and anxiety domains, and how this may lead to misinterpreted animal behavioral and drug-induced phenotypes (effects: ↑ increased, ↓ reduced behavior). Note that real animal models have multiple other factors and domains, and the complexity (and risks of incorrect interpretation) of their phenotypes is much higher.
| Domains | Anxiety | ||
|---|---|---|---|
| Memory, learning | Elevated | Unaltered | Reduced |
| Elevated | Likely phenotype: ↑ initial anxiety (↓ activity) with ↑ habituation (anxiolytics would ↓ hypoactivity and habituation). Possible misinterpretation of baseline phenotype: hyperanxiety; ↓ sensitivity to repeated stressors (while, in fact, having ↑ vulnerability to chronic stress) | Likely phenotype: ↑ habituation [anxiolytics would ↑ activity and ↓ habituation]. Possible misinterpretation: ↓ exploration (↑ anxiety). Anxiolytics would ↓ habituation (however, this may be mistaken for ↓ anxiety) | Likely phenotype: ↓ initial anxiety with ↑ habituation (anxiolytics would ↓ habituation) Possible misinterpretation: initial hyperactivity followed by ↑ freezing (“↑ anxiety”). Anxiolytics will ↓ habituation (however, this may be mistaken for mild psychostimulant action) |
|
| |||
| Unaltered | Likely phenotype: ↑ anxiety (↓ exploration), normal memory. Anxiolytics may ↓ anxiety and memory. In some tests phenotype may be misinterpreted as baseline hypolocomotion | Likely phenotype: reduced anxiety (↑ exploration), normal memory. Anxiolytics may impair memory without affecting (already low) anxiety In some tests baseline phenotype may be misinterpreted as hyperactivity | |
|
| |||
| Reduced | Likely phenotype: ↑ initial anxiety with ↓ habituation. Anxiolytics may ↓ anxiety and further impair memory. Possible misinterpretation of baseline phenotype: hypersensitivity to repeated stressors (while, in fact, having ↓ vulnerability to chronic stress). Effects of anxiolytics may be mistaken for psychostimulant action | Likely phenotype: ↓ habituation. Anxiolytics may further impair memory. Possible misinterpretation of baseline phenotype: ↑ exploration (↓ anxiety). Effects of anxiolytics may be mistaken for psychostimulant action | Likely phenotype: ↓ initial anxiety with ↓ habituation (anxiolytics may ↓ memory). In some tests may be misinterpreted as persistent hyperlocomotion. Effects of anxiolytics may be mistaken for psychostimulant action |
In fact, some of phenotypes that we do observe in different models strikingly parallel hypothetical situations modeled in Table 1 (see, for example, altered anxiety and cognitions in 5-HT1a and 5-HT1b receptor knockout mice, and the ways to dissect their possible interplay, in [30–32]). Adding further complexity to the problem, it is always important to consider potential heterogeneity of memory subtypes, as the same mutation (such as 5-HT1b receptor knockout) may impair one type of memory (e.g., habituation) while improving another (e.g., spatial memory) [30].
Several other interesting directions of research may be considered further, based on specific targeting of memory-anxiety interplay. For example, as some subtypes of anxiety problems, such as post-traumatic stress disorder (PTSD), are based on strong aversive memories, genetic and behavioral models with both high anxiety and memory components [41, 49, 50] may lead to more valid experimental models of PTSD. However, some difficulties may also be likely with such models, as PTSD-like hyperarousal, commonly observed both clinically and in animals [49], may possibly be misinterpreted as increased locomotion (suggestive of anxiolytic-like phenotype). In any case, researchers should be aware of such interpretational difficulties, and make their conclusions with necessary caution and after testing several alternative hypotheses (see Table 1 for examples).
Finally, genetic models may target reciprocal interplay between these domains that are potentially relevant to mechanisms of stress resistance. Likewise, mice with both reduced anxiety and memory (see [41] for review) may lead to genetic models focused on mechanisms of resistance to PTSD and other types of anxiety associated with recurrent negative cognitions (see [6, 47]).
3. MODELING MEMORY-DEPRESSION INTERPLAY
The importance of cognitive mechanisms in clinical depression has long been known in the literature [51]. Indeed, we need to remember our past traumas and frustrations in order to become properly depressed. Memory and learning have also been considered in animal models of depression (e.g., see[52]). How can we apply this understanding to our experimental models and do it correctly? Table 2 summarizes a hypothetical situation where two interplaying domains (depression and memory) may lead to multiple alternative states, whose misinterpretations in different behavioral tests seem to be highly likely.
Table 2.
Examples of possible interplay between memory and depression domains, that may lead to misinterpreted animal behavioral phenotypes (effects: ↑ increased, ↓ reduced behavior; OCD-obsessive-compulsive disorder). Given high research pressure on behavioral labs, consider the likelyhood of incorrect interpretation of behavioral data.
| Domains | Depression | ||
|---|---|---|---|
| Memory, learning | Elevated | Unaltered | Reduced |
| Elevated | Likely phenotype: hypoactivity (or stereotypic hyperactivity in some tests) but ↑ sensitivity to repeated stressors. Possible misinterpretation of baseline phenotype: ↑ anxiety/freezing (or ↓ habituation, spatial memory in acute stress models) | Likely phenotype: ↑ habituation and ↑ sensitivity to repeated stressors. Possible misinterpretations: ↓ exploration (↑ anxiety) and ↑ despair depression | Likely phenotype: active locomotion with ↑ habituation and sensitivity to repeated stressors. Possible misinterpretations: initial hyperactivity followed by gradually ↑ anxiety, or ↑ “despair” depression (which, in fact, reflects ↑ learning) |
|
| |||
| Unaltered | Likely phenotype: ↓ hypoactivity (or stereotypic hyperactivity in some tests). Possible misinterpretation: ↑ anxiety/freezing (or ↓ habituation, spatial memory) | Likely phenotype: active locomotion. Possible misinterpretation of this phenotype: no or ↓ anxiety | |
|
| |||
| Reduced | Likely phenotype: marked sustained hypoactivity (or stereotypic hyperactivity) with ↓ habituation and sensitivity to repeated stressors. Possible misinterpretations: ↑ anxiety (and/or OCD-like behavior) or ↓ despair depression | Likely phenotype: ↓ habituation. Possible misinterpretation: ↑ exploration (↓ anxiety) | Likely phenotype: active locomotion with ↓ habituation and sensitivity to repeated stressors. In some tests this may be misinterpreted as persistent hyperlocomotion |
Some interesting experimental models of neuropsychiatric disorders may arise from specific targeting of memory-depression interplay. For example, since recurrent intrusive negative memories frequently accompany clinical depression [53–56], animal models based on simultaneously increased memories and depression-like phenotypes [52, 57–59] may be clinically relevant to modeling affective disorders associated with negative cognitions. In contrast, mouse models with cooccurring memory deficits and reduced depression-related behaviors (such as 5-HT1a knockout mice, see [60]) may be potentially useful to understand mechanisms of resistance to depression associated with chronic negative memories [61].
4. MODELING WITHIN AND BEYOND
With recent strategies of behavioral modeling of anxiety and depression (see [62]) supporting expansion beyond “pure” anxiety and depression domains, experimental models based on targeting these plus cognitive domains represent further important directions of research. One strategy may be to apply more extensively the models and tests that simultaneously profile anxiety (or depression) and memory functions. Conceptualized as behavioral “models-hybrids” [62, 63], this approach allows minimization of the unwanted behavioral consequences of test batteries, and provides an extensive high-throughput phenotyping of animals with a fewer number of procedures. For example, increased anxiety in the elevated plus maze and the loss of benzodiazepine anxiolytic efficacy upon repeated testing [48] may be used to indirectly assess memory functions in different mutant or drug-treated animals, as evaluated by the presence or absence of the above-mentioned “one trial tolerance” phenomenon. Likewise, the forced swim test (measuring “despair” depression domain) may be used to assess within- and between-trial habituation (spatial working and long-term memory) and learned helplessness. Fear conditioning, including active avoidance tests [64, 65]) are highly relevant to both fear (anxiety-related) and cognitive (learning) domains. Y- and T-mazes allow parallel assessment of spatial memory, exploration (anxiety), and spontaneous alternation. Morris water maze, a traditional hippocampal memory test, can also be used to study depression-like traits (e.g., immobility in [66, 67]). Finally, various elevated mazes can be used to profile cognitive domains (memory, learning) as well as animal anxiety [68, 69].
In general, there may be other combinations of anxiety, depression and memory tests, or even more sophisticated hybrid models, that could be used more extensively for high-throughput behavioral phenotyping. However, another reason to use these models more widely in behavioral research is the possibility of performing an integrative (versus more traditional, domain-oriented) experimental modeling of brain disorders. This approach, based on targeting commonalities (rather than differences) of disorders, will allow researchers to parallel their animal models with recent trends in clinical psychiatry, where “continuum” or “spectrum” theories are beginning to challenge the existing “heterogeneous” Kraepelinian paradigms [70–72].
An important step in this direction may be the use of rodent models that simultaneously evaluate “comorbid” anxiety and depression and also focus on cognitive (dys)functions in these models. For example, selectively bred HAB mice [52] and thyroid hormone receptor knockout mice [9] display inherited anxiety- and depression-like phenotypes, and their cognitive functions merit further studies (see, e.g., aberrant memory in the latter model). Similarly, olfactory bulbectomy, traditionally known to produce depression in rodents, has been recently reported to be relevant to comorbidity of anxiety and depression, and is accompanied by specific memory deficits in animals that resemble cognitive dysfunctions in humans with comorbid anxiety and depression [5].
Further important information can also be obtained through in-depth ethological analyses of behavioral strategies, including cross-species and cross-strain comparisons [73, 74] of animal behaviors in different tests—an approach consistent with recent endophenotyping and cross-species trait genetics concepts in animal behavioral modeling [75, 76]. Finally, expanding far beyond anxiety and depression domains may also be a rational strategy of research, as it allows modeling of complex schizo-affective and neurodevelopmental disorders based on increased anxiety, depression and altered memory, and other cognitions [77–80].
5. CONCLUDING REMARKS
To optimize behavioral phenotyping research, the neuroscientific community may need to encourage behavioral neuroscientists to produce data on memory and learning phenotypes in their papers that report anxiety- and depression-related behaviors (e.g., [30, 31, 60]). As a practical solution, “can my findings be a result of merely altered memory or learning?” should be one of the first questions asked in studies on animal emotionality and affective behaviors. In cases when both cognitive and emotionality domains seem to be affected (e.g., [81, 82]), we next need to establish the nature of their interactions, and how they might codetermine the behavioral phenotype observed. Finally, in addition to studying behavior x gene x environment interactions, we may benefit from focusing on behavior x cognitions x gene x environment interactions. “Work hard and marry a talent”—advised R. Blanchard in one of his interviews, sharing with fellow colleagues the recipe for a successful career in science. Following such wise advice, diligent behavioral neuroscientists working with anxiety and depression may benefit from joining forces with (and even perhaps marrying) their talented colleagues studying memory and learning.
ACKNOWLEDGMENT
This research is supported by the NIMH/NIH Intramural Research Program.
References
- 1.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews. 2005;29(3):399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Miles H, MacLeod AK, Pote H. Retrospective and prospective cognitions in adolescents: anxiety, depression, and positive and negative affect. Journal of Adolescence. 2004;27(6):691–701. doi: 10.1016/j.adolescence.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Waikar SV, Craske MG. Cognitive correlates of anxious and depressive symptomatology: an examination of the Helplessness/Hopelessness model. Journal of Anxiety Disorders. 1997;11(1):1–16. doi: 10.1016/s0887-6185(96)00031-x. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Noda Y, Tsunekawa H, et al. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behavioural Brain Research. 2007;178(2):262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. doi: 10.1002/da.20262. to appear in Depression and Anxiety . [DOI] [PubMed] [Google Scholar]
- 7.Rustay NR, Wrenn CC, Kinney JW, et al. Galanin impairs performance on learning and memory tasks: findings from galanin transgenic and GAL-R1 knockout mice. Neuropeptides. 2005;39(3):239–243. doi: 10.1016/j.npep.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behavioural Brain Research. 2006;170(1):126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor . Behavioural Brain Research. 2007;177(1):109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. The Mount Sinai Journal of Medicine. 2006;73(7):941–949. [PubMed] [Google Scholar]
- 11.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. Journal of Psychiatric Research. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel A, Cochran CK. Autobiographical memories prompted by automatic thoughts in panic disorder and social phobia. Cognitive Behaviour Therapy. 2006;35(3):129–137. doi: 10.1080/16506070600583130. [DOI] [PubMed] [Google Scholar]
- 13.Vasa RA, Roberson-Nay R, Klein RG, et al. Memory deficits in children with and at risk for anxiety disorders. Depression and Anxiety. 2007;24(2):85–94. doi: 10.1002/da.20193. [DOI] [PubMed] [Google Scholar]
- 14.D'Argembeau A, van der Linden M, d'Acremont M, Mayers I. Phenomenal characteristics of autobiographical memories for social and non-social events in social phobia. Memory. 2006;14(5):637–647. doi: 10.1080/09658210600747183. [DOI] [PubMed] [Google Scholar]
- 15.Pine DS, Lissek S, Klein RG, et al. Face-memory and emotion: associations with major depression in children and adolescents. Journal of Child Psychology and Psychiatry. 2004;45(7):1199–1208. doi: 10.1111/j.1469-7610.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- 16.Gilboa-Schechtman E, Erhard-Weiss D, Jeczemien P. Interpersonal deficits meet cognitive biases: memory for facial expressions in depressed and anxious men and women. Psychiatry Research. 2002;113(3):279–293. doi: 10.1016/s0165-1781(02)00266-4. [DOI] [PubMed] [Google Scholar]
- 17.Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Current Opinion in Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 18.Gould NF, Holmes MK, Fantie BD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. American Journal of Psychiatry. 2007;164(3):516–519. doi: 10.1176/ajp.2007.164.3.516. [DOI] [PubMed] [Google Scholar]
- 19.Pendse BP, Engström G, Träskman-Bendz L. Psychopathology of seasonal affective disorder patients in comparison with major depression patients who have attempted suicide. Journal of Clinical Psychiatry. 2004;65(3):322–327. doi: 10.4088/jcp.v65n0306. [DOI] [PubMed] [Google Scholar]
- 20.Williams JMG, Scott J. Autobiographical memory in depression. Psychological Medicine. 1988;18(3):689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
- 21.Payton A. Investigating cognitive genetics and its implications for the treatment of cognitive deficit. Genes, Brain, and Behavior. 2006;5(1):44–53. doi: 10.1111/j.1601-183X.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 22.Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of Disc1—an emerging role in psychosis and cognition. Biological Psychiatry. 2006;60(2):123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Kalueff AV, Wheaton M, Ren-Patterson R, Murphy DL. Brain-derived neurotrophic factor, serotonin transporter, and depression: comment on Kaufman et al. Biological Psychiatry. 2007;61(9):1112–1113. doi: 10.1016/j.biopsych.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Savitz JB, Solms M, Ramesar RS. Neurocognitive function as an endophenotype for genetic studies of bipolar affective disorder. NeuroMolecular Medicine. 2005;7(4):275–286. doi: 10.1385/NMM:7:4:275. [DOI] [PubMed] [Google Scholar]
- 25.Akiskal HS, Akiskal KK, Perugi G, Toni C, Ruffolo G, Tusini G. Bipolar II and anxious reactive “comorbidity”: toward better phenotypic characterization suitable for genotyping. Journal of Affective Disorders. 2006;96(3):239–247. doi: 10.1016/j.jad.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. Journal of Affective Disorders. 2006;94(1–3):219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Roberson-Nay R, McClure EB, Monk CS, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an FMRI study. Biological Psychiatry. 2006;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Frodl T, Schaub A, Banac S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. Journal of Psychiatry and Neuroscience. 2006;31(5):316–325. [PMC free article] [PubMed] [Google Scholar]
- 29.Emdad R, Bonekamp D, Söndergaard HP, et al. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychotherapy and Psychosomatics. 2006;75(2):122–132. doi: 10.1159/000090897. [DOI] [PubMed] [Google Scholar]
- 30.Malleret G, Hen R, Guillou J-L, Segu L, Buhot M-C. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. The Journal of Neuroscience. 1999;19(14):6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT1A receptor KO mice. Biological Psychiatry. 2000;48(12):1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- 32.Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31(1):101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- 33.Clement Y, Chapouthier G. Biological bases of anxiety. Neuroscience and Biobehavioral Reviews. 1998;22(5):623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 34.Chapouthier G, Venault P. GABA-A receptor complex and memory processes. Current Topics in Medicinal Chemistry. 2002;2(8):841–851. doi: 10.2174/1568026023393552. [DOI] [PubMed] [Google Scholar]
- 35.Beuzen A, Belzung C. Link between emotional memory and anxiety states: a study by principal component analysis. Physiology and Behavior. 1995;58(1):111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 36.Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends in Cognitive Sciences. 2007;11(2):70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Crawley JN. What's Wrong with My Mouse? Behavioural Phenotyping of Transgenic and Knockout Mice. New York, NY, USA: John Wiley & Sons; 2000. [Google Scholar]
- 38.Hunter AJ, Nolan PM, Brown SDM. Towards new models of disease and physiology in the neurosciences: the role of induced and naturally occurring mutations. Human Molecular Genetics. 2000;9(6):893–900. doi: 10.1093/hmg/9.6.893. [DOI] [PubMed] [Google Scholar]
- 39.Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behavioural Brain Research. 2007;179(2):192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121(2):341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 41.Kalueff AV. Neurobiology of memory and anxiety: from genes to behavior. Neural Plasticity. 2007;2007:12 pages. doi: 10.1155/2007/78171.78171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiology and Behavior. 2001;73(5):705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 43.Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries—II: effect of test interval. Physiology and Behavior. 2006;87(1):95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Võikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes, Brain, and Behavior. 2004;3(1):27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- 45.Tucci V, Lad HV, Parker A, Polley S, Brown SDM, Nolan PM. Gene-environment interactions differentially affect mouse strain behavioral parameters. Mammalian Genome. 2006;17(11):1113–1120. doi: 10.1007/s00335-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 46.Andreatini R, Bacellar LFS. Animal models: trait or state measure? The test-retest reliability of the elevated plus-maze and behavioral despair. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2000;24(4):549–560. doi: 10.1016/s0278-5846(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 47.Kalueff AV, Nutt DJ. Role of GABA in memory and anxiety. Depression and Anxiety. 1997;4(3):100–110. doi: 10.1002/(SICI)1520-6394(1996)4:3<100::AID-DA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 48.File SE. The interplay of learning and anxiety in the elevated plus maze. Behavioural Brain Research. 1993;58(1-2):199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- 49.Siegmund A, Wotjak CT. Hyperarousal does not depend on trauma-related contextual memory in an animal model of posttraumatic stress disorder. Physiology and Behavior. 2007;90(1):103–107. doi: 10.1016/j.physbeh.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 50.Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006;1071(1):324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- 51.McBride C, Segal Z, Kennedy S, Gemar M. Changes in autobiographical memory specificity following cognitive behavior therapy and pharmacotherapy for major depression. Psychopathology. 2007;40(3):147–152. doi: 10.1159/000100003. [DOI] [PubMed] [Google Scholar]
- 52.El Yacoubi M, Vaugeois J-M. Genetic rodent models of depression. Current Opinion in Pharmacology. 2007;7(1):3–7. doi: 10.1016/j.coph.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biological Psychiatry. 2007;61(2):231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Brewin CR, Hunter E, Carroll F, Tata P. Intrusive memories in depression: an index of schema activation? Psychological Medicine. 1996;26(6):1271–1276. doi: 10.1017/s0033291700035996. [DOI] [PubMed] [Google Scholar]
- 55.Kuyken W, Brewin CR. Intrusive memories of childhood abuse during depressive episodes. Behaviour Research and Therapy. 1994;32(5):525–528. doi: 10.1016/0005-7967(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 56.Peeters F, Wessel I, Merckelbach H, Boon-Vermeeren M. Autobiographical memory specificity and the course of major depressive disorder. Comprehensive Psychiatry. 2002;43(5):344–350. doi: 10.1053/comp.2002.34635. [DOI] [PubMed] [Google Scholar]
- 57.Dubrovina NI, Tomilenko RA. Extinction of a passive avoidance response of mice with a depressive-like state. Russian Physiological Journal. 2006;92(9):1092–1099. [PubMed] [Google Scholar]
- 58.Wu DW, Shen XY, Dong Q, Wang SP, Cheng ZH, Zhang SJ. Effects of tail suspension on learning and memory function of mice. Space Medicine & Medical Engineering. 2000;13(4):244–248. [PubMed] [Google Scholar]
- 59.Dubrovina NI, Loskutova LV, Savost'ianova DA. Effect of forced swimming on the memory track retention in mice with various behavioral stereotypes. Russian Physiological Journal. 2003;89(8):935–942. [PubMed] [Google Scholar]
- 60.Pattij T, Broersen LM, van der Linde J, et al. Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behavioural Brain Research. 2003;141(2):137–145. doi: 10.1016/s0166-4328(02)00345-5. [DOI] [PubMed] [Google Scholar]
- 61.Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63(7):749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- 62.Kalueff AV, Wheaton M, Murphy DL. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behavioural Brain Research. 2007;179(1):1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 63.Kalueff AV. Animal Modeling of Anxiety and Depression. Moscow, Russia: RSBP; 2003. (K.C. Montgomery Memorial Lecture). [Google Scholar]
- 64.Sierra-Mercado D, Jr, Corcoran KA, Lebrón-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. European Journal of Neuroscience. 2006;24(6):1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- 65.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85(3):213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Schulz D, Buddenberg T, Huston JP. Extinction-induced “despair” in the water maze, exploratory behavior and fear: effects of chronic antidepressant treatment. Neurobiology of Learning and Memory. 2007;87(4):624–634. doi: 10.1016/j.nlm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Schulz D, Huston JP, Buddenberg T, Topic B. “Despair” induced by extinction trials in the water maze: relationship with measures of anxiety in aged and adult rats. Neurobiology of Learning and Memory. 2007;87(3):309–323. doi: 10.1016/j.nlm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behavioural Brain Research. 2006;171(1):26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. Models of anxiety: responses of mice to novelty and open spaces in a 3D maze. Behavioural Brain Research. 2006;174(1):9–38. doi: 10.1016/j.bbr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Akiskal HS, Vázquez GH. Widening the borders of the bipolar disorder: validation of the concept of bipolar spectrum. Vertex. 2006;17(69):340–346. [PubMed] [Google Scholar]
- 71.Lara DR, Pinto O, Akiskal K, Akiskal HS. Toward an integrative model of the spectrum of mood, behavioral and personality disorders based on fear and anger traits—I: clinical implications. Journal of Affective Disorders. 2006;94(1–3):67–87. doi: 10.1016/j.jad.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biological Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Cressant A, Besson M, Suarez S, Cormier A, Granon S. Spatial learning in Long-Evans Hooded rats and C57BL/6J mice: different strategies for different performance. Behavioural Brain Research. 2007;177(1):22–29. doi: 10.1016/j.bbr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Deacon RMJ, Thomas CL, Rawlins JNP, Morley BJ. A comparison of the behavior of C57BL/6 and C57BL/10 mice. Behavioural Brain Research. 2007;179(2):239–247. doi: 10.1016/j.bbr.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Kas MJH, Fernandes C, Schalkwyk LC, Collier DA. Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Molecular Psychiatry. 2007;12(4):324–330. doi: 10.1038/sj.mp.4001979. [DOI] [PubMed] [Google Scholar]
- 76.Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes, Brain, and Behavior. 2006;5(2):113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 77.Szumlinski KK, Lominac KD, Kleschen MJ, et al. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes, Brain, and Behavior. 2005;4(5):273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 78.Pelka GJ, Watson CM, Radziewic T, et al. MeCP2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129(4):887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 79.Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Current Opinion in Genetics & Development. 2006;16(3):276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Glynn D, Drew CJ, Reim K, Brose N, Morton AJ. Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Human Molecular Genetics. 2005;14(16):2369–2385. doi: 10.1093/hmg/ddi239. [DOI] [PubMed] [Google Scholar]
- 81.Callaerts-Vegh Z, Beckers T, Ball SM, et al. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. Journal of Neuroscience. 2006;26(24):6573–6582. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koponen E, Võikar V, Riekki R, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLC pathway, reduced anxiety, and facilitated learning. Molecular and Cellular Neuroscience. 2004;26(1):166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]