Abstract
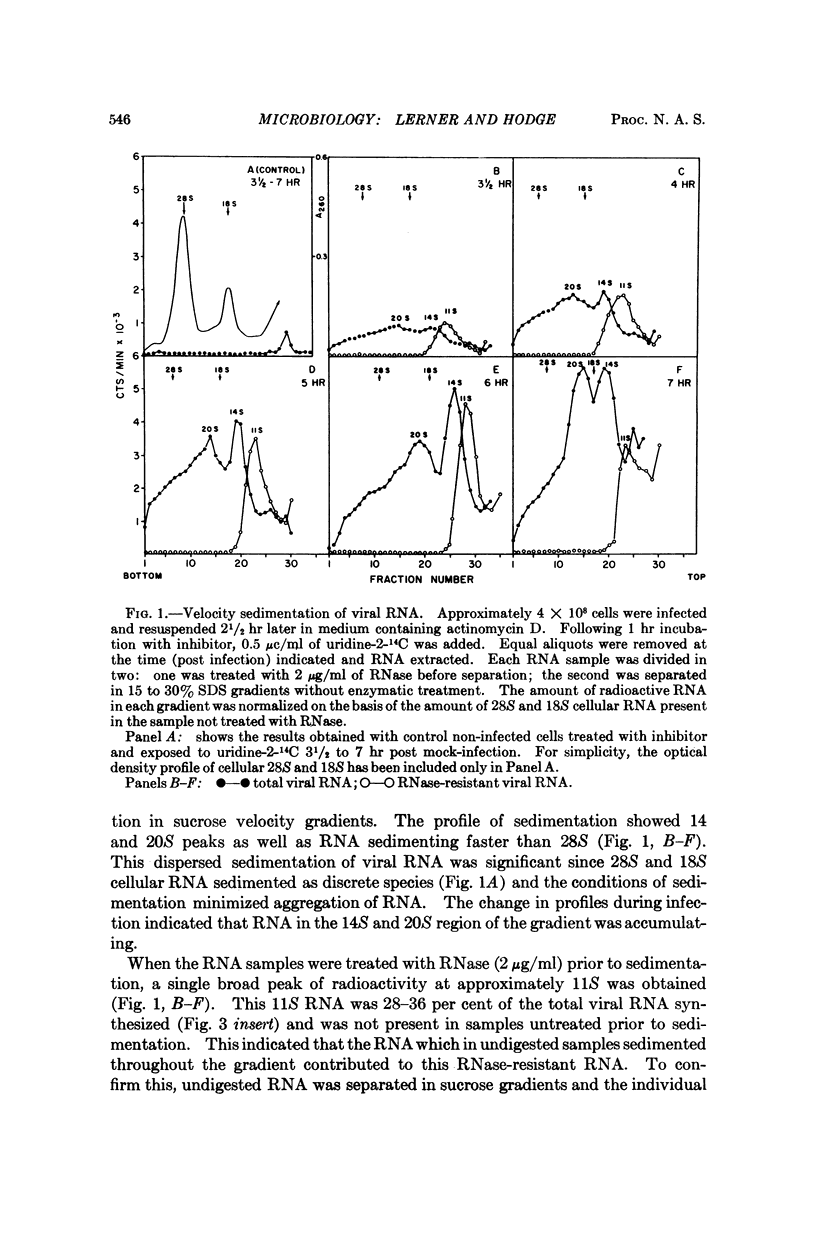
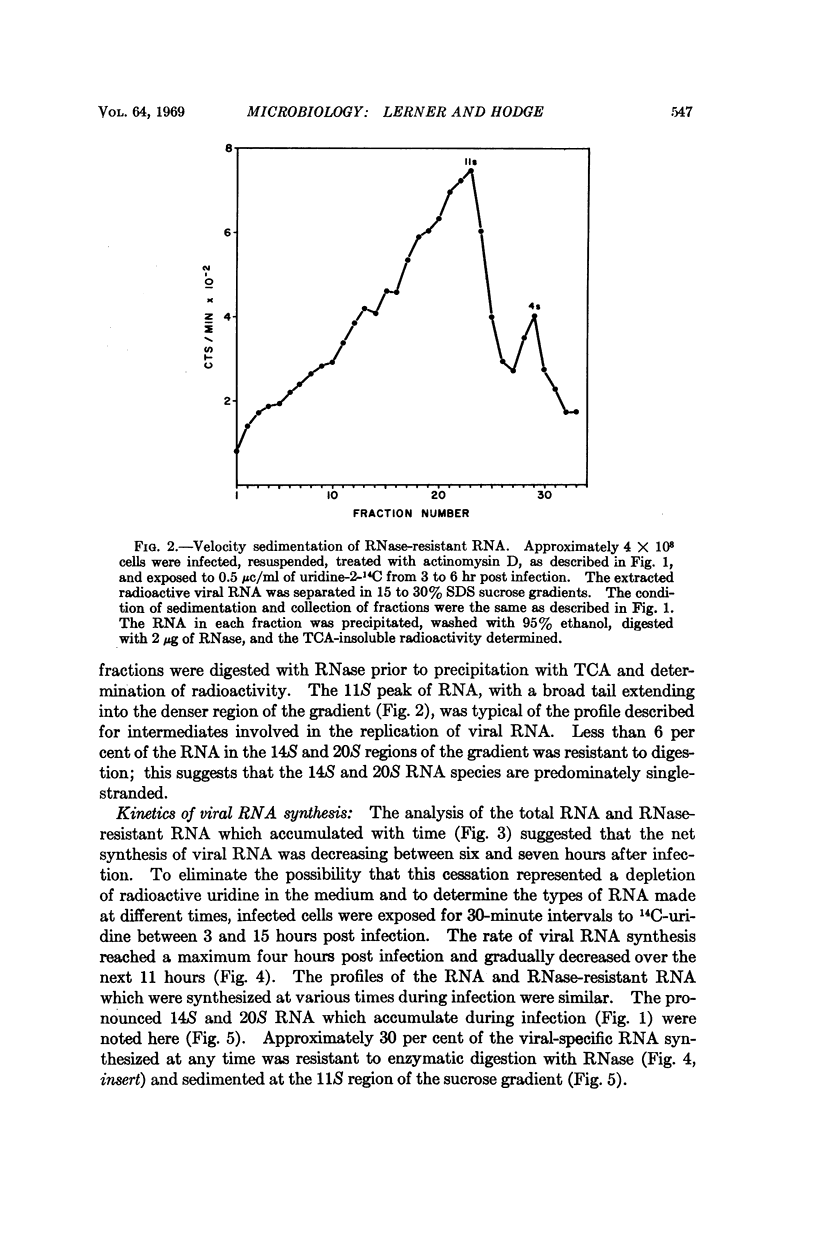
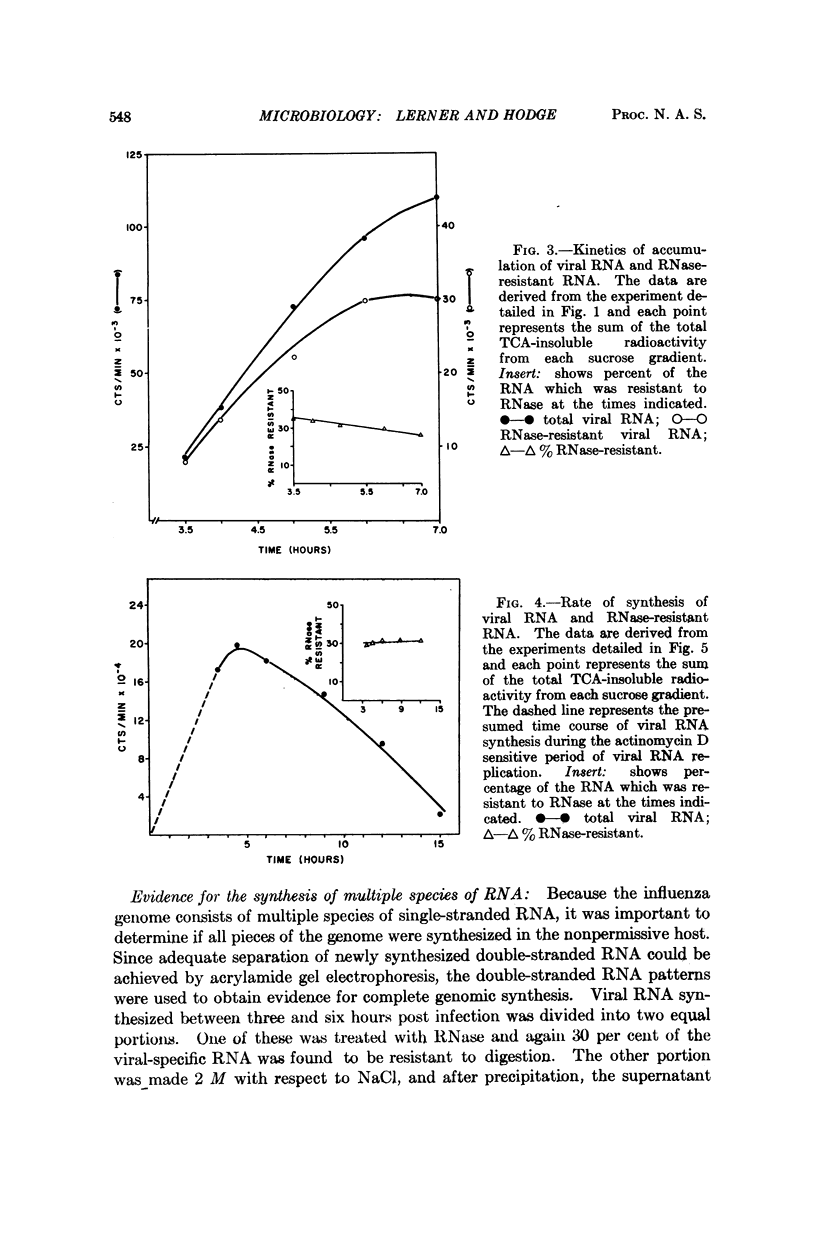
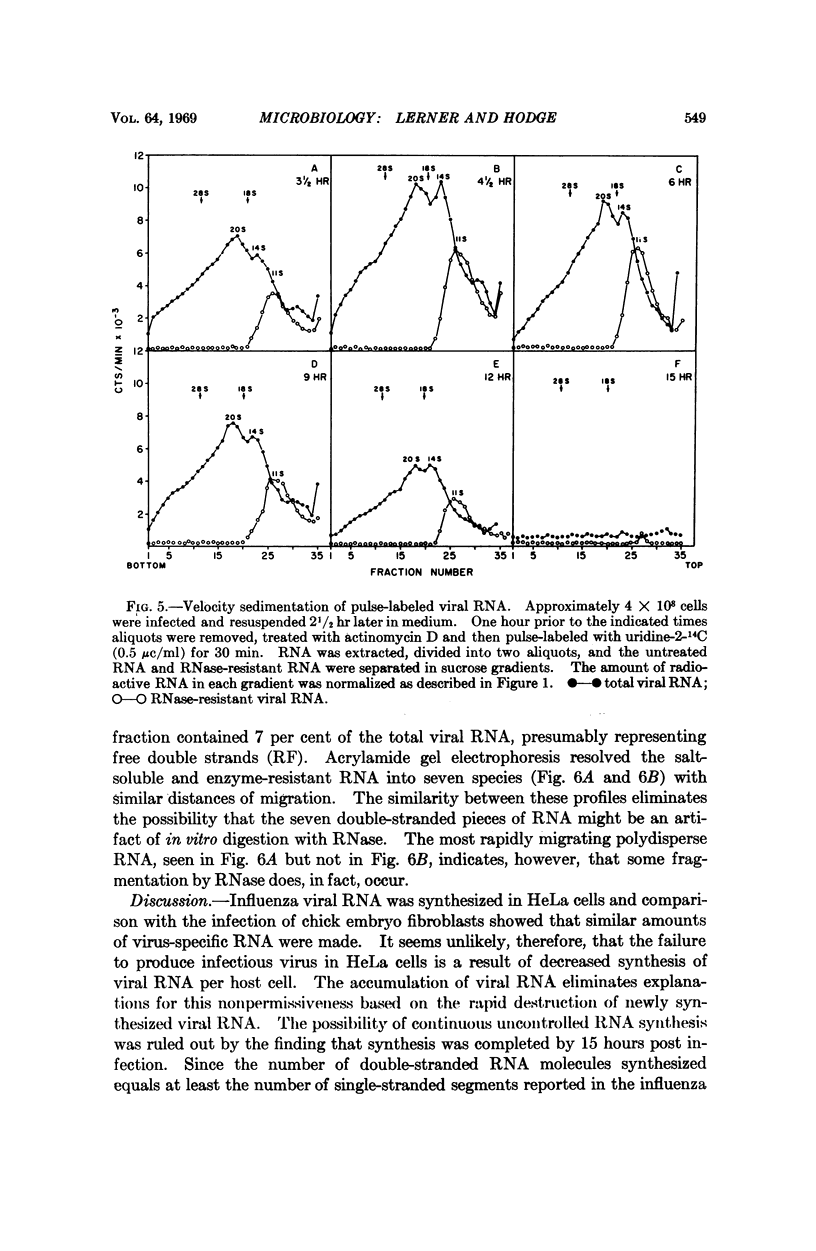
The RNA synthesis of a single-stranded, multisegmented viral genome has been investigated in nonpermissive host cells. In HeLa cells, both single- and double-stranded influenza viral RNA were made and accumulated, and synthesis was completed by 15 hours. The evidence indicated that all pieces of the genome were synthesized. At least as much viral RNA was synthesized in HeLa cells as in permissive chick embryo fibroblasts cells. Explanations for this nonpermissive infection based on reduced synthesis of viral RNA or rapid destruction of newly synthesized RNA, uncontrolled synthesis of RNA, or partial synthesis of the genome appear to have been ruled out by this investigation. The persistently high amounts of double-stranded RNA, combined with the reported lack of dispersion of nucleocapsid protein from the nuclear region of the HeLa cell are consistent with a failure of assembly of the virion in this host. This nonpermissive infection of HeLa cells with influenza virus may offer a unique system for the accumulation of the intermediates involved in the assembly of a complex RNA viral genome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deusberg P. H., Robinson W. S. On the structure and replication of influenza virus. J Mol Biol. 1967 May 14;25(3):383–405. doi: 10.1016/0022-2836(67)90193-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BREITENFELD P. M. The abortive infection of Earle's L-cells by fowl plague virus. Virology. 1959 Jul;8(3):293–307. doi: 10.1016/0042-6822(59)90031-5. [DOI] [PubMed] [Google Scholar]
- HENLE G., GIRARDI A., HENLE W. A non-transmissible cytopathogenic effect of influenza virus in tissue culture accompanied by formation of non-infectious hemagglutinins. J Exp Med. 1955 Jan 1;101(1):25–41. doi: 10.1084/jem.101.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLIS W. D., MOFFAT M. A., HOLTERMANN O. A. The development of soluble (S) and viral (V) antigens of influenza A virus in tissue culture as studied by the fluorescent antibody technique. 3. Studies on the abortive cycle of replication in HeLa cells. Acta Pathol Microbiol Scand. 1960;50:419–429. doi: 10.1111/j.1699-0463.1960.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Hecht L. I., Stephenson M. L., Zamecnik P. C. BINDING OF AMINO ACIDS TO THE END GROUP OF A SOLUBLE RIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1959 Apr;45(4):505–518. doi: 10.1073/pnas.45.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Replication of the RNA of Bacteriophage f2. Science. 1966 Apr 15;152(3720):372–377. doi: 10.1126/science.152.3720.372. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Pons M. Effect of actinomycin D on the replication of influenza virus and influenza virus RNA. Virology. 1967 Sep;33(1):150–154. doi: 10.1016/0042-6822(67)90104-3. [DOI] [PubMed] [Google Scholar]
- Pons M., Hirst G. K. The single- and double-stranded RNA's and the proteins of incomplete influenza virus. Virology. 1969 May;38(1):68–72. doi: 10.1016/0042-6822(69)90128-7. [DOI] [PubMed] [Google Scholar]
- TAMAOKI T., MUELLER G. C. Synthesis of nuclear and cytoplasmic RNA of HeLa cells and the effect of actinomycin D. Biochem Biophys Res Commun. 1962 Nov 27;9:451–454. doi: 10.1016/0006-291x(62)90033-5. [DOI] [PubMed] [Google Scholar]
- Ter Meulen V., Love R. Virological, immunochemical, and cytochemical studies of four HeLa cell lines infected with two strains of influenza virus. J Virol. 1967 Jun;1(3):626–639. doi: 10.1128/jvi.1.3.626-639.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. O., DAY H. M., BATCHELDER E. J., CHEYNE I. M., WANSBROUGH A. J. DELAY IN THE MULTIPLICATION OF INFLUENZA VIRUS. Virology. 1965 Feb;25:289–302. doi: 10.1016/0042-6822(65)90207-2. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]



