Abstract
Several cloned ClC-type Cl− channels open and close in a voltage-dependent manner. The Torpedo electric organ Cl− channel, ClC-0, is the best studied member of this gene family. ClC-0 is gated by a fast and a slow gating mechanism of opposite voltage direction. Fast gating is dependent on voltage and on the external and internal Cl− concentration, and it has been proposed that the permeant anion serves as the gating charge in ClC-0 (Pusch, M., U. Ludewig, A. Rehfeldt, and T.J. Jentsch. 1995. Nature (Lond.). 373:527–531). The deactivation at negative voltages of the muscular ClC-1 channel is similar but not identical to ClC-0. Different from the extrinsic voltage dependence suggested for ClC-0, an intrinsic voltage sensor had been proposed to underlie the voltage dependence in ClC-1 (Fahlke, C., R. Rüdel, N. Mitrovic, M. Zhou, and A.L. George. 1995. Neuron. 15:463–472; Fahlke, C., A. Rosenbohm, N. Mitrovic, A.L. George, and R. Rüdel. 1996. Biophys. J. 71:695–706). The gating model for ClC-1 was partially based on the properties of a point-mutation found in recessice myotonia (D136G). Here we investigate the functional effects of mutating the corresponding residue in ClC-0 (D70). Both the corresponding charge neutralization (D70G) and a charge conserving mutation (D70E) led to an inwardly rectifying phenotype resembling that of ClC-1 (D136G). Several other mutations at very different positions in ClC-0 (K165R, H472K, S475T, E482D, T484S, T484Q), however, also led to a similar phenotype. In one of these mutants (T484S) the typical wild-type gating, characterized by a deactivation at negative voltages, can be partially restored by using external perchlorate (ClO4 −) solutions. We conclude that gating in ClC-0 and ClC-1 is due to similar mechanisms. The negative charge at position 70 in ClC-0 does not specifically confer the voltage sensitivity in ClC-channels, and there is no need to postulate an intrinsic voltage sensor in ClC-channels.
Keywords: gating, myotonia, voltage dependence, anion
introduction
In contrast to a large family of cation channels, in which voltage-sensing elements (probably involving the “S4” segment) are responsible for voltage dependence of gating (for review, see Sigworth, 1994), there is no similar molecular structure in ClC-proteins (for review, see Jentsch, 1996). Yet ClC-channels are clearly voltage dependent. While the gating charge for a single potassium channel was estimated to involve about 12 elementary charges (Schoppa et al., 1992), the voltage dependence in Cl− channels is rather weak, involving only 1–2.2 elementary charges for the different gating processes.
The best characterized member of the ClC family is the voltage-gated Cl− channel from Torpedo electric organ (ClC-0, Jentsch et al., 1990). This channel has two different gating mechanisms of opposite voltage dependence. A fast gate (in the millisecond time range) opens ClC-0 at depolarized voltages with an apparent gating-valence of ≈1 elementary charge (Miller, 1982). A much slower gating mechanism opens ClC-0 at hyperpolarizing voltages with an apparent gating-valence of ≈2.2 elementary charge (Ludewig et al., 1997; Pusch et al., 1997; White and Miller, 1979). ClC-0 wild-type (WT)1 channels are dimers of two identical subunits (Ludewig et al., 1996; Middleton et al., 1996). Each subunit probably forms a single pore (Ludewig et al., 1996). Both pores (subunits) have their own fast gating mechanism (Miller, 1982; Ludewig et al., 1996). The fast gating of single pores is strongly dependent on the extracellular Cl− concentration, and external permeant anions can effectively open the channel. Recent detailed biophysical studies established that the voltage dependence is conferred by the extracellular permeant anion itself (Pusch et al., 1995; Chen and Miller, 1996; Pusch, 1996). In this model, voltage-dependent binding of external Cl− to the closed channel leads to an increase of the opening rate compared with the Cl−-free channel. Such a mechanism differs fundamentally from gating models proposed for voltage-dependent cation channels in which a voltage-dependent movement of charged amino acids through the membrane is thought to confer voltage dependence to the channel proteins. Also the slow gating seems to be affected by the Cl− concentration (Richard and Miller, 1990; Chen and Miller, 1996).
Gating of the homolog ClC-1 looks similar to the fast gating of ClC-0: it acts in a similar time range, has a similar apparent gating-valence of ≈1 elementary charge, and closes the channels at hyperpolarized voltages. The time course of deactivation, however, is not monoexponential as in ClC-0 but involves more than 2 exponential components (Pusch et al., 1994; Fahlke et al., 1996; Rychkov et al., 1996). Despite these similarities, Fahlke et al. (1995, 1996) concluded that fast gating of ClC-1 differs from ClC-0 in the sensitivity to external Cl−: when Cl− was reduced and replaced by MeSO3 −, the open probability was increased compared with the pure Cl− solution. Similar experiments by Rychkov et al. (1996) and by us (unpublished observation) show, however, that the increase of open probability with MeSO3 − is a direct effect of MeSO3 − on the channel, rather than an effect of reduced Cl− concentration. Similar to several external anions, MeSO3 − is able to activate ClC-1 channels. By contrast, replacing external Cl− (e.g., by the “inert” anion glutamate or by glucose) decreases the open probability of ClC-1 (Rychkov et al., 1996), which closely resembles findings with ClC-0. Thus, it is reasonable to conclude that the mechanism(s) of activation are similar in ClC-0 and ClC-1 channels.
Fahlke et al. (1995) analyzed a mutation of ClC-1 (D136G) found in patients affected by recessive Becker-type myotonia (Heine et al., 1994). This mutation drastically altered gating properties: D136G channels activate at negative potentials, and the steady-state current voltage relationship is inwardly rectifying (Fahlke et al., 1995). The negatively charged aspartate that is neutralized in the mutant (D136) was proposed to be a voltage sensor of ClC-1 (Fahlke et al., 1995, 1996). Because of the close homology of ClC-0 and ClC-1, the highly conserved aspartate in domain D1 should then serve as a voltage sensor also in ClC-0.
To test this hypothesis we introduced the corresponding mutation in ClC-0 (D70G in ClC-0). This mutant has a similar phenotype: currents activate at negative potentials, and the steady-state current voltage relation is inwardly rectifying. Very similar characteristics are observed when a charge conserving amino acid is introduced at this position (D70E). In addition, we found that mutations in various different protein regions of ClC-0 lead to a very similar phenotype. We conclude that (a) gating in ClC-0 and ClC-1 involves similar mechanisms, (b) the charge at position D70 in ClC-0 does not specifically confer voltage sensitivity in ClC-channels, (c) several mutations lead to an activating phenotype at hyperpolarized potentials in ClC0, (d) gating and permeation are strictly coupled in these channels, and (e) gating of ClC-channels appears not to involve an intrinsic voltage sensor.
materials and methods
Molecular Biology and Oocyte Injection
Mutations were introduced by recombinant PCR and verified by sequencing. Mutants were cloned into a vector containing Xenopus β-globin untranslated sequences (Lorenz et al., 1996). The constructs were linearized and capped cRNA was transcribed in vitro using SP6 RNA polymerase and the mMessage mMachine kit (Ambion Inc., Austin TX). cRNA was diluted to about 30–100 mg/liter before injection. Stage V and VI Xenopus oocytes were manually defolliculated and injected with 50 nl of cRNA solution. For patch clamping, defolliculated oocytes were collagenase treated (1 g/liter Sigma type II for 10–15 min) before injection, and the vitelline membrane was removed manually after incubation of oocytes in hypertonic medium directly before the experiment. Oocytes were kept in Barth's solution at 14–19°C.
Electrophysiology
Currents were measured at room temperature 1–3 d after injection using two-electrode voltage-clamp and pCLAMP5.5 software. Microelectrodes were filled with 3 M KCl and had resistances of 0.3–0.6 MΩ. Leakage or capacitative currents were not subtracted. Capacitative transients were cut off in some figures for clarity. In all figures zero current is indicated by dashed lines. Currents were recorded in ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Na-N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES), pH 7.4). For inside-out patch recording oocytes were bathed in 100 mM N-methyl-d-glucamine (NMDG)-Cl solutions, with 2 mM MgCl2, 5 mM Na-HEPES, 5 mM Na-ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), pH 7.4 or solutions in which NMDG-Cl was partially replaced by NMDG-glutamate. Pipettes were filled with 100 mM NMDG-Cl, 1.8 mM CaCl2, 5 mM Na-Hepes, pH 7.4. Data were low pass filtered at 5 kHz.
Pulse Protocols
The usual pulse-protocol is displayed in Fig. 1 A. From a pre-potential of +40 mV, where WT ClC-0 is fully activated (at normal external Cl−), the voltage was stepped to progressively more negative values in 20-mV steps for various durations for the different mutations (depending on the kinetics of the mutants). A tail potential of −100 mV was chosen to monitor the degree of activation of the channel. In some experiments a tail-potential of +40 mV was chosen in order to test for the rectification and/or deactivation properties of the mutants at positive voltages.
Figure 1.

Effect of mutant D70G on gating of ClC-0. (A) Voltage protocol for all traces shown in this paper. (B) Macroscopic currents from ClC-0 WT deactivate after a pulse to +40 mV. (C) Macroscopic currents from mutant D70G. Channels open at negative potentials after a step from a positive pre-potential.
To avoid large holding currents, the holding potential was chosen at −30 mV, close to the Cl−-equilibrium potential of oocytes. Between individual pulses the membrane potential was held for at least 0.5 s at the holding potential.
results
Mutant D70G in ClC-0 Is Similar to the Corresponding ClC-1 Mutant D136G
ClC-0 fast gating is similar to ClC-1 in its sensitivity to voltage: when a typical pulse protocol (Fig. 1 A) is applied, channels are open at depolarized potentials and deactivate at negative potentials (Fig. 1 B). In ClC-1, the D136G mutant has drastically changed currents. In contrast to WT, steady-state currents are inwardly rectifying and activate with hyperpolarizing voltages (Fahlke et al., 1995).
To search for similarities in gating between ClC-1 and ClC-0, we mutated the corresponding ClC-0 residue (D70G). An aspartate at this position is highly conserved among CLC channels. This gave a similar phenotype as in ClC-1: currents are inwardly rectifying and channels do open even at voltages as negative as −160 mV (Fig. 1 C). Fast ClC-0 gating differs from ClC-1 as it activates and deactivates with a monoexponential time course. The D70G ClC-0 mutant also differs from the corresponding D136G ClC-1 mutant: gating is faster to the degree that it is not possible to fully separate instantaneous and steady-state inward rectification in 2-electrode voltage-clamp measurements. However, the similar inward rectification of the ClC-0 mutant supports the idea that fast gating in ClC-0 and ClC-1 are fundamentally similar.
The Charge Conserving Mutation D70E Causes a Similar Phenotype
Since the D136G mutation involved a neutralization of a charge in a transmembrane domain, Fahlke et al. (1995) suggested D136 to be a ClC-1 voltage sensor. To test this idea, we introduced a charge-conserving mutation at the corresponding position in ClC-0 (D70E). However, its phenotype (Fig. 2 A) closely resembled the one of the neutralizing D70G mutation. Currents are again strongly inward rectifying and channels also open at negative potentials (Fig. 2 A). Hence, the characteristics of the D70G mutant is not specifically due to the reduced charge, but to other properties of the side chain. We cannot strictly rule out, however, that the glutamate residue at position 70 in the mutant is not fully deprotonated, although this is unlikely given the similar pKs of glutamate and aspartate.
Figure 2.
Several point mutations convert ClC-0 into inwardly rectifying channels. Macroscopic currents from 2-electrode voltage clamp experiments are shown for mutants D70E (A), K165R (B), H472K (C), S475T (D), E482D (E), and T484Q (F).
Several Mutants Have Characteristics Similar to Mutant D70G in ClC-0
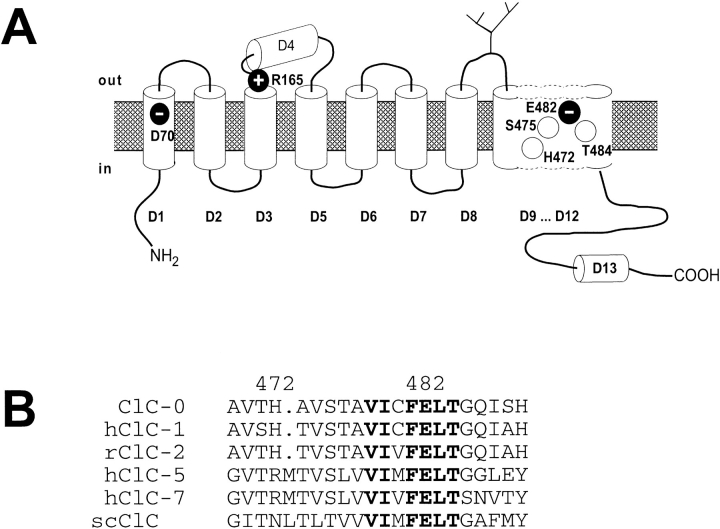
To investigate whether the phenotype observed with the mutations described above is specific to position 70, we mutated some other conserved residues in ClC-0. Several of those, including positively charged, negatively charged, or uncharged residues, display a similar characteristic as the mutants at position D70 (Fig. 2). In mutant K165R (located between domains 3 and 4) currents are inwardly rectifying (Fig. 2 B) similar to D70G but no activation phase is visible at the start of the hyperpolarizing pulses. Rather a small deactivating component is present. Mutating a histidine residue to lysine at position 472 again results in an inwardly rectifying phenotype (Fig. 2 C) with activating currents, similar to S475T, where a conserved serine is exchanged to threonine (Fig. 2 D). A charge-conserving mutation of glutamate E482 (E482D) that is located between domains 11 and 12 has an intermediate phenotype (Fig. 2 E). Mutating E482 to glutamine leads to nonfunctional channels (Pusch et al., 1995). Substituting a neutral residue two positions carboxy-terminal (T484) by glutamine or serine gives a very similar phenotype and is shown in Fig. 2 F and Fig. 5. Since these last mentioned mutants are very close to each other in the linear amino acid sequence (Fig. 3), we also investigated functional effects of exchanging neighboring residues. Mutants F481Y, F481Q, L483I, G485A, and Q486S were found to be indistinguishable from WT.
Figure 5.
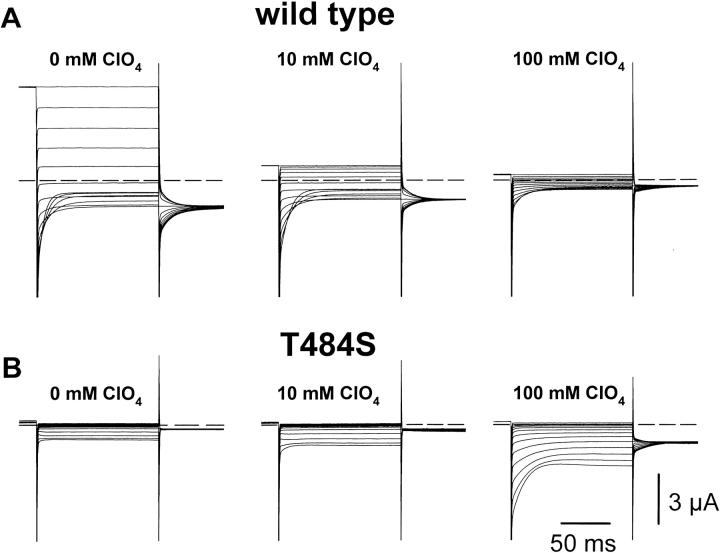
Analysis of effects of external ClO4 − on WT and mutant T484S channels. (A) Macroscopic 2-electrode-voltage-clamp data from WT in 100 mM Cl− (left), a mixture of 90 mM Cl− and 10 mM ClO4 − (center), and 100 mM ClO4 − (right). ClO4 − blocks ClC-0 in a voltage dependent manner with the following parameters: δ = 0.30 ± 0.02; K d(0 mV) = 2.2 ± 0.5 mM (n = 3). (B) Stimulation of gating in T484S mutant channels by ClO4 −. Macroscopic currents from T484S in 100 mM Cl− (left), a mixture of 90 mM Cl− and 10 mM ClO4 − (center), and 100 mM ClO4 − (right).
Figure 3.
(A) Location of mutations that lead to inwardly rectifying channels. They are located in domain 1 (D70), at the carboxy-terminal end of domain 3 (K165), in domain 11 (H472, S475) and between domain 11 and domain 12 (E482, T484). (B) Alignment of homologous ClC proteins in the region of the domains D11/D12. hClC-1 is the human muscular chloride channel (Steinmeyer et al., 1991; Koch et al., 1992), ClC-2 is a ubiquitous swelling-activated Cl− channel from rat (Thiemann et al., 1992), ClC-5 is predominantly expressed in kidney (Steinmeyer et al., 1995; Lloyd et al., 1996), ClC-7 is a rather broadly expressed CLC-protein (Brandt and Jentsch, 1995), and scClC is the S. cerevisiae CLC protein GEF-1 (Greene et al., 1993). The prefix r in front of ClC means rat, h means human, and sc means yeast.
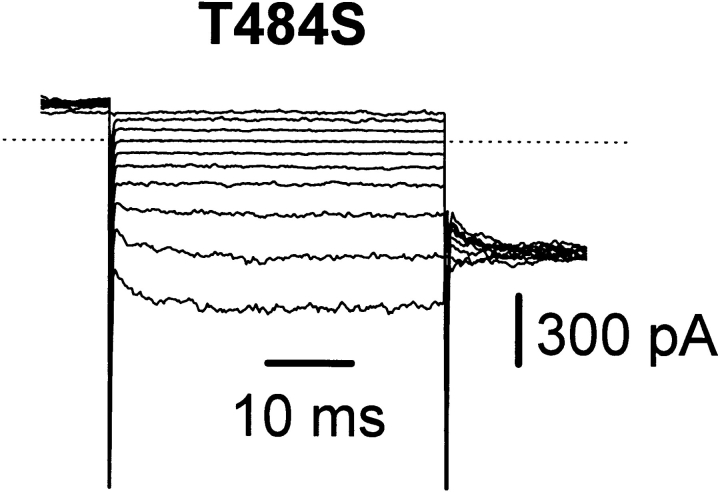
Since gating in all these mutants is apparently very fast, we sought to investigate some of them with the higher time resolution of the patch clamp technique. Results are shown for mutant T484S in Fig. 4. The macroscopic behavior is similar to 2-electrode voltage clamp measurements (Fig. 5 B). Even in these recordings the time resolution is not sufficient to resolve details of the gating.
Figure 4.
Macroscopic currents from T484S mutant channels in an inside-out patch clamp experiment.
Gating of Mutant T484S Can Be Partially Restored by Use of External Perchlorate
Gating of ClC-0 chloride channels depends on the external anion concentration, and it was proposed that ClC-0 is gated by the permeant anion (Pusch et al., 1995). With some anions, we found an anomalous mole fraction behavior and a different slope of voltage dependence (Pusch et al., 1995). If voltage sensitivity is conferred by an ion inside the pore, one might indeed expect a different gating with different anion species. The most drastic changes are expected for an anion that has a very different residence time in the pore compared with Cl−. The strong blocking effect seen with low external ClO4 − suggests that ClO4 − has a long residence time in the pore: external ClO4 − blocks ClC-0 WT in a voltage-dependent manner (Fig. 5 A) with a relative voltage dependence of δ = 0.30 ± 0.02 and a half-maximal blocking concentration at 0 mV of 2.2 ± 0.5 mM (n = 3) (data not shown). Gating is also affected by ClO4 −: though the Cl− concentration is reduced with 10 mM ClO4 − solutions, the voltage dependence is similar as in pure Cl−. In pure ClO4 −, however, the steady state open probability is shifted drastically to more positive potentials (data not shown).
As with WT ClC-0, gating of mutant T484S is also influenced by ClO4 − (Fig. 5 B). Since only small outward currents are detectable in this mutant, a potential block of outward currents by external ClO4 − cannot be investigated. ClO4 − exerts, however, an interesting effect on the gating of this mutant: inward currents are increased by ClO4 − and the activation at hyperpolarization seen in pure Cl− is replaced by a WT-like deactivation in high ClO4 −. This proves that the WT gating mechanism is still working in this mutant; the “normal” gating can be restored using a specific anion mixture.
discussion
Several studies have shown that fast gating of ClC-0 is tightly coupled to the permeating anion that probably serves as the gating charge (Pusch et al., 1995; Chen and Miller, 1996; Ludewig et al., 1997; Pusch, 1996). Even though the gating of the homologous ClC-1 channel looks very similar to ClC-0 (Steinmeyer et al., 1991), Fahlke et al. (1995, 1996) suggested that, in contrast to ClC-0, an intrinsic voltage sensor is responsible for voltage-sensitivity in ClC-1. This conclusion was drawn on the basis of two findings: (a) Replacement of [Cl−]ext by MeSO3 − does not decrease popen in ClC-1. (b) The mutation D136G found in recessive myotonia has a drastically altered voltage sensitivity, being inwardly rectifying and lacking a very fast gating component. It is now clear, however, that the effect of MeSO3 − is due to the stimulating effect of this anion on ClC-1 channel opening but not due to the reduction of [Cl−]ext (Rychkov et al., 1996; our unpublished observation).
Here we show that mutations in various protein regions of ClC-0 lead to a macroscopic phenotype similar to that observed by Fahlke et al. for the D136G mutant. The corresponding mutant D70G as well as the charge conserving mutation D70E are both inwardly rectifying. Mutations at several other amino acid positions, including a positively charged residue between domains 3 and 4, a negatively charged residue, and uncharged residues in and after domain 11, show a very similar phenotype. In the case of mutant T484S the deactivating WT phenotype can even be partially restored by replacing external Cl− by ClO4 −, indicating that no previously present “voltage-sensor” is lost in the mutant.
Interestingly, a similar inward rectification as seen with these mutants in ClC-0 can be induced in ClC-1 by low external pH and positive holding potentials (Rychkov et al., 1996). It would be interesting to insert the analogous mutations described here in the ClC-1 channel and to test for effects on the pH-sensitivity.
Four of the inwardly rectifying mutations are located in the highly conserved D11/D12 stretch (Fig. 3 B), demonstrating the functional importance of this broad hydrophobic region. Interestingly, a mutation equivalent to E482D has been found in the ClC-5 gene of patients suffering from an inherited hypercalciuric kidney stone disease (Lloyd et al., 1997). In contrast to the ClC-0 mutation, however, the E527D mutation in ClC-5 did not express currents in Xenopus oocytes (Lloyd et al., 1997).
One could assume that all of the amino acids that lead to the inwardly rectifying phenotype are involved in the gating and/or conduction process of the channel. For instance, they might be involved in ion binding steps that are important for gating. Alternatively, and maybe more likely, changing these amino acids could also affect one of the rate constants of the gating transitions, thereby causing inward rectification. It seems clear, however, that the aspartate in domain D1 (D70 in ClC-0, D136 in ClC-1) is not special among the mutated amino acids, and one cannot conclude that it represents the voltage sensor. Although we cannot completely rule out the presence of an intrinsic voltage sensor, our results are consistent with the idea that no voltage sensor is present in CLC channels, but that the voltage dependence is conferred by the permeating ions.
Acknowledgments
We thank Christine Neff for technical assistance.
This work was supported by grants of the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Abbreviation used in this paper
- WT
wild-type
references
- Brandt S, Jentsch TJ. ClC-6 and ClC-7 are two novel broadly expressed members of the ClC chloride channel family. FEBS Lett. 1995;377:15–20. doi: 10.1016/0014-5793(95)01298-2. [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Miller C. Nonequilibrium gating and voltage dependence of ClC-0 Cl−channel. J Gen Physiol. 1996;108:237–250. doi: 10.1085/jgp.108.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Rüdel R, Mitrovic N, Zhou M, George AL. An aspartic residue important for voltage-dependent gating of human muscle chloride channels. Neuron. 1995;15:463–472. doi: 10.1016/0896-6273(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Rosenbohm A, Mitrovic N, George AL, Rüdel R. Mechanism of voltage-dependent gating in skeletal muscle chloride channels. Biophys J. 1996;71:695–706. doi: 10.1016/S0006-3495(96)79269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JR, Brown NH, DiDomenico BJ, Kaplan J, Eide DJ. The GEF-1 gene of Saccharomyces cerevisiaeencodes an integral membrane protein; mutations in which have effects on respiration and iron-limited growth. Mol Gen Genet. 1993;241:542–553. doi: 10.1007/BF00279896. [DOI] [PubMed] [Google Scholar]
- Heine R, George AL, Jr, Pika U, Deymeer F, Rüdel R, Lehmann-Horn F. Proof of a non-functional muscle chloride channel in recessive myotonia congenita (Becker) by detection of a 4 base pair deletion. Hum Mol Genet. 1994;3:1123–1128. doi: 10.1093/hmg/3.7.1123. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Chloride channels: a molecular perspective. Curr Opin Neurobiol. 1996;6:303–310. doi: 10.1016/s0959-4388(96)80112-7. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopusoocytes. Nature (Lond) 1990;348:510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik K-H, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science (Wash DC) 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Lloyd SE, Pearce SHS, Fisher SE, Steinmeyer K, Schwappach B, Scheinmann SJ, Harding B, Bolino A, Devoto M, Goodyer P, et al. Mutations in the chloride channel ClC-5 are associated with X-linked hypercalciuric nephrolithiasis. Nature (Lond) 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- Lloyd, S.E., W. Günther, S.H.S. Pearce, A. Thomson, M.L. Bianchi, M. Bosio, I.W. Craig, S.E. Fisher, S.J. Scheinman, O. Wrong, T.J. Jentsch, and R.V. Thakker. 1997. Characterisation of renal chloride channel, CLCN5, mutations in hypercalciuric nephrolithiasis disorders. Hum. Mol. Genet. In press. [DOI] [PubMed]
- Lorenz C, Pusch M, Jentsch TJ. Heteromeric ClC chloride channels with novel properties. Proc Natl Acad Sci USA. 1996;93:13362–13366. doi: 10.1073/pnas.93.23.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Jentsch TJ, Pusch M. Analysis of a protein region involved in permeation and gating in the ClC-0 chloride channel. J Physiol (Lond) 1997;498:691–702. doi: 10.1113/jphysiol.1997.sp021893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Pusch M, Jentsch TJ. Two physically distinct pores in the dimeric chloride channels ClC-0. Nature (Lond) 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- Middleton RE, Pheasant DJ, Miller C. Homodimeric architecture of a ClC-type chloride ion channel. Nature (Lond) 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- Miller, C. 1982. Open-state substructure of single chloride channels from Torpedo electroplax. Phil. Trans. R. Soc. London. B 299:401– 411. [DOI] [PubMed]
- Pusch M. Knocking on channel's door: the permeating anion acts as the gating charge in ClC-0. J Gen Physiol. 1996;108:233–236. doi: 10.1085/jgp.108.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. J Gen Physiol. 1997;109:105–116. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Gating of the voltage-dependent chloride channel ClC-0 by the permeant anion. Nature (Lond) 1995;373:527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- Pusch M, Steinmeyer K, Jentsch TJ. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys J. 1994;66:149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard EA, Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science (Wash DC) 1990;247:1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- Rychkov GY, Pusch M, St D, Astill J, Roberts ML, Jentsch TJ, Bretag AH. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. J Physiol (Lond) 1996;497:423–435. doi: 10.1113/jphysiol.1996.sp021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science (Wash DC) 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1994;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature (Lond) 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ. Cloning and functional expression of rat ClC-5, a chloride channel related to kidney disease. J Biol Chem. 1995;270:31172–31177. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- Thiemann A, Gründer S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature (Lond) 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- White MM, Miller C. A voltage-gated anion channel from the electric organ of Torpedo californica. . J Biol Chem. 1979;254:10161–10166. [PubMed] [Google Scholar]