Abstract
K+ channel gating currents are usually measured in the absence of permeating ions, when a common feature of channel closing is a rising phase of off-gating current and slow subsequent decay. Current models of gating invoke a concerted rearrangement of subunits just before the open state to explain this very slow charge return from opening potentials. We have measured gating currents from the voltage-gated K+ channel, Kv1.5, highly overexpressed in human embryonic kidney cells. In the presence of permeating K+ or Cs+, we show, by comparison with data obtained in the absence of permeant ions, that there is a rapid return of charge after depolarizations. Measurement of off-gating currents on repolarization before and after K+ dialysis from cells allowed a comparison of off-gating current amplitudes and time course in the same cells. Parallel experiments utilizing the low permeability of Cs+ through Kv1.5 revealed similar rapid charge return during measurements of off-gating currents at ECs. Such effects could not be reproduced in a nonconducting mutant (W472F) of Kv1.5, in which, by definition, ion permeation was macroscopically absent. This preservation of a fast kinetic structure of off-gating currents on return from potentials at which channels open suggests an allosteric modulation by permeant cations. This may arise from a direct action on a slow step late in the activation pathway, or via a retardation in the rate of C-type inactivation. The activation energy barrier for K+ channel closing is reduced, which may be important during repetitive action potential spiking where ion channels characteristically undergo continuous cyclical activation and deactivation.
Keywords: potassium channel, Kv1.5, gating current
introduction
K+ channels are an extremely diverse group of proteins that are responsible for controlling excitation and membrane potential in many different cell types. Kv channels form part of a superfamily of ion channel proteins that encode voltage-gated Na+, K+, and Ca2+ channels. Voltage sensing in these channels is mediated by regions of repeating positively charged residues primarily in the fourth transmembrane domain (Papazian et al., 1991; Schoppa et al., 1992; Perozo et al., 1994; Goldstein, 1996; Larsson et al., 1996; Seoh et al., 1996) of each subunit. The opening and closing of voltage-gated channels is characterized by gating currents (Armstrong and Bezanilla, 1973; Keynes and Rojas, 1974), predicted by Hodgkin and Huxley (1952), that reflect displacement of the charged domains (Larsson et al., 1996; Mannuzzu et al., 1996; Yang et al., 1996) sensing the transmembrane electric field (Sigworth, 1993; Goldstein, 1996). The cloning of voltage-gated ion channels has facilitated the study of Kv gating currents, initially in Xenopus oocytes using high levels of channel expression and signal averaging (Bezanilla et al., 1991; Stühmer et al., 1991), in whole oocytes or using cut-open oocyte methods (Taglialatela and Stefani, 1993; Stefani et al., 1994), or in membrane patches (McCormack et al., 1994), and expression in small mammalian cells (Bouchard and Fedida, 1995; Fedida et al., 1996).
In almost every case, ion permeation has been abolished through the replacement of permeant ions with nonpermeant cations like NMG (Zagotta et al., 1994 b; Fedida et al., 1996), the use of blockers like intracellular TEA (Bezanilla et al., 1991; Stühmer et al., 1991) or charybdotoxin (Schoppa et al., 1992), or the engineering of nonconducting mutants (Perozo et al., 1993; Stefani et al., 1994). In the absence of permeating ions, a fundamental feature of Kv channel gating is that the return of gating charge after channels have opened is delayed (Taglialatela and Stefani, 1993; Stefani et al., 1994). On repolarization there is often a detectable rising phase of off-gating current and slow subsequent decay (Perozo et al., 1993; Stefani et al., 1994). Some slowing is expected due to the relative voltage independence of the last closed-open transition (Zagotta and Aldrich, 1990), as shown by single channel and whole cell studies (Zagotta and Aldrich, 1990; Hoshi et al., 1994), but longer depolarizations, even those beyond the time required to move most channels into the open state, continue to slow charge return on repolarization (Bezanilla et al., 1994; Zagotta et al., 1994 a; Fedida et al., 1996). To account for the delayed return, gating models for K+ channels have been proposed for depolarizations to positive potentials which include additional transitions, carrying little charge, around the open state in order to produce slowed off-gating currents on repolarization (Taglialatela and Stefani, 1993; Bezanilla et al., 1994; Zagotta et al., 1994 b). It is suggested that there is a concerted rearrangement of subunits before the open state (Bezanilla et al., 1994; McCormack et al., 1994) and that reversal of this process accounts for the subsequent very slow charge return from opening potentials (McCormack et al., 1994; Stefani et al., 1994). This observation is not universal across other voltage-gated channels (Neely et al., 1993; Jones et al., 1997), and even in K+ channels, the Shaker mutant V2 lacks such off-charge slowing at depolarizations that move channels into the open state (Schoppa et al., 1992; McCormack et al., 1994). Small depolarizations in this channel above the activation threshold give ionic currents with fast off-gating currents upon repolarization (Schoppa et al., 1992). Thus, it is a particularly important issue to understand why off-gating currents are slow in most Shaker channels, and whether K+ channel gating functions in the same manner in the presence of permeating ions as in their absence. Here we have investigated the problem by comparing off-gating currents in the absence and presence of permeating cations. We demonstrate that when ions are allowed to permeate through the K+ channel, Kv1.5, in a physiological manner, slowing of off-gating currents on repolarization is largely prevented.
materials and methods
Cells and Solutions
HEK-293 cells were transiently transfected with Kv1.5 cDNA in pRC/CMV, using LipofectACE reagent (Canadian Life Technologies, Bramalea, Canada) in a 1:10 (wt:vol) ratio. Transfectants were detected using the phOx system (Invitrogen Corp., San Diego, CA) as described previously (Fedida, 1997). Kv1.5 in the plasmid expression vector, pRC/CMV was mutagenized using the Stratagene Chameleon Kit (Stratagene Inc., La Jolla, CA) such that tryptophan 472 was converted to phenylalanine (W472F). This mutation is analogous to the ShH4-IR W434F (Perozo et al., 1993). Patch pipettes contained 140 mM N-methyl-d-glucamine (NMG), 1 mM MgCl2, 10 mM HEPES, 10 mM EGTA, adjusted to pH 7.2 with HCl. The bath solution contained 140 mM NMG, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM dextrose, adjusted to pH 7.4 with HCl. For Cs+ experiments, pipettes contained 130 mM CsCl, 4 mM Na2ATP, 1 mM MgCl2, 0.1 mM GTP, 5 mM HEPES, 10 mM EGTA, adjusted to pH 7.2 with CsOH. The bath solution contained 140 mM NMG, 2.5 mM CsCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM dextrose, adjusted to pH 7.4 with HCl. For the nonconducting mutant, the bath and pipette solutions are as described for the Cs+ experiments. All chemicals were from Sigma Chemical Co. (St. Louis, MO).
Electrophysiology
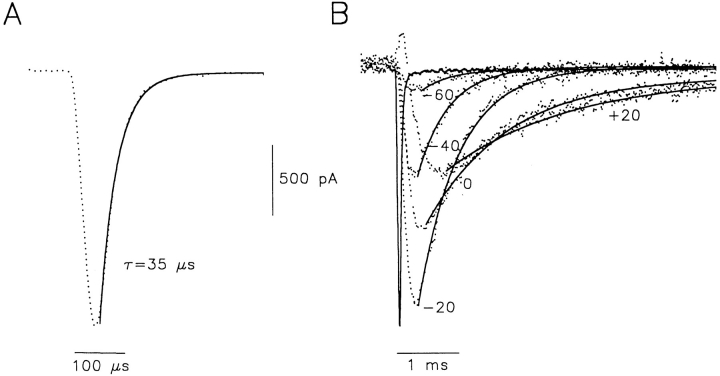
Current recording and data analysis were done using an Axopatch 200A amplifier and pClamp 6 software (Axon Instruments, Foster City, CA). Patch electrodes were fabricated using thin-walled borosilicate glass (World Precision Instruments, Sarasota, FL). After sylgarding and fire polishing, pipettes used to measure current had resistances of 1.0–2.5 MΩ when filled with control filling solution. Mean series resistance was 3.6 ± 0.4 MΩ (n = 39), and cell capacitance was 19.9 ± 1.4 pF. Leakage and capacitative currents were subtracted on-line using a P/6 protocol (Zagotta et al., 1994 b). The absence of ionic current at negative membrane potentials in HEK cells allowed faithful leak subtraction of data. An uncorrected capacity transient is illustrated in Fig. 1 A for a 10-mV voltage step. A monoexponential fit to data gave a decay time constant of 35 μs with a mean value of 55.0 ± 5.2 μs (n = 10). When superimposed on typical off-gating current traces in Fig. 1 B, it is obvious that the capacity transient has settled well before the peak of off-gating currents and does not limit the τdecay measurements of off-gating currents which are shown by the monoexponential fits to data in Fig. 1 B. These capacity measurements are in keeping with those made by others from mouse L-cells for hKv1.5 (Snyders et al., 1993) and our own previous reports where the mean capacity transient decay rates were between 46 and 62 μs (Fedida et al., 1996; Fedida, 1997). During the present study we often report τdecay of off-gating currents (see Figs. 3–8) where the fastest decay was 0.27 ms, but most measurements were in the range of 0.4 ms and slower. Given the properties of the recording system that we describe, our recording bandwidth does not appear to be a limiting factor in our measurements. Capacity compensation and leak subtraction were routinely used, but series resistance compensation was only rarely used. No difference between results with and without Rs compensation was observed. Data were sampled at 100–330 kHz (except for data in Fig. 5, D and E, where sample rate was reduced to 20 kHz to allow for long recording times) and filtered at 5–10 kHz. All experiments were performed at 22°C, and cells were superfused continually at a flow rate of 1–2 ml/min. All Qoff measurements were obtained by integrating the off-gating currents until current waveforms decayed to the baseline. This was usually complete by 25 ms. Due to the high level of expression of Kv1.5 channels in HEK cells, there was no need for signal averaging. For a gating charge of 1 pC, assuming each channel moved 12.3 e 0 (Schoppa et al., 1992) we calculate approximately 508,000 channels per cell, or a density of 254 channels μm−1. This is comparable with the Na channel density of squid giant axon, but much less than that at the node of Ranvier (Hille, 1992).
Figure 1.
(A) Uncorrected capacity transient during a 10-mV hyperpolarization from −60 mV in an HEK cell. The cell capacitance was 13.4 pF. The data were filtered at 10 kHz for presentation and are represented with a dotted line. The decay phase was fit with a single exponential function, τ = 35 μs (solid line). (B) Off-gating currents during depolarizations to between −60 and +20 mV in 20-mV steps from a holding potential of −100 mV. Capacity compensation and leak subtraction have been applied to the gating currents, but superimposed is the uncorrected capacity transient from A. The decay of each off-gating current has been fit to a monoexponential decay function with τdecay = 0.47, 0.50, 0.64, 1.24, and 1.9 ms for pulses from −60 to +20 mV.
Figure 3.
(A) Mean normalized peak off-gating current amplitudes at −100 mV after depolarizations to between −100 and +60 mV (n = 5). Data (±SEM) obtained immediately on whole-cell access (•, n = 4) and after disappearance of any ionic current (▿, n = 5). (B) Normalized Qoff from off-gating currents as for A. Boltzmann fits gave a V0.5 of −18.2 ± 2.3 mV and z = 2.0 ± 0.1 e 0 (▿) and V0.5 of −16.7 ± 2.4 mV, z = 2.1 ± 0.2 e 0 (•). (C) τdecay of off-gating currents after prepulses to between −60 and +60 mV in cells with ionic K+ current (n = 5, •) and in cells with no ionic current (n = 4, ▿). (D) Normalized conductance-voltage (G-V, •, mean ± SEM, n = 11) curve obtained from K+ tail currents at −30 mV after 30-ms prepulses, in K+-containing intracellular and bath solutions. The Q-V (▿) curve was determined in NMG solutions (e.g., Fig. 2 A) from the sum of on- and off-gating charge movement for each amplitude of depolarizing pulse (n = 5, ±SEM). Fits to the Boltzmann equation gave V0.5 for G and Q curves of +2.9 mV and −16.3 mV, respectively, with slope factors (k) of 9.3 and 15.3 mV (1.7 e 0).
Figure 8.
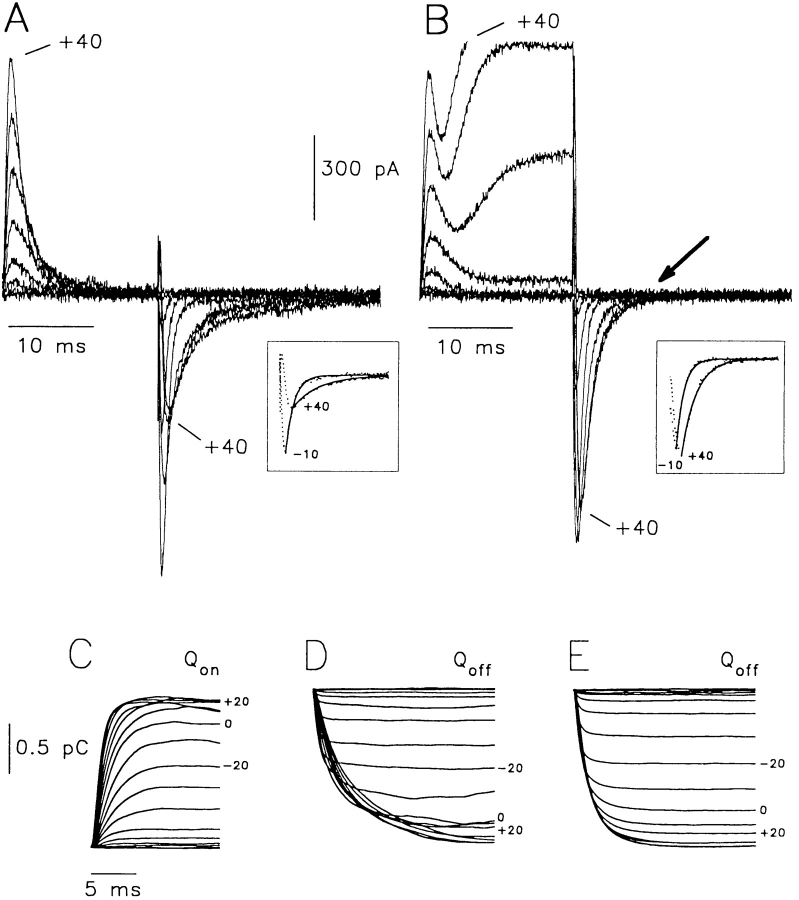
Gating currents from a nonconducting mutant of Kv1.5 channels, W472F. Permeant ions are present in the pipette and bath solutions but do not permeate through the pore as demonstrated by the lack of ionic currents present on depolarization. (A) Gating currents on depolarizations up to +60 mV from −100 mV in 20-mV steps. (Inset) Off-gating currents at −100 mV after depolarizing prepulses to −10 mV and +40 mV. τdecay was 0.53 and 1.6 ms at −10 and +40 mV, respectively. (B) Normalized Qoff from integration of off-gating currents (mean ± SEM, n = 4). V0.5 was −19.9 ± 3.6 mV and z = 2.5 ± 0.2 e 0. (C) τdecay of off-gating currents as a function of the voltage prepulse potential (mean ± SEM, n = 4). (D) Envelopes of on- and off-gating currents from Kv1.5 W472F. Protocol was as for data in Fig. 7 A. All on-gating currents are superimposed to show homogeneity. (E) Ratio of Qoff to Qon, as a function of pulse duration at +60 mV (mean ± SEM, n = 4). (F) Slowing of τdecay of off-gating currents with increased prepulse duration (mean ± SEM, n = 4).
Figure 5.
Validation of off-gating current measurements made in the presence of permeant Cs+. (A) Cells were depolarized to +40 mV from −100 mV, then repolarized to test potentials between −100 and +10 mV before returning to −100 mV. (Inset) Decaying outward Cs+ tails and off-gating currents for prepulses to +40, +10, and −20 mV. Solid lines show fit to monoexponential decay functions during subsequent current waveforms recorded at −100 mV. τdecay was 0.51, 0.4, and 0.32 ms for +40-, +10-, and −20-mV prepulses, respectively. (B) K+ Ionic tail currents from Kv1.5 expressed in HEK cells. EK was −70 mV, and the cell was pulsed to +40 mV for 100 ms before repolarization to a range of potentials between −40 and −100 mV. Only decaying ionic tail currents during the repolarizing pulse are shown in B. (C) Normalized peak off-gating current (Igoff), and charge (Qoff), and values for τdecay at −100 mV after repolarizations from +40 mV (τdecay, hatched bar), from +10 mV (solid bars), and from −20 mV (empty bars). Igoff and Qoff were normalized to values obtained from immediate repolarization from +40 mV to −100 mV (n = 3, ±SEM). (D) Mean normalized Q-V relation for off-gating current (▿) and G-V for Cs+ current (•) (±SEM, n = 4). V0.5 for Qoff and G were −15.0 ± 1.3 mV (z = 1.94 ± 0.2 e 0) and −1.2 mV (k = 15.6 mV), respectively.
results
Measurement of Off-gating Currents, with and without Permeating K+
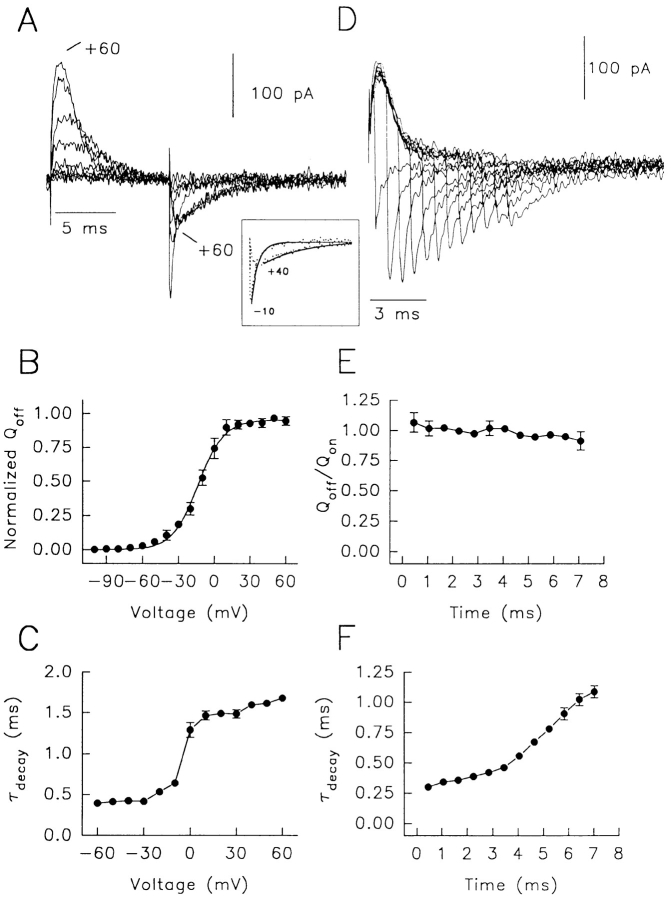
Gating currents from Shaker channels that lack N-type inactivation are of the general form illustrated in Fig. 2 A with data obtained from Kv1.5 overexpressed in HEK-293 cells using intracellular and extracellular NMG to prevent ionic flux. In oocytes where permeant ions were replaced by NMG in conducting channels, the gating current properties are similar to those described here (Perozo et al., 1993; Stefani et al., 1994). However, when tetraethylammonium or Tris were used as substitute cations, there was an enhancement of gating charge immobilization which is similar to that seen during the onset of N-type inactivation (Bezanilla et al., 1991; Stühmer et al., 1991). On-gating currents appear during depolarizations positive to −70 mV and increase in amplitude with larger depolarizations, then begin to decay more rapidly at positive potentials (Fig. 2 A). When on-gating currents are integrated, the waveforms represent the time- and voltage-dependent movement of gating charge as channels progress towards the open state. The time course of on-charge movement (Qon) is shown in Fig. 2 C. For larger depolarizations, total gating charge moved upon depolarization increases more rapidly (Fig. 2 C) and eventually saturates (Fig. 2 C and 3 D, ▿). When repolarized to −100 mV, off-gating currents represent the return of gating charge as channels deactivate. In Fig. 2, A and B, these are shown as the downward current deflections after 15-ms depolarizations to between −80 and +60 mV. In Fig. 2, D and E, the integrated off-gating currents depict the charge return with time. For small depolarizations to <−10 mV, off-gating currents reach a peak very rapidly and decay rapidly and monoexponentially. After depolarizations to more positive potentials, the peak off-gating current is reduced (Fig. 2 A and 3 A, ▿) and the time constant of relaxation of off-current (τdecay), while still monoexponential, slows 5–6-fold (Fig. 2 A, inset and Fig. 3 C, ▿). Depolarizations to +60 mV compared with −10 mV resulted in a decrease in the peak off-gating current to 40.8% of maximum (n = 5), and a slowing of the decay time constant from 0.59 ± 0.06 to 2.24 ± 0.09 ms. A clear threshold for off-gating current slowing occurs at ∼−10 mV, where the pore would normally be open for the channel to conduct ions (Fedida et al., 1993). The slow return of charge in this situation is clearly seen in the integrals (Fig. 2 D), where the rise of Qoff is progressively delayed for stronger depolarizations. This slow return of charge thus reflects delayed return from the open state, as described in recent detailed studies on Shaker channels (Perozo et al., 1993; Stefani et al., 1994; Zagotta et al., 1994 a). There is no immobilization of charge as integration of on- and off-gating currents show conservation of charge moved and returned. It can clearly be seen that in the example shown that Qon saturated at around 1.4 pC (Fig. 2 C) and Qoff saturated at exactly the same charge (Fig. 2 D). For 12-ms depolarizations from −100 to +50 mV, the mean gating charge ratio (Qoff/Qon) was 1.0 ± 0.04 (mean ± SEM, n = 12). Integration of off-gating currents was carried out for sufficient time to allow relaxation to the zero current level (usually <25 ms). Off-gating charge showed saturation (mean Qoff = 1.4 ± 0.3 pC after depolarization to +40 mV and 1.38 ± 0.3 pC after +60 mV) and a smooth off-gating charge voltage dependence (Q-V curve, Fig. 3 B, ▿). These results indicate our ability to accurately measure gating currents from Kv1.5 and that our results in the absence of permeating ions are comparable with those of others (Stefani et al., 1994; Zagotta et al., 1994 a).
Figure 2.
Voltage-dependent on- and off-gating currents of Kv1.5 in the absence and presence of permeant K+. Currents are from −100 mV for pulses to +40 mV in 20-mV steps. (A) Steady-state gating currents, 3 min after patch rupture; inset, off-gating currents at −100 mV after depolarizing prepulses to −10 and +40 mV. Solid lines indicate monoexponential fits with τ's of 0.75 ms at −10 and 2.3 ms at +40 mV. (B) Gating and ionic K+ currents immediately on achievement of whole-cell recording. Arrow indicates current decay to baseline; inset, off-gating currents at −100 mV after prepulses to −10 and +40 mV. τdecay was 0.73 ms at −10 mV and 1.11 ms at +40 mV. Data in both A and B are from the same cell. Subtraction of on-gating current waveforms before and after K+ dialysis gave faithful ionic currents on depolarization (data not shown). (C–E) On- (Qon) and off-gating (Qoff) charge time course calculated by integration of on-gating current in A (C), and off-gating currents in A (D) and B (E). Note that for all data where gating charge was measured by integration of off-gating current transients, integration times were sufficient to allow currents to relax to baseline. This was usually complete within 25 ms.
The measurements of gating currents in the absence of ionic currents depend upon the dialysis of K+ from cells on the attainment of the whole cell recording configuration. I-V voltage protocols performed immediately on patch rupture allowed measurement of gating currents in the presence of residual cell K+, as shown by outward ionic currents on depolarization (Fig. 2 B). The usual problem with the measurement of ionic and gating currents together is that extremely large ionic K+ currents dwarf and prevent resolution of gating currents. Here, due to the dialysis of K+ from the cells, only small outward K+ currents were present, and in the absence of bath permeant ions, off-gating currents are uncontaminated by ionic current deactivation (which would be absent at −100 mV, or extremely small due to Goldman-Hodgkin-Katz rectification). This was confirmed by integration of records from the same cell before (Fig. 2 E) and after K+ dialysis (Fig. 2 D). Qoff waveforms show a different time course but show clear identity of charge returned before and after K+ dialysis, and exactly the same charge as that moved during depolarizations (Qon, Fig. 2 C). For the data in Fig. 2, A and B, charge returned at −100 mV after prepulses to >10 mV was almost identical (i.e., after a prepulse to +40 mV, Qoff was 1.4 pC in both cases; after +60 mV, 1.3 pC and 1.4 pC). The identity of charge returned during off-gating currents from the same cell in Fig. 2, A and B, confirms that off-gating currents in Fig. 2 B accurately represent pure Kv1.5 off-gating currents, and that no significant contamination from ionic tail currents has occurred. In the presence of K+, off-gating currents showed little decrease in peak or slowing of decay. The essential finding is illustrated by the arrow (Fig. 2 B) showing rapid relaxation of off-gating current to the zero line. Here, no slow component of gating current is present, unlike off-gating currents recorded in the absence of permeant K+ (Fig. 2 A). There is no large decrease in peak off-gating current after prepulses to positive potentials (mean data, •; n = 5 in Fig. 3 A), and much less slowing of τdecay (Fig. 3 C, •; τ = 0.53 ± 0.1 ms at −10 mV and 0.98 ± 0.1 ms at +60 mV) which are still monoexponential (Fig. 2 B, inset). We believe that the lack of residual inward gating current (indicated by the arrow) in Fig. 2 B must indicate that at −100 mV the gating current has decayed more rapidly and completely than off-gating currents in Fig. 2 A. Any ionic tail, even if it was present, would have deactivated by this time given ionic tail decay time constants of ∼1 ms at this potential. Similarly, if ionic currents were contributing significantly to the off-gating currents, there should be a shift in the charge relation (Qoff, Fig. 3 B, •) to the right along the voltage axis reflecting a contribution from ion flux activating at more positive potentials than movement of gating charge (Fig. 3 D, •). This does not occur as shown by the overlay of the symbols in Fig. 3 B (•, ▿), and the smooth Qoff relation in the presence of permeant K+ (Fig. 3 B, •) which does not show any change of slope or discontinuity positive to −20 mV (where conductance activates, Fig. 3 D), is further evidence for the lack of contamination of off-gating currents in Fig. 2 B by ionic current tails at potentials at which channels open.
Off-gating Currents in the Presence of Permeant Cs+ Decay Rapidly
We suggest that both ionic and gating currents can be measured together from the same cell and propose that permeant K+ in some way speeds the return of gating charge on repolarization. To reproduce a low ionic permeability which allowed simultaneous measurements of off-gating currents, we replaced NMG in the pipette solution with Cs+ and added low [Cs+] to the bath to make continuous measurements of gating current in the presence of permeating Cs+ without the possibility of any transient effects during cell dialysis. Cs+ has a low permeability through K+ channels, so that it is usually thought of as a channel blocker (Hille, 1992). However, at high levels of K+ channel expression, and with a permeability ratio of 0.11 ± 0.01 obtained from biionic reversal potential measurements (Zhang and Fedida, unpublished data), Cs+ currents are recordable (De Biasi et al., 1993) at the same time as on- and off-gating currents (see Figs. 4, 5, and 7).
Figure 4.
Measurement of Kv1.5 off-gating currents with permeant Cs+. (A) On-gating and outward Cs+ currents during depolarizing prepulses from −100 to +60 mV in 20-mV steps, and off-gating currents at −100 mV (ECs); the arrow indicates the complete current decay. (Inset) Off-gating currents at ECs, −100 mV, after prepulses to +10 mV and +60 mV. τdecay was 0.48 ms on repolarization from +10 mV and 0.63 ms from +60 mV. (B) τdecay of off-gating currents after prepulses to between −60 and +60 mV in cells with no permeant ions present (data from experiments as shown in Fig. 2 A, n = 4, ▿) and in cells with Cs+ present as the permeant cation (n = 4, ▾). (C and D) Time course of off-gating charge integrated from the off-current data in Fig. 4 A with Cs+ present (C) and from a cell where no permeant ions were present (D). Traces are integrated off-gating currents after prepulses in 10-mV steps, indicated by the potentials adjacent to the tracings.
Figure 7.
Envelopes of on- and off-gating currents in the presence of permeant Cs+. Gating and ionic currents during depolarizations from −100 to +60 mV and then to −100 mV (ECs) to record off-gating currents. Pulse durations (frequency 0.5 Hz) were incremented 0.6 ms after the first duration of 0.45 ms to give a maximum pulse duration of 7.05 ms. Multiple sweeps of on-gating currents are superimposed in the upper left segment to show homogeneity. (B) Mean (±SEM) τdecay of off-gating currents after different prepulse durations in Cs+ (n = 6, •). For off-gating current data in C, pulse duration was incremented 50 ms to give 8–358 ms durations. Currents were recorded after depolarization indicated above each segment. (D) Change in mean off-gating charge returned during the first 20 ms of repolarization after different duration depolarizations in Cs+ (n = 8, •).
In an extension of our K+ data, in the presence of permeating Cs+, little slowing of decay of the off-gating current was observed on repolarization to −100 mV (Fig. 4 A, arrow), with a mean change in τdecay of the off-gating current from 0.48 ± 0.06 at −10 mV to 0.65 ± 0.06 ms at +60 mV (Fig. 4 B, ▾). These may be compared with the τdecay of off-gating currents in the absence of permeating ions (Fig. 4 B, ▿). The off-gating currents in the inset to Fig. 4 A illustrate such monoexponential fits to off-gating current decay in the presence of Cs+, at +10 and +60 mV. Clearly, little off-gating current slowing has occurred over this potential range (compare with Fig. 2 A, inset). A dramatic demonstration of the lack of slowing of off-gating current in the presence of permeant ions is provided by calculating the development of Qoff with time of repolarization. These data are shown in Fig. 4 C and can be compared with data obtained with only NMG present (Fig. 4 D). Off-gating transients rise smoothly and rapidly to a peak when Cs+ is present (C), but for depolarizations positive to −10 mV in the absence of ions (D), the rise time is increasingly slowed.
Validation of Off-gating Currents in the Presence of Permeant Cs+
To validate the measurement of off-gating current in the presence of permeating Cs+, we used a double pulse voltage protocol, which allowed comparison of inward currents at −100 mV before and after ionic current deactivation. Results from the protocol are illustrated in Fig. 5 A. Here, the calculated ECs was −100 mV (2.5 mM [Cs+]o). Cells were first pulsed to +40 mV to move channels into the open state, and then repolarized to a range of potentials between +10 and −100 mV. Outward ionic current tails were visible, at repolarization potentials from +10 to −20 mV, but then all tails were inwardly directed (although still 80 mV positive to ECs) and reflected off-gating current as the predominant component of the observed current. At potentials positive to ECs (between −30 and −100 mV) ionic current tails should be outwardly decaying, so the inward current tails must represent the dominance of rapidly decaying off-gating current—the question is, how much are the amplitude and time course of inward off-gating currents distorted by ionic tail currents? At ECs itself (−100 mV in our experiments), the tail should represent only off-gating currents as the ionic flux should be at equilibrium. From the repolarization data in Fig. 5 A we can calculate the magnitude of ionic tail currents at the different repolarizing potentials. The slope conductance of the channel conducting Cs+ can be calculated from the peak current at +40 mV, or instantaneous and steady tail amplitudes at +10 or 0 mV, given the G-V curve in Fig. 5 D. The mean value for GCs from the cell in Fig. 5 A was 2.8 ± 0.3 nS, which means that the peak outward tail current amplitude during repolarization to −80 mV would be 56 pA, out of a peak tail amplitude of 343 pA (16%). As ECs is approached, for pulses to −90 or −100 mV, any ionic component to the peak tail current measured diminished to 7% and zero, respectively. This calculation suggests that close to the reversal potential, the large gating currents relative to Cs+ conductance allow measurement of a peak value for off-gating current contaminated by less than 10% of ionic tail current. The data also indicate that accumulation of Cs+ close to the external membrane surface during outward currents is unlikely to affect ECs enough to give rise to significant ionic tail currents. The clear monoexponential nature of the decay of off-gating currents observed at ECs (Fig. 5 A, inset) supports this conclusion that off-gating currents are uncontaminated by a component of ionic current decay.
After 10 ms of the first repolarizing pulse, tail decay reached a steady state, and the membrane was subsequently pulsed to ECs (−100 mV). Here, at ECs no ionic current should be present, and tails should represent pure off-gating current which show rapid decay to the baseline (Fig. 5 A). We have compared off-current amplitude (Igoff), charge (Qoff), and the τdecay of the tails at ECs before and after repolarization to +10, and −20 mV (Fig. 5 A, inset and C). The data displayed in the bar graph (except τdecay) have been normalized to the values obtained from the initial tail at −100 mV in Fig. 5 A. After a prepulse to +10 mV, on return to −100 mV, there was no change in peak off-gating current, and less than a 10% reduction in charge returned, compared with no prepulse (Fig. 5 C, solid bars). From the G-V and Q-V relations (Fig. 5 D), there would be a predicted ∼45% decrease in the current if it was predominantly an ionic tail (Fig. 5 C), but only a predicted 12% reduction of charge returned if it represented off-gating current. The data are thus much more consistent with the tail comprising off-gating rather than ionic current. During the prepulse to −20 mV, the G-V relation in Fig. 5 D (•) predicts that complete ionic current deactivation should occur, but from the Q-V relation (▿) only 60% of the gating charge should have returned. The normalized tail amplitude was still 50% of that without the −20 mV prepulse (Fig. 5 C), and the normalized charge in the tail after the −20-mV prepulse (Fig. 5 C, empty bars) was 37% (mean, n = 3) of the total charge moved on depolarization. These values were almost exactly what was expected if the tail comprised off-gating current rather than ionic current.
It appears then, that the peak off-gating current and charge content of inward currents at the ionic reversal potential will not be much affected by permeating Cs+. A second issue is whether the time course of decay of the off-gating current will be affected by a decaying inward or outward ionic tail current close to ECs. Representative ionic tail currents for Kv1.5 in K+-containing pipette and extracellular solutions are as shown in Fig. 5 B for repolarization potentials between −40 and −110 mV (EK in this case was −70 mV). The time constants of decay (shown by the monoexponential fits) decreased from 1.48 ms at −40 mV to 1.07 ms at −100 mV. Values of ∼1 ms for the ionic tail decay around −100 mV are ∼2× slower than measured gating current τdecay (Fig. 5 C, Fig. 4 B). Using the double pulse protocol illustrated in Fig. 5 A, tail decay at −100 mV accelerated from 0.52 ± 0.04 ms (repolarization from +40 mV) to 0.37 ± 0.03 ms (repolarization from −20 mV after complete ionic current deactivation). Data obtained over the entire depolarization voltage range (Fig. 4 B, ▾) suggested a mild voltage dependence to off-gating current τdecay, in the presence of permeating ions. We believe that this reflects the minor voltage dependence to the last closed-open transition that has been described before (Zagotta and Aldrich, 1990). The measurements of τdecay support the idea that off-gating currents at −100 mV measured in the presence of permeating Cs+ are free of contamination by ionic tail currents. In any case, the unequivocal action of the presence of permeant ions is to cause an acceleration of the return of off-gating current and the disappearance of the very slowly decaying off-gating currents on repolarization from positive potentials (Figs. 2, 4, and 5). This slow decay phase takes 10–15 ms to reach completion and, therefore, is too slow to be concealed by overlap from rapidly decaying ionic tail currents.
Time-dependent Slowing of Off-gating Currents Is Prevented by the Presence of Permeant Ions
An important feature of off-gating currents in the Shaker H4-IR construct (Bezanilla et al., 1994) measured in the absence of permeant ions is a slowed decay and decreased peak current with longer prior depolarizations, up to about 8 ms. This property is also shown by Kv1.5 and is illustrated in Fig. 6 A with data from an envelope test of on- and off-gating currents. From −100 mV, cells were depolarized to +60 mV for varying lengths of time. The first pulse was 0.25 ms long, and each subsequent pulse was incremented by 0.50 ms. A cumulative waveform of on-gating current was built up which provided a control for cell stability and the constant availability of on-gating current. Off-gating current transients after brief depolarizations rose rapidly to a peak and initially decayed quickly. After ∼2 ms depolarizations, off-gating transients were smaller and slower as summarized by the graph of τdecay against pulse duration (Fig. 6 B, •). This represents the time dependence of the slowing of off-gating current described earlier (Fig. 2 A) and known to occur in various K+ channels after large depolarizations (Stefani et al., 1994; Fedida et al., 1996). The slowing shows a wide voltage dependence, becoming apparent for depolarizations to potentials greater than those required to open channels and persisting up to large depolarizations (Fedida et al., 1996). Despite the slowing, charge was conserved for short depolarizations allowing about 25 ms to integrate returning charge as reported previously (Bezanilla et al., 1994; Fedida et al., 1996). However, for longer depolarizations, up to 358 ms (Fig. 6 C) the off-gating transients became progressively smaller, and the off-gating current immobilized such that off-gating charge was not conserved when integrated over 20 ms (Fig. 6 D, •). Most of the changes described above in the off-gating current and charge were prevented when Cs+ was present in the pipette and bath. There was little decrease in the peak off-gating current for depolarizations of up to 8 ms duration (Fig. 7 A) and little slowing of τdecay (Fig. 7 B, •), although there was some slowing at very long pulse durations (Fig. 7 C). When integrated, mean data showed that off-gating charge was extremely well conserved when Cs+ was present as a permeant ion (Fig. 7 D, •).
Figure 6.
Envelopes of on- and off- gating currents in the absence of permeant ions with NMG as the substitute cation. (A) Gating currents during depolarizations from −100 to +60 mV and then to −100 mV to record off-gating currents. Pulse durations (frequency 0.5 Hz) were incremented 0.5 ms after the first duration of 0.25 ms to give a maximum pulse duration of 7.75 ms. Multiple sweeps of on-gating currents are superimposed in the upper left segment to show homogeneity. A decrease in off-gating current was apparent after pulse durations longer than a few ms. (B) Mean (±SEM) τdecay of off-gating currents after different prepulse durations (n = 4, •). (C) Off-gating current data when pulse duration was incremented 50 ms to give 8–358 ms durations. Currents were recorded after depolarization indicated above each segment. (D) Change in mean off-gating charge returned during the first 20 ms of repolarization after different duration depolarizations (n = 4, •).
Off-gating Current Slowing in a Nonconducting Mutant of Kv1.5
The decreased peak and slowing of off-gating currents with depolarization, and the progressive slowing of gating charge return are not reproduced when permeant cations such as K+ and Cs+ are present in the pipette filling solution and/or bath. Permeant ions passing through or within the pore of the K+ channels appear to facilitate a more rapid off-gating of the channel. We have tested this requirement for permeating ions with a nonconducting mutant of Kv1.5. The mutation W472F, analogous to the ShH4-IR W434F (Perozo et al., 1993), when incorporated into Kv1.5, prevented measurable ion conduction when channels were transiently expressed in HEK cells, despite high transfection levels determined by adherence of multiple antigen coated beads. In this situation we measured only gating currents from Kv1.5 W472F channels using Cs+ in the pipette and Cs+ plus NMG in the bath solution (Fig. 8). However, gating currents were similar to those observed with NMG present in bath and pipette filling solutions (Fig. 2 A), and quite unlike those recorded from conducting channels in the presence of Cs+ or K+ (Figs. 4, 5, and 2 B). During depolarizations, on-gating currents appeared identical to those observed before (Fig. 2), and off-gating currents on repolarization were slow to peak and to decay for depolarizations positive to −10 mV (Fig. 8 A, inset and C). Despite the slowing, the Q-V relation appeared unchanged from that observed in the wild-type channel with a V0.5 of about −19 mV and z = 2.5 (Fig. 8 B). The slowing of off-gating currents, even in the presence of Cs+ was seen more dramatically with the envelope test protocol (Fig. 8 D). Here, increased duration of depolarization did not decrease the amount of charge returned (Fig. 8 E) but reduced the peak and slowed off-gating current decay (Fig. 8 F ). These data are very similar to those observed by others (Bezanilla et al., 1994) for nonconducting Shaker mutants but fundamentally different from data obtained when permeant ions are present within the pore of the channel (Figs. 2, 4, 5, and 7).
discussion
The data indicate a central role for the K+ channel pore in the physiological gating properties of K+ channels as they close. These direct measurements of K+ channel gating currents along with ionic currents suggest that permeating K+ or Cs+ can accelerate the return of gating charge on repolarization.
Measurement of Off-gating Currents in the Presence of K+ or Cs+
The major methodological hurdle in this study was to make measurements of off-gating currents without contamination from deactivating ionic tail currents. This goal was realized in two ways. In the first, simultaneous measurements of off-gating currents before and after K+ dialysis allowed a direct comparison of charge return in the same cells (Figs. 2 and 3). The data showed no extra charge return (and Qoff/Qon remained at 1.0) or change in voltage dependence when permeating K+ was present. This experiment virtually excluded a contribution of ionic K+ tail currents to the charge return, and the data clearly demonstrated a rapid charge return in the presence of intracellular K+. The second method was to utilize Cs+, an ion of low permeability, to measure off-gating currents in a controlled manner on repolarization to ECs to reduce contamination of off-gating charge by ionic charge at measurement potentials (Figs. 4 and 5). It was clearly demonstrated that on repolarization after a depolarizing prepulse, off-gating currents predominated over deactivating Cs+ ionic tail currents, at repolarizing potentials as positive as −30 mV (Fig. 5 A). As repolarizing potentials were made more negative, reducing Cs+ driving force nearer ECs, we calculated that off-currents were contaminated by <7% ionic current at ±10 mV either side of the test voltage of −100 mV (ECs, where no contamination was expected). We believe that this gave us sufficient margin for safety, and on repolarization to ECs, the amplitudes of off-gating currents and charge returned were entirely consistent with gating current rather than ionic current tail decay, from a comparison of tail amplitudes and consideration of the steady-state Q-V and G-V curves (Fig. 5, C and D). Consistently, in the presence of Cs+ (Fig. 4) the τdecay of off-gating currents at −100 mV remained fast, despite time constants for decay of ionic tail currents that were ∼2× slower (Fig. 5 B). Our data suggest that when permeating ions are present at physiological concentrations within a conducting pore, off-gating currents maintain peak values and decay rapidly, as indicated by the arrows in Fig. 2 B and Fig. 4 A. Below we consider mechanisms for this acceleration of charge return compared with data obtained in the absence of permeating ions.
Acceleration of a Rate-limiting Step Near the Open State
Two other interventions accelerate off-gating currents; 4-aminopyridine (McCormack et al., 1994; Bouchard and Fedida, 1995) and Ba2+ (Hurst et al., 1996) by different mechanisms in each case. 4-aminopyridine prevents slowing (and blocks the channel) by preventing a final transition to channel opening (McCormack et al., 1994), whereas it is suggested that Ba2+ acts by destabilizing the open state (Hurst et al., 1997). These data have been interpreted in terms of existing models of Shaker K+ channel gating (Bezanilla et al., 1994; Zagotta et al., 1994 a) and allosterism (McCormack et al., 1994). In all cases it is a rate-limiting concerted transition carrying little charge, near the open state that is affected (Bezanilla et al., 1994; Zagotta et al., 1994 a). Here we can explain our observations if the rate of this transition is different in the absence or presence of K+ or Cs+. This is depicted by the left transitions of the model in Fig. 9. For simplicity, the closed transitions have been grouped into one closed state (C). The channel can exist in an open state without ions (O), or with ions permeating through the channel (OK). Any model will predict fast on-gating and slowed off-gating currents with a rate limiting slow transition, close to the open state that carries little charge. After voltage-dependent conformational movement of the charged domains, in the absence of permeating cations, the final transition to the open state carries little charge, but is slow in both directions (C ↔ O, filled arrow in Fig. 9). This provides a rate-limiting step on deactivation that visibly slows returning charge (dashed box). Accelerated return of off-gating charge requires a speeding of this returning transition. In the presence of physiological concentrations of intracellular and extracellular ions, our data suggest that the final transition is fast, and thus no longer rate-limiting (OK ↔ C, open arrow). In the absence of permeant ions (Fig. 2) or when a nonconducting mutant was used, which may prevent free movement of permeating ions within the pore (Fig. 8), return of gating charge followed a fundamentally different and slower time course than activation, as has been observed previously (Stefani et al., 1994).
Figure 9.
Schematic diagram of two pathways by which allo-steric modulation of channel gating may occur during short depolarizations. Channels may traverse the path outlined by the dashed box in the absence of permeating cations or in a nonconducting mutant, W472F. Immediately preceding the open state (O) is a late closed state (C) in the activation pathway from which channels proceed slowly to O and rapidly to an absorbing inactivated state (I) from which return is slow. With permeating K+ or Cs+, a state where cations are bound within the pore (OK) exists that shows rapid transitions to and from C (open arrow). Access to the inactivated state is not significant during short depolarizations when permeant ions are present. The scheme with permeating ions is then defined by the solid box.
In the absence of permeant ions, the activation energy barrier that the channel has to overcome to deactivate is greater. We calculate (from data in Fig. 4, C and D) at +60 mV, a difference in the activation energies of ∼1.2 kcal/mol in the absence and presence of permeant ions, respectively. This difference is small but facilitates rapid return of charge when permeant ions are present (Fig. 9, solid box). The relative independence of rate of charge return on pulse duration or amplitude that we have observed can then support gating models which utilize independence of subunit movement during activation (and deactivation) (Zagotta et al., 1994 a). Such an allosteric role for permeating ions in K+ channel gating may then be of fundamental importance in the maintenance of faster cycling as the channel moves from depolarization to repolarization, particularly for channels like Kv1.5 from excitable tissues (heart, brain) where repetitive activation and deactivation is the norm.
Role of Permeating Ions as Gating Modifiers
Permeating ions within ion channel pores are increasingly thought to be involved as modulators of gating. Permeating ions can influence the direction of a cyclical gating reaction in NMDA channels (Schneggenburger and Ascher, 1997). In Shaker channels, high external [K+] (in some channels; Matteson and Swenson, 1986), and [Cs+] or [Rb+] (in most channels; Zagotta et al., 1994 b; Clay, 1996) slow deactivating ionic tail currents in a “foot-in-the-door” manner (Matteson and Swenson, 1986; Demo and Yellen, 1992) and slows the return of gating charge (Loboda and Armstrong, 1997). Inactivation can also be modified by the presence of small cations and recent studies have shown that the rate of C-type inactivation is modulated by different [K+] at the outer pore mouth (Baukrowitz and Yellen, 1995, 1996). In the extreme case, in the absence of external K+ or in the nonconducting Shaker mutant W434F, it has been suggested that the channel exists in a permanently C-type inactivated state (Yan et al., 1996). These studies all describe situations where the channel conformational changes of gating can be seen to be coupled to ion concentrations within the vicinity of the pore. Here we have shown that the rate of return of charge after channel opening depends on the presence of permeating ions at physiological concentrations within the pore.
A provocative suggestion from a consideration of the above literature is that our data reflect a prevention of C-type inactivation by small cations somewhere in the permeation pathway. This would provide an explanation of our results that fits neatly with data on the rate of C-type inactivation being dependent on the cation occupancy of a site in the external mouth of the pore, when there is an acceleration in the rate of C-type inactivation with a decrease in K+ flux (Baukrowitz and Yellen, 1995, 1996). Furthermore, it has been shown that the time course of C-type inactivation is slowed by increasing external K+, Rb+, and Cs+ (Lopez-Barneo et al., 1993) through a foot-in-the-door manner which is dependent on the residue at site 449 in Shaker channels lacking N-type inactivation. In the model in Fig. 9, since C-type inactivation depends on the occupancy of cations in the external mouth of the pore, a single inactivated state (I) is shown as coupled directly to the open state. We have ignored the possibilities of closed-state inactivation (Marom and Levitan, 1994) and an inactivated state which has K+ bound to explain faster recovery from inactivation by elevated [K+ o] (Levy and Deutsch, 1996). In the case where there is NMG alone, upon activation, the channel would follow the upper pathway (dashed box) but with a fast C → O transition. Since there would not be any ions occupying the binding sites within the pore, the O → I transition would be rapid and preferred. Upon repolarization, the I → O transition would be the slow, rate-limiting step which would explain the slow return of gating charge. The O → C transition would remain fast and be rate-limited by the speed of the I → O transition.
When pulses of increasing duration were given (Fig. 6 A), the off-gating currents that resulted initially decayed rapidly (Fedida et al., 1996), but after depolarizations longer than 1–2 ms they began to slow and then became increasingly slower (Zagotta et al., 1994 a; Fedida et al., 1996). These effects were prevented by the presence of K+ (data not shown) or Cs+ (Fig. 7 A). With permeating K+ or Cs+, there would be an extra transition which is fast (O → OK, open arrow) that channels prefer to the inactivated state (due to the lower activation energy barrier). The majority of channels would then prefer the sequence outlined in the solid box which would not result in the conformational changes at the outer mouth of the channel associated with inactivation (Liu et al., 1996). Return of gating charge would remain fast because the OK → C or OK → O → C pathways avoid the rate-limiting I → O step. In the definitive case, inactivation should lead to charge immobilization similar to N-type inactivation in Shaker channels (Stühmer et al., 1991; Perozo et al., 1992). However, charge return was well-conserved both when return was slow in NMG alone and when return was rapid in the presence of K+ or Cs+. There was also no change in Q-V relations nor the Qoff/Qon ratios (Figs. 2 and 3). A more subtle initial shift in the Q-V curve to more negative potentials without reduction in the total charge moved has been suggested to result from C-type inactivation (Olcese et al., 1994). This inactivation becomes more obvious when the preparation is held for several seconds, and there is a reduction in charge. In Figs. 6 and 7 we showed that pulses of increasingly longer duration (up to 400 ms) did lead to charge immobilization that was also prevented, or slowed considerably, by permeating cations, so it is possible that the very early slowing of off-gating charge represents the very earliest steps of inactivation, one end of a continuum that will eventually lead to measurable charge immobilization. This also provides a possible explanation of our data from the nonconducting mutant, W472F. If this channel predominantly exists in a C-type inactivated state, as suggested previously (Yan et al., 1996), then permeating cations may not be able to access the pore quickly or deeply enough to prevent off-gating current slowing. The channel would show rapid C-type inactivation which would bypass the O → OK transition and follow the sequence outlined by the dashed box. In the same way as in NMG solutions alone, the I → O transition would be the rate-limiting step and return of gating charge would be slow.
A Site for the Action of Cations
Although it is possible that the K+ or Cs+ binds to an intracellular site outside the pore itself to produce the effects on off-gating currents, data from the nonconducting mutant with high intracellular Cs+ (Fig. 8) do not show the same acceleration in the return of gating charge seen in conducting Kv1.5 channels with identical Cs+ concentrations (Figs. 5 and 7). This result suggests that the single W472F mutation in the nonconducting mutant might disrupt a binding site for a permeating monovalent cation within the pore of the channel or prevent access to such a site. A putative structural explanation would involve sites near the equivalent of the Shaker 449 site. In addition to its involvement in C-type inactivation (Lopez-Barneo et al., 1993) and TEA block (MacKinnon and Yellen, 1990), this site has been suggested to be an external K+ binding site in DRK1 (Krovetz et al., 1997). Ba2+ block in Shaker channels also speeds the return of off-gating charge and an adjacent residue, D447, has been shown to contribute to Ba2+ binding while T449 is a barrier to the site from the outside of the pore (Hurst et al., 1996). Hurst et al. also found that a D447N substitution is nonconducting. Residues near the 434 equivalent site in Kv2.1 have recently been shown to affect C-type inactivation and TEA sensitivity (Kirsch and Shieh, 1997). It is therefore possible that the 434 and 449 sites are in close physical proximity in the external mouth of the pore and together affect TEA binding, C-type inactivation, and are responsible for producing nonconducting mutants. In addition, they may cooperate to confer a cation binding site which is also affected by both Cs+ and Ba2+. When there are no permeating ions (or ions at the binding site), return of gating charge will be slowed after short depolarizations. However, having K+, Cs+, or Ba2+ at the binding site prevents off-gating current slowing, either via allosteric actions on channel closing or via a prevention of C-type inactivation (or, of course, a combination of both). In a situation where the binding site is disrupted by a mutation in the 434 residue, it is possible that K+ cannot pass through the pore and C-type inactivation is strongly accelerated, causing a slow return of gating charge, which cannot be prevented by Cs+ (Fig. 8). However, these suggestions are speculative and in the present study we cannot distinguish whether the rapid return of gating charge is due to a slow step near the open state which is facilitated by permeant ions or due to a rate-limiting step resulting from C-type inactivation which is prevented (or slowed considerably) by permeant ions.
Acknowledgments
Supported by grants from the Heart and Stroke Foundation of Ontario and the Medical Research Council of Canada to D. Fedida.
Footnotes
A preliminary report of this work has appeared in abstract form (Chen, F.S.P., and D. Fedida. 1997. Biophys. J. 72:A27).
references
- Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature (Lond) 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Baukrowitz, T., and G. Yellen. 1995. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron .15:951–960. [DOI] [PubMed]
- Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+channel. Science (Wash DC) 1996;271:653–656. doi: 10.1126/science.271.5249.653. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Perozo E, Papazian DM, Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science (Wash DC) 1991;254:679–683. doi: 10.1126/science.1948047. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Perozo E, Stefani E. Gating of Shaker K+channels. II. The components of gating currents and a model of channel activation. Biophys J. 1994;66:1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard RA, Fedida D. Closed and open state binding of 4-aminopyridine to the cloned human potassium channel Kv1.5. J Pharmacol Exp Ther. 1995;275:864–876. [PubMed] [Google Scholar]
- Clay JR. Effects of permeant cations on K+channel gating in nerve axons revisited. J Membr Biol. 1996;153:195–201. doi: 10.1007/s002329900122. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Drewe JA, Kirsch GE, Brown AM. Histidine substitution identifies a surface position and confers Cs+ selectivity on a K+pore. Biophys J. 1993;65:1235–1242. doi: 10.1016/S0006-3495(93)81154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo SD, Yellen G. Ion effects on gating of the Ca2+-activated K+channel correlates with occupancy of the pore. Biophys J. 1992;61:639–648. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D. Gating charge and ionic currents associated with quinidine block of hKv1.5. J Physiol (Lond) 1997;499.3:661–675. doi: 10.1113/jphysiol.1997.sp021959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Bouchard R, Chen FSP. Slow gating charge immobilization in the human potassium channel Kv1.5 and its prevention by 4-aminopyridine. J Physiol (Lond) 1996;494:377–387. doi: 10.1113/jphysiol.1996.sp021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Wible B, Wang Z, Fermini B, Faust F, Nattel S, Brown AM. Identity of a novel delayed rectifier current from human heart with a cloned K+channel current. Circ Res. 1993;73:210–216. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- Goldstein SNA. A structural vignette common to voltage sensors and conduction pores: Canaliculi. Neuron. 1996;16:717–722. doi: 10.1016/s0896-6273(00)80092-6. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic channels of excitable membranes. Sinauer Associates Inc. Sunderland, MA.
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Shakerpotassium channel gating. I. Transitions near the open state. J Gen Physiol. 1994;103:249–278. doi: 10.1085/jgp.103.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Toro L, Stefani E. Molecular determinants of external barium block in Shakerpotassium channels. FEBS Lett. 1996;388:59–65. doi: 10.1016/0014-5793(96)00516-9. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Roux MJ, Toro L, Stefani E. External barium influences the gating charge movement of Shakerpotassium channels. Biophys J. 1997;72:77–84. doi: 10.1016/S0006-3495(97)78648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Patil PG, Snutch TP, Yue DT. G-protein modulation of N-type calcium channel gating current in human embryonic kidney cells (HEK 293) J Physiol (Lond) 1997;498:601–610. doi: 10.1113/jphysiol.1997.sp021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes RD, Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol (Lond) 1974;239:393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch GE, Shieh C-C. Regulation of ion-dependent gating and block by conserved aromatic amino acid residues in the pore of voltage gated K+channels. Biophys J. 1997;72:A232. [Google Scholar]
- Krovetz HS, VanDongen HMA, VanDongen AMJ. An external potassium binding site revealed in DRK1, a voltage-gated K channel. Biophys J. 1997;72:A232. [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the Shaker K+channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Levy DI, Deutsch C. A voltage-dependent role for K+in recovery from C-type inactivation. Biophys J. 1996;71:3157–3166. doi: 10.1016/S0006-3495(96)79509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+channel during gating. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Loboda A, Armstrong CM. External potassium and rubidium slow down the off gating charge movement of non-conducting Shaker K+channel. Biophys J. 1997;72:A28. [Google Scholar]
- Lopez-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors and Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- MacKinnon R, Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+channels. Science (Wash DC) 1990;250:276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Isacoff EY. Direct physical measurement of conformational rearrangement underlying potassium channel gating. Science (Wash DC) 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Marom S, Levitan IB. State-dependent inactivation of the Kv3 potassium channel. Biophys J. 1994;67:579–589. doi: 10.1016/S0006-3495(94)80517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson DR, Swenson RP. External monovalent cations that impede the closing of K+channels. J Gen Physiol. 1986;87:795–816. doi: 10.1085/jgp.87.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Joiner WJ, Heinemann SH. A characterization of the activating structural rearrangements in voltage-dependent Shaker K+channels. Neuron. 1994;12:301–315. doi: 10.1016/0896-6273(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Potentiation by the β subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Nature (Lond) 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- Olcese R, Toro L, Perozo E, Bezanilla F, Stefani E. Prolonged depolarization changes charge movement properties in Shaker-IR W434F K+channels. Biophys J. 1994;66:A107. [Google Scholar]
- Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shakerpotassium channel by mutations in the S4 sequence. Nature (Lond) 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Perozo E, MacKinnon R, Bezanilla F, Stefani E. Gating currents from a non-conducting mutant reveal open-closed conformation in Shaker K+channels. Neuron. 1993;11:353–358. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- Perozo E, Papazian DM, Stefani E, Bezanilla F. Gating currents in Shaker K+channels. Implications for activation and inactivation models. Biophys J. 1992;62:160–171. doi: 10.1016/S0006-3495(92)81802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Santacruz-Toloza L, Stefani E, Bezanilla F, Papazian DM. S4 mutations alter gating currents of Shaker K channels. Biophys J. 1994;66:345–354. doi: 10.1016/s0006-3495(94)80783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shakerpotassium channels. Science (Wash DC) 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1993;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- Snyders DJ, Tamkun MM, Bennett PB. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol. 1993;101:513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E, Toro L, Perozo E, Bezanilla F. Gating of Shaker K+channels. I. Ionic and gating currents. Biophys J. 1994;66:996–1010. doi: 10.1016/S0006-3495(94)80881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W, Conti F, Stocker M, Pongs O, Heinemann SH. Gating currents of inactivating and non-inactivating potassium channel expressed in Xenopus oocytes. Pflüg Arch. 1991;410:423–429. doi: 10.1007/BF00550881. [DOI] [PubMed] [Google Scholar]
- Taglialatela M, Stefani E. Gating currents of the cloned delayed-rectifier K+channel DRK1. Proc Natl Acad Sci USA. 1993;90:4758–4762. doi: 10.1073/pnas.90.10.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang Y, Sigworth FJ. How does W434F block Shaker channel current? . Biophys J. 1996;71:A190. [Google Scholar]
- Yang NB, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Aldrich RW. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990;95:29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. a. Shakerpotassium channel gating. III. Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Dittman J, Aldrich RW. b. Shakerpotassium channel gating. II. Transitions in the activation pathway. J Gen Physiol. 1994;103:279–319. doi: 10.1085/jgp.103.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]