Abstract
Systemically administered fluoride at a concentration of 75 ppm increases the surface roughness of developing enamel crystals in rats, which may be significant in advancing our understanding of the biological mechanism of fluorosis. Thus, the aim of this study was to investigate whether the increased surface roughness may be a result of surface restructuring by the direct action of fluoride at the crystal surface. We examined the fluoride dose-dependent roughening of enamel crystal surfaces in vivo, in the rat, and whether this roughening could be mimicked by the in vitro treatment of rat enamel crystals with neutral pH fluoride solutions. Our results showed that enamel crystal surface roughness increased after treatment with increasing fluoride ion concentrations, whether applied in vitro or administered systemically. This suggests a mechanism, alongside others, for the increased surface roughness of crystals in fluorotic enamel.
Keywords: dental fluorosis, enamel crystals, fluoride, AFM
Introduction
With the introduction of fluoride as the main anticaries agent used in preventive dentistry, and perhaps an increase in fluoride in our food chain, dental fluorosis has become an increasing world-wide problem (Aoba and Fejerskov, 2002). There is still debate regarding the precise mechanisms causing dental and skeletal fluorosis (Bawden et al., 1995; Aoba and Fejerskov, 2002; Robinson et al., 2004a,b).
Recently, increases in the roughness of crystal surfaces in developing fluorosed enamel have been reported, and the implications of this with regard to fluorosis discussed (Kirkham et al., 2001; Robinson et al., 2003, 2004b). The proposed mechanism of how these roughened crystals may cause fluorosis involved impairment of crystal growth due to changes in crystal surface chemistry, and/or incomplete removal of modulating matrix proteins, which are known to hinder crystal development (Aoba and Fejerskov, 2002; Robinson et al., 2004b). Rougher crystals have been shown to bind increased amounts of protein in other tissues (Gathercole et al., 1996).
Reasons for the increased roughness were thought to be a change in kink and step-site density, reflecting a higher supersaturation level in tissue fluid for the deposition of mineral. This might arise due to the deposition of less soluble fluoridated mineral species—apatite. Retention of matrix proteins themselves, due to increased interaction with the altered crystal surfaces, might also generate altered growth patterns. Increased retention of matrix protein is highly likely, since proteins both bind more effectively to fluoridated apatite in vitro (Bawden et al., 1995; Aoba and Fejerskov, 2002) and are retained in naturally fluorotic enamel to a significant degree (Tanabe et al., 1988; DenBesten, 1999; Robinson et al., 2004b).
Recent studies in our laboratory have showed, with atomic force microscopy (AFM), that ameloblastin adsorbs to crystal surfaces (Misch et al., unpublished data), and that dentin phosphoprotein is bound more tightly to fluorotic enamel crystals than to non-fluorotic ones (Spencer et al., unpublished data). Thus, since fluorotic enamel crystals appear to have a greater binding capacity for the matrix proteins, which would lead to their impaired removal, this would offer an explanation for the greater concentration of proteins and impaired crystal growth in fluorotic enamel, which, in turn, results in the production of hypomineralized enamel.
It is well-established that fluoride administration in vivo generates rougher crystal surfaces, and we hypothesized that this particular effect could be due, at least in part, to interaction between crystal surfaces and fluoride in the surrounding tissue fluid. In view of this, we carried out in vivo studies to establish whether the degree of roughening of the surfaces was dependent on the amount of fluoride given. The in vitro studies were carried out to demonstrate that the crystal surface restructuring (roughness) could be caused by fluoride and, therefore, could be a mechanism, alongside others, in the development of the roughened crystal surface in fluorosed enamel. The crystal surfaces were visualized by AFM, which is capable of imaging features at the size of the apatite unit cell (Kirkham et al., 1998, 2001).
Materials & Methods
Fluorotic Enamel Preparation
Small particles (approximately 50-100 μg in weight) of maturation-stage enamel were microdissected free from the underlying dentin and used for the isolation of individual crystals. These particles were obtained from Wistar rats which had received 25, 50, or 75 ppm of fluoride (added as NaF) to the drinking water on day 22, and continuing for 21 days. The presence of fluorotic enamel was determined by the appearance, after eruption, of striae on the enamel. Matched littermates were the controls, which received non-fluoridated drinking water for the same period of time, and were fed the same laboratory diet. (The animal use protocol was reviewed and approved by the University's committee on Use and Care of Animals.)
Isolation of the Enamel Crystals
All detectable traces of matrix protein were removed from the enamel samples by a sequential extraction procedure described previously (Kirkham et al., 1998). The enamel crystals were sonicated for 2 min in HPLC-grade methanol to reduce aggregation. Approximately 5 μL of this suspension was then pipetted onto freshly cleaved mica. The methanol was removed by evaporation, leaving a coating of dispersed hydroxyapatite crystals on the surface. The specimens were dried in a desiccator for at least 12 hrs and subsequently examined by AFM.
Enamel Crystals Treated with NaF
Protein-free developing enamel crystals were obtained from the maturation stage of normal rat incisors. These enamel crystals were treated with: (a) 0 ppm, 25 ppm, 50 ppm, and 75 ppm NaF solutions [pH 7.4, phosphate-buffered saline (PBS), containing 0.01% NaN3) in a water bath held at 37°C for 21 days; or (b) 0, 200 ppm, 1000 ppm, 2000 ppm, 10,000 ppm, and 20,000 ppm NaF solutions (pH 7.4, PBS buffer plus NaN3) in a water bath held at 37°C for 18 hrs. The volume of NaF solution relative to the weight of enamel crystals was approximately 1.5 mL solution/0.1 mg enamel crystals. After centrifugation, the crystals were washed 6 times with de-ionized water (pH 7.4) and dispersed onto a mica surface prior to surface roughness measurements with a Nanoscope IIIa AFM.
Atomic Force Microscopy
All samples were imaged in tapping mode in air, with a Nanoscope IIIa Multimode AFM and controller (Digital Instruments, Santa Barbara, CA, USA) equipped with a 120 × 120 μm J-type scanner. Commercially available tapping mode cantilevers, TESP, were used (Digital Instruments, Santa Barbara, CA, USA) (thickness, 2.6-4.5 μm; width, 28-30 μm; height, 10-15 μm; length, 125 μm; resonant frequency, 297-378 kHz; spring constant, 29-61 N/m; nominal tip radius of curvature, 5-10 nm). Multiple images for each sample were obtained with scan sizes ranging from 500 × 500 nm to 10 × 10 μm at 70-80% of free cantilever amplitude. The phase image results from effective damping or alteration to the resonant frequency of the cantilever, depending on the modulus of the surface being examined. Therefore, it is more sensitive to the surface morphology change and gives much higher resolution.
Surface roughness measurements (Ra) were obtained over a length of 100-nm line approximately parallel to the crystal c-axis. Fifty measurements were obtained at different sites from approximately 10 typical individual crystals, at each fluoride concentration used, to achieve a final mean roughness value. Ra roughness was defined as:
and was calculated with the use of Nanoscope 5.12r3 software. Here, f(x) is the roughness curve relative to the center plane, and L is the length of the roughness curve. Therefore, the calculated surface roughness in this case is the mean value of the surface relative to the center plane, and takes into account any changes in the slope of the crystal surface. Statistical analysis of the results of the roughness measurements for the crystal surfaces were analyzed by ANOVA for multiple comparison, followed by a pairwise t test (Kirkham et al., 2001; Wallwork et al., 2002).
Results
To reduce the number of AFM images, we chose: maturation control (Fig. 1a); 50 ppm administered systemically (Fig. 1b); and 50 ppm (Fig. 1c) and 1000 ppm (Fig. 1d) applied in vitro as being representative of the crystal surface morphology.
Figure 1.
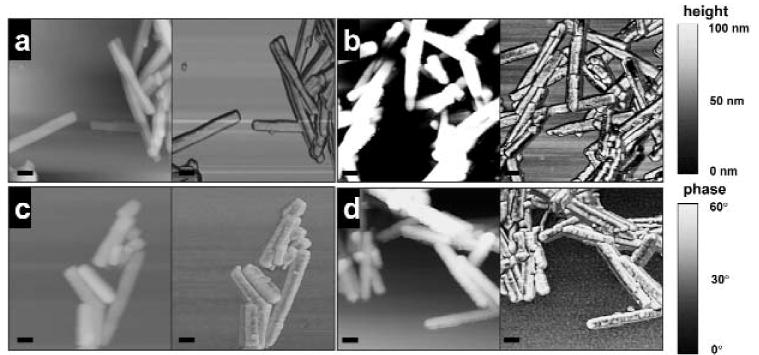
Tapping mode AFM images of enamel crystals on a mica surface, imaged in air. (a) Control (maturation stage); (b) maturation-stage enamel crystals from rats after systemic administration of 50 ppm fluoride for 21 days; (c) maturation-stage non-fluorotic enamel crystals after in vitro treatment with 50 ppm fluoride, pH 7.4, for 21 days at 37°C; (d) maturation-stage non-fluorotic enamel crystals after in vitro treatment with 1000 ppm fluoride, pH 7.4, for 18 hrs at 37°C. [a-d image sizes, 1 × 1 μm; bar = 100 nm; left, height image, z-range, 100 nm; right, phase image, z-range, 60°.]
Fluoride Administered in vivo
When phase images were viewed, crystal surface nanoscale changes became more evident as the concentration of fluoride increased (Fig. 1b). In crystals from rats receiving systemic fluoride for 21 days, this resulted in a significant change in surface roughness of the maturation crystals (Fig. 2), from Ra = 0.53 ± 0.18 in the control to 0.65 ± 0.21 (25 ppm F), 0.71 ± 0.20 (50 ppm F), and 0.85 ± 0.28 (75 ppm F). The Ra of the crystals from the rats receiving the 25 ppm and 50 ppm fluoride systemically were not significantly different from each other. Crystals from rats receiving 75 ppm were significantly rougher (P < 0.05) than crystals from those receiving 25 ppm or 50 ppm.
Figure 2.
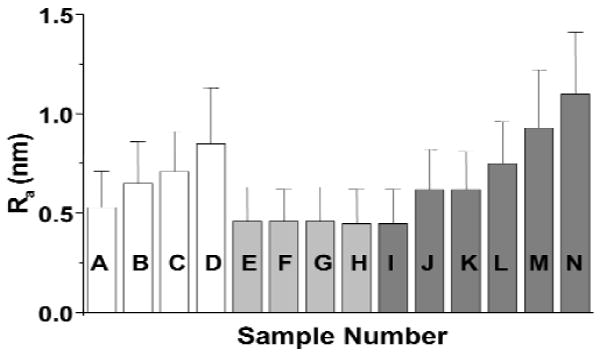
Surface roughness measurements (Ra ± SD) of maturation-stage enamel crystals after in vivo or in vitro treatment of fluoride. In vivo treatment (rats received systemically different concentrations of fluoride in their drinking water, added as NaF, for 21 days): A (control), B (25 ppm), C (50 ppm), D (75 ppm). [B and C are not significantly different from each other (P < 0.05).] Maturation-stage enamel crystals treated in vitro with NaF at different concentrations for 21 days at 37°C: E (control), F (25 ppm), G (50 ppm), H (75 ppm) [not significantly different from each other (P < 0.05)]. Maturation-stage enamel crystals treated in vitro with NaF in different concentrations for 18 hrs at 37°C: I (control), J (200 ppm), K (1000 ppm), L (2000 ppm), M (10,000 ppm), N (20,000 ppm) [J, K, L, M, N are significantly different from the control, I; J and K are not significantly different from each other but are significantly different from L, M, N; L, M, N are significantly different from each other (P < 0.05)].
Fluoride Administered in vitro
Fig. 1c shows the height and phase images of maturation-stage enamel crystals after in vitro treatment with 50 ppm F, pH 7.4, for 21 days at 37°C. There was no significant difference in the roughness of the crystals that were in vitro-treated with 25 ppm, 50 ppm, or 75 ppm fluoride (Ra, 0.46 ± 0.16, 0.46 ± 0.17, and 0.45 ± 0.17, respectively) for 21 days and the non-treated control (Ra = 0.46 ± 0.17) (Fig. 2). Treating the enamel crystals with higher concentrations of NaF (200 ppm, 1000 ppm, 2000 ppm, 10,000 ppm, 20,000 ppm fluoride for 18 hrs at 37°C) produced considerable visual nanoscale changes to crystal surface morphology (Fig. 1d). [A time-course experiment in which enamel crystals were treated in vitro with NaF at these higer concentrations showed that surface roughening occurred after 18 hrs (data not shown).] Surface roughness measurements confirmed this observation, with Ra 0.45 ± 0.17 (control) and 0.62 ± 0.20 (200 and 1000 ppm F) increasing progressively to 0.75 ± 0.21 (2000 ppm F), 0.93 ± 0.29 (10,000 ppm F), and 1.01 ± 0.31 (20,000 ppm F) (Fig. 2).
Discussion
Our results showed that fluoride, administered both systemically to rats and used as an in vitro treatment of rat enamel crystals, was able to change the surface structure of the crystals, resulting in increased surface roughness. When fluoride was administered systemically at 75 ppm, the maturation-stage enamel was significantly rougher than control maturation-stage enamel crystals, in agreement with Kirkham et al. (2001). However, we also showed that the roughness of maturation-stage enamel crystal surfaces increased at much lower fluoride concentrations (25 ppm), and, although, there was no significant difference in roughness between the 25-ppm and 50-ppm treatments, there was a further significant increase in roughness when 75 ppm was administered. This observation suggested a dose-dependence of crystal surface roughness by systemically administered fluoride, although this did not appear to be linear at the dosages used.
There is evidence that, during the systemic administration of fluoride, concentrations during the transition and maturation stage of enamel development may be very high (Weatherell et al, 1977; DenBesten and Thariani, 1992), and that this fluoride may be labile (Weatherell et al., 1977). In view of this, we also investigated the direct effect of fluoride on enamel crystal surfaces in vitro. Enamel crystals in vitro were exposed to F concentrations of 25 to 75 ppm for 21 days. Unlike systemic administration, this did not cause any significant increase in the surface roughness of the crystals.
At higher concentrations in vitro (> 200 ppm), however, increases in crystal surface roughness did occur. This raises the possibility that effective F ion activity in vivo may be very high in the immediate crystal environment. This, however, assumes that the roughness originates in precisely the same way both in vivo and in vitro.
It has been suggested that increased roughness in vivo was due to fluoride incorporation into depositing mineral, possibly fluoride substituting for hydroxyl ions in apatite, producing a less soluble phase (Robinson et al., 2004a,b). The effective increase in supersaturation could lead to increased density of kink and step growth sites on the crystal surface. However, analysis of the in vitro data suggests another possibility.
Since the in vitro treatments were not carried out in the presence of solutions supersaturated with respect to apatite, surface roughness could not originate directly from growth-related phenomena. It might be that some restructuring of the crystal surface occurs, perhaps via ions in the Gouy-Chapman layers and those in the crystal surface. This process would include ion exchange, dissolution, and/or redeposition. While the precise chemistry is not known, this would result from fluoride altering the levels of supersaturation in the supernatant, with respect to dissolving and reprecipitating phases of the crystal surface. For example, incorporation of fluoride into the crystals by hetero-ionic exchange would effectively increase supersaturation with respect to the surface. This may in turn reduce surface carbonate. Calcium would also be released to maintain charge balance, resulting in reprecipitation of less soluble phases. There is also evidence that these crystals may be heterogeneous from a chemical viewpoint, which would give rise to growth on some areas but not in others (Robinson et al., 2004a). At high concentrations of fluoride in vitro, double decomposition could occur between fluoride and hydroxyapatite, resulting in calcium fluoride and various calcium fluoride complexes (Øgaard, 2001). Whether this precise mechanism occurs in vivo is difficult to say, since solution conditions in vivo are not precisely known. However, such changes cannot be ruled out, especially at the maturation stage of development, where fluoride is known to accumulate. Therefore, in vivo, these phenomena would occur if concentrations in the crystal environment reached such high levels.
On the basis of the known pharmacokinectics of fluoride (DenBesten and Crenshaw, 1984; Aoba and Fejerskov, 2002; Robinson et al., 2004b), it seems unlikely that, at 25 to 75 ppm, fluoride given systemically would generate greater than 200 ppm free fluoride in the enamel organ or enamel matrix. However, it is possible that the concentration of fluoride at the enamel crystal surface during growth could be very high. DenBesten and Crenshaw (1984) reported that the maturation stage of fluorosed enamel from rats that had received 75 ppm systemically contained approximately 405 ± 99 ppm of fluoride. These concentrations were obtained from analyses of whole enamel and are not indicative of even higher concentrations that might occur at the enamel crystal surface.
The precise concentrations of fluoride in supernatant solutions in vivo are unknown, particularly at this developmental stage, where it is known that fluoride concentrates. In addition, in the immediate vicinity of crystal surfaces, concentrations of all ions, including fluoride, would be considerably higher than in the solution, depending upon the surface zeta potential. We therefore suggest that if fluoride concentrations of > 200 ppm are reached at the enamel crystal surface after the systemic administration of fluoride, they could cause crystal surface changes similar to those seen after in vitro application. Crystal roughening in vivo may therefore be partly due to supersaturation effects on crystal growth, and partly a direct effect on surface restructuring.
In conclusion, this study has showed that both in vitro and systemically administered fluorides are able to restructure the surface of the enamel crystal. The topography of the crystal surfaces was altered by treatment with fluoride. The enamel crystal surface roughness increased after treatment with increasing fluoride ion concentrations.
Acknowledgments
This investigation was supported by USPHS Research Grant DE015599 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
References
- Aoba T, Fejerskov O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med. 2002;13:155–170. doi: 10.1177/154411130201300206. [DOI] [PubMed] [Google Scholar]
- Bawden JW, Crenshaw MA, Wright JT, LeGeros RZ. Consideration of possible biologic mechanisms of fluorosis. J Dent Res. 1995;74:1349–1352. doi: 10.1177/00220345950740070501. [DOI] [PubMed] [Google Scholar]
- DenBesten PK. Biological mechanisms of dental fluorosis relevant to the use of fluoride supplements. Community Dent Oral Epidemiol. 1999;27:41–47. doi: 10.1111/j.1600-0528.1999.tb01990.x. [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Crenshaw MA. The effects of chronic high fluoride levels on forming enamel in the rat. Arch Oral Biol. 1984;29:675–679. doi: 10.1016/0003-9969(84)90171-7. [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Thariani H. Biological mechanisms of fluorosis and level and timing of systemic exposure to fluoride with respect to fluorosis. J Dent Res. 1992;71:1238–1243. doi: 10.1177/00220345920710051701. [DOI] [PubMed] [Google Scholar]
- Gathercole LJ, Swan AJ, Price G, Dieppe PA. Nanometre-scale surface features of arthropathic microcrystals and their relation to protein adsorption: a study by scanning probe microscopy and wide angle x-ray diffraction. J Mater Sci Mater Med. 1996;7:511–516. [Google Scholar]
- Kirkham J, Brookes SJ, Shore RC, Bonass WA, Smith DA, Wallwork ML, et al. Atomic force microscopy studies of crystal surface topology during enamel development. Connect Tissue Res. 1998;38:91–100. doi: 10.3109/03008209809017025. [DOI] [PubMed] [Google Scholar]
- Kirkham J, Brookes SJ, Zhang J, Wood SR, Shore RC, Smith DA, et al. Effect of experimental fluorosis on the surface topography of developing enamel crystals. Caries Res. 2001;35:50–56. doi: 10.1159/000047431. [DOI] [PubMed] [Google Scholar]
- Øgaard B. CaF(2) formation: cariostatic properties and factors of enhancing the effect. Caries Res. 2001;35 1:40–44. doi: 10.1159/000049109. [DOI] [PubMed] [Google Scholar]
- Robinson C, Brookes SJ, Wood SR, Kirkham J, Shore RC. The effect of fluoride on the developing mineralised tissues: a brief review. Oralprophylaxe. 2003;25:33–38. [Google Scholar]
- Robinson C, Connell S, Kirkham J, Shore R, Smith A. Dental enamel—a biological ceramic: regular substructures in enamel hydroxyapatite crystals revealed by atomic force microscopy. J Mater Chem. 2004a;14:2242–2248. [Google Scholar]
- Robinson C, Connell S, Kirkham J, Brookes SJ, Shore RC, Smith AM. The effect of fluoride on the developing tooth. Caries Res. 2004b;38:268–276. doi: 10.1159/000077766. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Aoba T, Moreno EC, Fukae M. Effect of fluoride in the apatitic lattice on adsorption of enamel proteins onto calcium apatites. J Dent Res. 1988;67:536–542. doi: 10.1177/00220345880670030301. [DOI] [PubMed] [Google Scholar]
- Wallwork ML, Kirkham J, Chen H, Chang SX, Robinson C, Smith DA, et al. Binding of dentin noncollagenous matrix proteins to biological mineral crystals: an atomic force microscopy study. Calcif Tissue Int. 2002;71:249–255. doi: 10.1007/s00223-001-1011-4. [DOI] [PubMed] [Google Scholar]
- Weatherell JA, Deutsch D, Robinson C, Hallsworth AS. Assimilation of fluoride by enamel throughout the life of the tooth. Caries Res. 1977;11 1:85–115. doi: 10.1159/000260297. [DOI] [PubMed] [Google Scholar]


