Abstract
Autoimmune myocarditis, a chronic stage of myocardial inflammation, occurs in a small subset of patients after acute cardiotropic viral infection and can lead to dilated cardiomyopathy (DCM). This disease can be recapitulated in susceptible mouse strains by infection with coxsackievirus B3, or by immunization with cardiac myosin or cardiac troponin I. The etiologies of myocarditis are multifactorial and genetically complex. Genetic linkage between susceptibility to myocarditis/DCM and the major histocompatibility complex (MHC) genes have been reported in both humans and experimentally induced mouse models. However, unlike other autoimmune diseases, the non-MHC genes seem to have greater impact than MHC genes on disease susceptibility. Several myocarditis-related non-MHC loci have been identified by our laboratory and others in different models. Most of these loci overlap with other autoimmune disease susceptibility loci, suggesting common or shared genetic traits influencing general autoimmunity. For example, we have demonstrated that Eam1 and Eam 2 may influence disease susceptibility via regulating T cell apoptosis at different developmental stages. Blockade of signaling through specific genes, such as CTLA4, ICOS and PD-1, can either enhance or prevent the development of experimental autoimmune myocarditis, but it remains unclear whether functional polymorphisms in these genes are involved in predisposition to disease. In humans, mutations/deletions in immunologically important genes such as CD45, and genes encoding cardiac proteins, have been reported in patients with recurrent myocarditis or DCM. Identification of genetic polymorphisms controlling autoimmune myocarditis will help us understand the mechanisms underlying autoimmune diseases in general, thereby improving potential therapies in patients.
Keywords: genetics, genetic polymorphism, autoimmune myocarditis, MHC and myocarditis
1. Autoimmune Myocarditis in Humans
Myocarditis, pathologic inflammation of the myocardium, is the leading cause of heart failure in patients less than 40 years of age [1]. About 10-20% of people with histological evidence of myocarditis, even in asymptomatic patients, will develop chronic disease eventually leading to dilated cardiomyopathy (DCM) [2]. Despite the well-established morbidity and mortality associated with myocarditis, its clinical etiologies and presentations are broad and difficult to evaluate. Cardiotropic viruses, such as coxsackievirus B3 (CVB3), are well known agents that can induce clinical myocarditis [3]. After the acute phase caused by direct cytopathic effects of the virus, a small proportion of patients subsequently develop autoimmune-mediated, chronic myocarditis [4], accompanied by circulating autoantibodies to cardiac myosin and other heart antigens, which can sometimes lead to heart failure and death associated with DCM.
2. Murine models of autoimmune myocarditis
Animal models resembling human myocarditis can be produced in susceptible mice by intraperitoneal inoculation of heart-passaged CVB3 [5] or immunization with purified cardiac myosin or cardiac α-myosin heavy chain peptide in complete Freund’s adjuvant [6]. After the acute phase of viral myocarditis has subsided, a few strains of mice, especially A/J and other strains sharing the A background, develop a second phase characterized by diffuse intra-heart infiltrates and large areas of myocyte necrosis and fibrosis. This phase occurs in the absence of infectious virus [7] and is believed to be mediated by cardiac-specific autoimmune responses, as suggested by the presence of IgG autoantibodies to cardiac myosin [8, 9]. Myocarditis induced by immunization with cardiac myosin or its peptide provides a virus-free mouse model, which simulates the second phase of the viral disease. Most important, cardiac myosin immunization produces pathology only in strains of mice that are susceptible to the second phase of CVB3-induced myocarditis, showing that the genetic predisposition to autoimmune myocarditis is similar in both models [10]. Furthermore, adoptive transfer of activated autoreactive T cells [11, 12] or autoantibodies [13] derived from cardiac myosin - immunized mice are able to induce disease in recipients of different strains, suggesting that myocarditis with similar phenotypic characteristics may develop via different mechanisms. In addition, immunization with another cardiac-specific autoantigen, troponin I, was also able to elicit severe autoimmune myocarditis in A/J mice followed by cardiomegaly, fibrosis, reduced fractional shortening, and reduced survival rate [14].
Very recently, Tanejia V. et al. developed a spontaneous myocarditis model in NOD mice (Ag7-/-) carrying the human DQ8 molecule (NOD.DQ8.Aβo) [15]. In this model, a high mortality was observed in female NOD.DQ8.Aβo mice at 16 weeks or older, associated with severe systolic dysfunction, enlarged heart with mononuclear infiltrates, and autoreactive T and B cells. Yet, NOD.DR3.Aβo, the trangene negative littermates, NOD and B10. DQ8 mice had no sign of cardiac pathology. Interestingly, in the spontaneous model females were predominantly affected, in contrast to human cases and the induced models where males seem to have a somewhat greater incidence [1, 16]. These results may imply different roles of sex-related genes in pathogenesis of spontaneous versus induced myocarditis.
3. Genetic linage between MHC and autoimmune myocarditis
Autoimmune myocarditis is a disease of heterogeneous etiology, involving interplay between predisposing genes and triggering environmental factors. Polymorphisms in the major histocompability complex (MHC) are among the strongest predisposing genetic factors in autoimmune diseases in general [17]. Most genetic studies in humans have been performed in patients with DCM, in which only some cases may represent an end stage of autoimmune myocarditis. In these studies, both serologic analyses [18-21] and recent molecular analyses [22, 23] have suggested that a significant correlation of DCM with MHC class II antigens, particularly HLA-DR4. Yet, this association was not observed in a small populational study with 36 patients (32 DCM and 4 myocarditis) [24]. Other positive (DR12, DR15, DPB*0601) [22-24] and negative (DR11, DQB1*0301) [23] associations have also been reported, but are also inconsistent between populations. The discrepancy in MHC association with DCM between different groups may be relevant to the differencing influences of ethnic origin, sex, age and geographic parameters of the populations under study. In one of the above studies [24], 4 myocarditis patients had been analyzed separately. Although no conclusion with statistical significance could be drawn due to the limited number of cases, all 4 patients were DQ5 positive and 3 possessed the allele DQB1*0501. In addition, the development of spontaneous myocarditis in NOD mice (Ag7-/-) carrying DQ8 but not DR3 transgene suggests polymorphisms in MHC class II is important in predisposition to myocarditis and HLA-DQ8 may be a risk factor in humans [15].
In studies of mice, determination of the MHC contribution to susceptibility to myocarditis were made among the H-2 congenic mice on either A/J (A/J, H-2a; A.BY, H-2b; A.CA, H-2f and A.SW, H-2s) or C57BL/10J (B10.A, H-2a; B10.S, H-2s and B10.PL, H-2u) backgrounds [10, 25]. Interestingly, in both viral and myosin-induced models, the s, a and f haplotypes are associated with increased morbidity of autoimmune-based myocarditis, whereas the b haplotype is associated with a relatively low responsiveness. Notably, the MHC polymorphisms also influence the prevalence and titer of cardiac-specific autoantibodies. For example, the A.BY mice are poor producers of myocardial autoantibodies, whereas A.SW and A.CA are good producers after either viral infection or myosin immunization [10, 25].
The role of MHC class I in autoimmune myocarditis has not been well elucidated in either humans or animals. Lin A et al. have reported that a 14bp deletion in the 3’-untranslated region of human nonclassical MHC class I genes, HLA-G, is a risk factor for DCM in a Chinese Han population [26]. Strong linkage disequilibrium between HLA-G and, in particular HLA-DR and DQ alleles, was also observed [27], indicating that HLA-G alone or together with the linked HLA-DR or DQ allelic products could play a role in the development of autoimmune myocarditis.
4. Genetic linage between non-MHC loci and autoimmune myocarditis
Progress in delineating the role of non-MHC genes in autoimmune diseases has been more problematic, probably because of the overriding influence of MHC in most instances [18]. In humans, several DCM-related non MHC loci have been defined on chromosomes 1q32, 2q11-22, 2q31, 9q13-22 and 10q21-23 [28], but it is unclear whether they are associated with autoimmune myocarditis.
The murine model of autoimmune myocarditis has unique advantages in mapping non-MHC loci because the major determinants of disease susceptibility seem to reside outside the MHC. We previously reported that most A congenic mouse strains, such as A/J, A.CA and A.SW differing only at the MHC locus (H-2 a, f, s, respectively), are susceptible to chronic autoimmune myocarditis after infection with CBV3. In contrast, mouse strains with a B10 background such as C57BL/10J, B10.A, B10.S and B10.PL (H-2 b, a, s, u, respectively) are largely resistant [25]. The similar pattern was confirmed in myosin-induced myocarditis model [10]. These results highlight the importance of genes that are not closely linked to MHC in the susceptibility to autoimmune myocarditis. To focus on the role of non-MHC genes on susceptibility to myosin-induced myocarditis, we used two prototypic mouse strains – A.SW and B10.S - that share the same MHC genes but have either A or B background. A.SW is susceptible and B10.S is resistant to autoimmune myocarditis, respectively. Utilizing polymorphic SSLP markers throughout the murine genome, linkage analysis of F1 × F1 (F2) mice revealed two non-MHC loci – the Eam1 on proximal chromosome l and Eam2 on distal region of chromosomes 6 – that are either significant or highly suggestive linkage to autoimmune myocarditis [16]. Both loci or their human counterparts have been previously identified in other autoimmune diseases such as lupus, diabetes, autoimmune encephalomyelitis and autoimmune orchitis in mouse [29-32], as well as diabetes and autoimmune thyroid disease in humans [33-35] (Fig.1). This observation suggests that although autoimmune diseases display a large variety of clinical manifestations, there are likely controlled by a number of shared inherited genetic abnormalities and pathogenic mechanisms. It also highlights the importance of understanding the genetic mechanisms leading to autoimmunity in any model system, including myocarditis, since knowledge gained will likely have wider applicability. Interestingly, we have found that both Eam1 and Eam2 influence the apoptosis of T cells at different stages of development [16] (Fig.1). It remains to be demonstrated that the genetic elements controlling sensitivity to apoptosis maps to the same location as those controlling disease susceptibility, and how these two phenomena are related.
Figure 1. Loci important in autoimmune myocarditis also play a role in other autoimmune diseases.
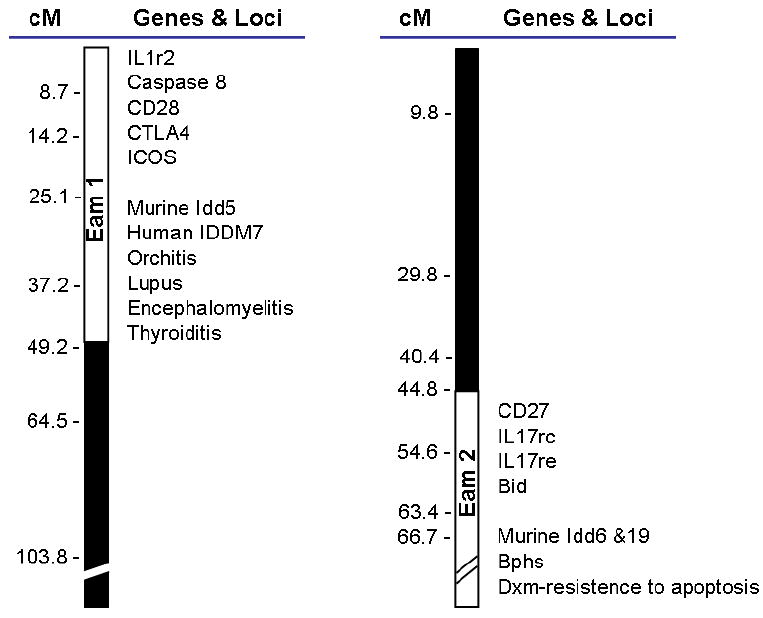
Genetic mapping data suggest that loci on Chrs. 1 and 6 play a role in susceptibility to experimental autoimmune myocarditis. These loci have been tentatively named Eam1 and Eam2. Eam1 overlaps with loci found to be important in human diabetes (IDDM7), and autoimmune thyroid disease, and murine diabetes (Idd5). This locus harbors important immune genes like IL1r2, caspases 8, CD28, CTLA4 and ICOS. Eam2 harbors genes like CD27, IL17rc, IL17re and Bid, as well as overlaps with other loci important for murine diabetes (idd6 and idd19), Bphs (Bordetella pertussis-induced histamine sensitization) and Dxm-resistance to apoptosis.
Very similar to our approach, a Canadian group recently reported three loci in A/J --H2a mice that control susceptibility to CVB3-induced myocarditis in comparison to B10.A-H2a mouse strain [36]. These three loci include one locus (Vms1) on chromosome 1 centered on D1Mit200 (80 cM), a second locus (Vms2) on chromosome 4 centered on D4Mit81 (38 cM) and a third locus (Vms3) on chromosome 3 centered on D3Mit19 (87.6 cM). Vms1 was linked to both myocardial infiltration and sarcolemmal damage in females, whereas Vms 2 and Vms3 were segregated only with sarcolemmal damage in males. None of them overlap with our loci, but Vms1 region was previously identified as heart failure modifier locus and Vms2 was defined for initial spontaneous loss of immunologic tolerance [36].
Other strains of mouse, such as BALB/c and DBA/2, also demonstrate susceptibility to autoimmune myocarditis induced by CBV3 infection or cardiac myosin immunization. However, the pathogenic mechanisms may be different. Myocarditis in A/J and Balb/c mice is transferable only with activated lymphocytes, whereas disease can be induced in DBA/2 mice by passive transfer of myosin-specific autoantibodies [13]. Analysis of DBA/2xCByD1F1 backcross mice has suggested that a locus on chromosome 12 is strongly linked with autoantibody-induced myocarditis and a second region on chromosome 1 that contributes to disease susceptibility only in male mice [13].
5. Specific genes that relate to autoimmune myocarditis
Despite the progress in identification of potential myocarditis-related loci in both human and mouse, disease-causing mutations are rarely defined. A case report on a 31-year-old female suggested that myocarditis may be associated with C77G polymorphism of CD45 exon 4, which alters the splicing and CD45RA/CD45RO phenotype of lymphocytes [37]. Mutations/deletions in genes encoding cardiac proteins, including cardiac actin [38], cardiac β-myosin heavy chain and cardiac troponin T [28], have also been found in patients with DCM; again their significance in autoimmune myocarditis in particular is not specified.
Polymorphisms in costimulatory molecule CTLA-4 have been reported to influence the development of autoimmune diseases in general [17]. Blocking of CTLA-4 function exacerbated autoimmune myocarditis in both A/J and C57BL/6 mice, but no polymorphisms in CTLA-4 have been found between the two strains (Ligons D. and Cihakova D., unpublished data). Similarly, manipulation of other immunologically important regulatory genes, such as ICOS [39] and PD-1 [40], is able to influence the development of autoimmune myocarditis in animal models. For example, administration of anti-ICOS blocking antibodies attenuates the incidence and severity of the disease [39], whereas blocking of PD-1/PD-1 ligands pathway increased the myocardial inflammation [40]. However, it remains unclear whether genetic polymorphisms distinguish susceptible and resistant human subjects or mouse strains.
6. Concluding Remarks
Genetic mapping of complex disease traits and, in particular, those associated with autoimmune disease, is complicated, since most of them have a polygenic basis. Susceptibility to autoimmune myocarditis varies among populations and strains of mice, and possibly is controlled by different genetic factors associated with distinct etiology. Among multiple autoimmune diseases including diabetes, lupus, myocarditis and thyroiditis, several defined non-MHC susceptibility loci overlap or are shared, suggesting common genetic predisposition. Further identification of disease-causing mutations will not only facilitate the discovery of populations in risk, but also help in establishment of therapeutic strategies through unraveling the pathogenic mechanisms of autoimmune diseases.
Acknowledgments
The authors’ research is supported by NIH research grants R01 HL-077611 and R01 HL067290.
Footnotes
- Chronic myocarditis in humans after acute viral infection invloves autoimmune-mediated cardiac impairment.
- Autoimmune myocarditis can be induced by viral infection or cardiac antigen immunization in susceptible mouse strains, or spontaneous develop in NOD.DQ8 transgenic mouse.
- Susceptibility to experimental autoimmune myocarditis varies among different strains of mice. It is mainly controlled by non-MHC genes, but MHC class II genes modify the severity of the disease
- Loci controlling autoimmune myocarditis overlap with other autoimmune disease loci, suggesting common or shared genetic traits.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drory Y, Turetz Y, Hiss Y, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol. 1991;68:1388–92. doi: 10.1016/0002-9149(91)90251-f. [DOI] [PubMed] [Google Scholar]
- 2.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–98. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270–83. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Rose NR, Herskowitz A, Neumann DA, Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988;9:117–20. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- 5.Rose NR, Wolfgram LJ, Herskowitz A, Beisel KW. Postinfectious autoimmunity: two distinct phases of coxsackievirus B3-induced myocarditis. Ann N Y Acad Sci. 1986;475:146–56. doi: 10.1111/j.1749-6632.1986.tb20864.x. [DOI] [PubMed] [Google Scholar]
- 6.Cihakova D, Sharma RB, Fairweather D, Afanasyeva M, Rose NR. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol Med. 2004;102:175–93. doi: 10.1385/1-59259-805-6:175. [DOI] [PubMed] [Google Scholar]
- 7.Reetoo KN, Osman SA, Illavia SJ, Cameron-Wilson CL, Banatvala JE, Muir P. Quantitative analysis of viral RNA kinetics in coxsackievirus B3-induced murine myocarditis: biphasic pattern of clearance following acute infection, with persistence of residual viral RNA throughout and beyond the inflammatory phase of disease. J Gen Virol. 2000;81:2755–62. doi: 10.1099/0022-1317-81-11-2755. [DOI] [PubMed] [Google Scholar]
- 8.Neu N, Beisel KW, Traystman MD, Rose NR, Craig SW. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987;138:2488–92. [PubMed] [Google Scholar]
- 9.Traystman MD, Beisel KW. Genetic control of Coxsackievirus B3-induced heart-specific autoantibodies associated with chronic myocarditis. Clin Exp Immunol. 1991;86:291–8. doi: 10.1111/j.1365-2249.1991.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–6. [PubMed] [Google Scholar]
- 11.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–7. [PubMed] [Google Scholar]
- 12.Smith SC, Allen PM. The role of T cells in myosin-induced autoimmune myocarditis. Clin Immunol Immunopathol. 1993;68:100–6. doi: 10.1006/clin.1993.1103. [DOI] [PubMed] [Google Scholar]
- 13.Kuan AP, Chamberlain W, Malkiel S, et al. Genetic control of autoimmune myocarditis mediated by myosin-specific antibodies. Immunogenetics. 1999;49:79–85. doi: 10.1007/s002510050466. [DOI] [PubMed] [Google Scholar]
- 14.Goser S, Andrassy M, Buss SJ, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693–702. doi: 10.1161/CIRCULATIONAHA.106.635664. [DOI] [PubMed] [Google Scholar]
- 15.Taneja V, Behrens M, Cooper LT, et al. Spontaneous myocarditis mimicking human disease occurs in the presence of an appropriate MHC and non-MHC background in transgenic mice. J Mol Cell Cardiol. 2007;42:1054–64. doi: 10.1016/j.yjmcc.2007.03.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guler ML, Ligons DL, Wang Y, Bianco M, Broman KW, Rose NR. Two autoimmune diabetes loci influencing T cell apoptosis control susceptibility to experimental autoimmune myocarditis. J Immunol. 2005;174:2167–73. doi: 10.4049/jimmunol.174.4.2167. [DOI] [PubMed] [Google Scholar]
- 17.Simmonds MJ, Gough SC. Genetic insights into disease mechanisms of autoimmunity. Br Med Bull. 2004;71:93–113. doi: 10.1093/bmb/ldh032. [DOI] [PubMed] [Google Scholar]
- 18.Carlquist JF, Menlove RL, Murray MB, O’Connell JB, Anderson JL. HLA class II (DR and DQ) antigen associations in idiopathic dilated cardiomyopathy. Validation study and meta-analysis of published HLA association studies. Circulation. 1991;83:515–22. doi: 10.1161/01.cir.83.2.515. [DOI] [PubMed] [Google Scholar]
- 19.Carlquist JF, Ward RH, Husebye D, Feolo M, Anderson JL. Major histocompatibility complex class II gene frequencies by serologic and deoxyribonucleic acid genomic typing in idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;74:918–20. doi: 10.1016/0002-9149(94)90586-x. [DOI] [PubMed] [Google Scholar]
- 20.Limas CJ, Limas C. HLA antigens in idiopathic dilated cardiomyopathy. Br Heart J. 1989;62:379–83. doi: 10.1136/hrt.62.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinetti M, Dugoujon JM, Caforio AL, et al. HLA and immunoglobulin polymorphisms in idiopathic dilated cardiomyopathy. Hum Immunol. 1992;35:193–9. doi: 10.1016/0198-8859(92)90105-v. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Li WM, Yang SS, et al. Association of HLA class II DRB1, DPA1 and DPB1 polymorphism with genetic susceptibility to idiopathic dilated cardiomyopathy in Chinese Han nationality. Autoimmunity. 2006;39:461–7. doi: 10.1080/08916930600893709. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Perez JM, Fragoso JM, varez-Leon E, et al. MHC class II genes in Mexican patients with idiopathic dilated cardiomyopathy. Exp Mol Pathol. 2007;82:49–52. doi: 10.1016/j.yexmp.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Lozano MD, Rubocki RJ, Wilson JE, McManus BM, Wisecarver JL. Human leukocyte antigen class II associations in patients with idiopathic dilated cardiomyopathy. Myocarditis Treatment Trial Investigators. J Card Fail. 1997;3:97–103. doi: 10.1016/s1071-9164(97)90041-5. [DOI] [PubMed] [Google Scholar]
- 25.Wolfgram LJ, Beisel KW, Herskowitz A, Rose NR. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986;136:1846–52. [PubMed] [Google Scholar]
- 26.Lin A, Yan WH, Xu HH, et al. 14 bp deletion polymorphism in the HLA-G gene is a risk factor for idiopathic dilated cardiomyopathy in a Chinese Han population. Tissue Antigens. 2007 doi: 10.1111/j.1399-0039.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 27.Hviid TV, Christiansen OB. Linkage disequilibrium between human leukocyte antigen (HLA) class II and HLA-G--possible implications for human reproduction and autoimmune disease. Hum Immunol. 2005;66:688–99. doi: 10.1016/j.humimm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Kamisago M, Sharma SD, DePalma SR, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–96. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 29.Haywood ME, Rose SJ, Horswell S, et al. Overlapping BXSB congenic intervals, in combination with microarray gene expression, reveal novel lupus candidate genes. Genes Immun. 2006;7:250–63. doi: 10.1038/sj.gene.6364294. [DOI] [PubMed] [Google Scholar]
- 30.Meeker ND, Hickey WF, Korngold R, et al. Multiple loci govern the bone marrow-derived immunoregulatory mechanism controlling dominant resistance to autoimmune orchitis. Proc Natl Acad Sci U S A. 1995;92:5684–8. doi: 10.1073/pnas.92.12.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicker LS, Chamberlain G, Hunter K, et al. Fine mapping, gene content, comparative sequencing, and expression analyses support Ctla4 and Nramp1 as candidates for Idd5.1 and Idd5.2 in the nonobese diabetic mouse. J Immunol. 2004;173:164–73. doi: 10.4049/jimmunol.173.1.164. [DOI] [PubMed] [Google Scholar]
- 32.Greve B, Vijayakrishnan L, Kubal A, et al. The diabetes susceptibility locus Idd5.1 on mouse chromosome 1 regulates ICOS expression and modulates murine experimental autoimmune encephalomyelitis. J Immunol. 2004;173:157–63. doi: 10.4049/jimmunol.173.1.157. [DOI] [PubMed] [Google Scholar]
- 33.Copeman JB, Cucca F, Hearne CM, et al. Linkage disequilibrium mapping of a type 1 diabetes susceptibility gene (IDDM7) to chromosome 2q31-q33. Nat Genet. 1995;9:80–5. doi: 10.1038/ng0195-80. [DOI] [PubMed] [Google Scholar]
- 34.Dittmar M, Kahaly GJ. Immunoregulatory and susceptibility genes in thyroid and polyglandular autoimmunity. Thyroid. 2005;15:239–50. doi: 10.1089/thy.2005.15.239. [DOI] [PubMed] [Google Scholar]
- 35.Rogner UC, Boitard C, Morin J, Melanitou E, Avner P. Three loci on mouse chromosome 6 influence onset and final incidence of type I diabetes in NOD.C3H congenic strains. Genomics. 2001;74:163–71. doi: 10.1006/geno.2001.6508. [DOI] [PubMed] [Google Scholar]
- 36.Aly M, Wiltshire S, Chahrour G, Osti JC, Vidal SM. Complex genetic control of host susceptibility to coxsackievirus B3-induced myocarditis. Genes Immun. 2007;8:193–204. doi: 10.1038/sj.gene.6364374. [DOI] [PubMed] [Google Scholar]
- 37.Tchilian EZ, Gil J, Navarro ML, et al. Unusual case presentations associated with the CD45 C77G polymorphism. Clin Exp Immunol. 2006;146:448–54. doi: 10.1111/j.1365-2249.2006.03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–2. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- 39.Futamatsu H, Suzuki J, Kosuge H, et al. Attenuation of experimental autoimmune myocarditis by blocking activated T cells through inducible costimulatory molecule pathway. Cardiovasc Res. 2003;59:95–104. doi: 10.1016/s0008-6363(03)00334-1. [DOI] [PubMed] [Google Scholar]
- 40.Seko Y, Yagita H, Okumura K, Azuma M, Nagai R. Roles of programmed death-1 (PD-1)/PD-1 ligands pathway in the development of murine acute myocarditis caused by coxsackievirus B3. Cardiovasc Res. 2007;75:158–67. doi: 10.1016/j.cardiores.2007.03.012. [DOI] [PubMed] [Google Scholar]


