Abstract
Environmental enrichment reduces reactivity to stressor and could also modulate pain perception. In this study we sought to compare the effects of enriched and standard housing on temperature perception. In an operant assay, rats housed in an enriched environment exhibited significantly lower sensitivities to thermal stimuli and displayed less exploratory behavior in a rearing chamber. These findings indicate that environmental enrichment can significantly affect temperature perception, likely through stress-related mechanisms.
Keywords: environmental enrichment, operant, stress, cold, allodynia, orofacial
It is well known that stress and pain have a synergistic relationship, one enhancing the impact of the other through shared pathways [1]. Several groups have found that acute and chronic stress increase nociceptive responses in rats [8, 9, 11, 12]. However, little research has been done to examine the effects of non-pharmacological, stress-reducing methods on nociceptive responses in animal models. Environmental enrichment produces neural and hormonal changes that are thought to reduce reactivity to stressors [7]. It may also reduce pain perception by strengthening endogenous inhibitory controls. To date, only a few studies examined the relationship between environmental enrichment and pain perception [21, 22]. These studies suggest that housing environment had no effect on baseline pain perception. However, pain perception was assayed by measuring tail withdrawal from hot water at only one or two temperatures. Because the anxiolytic effects of environmental enrichment are likely to occur at the cortical level, use of reflex-based assays may not be the most effective way to evaluate the effect of enrichment on supraspinal processing of painful stimuli. It is also important to examine the effects of enrichment on responses to a range of temperatures since the perception of these stimuli could be differentially modulated by affective input.
The goal of this study was to determine if environmental enrichment alters thermal pain perception in rats. Unlike previous studies, we used an operant orofacial assay to measure the sensitivity of the rats to temperatures ranging from uncomfortably cold (2°C) to painfully hot (48°C). In addition, the general activity levels of the rats were assessed by monitoring rearing behavior.
Seven week-old male hairless Sprague-Dawley rats (Charles River, Raleigh, NC) were housed in either an enriched environment (n=6) or in a standard environment (n=6) at separate testing facilities. Rats in the enriched group were housed three per cage. Enriched housing consisted of a metal cage (81.3 X 45.7 X 61 cm) containing cardboard boxes, two shelves (17.8 X 43.2 cm), a hammock, PVC tubing, various chew toys and an exercise wheel. Enrichment was constant when rats were not being tested and objects were changed once a week. Animals in the standard group were housed in pairs in Plexiglas cages (20.3 × 45.7 × 25.4 cm) with no additional objects. All rats had ad libitum access to food and water between testing sessions, and their weights were monitored weekly.
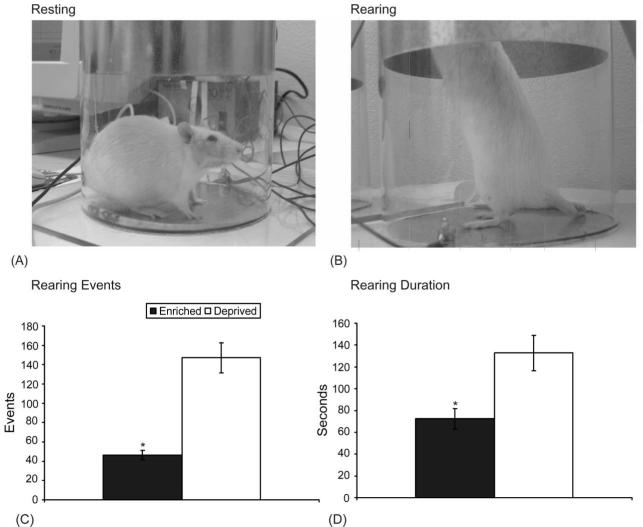
General activity levels of all animals were assessed by measuring their vertical locomotor (rearing) behavior when placed in a cylindrical chamber (19.5 cm diameter × 40.5 cm height). Rats were allowed to acclimate to the chamber on the first day of testing and both groups were tested in tandem at the same time of day. The level of activity of each animal was monitored electronically by recording the number of events and length of time the rats reared on their hind legs with their forepaws in contact with the aluminum sheet 13.5-cm above the floor chamber (Fig. 1A,B). Each testing session lasted 15 minutes.
Fig. 1. Effect of housing on vertical locomotion (rearing) behavior in rats.
The top two panels show a rat at rest (A) and rearing (B) in the cyclinder. Rats in enriched housing (black bars) had significantly fewer rearing events (C) and a significantly lower total rearing duration (D) as compared to rats in standard housing (white bars). Error bars represent SEM. *P<0.05.
Thermal sensitivity was evaluated using an operant orofacial pain assay [15]. Briefly, rats were trained to drink sweetened condensed milk while making facial contact with a thermal probe, set at 37°C. The rats were moved to enriched housing when training began. Following the two-week training period, the ability of the animals to obtain the milk reward in the presence of various thermal stimuli was recorded. Enriched and standard rats were tested using a range of temperatures (2 - 48°C), from cold to very hot. Four behavioral outcome measures were recorded for each 20-minute testing session: intake (grams of milk consumed), licks (number of contacts with the sipper tube, which cannot be made without contacting the thermal probe), stimulus contacts (number of contacts with the thermal probe), and duration (total time in seconds spent in contact with the thermal probe). Two additional outcomes were also calculated for each rat: the success ratio (licks/stimulus contact) and the tolerance ratio (duration/stimulus contact). The success ratio is the number of licks (i.e. successful attempts) divided by the stimulus contacts (total number of attempts). The tolerance ratio is the duration divided by the stimulus contacts, which indicates how long the rats were able to remain in contact with the thermal probe during each individual contact event. All statistical evaluations were made using SPSS (v. 14.0, SPSS, Inc.).
The activity levels of rats housed in either enriched or standard environments were measured on four consecutive days. A general linear model was used to perform a two-way ANOVA, evaluating the individual effect of testing day and housing conditions on rearing events and duration. There was no significant effect of test day on rearing behavior, but there was a significant effect of housing on rearing events (F1,48 = 39.168) and duration (F1,48 = 10.238). The activity levels of animals housed in the enriched environment, as measured by rearing events and duration, were significantly less than those exhibited by rats in standard housing (Fig.1C,D).
Exposing animals to enriched housing environments is thought to improve the efficiency of exploratory behavior [26]. The reduction in rearing activity that we observed in rats housed in enriched environments may reflect a reduction in exploratory behavior, perhaps as a consequence of reduced anxiety. Additionally, voluntary wheel running, which is also a component of our enrichment, has also been shown to reduce activity in the open field test and in the elevated plus maze [4]. Thus, access to exercise may lead to a decrease in rearing activity. A formal comparison of enriched rats with and without wheel access is needed to separate the effects of enrichment and exercise on rearing behavior.
The reduced levels of activity exhibited by animals housed in enriched environments were accompanied by greater success in the orofacial pain assay, as indicated by the differences in licks, facial contacts, and success ratios for the two housing groups. A general linear model was used to perform a two-way ANOVA, evaluating the individual effect of temperature and housing conditions on behavior. There was a significant main effect of temperature on all outcome measures for each group as we have demonstrated previously [15, 18]. There was also a significant main effect of housing on all outcome measures (intake F1,72 = 30.612, licks F1,72 = 8.955, stimulus contacts F1,72 = 8.759, duration F1,72 = 5.425, success ratio F1,72 = 16.482, tolerance ratio F1,72 = 16.145). Post-hoc unpaired t-tests were used to determine significance between the housing groups at individual temperatures. The patterns of responses for intake, licking events and duration are similar, so only licking events are shown (Fig. 2). The same is true of the success and tolerance ratios, so only the success ratios are shown. Overall, licking events, success ratio, and the tolerance ratio were higher for the enriched rats (Fig. 2). The success ratio (Fig. 2C), tolerance ratio, and intake were significantly increased at 2, 42, and 45°C. Licking events (Fig. 2A) and duration were only significantly increased at 2°C. The opposite was true for stimulus contacts (Fig. 2B); they were generally lower for enriched animals at all temperatures below 48°C and this decrease was significant at 24°C and 45°C. This is consistent with a reduction in pain behavior, as animals are able to tolerate the stimulus and keep their face contacted on the thermode longer. There were no significant differences between the two groups at 37°C and 48°C for any of the outcome measures.
Fig. 2. Effect of housing and temperature on operant behavior in rats.
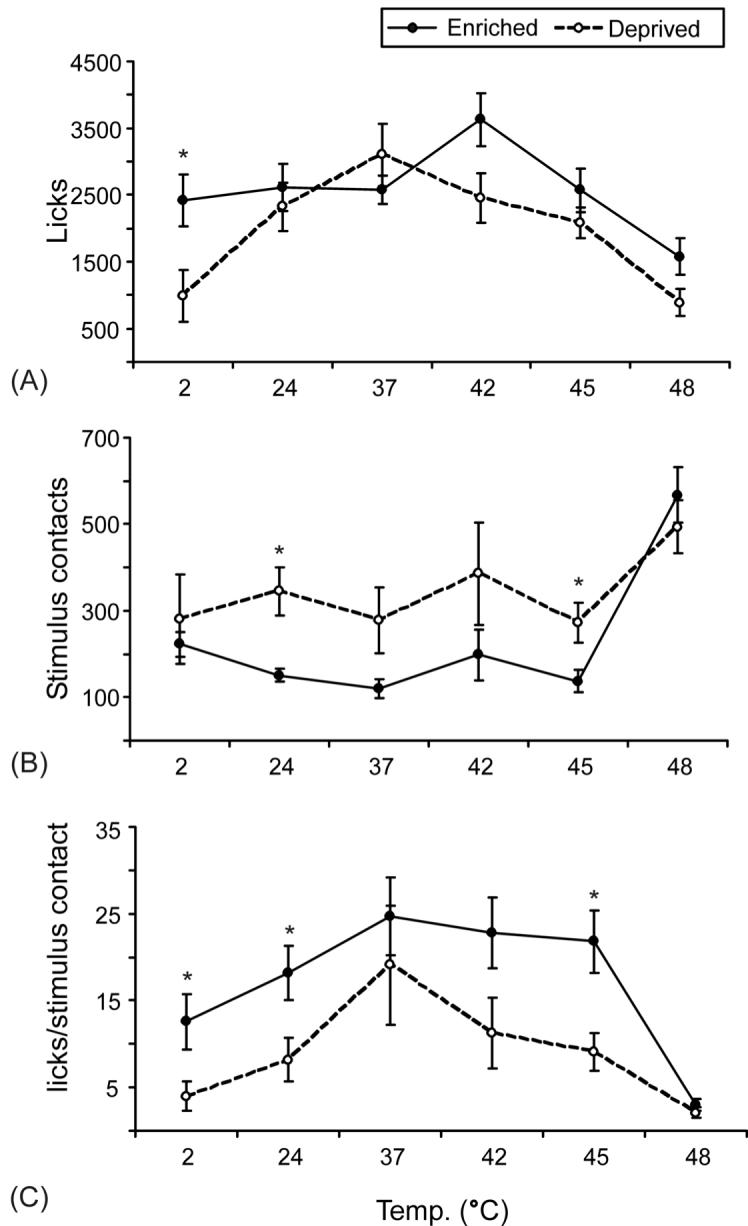
Rats in enriched (closed circles) and standard (open circles) housing were tested at a range of cold to hot temperatures. There was a significant main effect of housing on all outcomes. Three of the six outcome measures are shown: licks (A), stimulus contacts (B), and the success ratio (licks/stimulus contact, C). Error bars represent SEM. *P<0.05, unpaired t-test comparing enriched and standard groups at each temperature.
Previous studies have shown that a variety of stressors can increase nociceptive responses in chronically stressed laboratory animals and humans [1, 6, 8, 9, 11, 12]. In this study, we sought to examine the effect of environmental enrichment, an application that reduces reactivity to stress, on operant response to a range of thermal stimuli. The enrichment applied in this study led to decreased activity levels as measured by rearing behavior and also let to increased success and tolerance ratios in the presence of 2, 24, and 45°C stimulation.
There are several factors that could underlie the differences displayed by the two groups of animals that are independent of stimulation. One is that object enrichment is known to improve spatial learning [20] and to cause rats to habituate more quickly to novel objects and environments [19, 26]. Thus, the enriched environment may lead to changes that allow the rats to attend better to the task and adopt strategies that render them more efficient at completing the operant task as compared to rats in standard housing. A second possibility is that greater activity levels of rats in standard housing may reflect an increased tendency to explore the thermodes. Third, there could be a difference in the motivational potential of the milk reward for enriched rats versus rats in standard housing. Some studies suggest that rats raised in an enriched environment do not seek sucrose or drug reward as readily as socially housed controls [17, 23, 25]. However, exercise can increase feeding behavior [14], so it is difficult to determine if enriched rats would be more or less motivated to seek reward.
The fact that there is no significant difference between rats in enriched and standard housing with a neutral stimulus argues against the first two possibilities mentioned above. As to the third possibility, one would expect that if one group were more or less motivated to seek and consume the reward that there would be a difference in intake and licks maintained between the two groups throughout the range of temperatures tested. However, this was not the case; the two groups had significant differences in both intake and licks only at 2°C. If motivational differences do exist, they appear to be dependent on the stimulus accompanying the reward.
Therefore, in this study we provide evidence to suggest that environmental enrichment does effect operant responses to thermal stimuli in rats, and that these responses are stimulus-dependent. We show here that enriched rats performed significantly better than rats in standard housing at 2, 24, and 45°C, as measured by the success ratio and the tolerance ratio. However, at 48°C there is no difference in performance. This is likely due to the fact that a stimulus of this intensity activates A-δ nerve fibers that sense high threshold inputs, which can be imminently more damaging to tissue if the animal remains on it too long. This may explain why others failed to find significant effect of enrichment on heat sensitivity using reflexive withdrawal from a single hot stimulus [5, 21, 22]. Thus, enriched housing can improve rats’ tolerance of temperatures that are not immediately damaging, but are still strongly aversive, such as cold stimuli, or on the threshold of what is perceived as painful, such as 45°C in this case.
Part of our enrichment included an exercise component. Exercise is known to engage the reward system [3] and is also known to reduce pain in healthy subjects [13, 24]. There is also evidence to suggest that the reward-avoidance circuitry in the brain is involved in the processing of painful stimuli [2]. Therefore, it is possible that exercise may lead to changes in this circuitry that can effect reactivity to painful, aversive stimuli. Such changes may underlie the enriched rats’ decreased sensitivity to cold and hot stimuli.
To date none have examined the effect of housing on cold sensitivity. There are several lines of evidence to suggest that the affective component of sensory processing is stronger for cold stimuli than for hot stimuli. Human subjects rate cold stimuli as more unpleasant relative to their intensity than hot stimuli [10, 16]. We recently demonstrated that rats prefer a 48°C stimulus to a -4°C, despite the fact that the success and tolerance ratios were not significantly different for the two temperatures [18]. That enriched rats were more successful in the presence of a cold stimulus also supports a strong role for affect-mediated central modulation of peripheral cold sensitivity.
This study provides evidence that housing conditions can have significant effects on thermal sensitivity, although further studies are needed to verify the role of stress-modulated systems, such as the opioid and serotoninergic systems as they related to these changes. The effect of housing on cold sensitivity in particular supports the idea that cold sensitivity may be predominantly vulnerable to stress-related changes in descending inhibitory controls. This may be one explanation for the prevalence of cold sensitivity in neuropathic pain, where these descending inhibitory controls are impaired. Traditional behavioral assessment of pain has been based on reflex or innate behaviors of animals housed in standard housing conditions, equivalent to our standard housing. The results reported here may have implications for future evaluation of pain-mediated behavior in animal models and the translation to the clinic.
Acknowledgements
Funding for this work was provided by the National Institute of Dental and Craniofacial Research, NIH grant 5R21DE016704-02. We thank Dr. Charles Widmer for providing the custom-written Labview software routines used to analyze rearing and operant facial data. We also thank Jean Kauffman for care and testing of the enriched rats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blackburn-Munro G, Blackburn-Munro RE. Chronic Pain, Chronic Stress and Depression: Coincidence or Consequence? J Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 2.Borsook D, Becerra L, Carlezon JWA, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. European Journal of Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 5.Clausing P, Mottles HK, Opitz B, Kormann S. Differential effects of communal rearing and preweaning handling on open-field behavior and hot-plate latencies in mice. Behav Brain Res. 1997;82:179–184. doi: 10.1016/s0166-4328(97)80987-4. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126:132–138. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Gameiro GH, da Silva Andrade A, de Castro M, Pereira LF, Tambeli CH, Ferraz de Arruda Veiga MC. The effects of restraint stress on nociceptive responses induced by formalin injected in rat’s TMJ. Pharmacology Biochemistry and Behavior. 2005;82:338–344. doi: 10.1016/j.pbb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Gameiro GH, Gameiro PH, da Silva Andrade A, Pereira LF, Arthuri MT, Marcondes FK, de Arruda Veiga MCF. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol Behav. 2006;87:643–649. doi: 10.1016/j.physbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan JD, Roy EA, Caldwell PA, Farooq NS. Thermosensory intensity and affect throughout the perceptible range. Somatosens Mot Res. 2003;20:19. doi: 10.1080/0899022031000083807. [DOI] [PubMed] [Google Scholar]
- 11.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 12.King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- 13.Koltyn KF. Analgesia Following Exercise: A Review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Lett BT, Grant VL, Gaborko LL. Wheel Running Simultaneously Induces CTA and Facilitates Feeding in Non-deprived Rats. Appetite. 1998;31:351–360. doi: 10.1006/appe.1998.0171. [DOI] [PubMed] [Google Scholar]
- 15.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Rainville P, Feine J, Bushnell M, Duncan G. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res. 1992;9:265–77. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- 17.Rose F, Love S, Dell P. Differential reinforcement effects in rats reared in enriched and impoverished environments. Physiol Behav. 1986;36:1139–45. doi: 10.1016/0031-9384(86)90491-9. [DOI] [PubMed] [Google Scholar]
- 18.Rossi HL, Vierck JCJ, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Molecular Pain. 2006:2. doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrijver NCA, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacology Biochemistry and Behavior. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- 20.Schrijver NCA, Pallier PN, Brown VJ, Wurbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152:307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA, Bryant PA, McClean JM. Social and environmental enrichment enhances sensitivity to the effects of kappa opioids: studies on antinociception, diuresis and conditioned place preference. Pharmacology Biochemistry and Behavior. 2003;76:93–101. doi: 10.1016/s0091-3057(03)00189-8. [DOI] [PubMed] [Google Scholar]
- 22.Smith MA, Chisholm KA, Bryant PA, Greene JL, McClean JM, Stoops WW, Yancey DL. Social and environmental influences on opioid sensitivity in rats: importance of an opioid’s relative efficacy at the mu-receptor. Psychopharmacology (Berl) 2005;181:27–37. doi: 10.1007/s00213-005-2218-2. [DOI] [PubMed] [Google Scholar]
- 23.Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- 24.Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. The Journal of Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 25.Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann A, Stauffacher M, Langhans W, Wurbel H. Enrichment-dependent differences in novelty exploration in rats can be explained by habituation. Behav Brain Res. 2001;121:11–20. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]