Abstract
Comparisons were made of differences in the hormonal sensitivity of preterm versus full-term infants to maternal depression, as reflected in children's cortisol levels. In Study 1 (N = 25), a comparison was made between preterm versus healthy full-term children. In Study 2 (N = 80), a comparison was made between preterm infants and full-term infants with mild or moderate medical problems. Preterm infants were found be highly reactive to maternal depression (as measured by the Beck Depression Inventory). That is, they demonstrated higher cortisol levels when paired with depressed mothers and lower cortisol levels when paired with non-depressed mothers. No equivalent effects were found for children who were full-term, even when they had experienced other medical problems at birth. It was concluded that premature infants are exceptionally sensitive to the “emotional climate” in their home environment. As a result, they may manifest very different hormonal outcomes -- with implications for their later development.
Considerable attention has been given historically to the fact that high levels of stress early in life predict later problems. More recently, such effects have been interpreted in terms of biological changes as mediators of this process. Researchers concerned with developmental psychopathology have given increasing attention to the neurohormonal responses of very young children to early trauma or stress (e.g., Bremner & Narayan, 1998; Gunnar, 2000; Nelson, 2000). However, interests have expanded beyond concern with trauma to a broader consideration of early experiences that predict children's later outcomes. It has also extended to consider possible variations in child vulnerability. Repetti, Taylor and Seeman (2000), in a review of literature concerning the biological outcomes of children in “risky” families (e.g., those characterized by aggression/conflict or coldness/neglect), concluded that child vulnerability and family risk combine to predict dysregulation of the child's stress response system -- a consequence that ultimately has negative implications for their later health and well-being. In this study, we focused attention on maternal depression as a potential source of early stress for infants. Our specific concern here was on the differential vulnerability of preterm versus full-term infants to maternal depression – in terms of their stress-relevant hormonal responses.
Maternal Depression
Maternal depression has repeatedly been found to have negative implications for maternal responsiveness and sensitivity to infants – which in turn has implications for infant development. For example, Zlochower and Cohn (1996) demonstrated that depressed mothers were less responsive to their infants – leading to a reduction in synchrony of the interaction within the dyad. Donovan, Leavitt, and Walsh (1998) observed that depressed mothers manifested deficits in their ability to detect differences in infant cries – communication patterns that have significance for accurate signal detection within the relationship. Supporting this finding, Broth, Goodman, Hall and Raynor (2004) found that depressed mothers were less accurate than non-depressed mothers in interpreting infant emotions, as reflected in their facial expressions.
The response patterns typically shown by depressed mothers, in turn, predict more negative outcomes for their children. Field and her colleagues have conducted extensive research concerned with interaction between depressed mothers and their children. In reviewing the literature, Field (1994) concluded that disruptions are shown at many levels among infants of depressed mothers. When mothers are relatively emotionally and socially unavailable, their infants are more likely to show negative affect and disrupted emotional regulation abilities, as well as dysregulated neurobehavioral development at later ages (Ashman, Dawson, Panagiotides, & Wilkinson, 2002; Dawson & Ashman, 2000; Field, 1994). Laboratory research has also demonstrated that disruptions can be experimentally produced in the affective responses of infants if they are exposed to a “still face” that simulates the behavior of depressed mothers (Gianino & Tronick, 1988). Extensive work making use of this paradigm has demonstrated that children manifest short-term responses to the still-face stimulus that are equivalent to those shown by the children of depressed mothers (as described by Field, 1994). For example, they show greater negative affect, increased heart rate and vagal tone (Moore & Calkins, 2004). In addition, infancy has been identified as a period in which maternal depression has the greatest effect on later development during early childhood (Sohr-Preston & Scaramella, 2006). However, less is known about the differential sensitivity of infants to maternal depression.
Variations in Children' Sensitivity to Context: Potential Moderators of Responses Shown to Maternal Depression
Whereas children are believed to be generally sensitive to their early environment, children may also vary in their level of sensitivity. For example, Belsky and his colleagues (Belsky, 2005; Belsky, Hsieh, & Crnic, 1998) have proposed that children differ in their degree of reactivity or responsiveness to parents' childrearing practices. Specifically, infants who show high negative emotionality are more (negatively) reactive to parenting practices than are those who show low negative emotionality; ultimately such children show a greater frequency of externalizing problems. Such children may be thought of as exceptionally vulnerable to the effects of harmful environments. Boyce and Ellis (2005) proposed that some children may be highly reactive to all aspects of their parenting history – with resultant implications for their later outcomes. In similar fashion, DeBellis (2004) proposed that some children have predispositions that lead them to show increased sensitivity to social cues in general.
Past research has focused on differences in children's temperament as a source of differential sensitivity to their social environment. However, there are suggestive indications that preterm infants may also provide an instance of children who are exceptionally vulerable to their early social-emotional environment. In some ways, they show heightened reactivity to their environment, for example, they show greater eye opening in response to infant-directed speech, and greater manifestations of distress when tactile stimulation (to the arms) is used in combination with infant-directed speech (Eckerman, Oehler, Medvin, & Hannan, 1994). However, they have more difficulty in sustaining attention to stimuli (Ruff, 1986). The attentional reactivity of preterm infants appears to pose a cost to their physiological stability (Lester, Boukydis, Zachariah, & LaGasse, 1996). In general, preterm infants show disorganization of their self-regulation ability, as well as attention and state disorganization (Als, Duffy, & McAnulty, 1988). They also show deficits in their processing of social stimuli. For example, preterm infants show auditory-visual deficits in their ability to detect face-voice synchrony in social stimuli (Pickens, Field, & Thomas, 1994). Possibly in the service of self-regulation, pre-term infants are also more likely to withdraw from social stimuli (Field, 1994). At the most general level, preterm infants have been found to show poorly developed social, communication, and joint attention initiation abilities (DeGroote, Roeyers & Warreyn, 2006). On a long-term basis, children born prematurely are less socially competent at later ages than are full-term children (Tessler, Nadeau, & Michel, 1997).
However, little is known about individual differences in infants' differential response to maternal depression (and resultant variations in their responsiveness). One of the few findings in connection with individual differences in response to maternal depression concerns gender effects. Infant sons appear to be more vulnerable than daughters to the depressive symptoms shown by mothers (Weinberg, Olson, Beeghly & Tronick, 2006). If indeed preterm infants have problems in self-regulation, any reduction in maternal responsiveness (generally associated with maternal depression) should pose a greater problem than is true for other infants. That is, mothers' relative unavailability could be expected to reduce their ability to facilitate the preterm infant's emotion regulation.
Hormonal Changes as Mediators of Children's Later Outcomes
In considering the effects of early experience on hormonal responses, our attention focused on chronic changes in children's cortisol levels. Chorpita and Barlow (1998), in reviewing the relevant literature, suggested that any early experience that causes the child to experience low perceptions of control will ultimately lead to hypercortisolism (chronically high levels of cortisol) at later ages. As one specific example, investigators have taken note of the fact that hypercortisolism appears among children who are exposed to poverty (Lupien, King, Meaney & McEwen, 2001). Relevant to the topic of this paper, maternal depression has also been found to be a source of stress – as evidenced by the fact that maternal depression predicts elevated cortisol levels in their children. For example, Ashman, Dawson, Panagiotides, and Wilkinson (2002) found that 7-8 year old children, whose mothers had a history of depression in the child's first two years of life, were more likely to manifest high baseline levels of cortisol. Bugental, Martorell, and Barraza (2003) demonstrated prospectively that children (as toddlers), whose mothers experienced post-partum depression, showed high baseline levels of cortisol.
Chronically elevated basal cortisol levels have been found to predict a wide variety of negative outcomes, for example, interference with normal immune function, metabolism, and reproduction, and loss of neuronal processes (e.g., Sapolsky, 1996). In children, elevated cortisol levels at younger ages are predictive of more negative psychological outcomes at older ages, for example, a higher presence of internalizing disorders and depressive symptoms (Post, Weiss, Li, Zang, Osuch, & McCann, 1998; Smider, Essex, Kalin, Buss, Davidson, & Goldsmith, 2002).
Predictions
Across two studies, we measured the differential sensitivity of children to maternal depressive symptoms, as a function of their birth history. It was predicted that preterm infants would manifest greater sensitivity to maternal depression than would full-term children. That is, preterm infants were expected to show higher basal cortisol levels than did full-term children in response to mothers who manifested depressive symptoms. No comparable differences between children were expected when mothers failed to show depressive symptoms. In Study 1 we compared the hormonal responses of preterm infants and healthy full-term infants. In Study 2, we compared the hormonal responses of preterm infants and full-term infants with other medical problems.
Study 1
Method
Participants
The sample included 25 mothers of toddlers recruited from the local community prior to the birth of the child (and involved an additional analysis of data obtained in another study (Bugental et al., 2003). Families were predominantly immigrants from Mexico and lived below the poverty line. As such, all children were exposed to stresses associated with early economic adversity. Mean maternal education was 8 years, SD = 3. The mean age of mothers was 25 years (SD = 6). The child sample included 17 boys and 8 girls. The mean age of children at the time of measurement was 16 months (SD = 5).
Families were referred to a home visitation program during the third trimester of the mother's pregnancy1. Although the families were not recruited on the basis of child medical risk, 11 of the children were identified as demonstrating mild or moderate prematurity (mean gestational age = 36 weeks, SD = 3). The remaining children were categorized as full-term (mean gestational age = 39 weeks, SD = 1).
A comparison was made of the demographic characteristics (age, education, and parity) of mothers of children in both groups. The only difference that approached significance was maternal age (t =2.03, p = .054). Mothers of preterm children were older (M = 27, SD = 6) than mothers of full-term children (M = 23, SD = 4).
Measures
Beck Depression Inventory (BDI)
The BDI provides a measure of depressive symptoms that has been validated against clinical ratings of depression (Beck, Steer, & Garbin, 1988). Coefficient alphas assessing internal consistency range from .73 to .92. Correlations between the BDI and clinical ratings of depression range from .55 to .73.
The BDI was translated into Spanish, as spoken regionally. In order to test the accuracy of the translation, it was back-translated (by another Spanish speaker) into English. The final translation was found to preserve the original meaning. In addition, this instrument was administered orally to facilitate its comprehension.
Cortisol production
Saliva samples (minimum of 400 μl) were scheduled for 10:00 A.M. in order to minimize circadian variation. Samples were taken from children approximately 20 minutes after entry to the lab. These samples provided a measure of children's basal cortisol levels.
Saliva samples were pipetted into cryogenic vials and stored at −20 degrees C. Assays were conducted on a monthly basis. Prior to assay, samples were thawed and centrifuged at 1500 RPM for 30 minutes to separate mucins. Cortisol was measured by utilizing a commercially-available enzyme immunoassay (EIA) kit (Salimetrics, LLC, State College, PA). Samples were assayed in duplicate, and were averaged for use in analyses. Samples from the same participants were run in the same assay. The lower limit of assay sensitivity was .007 μg/dl. Cortisol readings were examined for outliers and all values exceeding 3 SD were excluded. The average intra- and inter-assay coefficients of variation were 9.7% and 7.5%. The test-retest reliability on cortisol levels was .92.
Procedure
The BDI was administered to mothers at a “one-year” visit, that is, after the family had been in the program for a year. Subsequently, mothers and children visited the lab during the child's second year (as indicated earlier, the mean age of children was 16 months). Saliva samples were taken at the start of this visit.
Results and Discussion
The effects of maternal depression and child medical risk (entered as a dummy variable) were tested within a regression analysis. Maternal age was introduced at step 1. The main effects of maternal depression and child risk were entered in step 2, and the interaction between variables was entered at step 3.
Significant main effects were found for BDI scores: the higher the level of maternal depression, the higher the levels of children's baseline cortisol. The interaction between maternal depression and child birth status (preterm versus full-term) approached significance, β =1.93, p = .07.
Follow-up regression analyses (again including maternal age as a covariate) were conducted separately for preterm and full-term children. Within the preterm group, maternal depression was significantly related to children's cortisol levels (β = .76, p = .04). The relationship between these variables did not approach significance in the full-term group (β = -.08, ns).
The findings of this pilot study supported the predicted interaction between child birth history and maternal depression. Children who were premature were sensitive to maternal depressive symptoms -- as manifested in the reactivity of their basal cortisol levels. No comparable effect was found for children who were full-term. This observation extends previous work by demonstrating preliminary support for the role of child risk characteristics as a moderator of their reactions to maternal depression. The higher cortisol levels shown by preterm children suggest that they experience elevated stress levels in response to their mothers' depressive symptoms.
Limitations
Study 1 considered the possibility that preterm infants show a heightened level of sensitivity to the affective features of their parenting environment. However, such infants not only experienced prematurity, they also could have been expected to experience stresses comparable to those of other infants born at medical risk (e.g., spending time in a neonatal intensive care unit, NICU). As such, it is unclear whether the differential sensitivity shown is unique to those with preterm status. In addition, the distribution of ages at which measures were taken posed a limitation. In order to investigate these effects further, a second study was conducted that allowed for a comparison of the differential sensitivity of (a) preterm infants without any other kinds of medical problems versus (b) full term infants who experienced early medical problems.
Study 2
The central question asked in Study 2 concerned the differences in children's sensitivity to maternal depression among preterm children and medically at-risk full-term children. This allows a more stringent comparison in that both groups experienced some level of medical adversity early in life.
Children's stress levels as a result of the combined influences of birth history and maternal depression were measured, as reflected in their basal cortisol levels shortly after one year of age. As in Study 1, children's birth status (preterm versus full-term) was explored as a moderator of the effects of maternal depression on infants' cortisol levels.
Method
Research Participants
Research participants (N = 100) were recruited through an ongoing home visitation program providing services to families with medically at-risk children. All of the parental participants were mothers who were referred by health care professionals in the period soon after the birth of the child. Referrals were based on the presence of (mild or moderate) medical problems, including but not limited to prematurity. Children with more severe problems were not eligible as they were routinely referred to other community agencies. Thirty-four of the children were referred to the program on the basis of their preterm status (gestational age < 36 weeks, M = 33, SD = 2) and 46 were referred on the basis of another medical problem (mean gestational age = 39 weeks, SD = 2). Twelve children were referred on the basis of both conditions. Eight children were referred in the absence of either condition (e.g., they were described as preterm but did not meet our definition of preterm status). The final sample was restricted to children with only one basis of referral (34 preterm infants and 46 children with other medical problems), thus allowing a clearer test of any distinctions between groupings.
Eighty per cent of children in the final sample spent time in the NICU. The mean age of children at the “one-year” visit was 14.41 months (SD = 2.24). No significant differences or trends were found on either of these variables between the two referral categories.
Eighty-one per cent of preterm children and 91% of medically at-risk, full-term children were Latino. A Chi-square analysis (corrected for small samples) testing these differences revealed a trend-level difference (X2 = 3.56, p =.07). Families also participated in an ongoing home visitation program in which they had been randomly assigned to either a cognitive reframing condition, or a condition focused on parent training and social support (employing methods described in Bugental, Ellerson, Lin, Rainey, Kokotovic, & O'Hara, 2002). A Chi-square analysis testing these differences revealed a trend-level difference among those who completed the program (X2 = 3.30, p =.08). Whereas 59% of the families with preterm infants were involved in the cognitive reframing condition, 39% of the families with medically at-risk, full-term infants were involved in this condition.
Comparisons were also made of the two groupings on three other variables (see Table 1). There were significant differences between the two groups on maternal age and child weight for gestational age. Mothers of preterm children were older than were mothers of full-term children. Children who were preterm had a lower weight for their gestational age than did full term children. Differences did not reach significance on maternal education.
Table 1.
Characteristics of Families in Two Referral Categories
| Variable | Full-term infants (with medical problems) | Preterm infants | Difference |
|---|---|---|---|
| Maternal education | 9.08 (3.31) | 10.02 (9.50) | .94 |
| Maternal age | 26.44 (5.54) | 30.84 (6.86) | -4.40** |
| Weight for age | 82.57 (13.82) | 54.71 (70.01) | -27.86** |
Note. Education is last grade completed. Weight for age = birth weight (grams)/gestational age (weeks). SDs are shown in parenthesis.
p <.01.
Measures
Demographic variables
Demographic variables were taken during an intake assessment. Measures included: parental age, ethnicity, education, and family composition.
Birth records
Hospital birth records were accessed to obtain information on such variables as medical diagnosis, weight and gestational age (combined to provide a measure of weight for gestational age).
Maternal depression
Mothers completed the Beck Depression Inventory during a one-year home visit (as well as at an intake visit). The characteristics of the measure were described in Study 1. Test items were translated into Spanish as spoken regionally (and back-translated into English for accuracy). Bilingual participants were given the option of completing this task in either Spanish or English. The experimenter read test items aloud to research participants and recorded their answers on a laptop computer. The procedures employed allowed a uniform testing procedure for those participants who were literate (in either English or Spanish) and for those who had very limited literacy.
Cortisol levels
All measures were scheduled for 10 A.M in order to control for variability due to diurnal rhythms. On all visits, saliva samples were taken at the point the experimenter arrived at the research participant's home. Although there were some deviations from this time schedule, all samples were taken between 8 and 11 a.m.
Measures were taken during an intake visit and again one year later. Among young infants, saliva samples were taken by means of swipes with a dental roll inserted along the gums of the child's mouth. At older ages, resistance to the insertion of a dental roll (to collect saliva) was reduced by the addition of a few crystals of cherry Kool-Aid at the end of the dental roll. Although some concern has been expressed about elevations in cortisol levels as a result of the use of Kool-Aid (Schwartz, Granger, Susman, Gunnar, & Laird, 1998), the very low levels of Kool-aid used (not exceeding 1/16th of a tsp.) are unlikely to have interfered with the findings (nonetheless, this modification in salivary collection must be recognized as potential limitation). Visits were rescheduled if child participants were sick or if they were taking medication.
Assay procedures were the same as those described in the pilot study. Assay sensitivity was .03 μg/dl. The average intra- and inter-assay coefficients of variation were 6.6% and 5.2%.
Procedure
Initial measures of children's cortisol levels and maternal depression were taken during a visit to the family during the first 4 months of the child's life. In all cases, the home visitor (serving as part of a program providing services to these families) also served as the experimenter. Approximately one year later, the experimenter came to participants' homes to obtain self-report measures from the mother, and saliva samples from the child. Saliva samples were taken from the infant at the start of the visit.
Results
In testing predictions regarding children's cortisol levels (at the one-year visit), use was made of a regression analysis that included referral category (entered as a dummy variable) and maternal depression (as reflected in scores on the BDI). The child's earlier cortisol levels and maternal depression taken at intake were introduced as covariates in order to control for any initial differences on these key variables. In addition, maternal age, family ethnicity, type of intervention participation, and child weight for gestational age were introduced as covariates in order to control for (significant or trend-level) differences found between the two referral groups)2. The dependent variable was the child's baseline cortisol level at the one-year visit.
As shown in Table 3, the only significant effect obtained within the regression analysis was the interaction between child birth history and maternal depression. As can be seen in Figure 1, preterm children showed the same pattern of response (elevated basal cortisol levels as toddlers) in response to maternal depression as shown by preterm children in the pilot study.
Table 3.
Maternal Depression as a Predictor of Toddlers' Baseline Cortisol Levels
| Step | Variable | B | SE (B) | beta | R2 change |
|---|---|---|---|---|---|
| Step 1 | .10 | ||||
| Cortisol levels at intake | -.04 | .06 | -.09 | ||
| Maternal depression at intake | .00 | .00 | .11 | ||
| Ethnicity | .03 | .05 | .08 | ||
| Participation in intervention | .02 | .03 | .07 | ||
| Maternal age | .04 | .00 | .19 | ||
| Weight for gestational age | .00 | .00 | -.18 | ||
| Step 2 | .04 | ||||
| Referral Category | -.08 | .05 | -.32 | ||
| Maternal Depressive Symptoms | .00 | .00 | -.09 | ||
| Step 3 | .06* | ||||
| Referral Category × Maternal Depression | .01 | .00 | .28* |
p <.05
Note. On referral category, 1 = preterm status and 0 = full-term status (with medical complications).
Fig. 1.
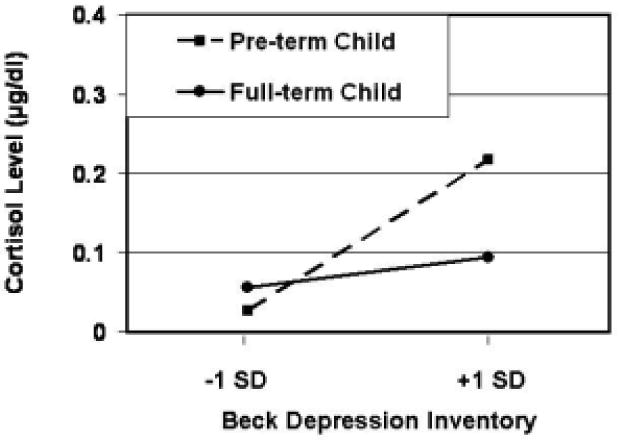
Cortisol levels (baseline) shown by toddlers as a function of child risk status (preterm vs. full-term) and maternal depressive symptoms. Regression lines are plotted for children of mothers scoring 1 SD above and below the mean on the BDI.
An additional analysis was conducted to rule out the possibility that the observed interaction effects were present at intake, that is, when measures were first taken. Making use of the same regression strategy, the interaction between variables did not approach significance.
General Discussion
Past research has provided a great deal of evidence that maternal depression produces problems in the mutual regulation system within the mother-infant dyad. In the current study, we demonstrated that infants are differentially vulnerable to these effects. Specifically, preterm infants, in comparison with full-term infants showed heightened hormonal sensitivity to maternal depressive symptoms. The emotional state and social responsiveness of mothers can be seen as particularly important for preterm children in view of their difficulty in self-regulation. As mothers typically provide the primary social environment for infants, the impact of depressive symptoms for exceptionally vulnerable children can be expected to have a large impact. Our findings demonstrate one of the ways in which children may respond very differently (at a neurohormonal level) to the emotional climate that exists within their families (as suggested by Cicchetti & Walker, 2001).
Our findings also add to the literature concerning variations in children's sensitivity to their social environment. Although children may be appropriately understood as quite generally responsive to their social environment, variations in their context-sensitivity have also been suggested (e.g., Belsky, 2005; Boyce & Ellis, 2005; Kochanska, 1997). Although other researchers have focused primarily on early trauma/adversity, temperament, or gender differences, we focused here on the effects of infants' birth history. Preterm infants showed dysregulation of the neuro-axis in terms of the high baseline levels of cortisol they manifested in response to maternal depression. It appears that the difficulty that preterm infants experience in state regulation leads to even greater problems when mothers are less likely to provide an external buffering role in the management of infant distress. In counter fashion, preterm infants manifested low baseline cortisol levels3 in response to mothers who failed to show signs of depression. With non-depressed mothers, the cortisol levels of preterm infants were equivalent to, or even lower than those shown by full-term infants. Healthy full-term infants showed generally low basal cortisol levels, and full-term infants with medical problems showed generally high cortisol levels. In both instances, however, full-term children showed no significant effects (or trends) with respect to their differential hormonal reactions to their mothers' depressive symptoms.
As a result of the sample measured, we were able to specify the role of preterm status per se. For example, we were able to rule out the effects of such early experiences as time in an NICU, the presence of others medical conditions, child gender, and family demographics. We also controlled for differences reflecting weight for gestational age. These findings will, of course, need to be replicated. As the sample of infants measured here were only moderately premature, it could be anticipated that even stronger findings might be obtained for infants at greater risk in terms of their preterm status.
A question may be raised concerning the age at which the effects of maternal depression first occurr. For example, evidence has been found that maternal depression prior to the child's birth may produce dysregulation of the infant's behavior, physiology and biochemistry soon after birth (Field, 2000). Thus it is important to note that the effects observed here for children's cortisol levels at intake (the first few months of life) were not associated with measures taken of maternal depression at intake (or the interaction between maternal depression and child referral category). The high sensitivity of preterm infants to maternal depression appears to have reflected a response to later levels of maternal depression (as measured when infants were toddlers) rather than the levels of maternal depression that were manifested earlier (in the first few months following the child's birth).
An overlap may be seen here with the literature on risk and resilience in the face of early adversity (e.g., Garmezy, 1993; Luthar & Cicchetti, 2000; Rutter, 1985). That is, children in both studies experienced poverty and/or early medical problems. The low levels of cortisol shown by preterm children -- if their mothers manifested no (or few) signs of depression -- may be thought of as a manifestation of resilience; that is, such children manifested hormonal levels equivalent to those of full-term children. However, it is also important to observe that these children manifested lower levels of stress hormones than did any other grouping of children. This suggests the possibility of thriving; that is, preterm children may experience exceptional advantages as a result of their relatively positive early history.
Limitations
A limitation in this research involves the biased sampling of families. Although the high representation of Latino families is unusual for the U.S. as a whole, it is not unusual in some portions of the country. In the county where this research was conducted, half of the children born are Latino, and their parents are typically poorly-educated recent immigrants to the U.S. Nonetheless, it will be useful in future research to replicate these findings in other kinds of populations.
Another limitation (primarily in Study 1) involves the relatively large age range of the children whose cortisol levels were measured as toddlers. There is also a potential limitation in having the BDI read to mothers. Although this was required as a result of mothers' limited literacy levels, it posed a greater chance of social desirability effects than would have been true if the measure had been completed in a less public fashion.
Implications
The long-term implications of our findings follow from the later outcomes of preterm infants whose mothers manifest depressive symptoms. It can be anticipated that children who have this combination of experiences early in life are more likely to show later problems consistent with hypercortisolism – including social-emotional problems, health problems, or cognitive deficits (Post et al., 1998, Sapolsky, 1996; Smider, et al., 2002). These findings suggest the importance of interventions directed to reducing maternal depression (and thus increasing the likelihood of maternal responsiveness), and/or reducing the arousal and reactivity of at-risk infants (Field, 1998).
Fig. 2.
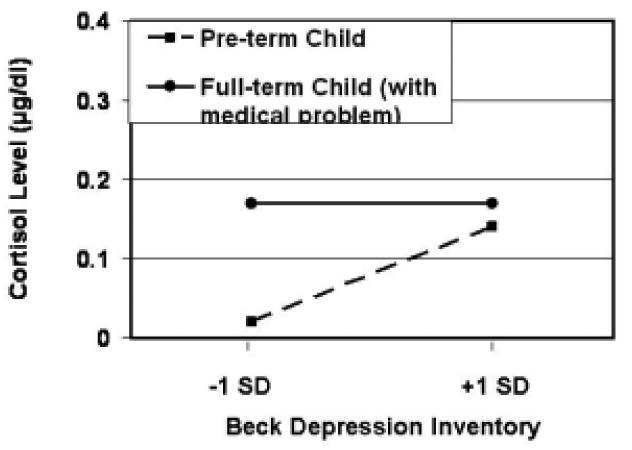
Cortisol levels (baseline) shown by toddlers as a function of infant referral category and maternal depressive symptoms. Regression lines are plotted for children of mothers scoring 1 SD above and below the mean on the BDI.
Table 2.
Maternal Depression as a Predictor of Toddlers' Baseline Cortisol Levels
| Step | Variable | B | SE (B) | beta | R2 change |
|---|---|---|---|---|---|
| Step 1 | .03 | ||||
| Maternal age | -.20 | .24 | -.18 | ||
| Step 2 | .45** | ||||
| Child Medical Risk at Birth | 4.52 | 2.32 | .37 | ||
| Maternal Depressive Symptoms | .45 | .14 | .56** | ||
| Step 3 | .10 | ||||
| Child Risk × Maternal Depression | .60 | .31 | 1.93 |
p <.01.
Acknowledgments
Research was supported by grants from the National Institutes of Health (RO1 MH19095) and the National Science Foundation (BNS 9021221) awarded to the first author, and the Elizabeth Munsterberg Koppitz Child Psychology Graduate Fellowship to the second author.
Footnotes
No significant overall differences were found in the cortisol levels shown by children whose mothers were referred to different types of home visitation programs (or a control condition).
A test was made of the differential hormonal reactivity of boys and girls to maternal depression. No significant effects or trends were found in either Study 1 or Study 2.
Hypocortisolism at older ages has been noted following very activation of the HPA axis early in life. The possibility has also been suggested that it may also occur very early in response to repeated activation of the HPA axis (Gunnar & Vazquez, 2001). In this investigation, however, the (low) levels of cortisol found here for preterm children with non-depressed mothers should be interpreted as following within a normative range. There is no reasonable basis for interpreting findings as reflecting pathological hypocortisolism. (e.g., a pattern that is more typically found with adults – either with prolonged pain or fatigue (Hellhammer, Schlotz, Stone, Pirke & Hellhammer, 2004).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Als H, Duffy FH, McAnulty GB. Behavioral differences between preterm and full-term newborn as measured with the APIB system scores. Infant Behavior and Development. 1988;11:305–318. [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Belsky J. Differential susceptibility in rearing influence: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. New York, NY: Guilford; 2005. pp. 139–163. [Google Scholar]
- Belsky J, Hsieh KH, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys' externalizing problems and inhibition at age 3 years: Differential susceptibility to rearing experience? Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: 1. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Development and Psychopathology. 1998;10:871–885. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- Broth MR, Goodman SH, Hall C, Raynor LC. Depressed and well mothers' emotion interpretation accuracy and the quality of mother-infant interaction. Infancy. 2004;6:37–55. [Google Scholar]
- Bugental DB, Ellerson PC, Lin EK, Rainey B, Kokotovic A, O'Hara N. A cognitive approach to child abuse prevention. Journal of Family Psychology. 2002;16:243–258. doi: 10.1037//0893-3200.16.3.243. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior. 2003;43:237–244. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: The role of control in the early environment. Psychological Bulletin. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Walker EF. Editorial: Stress and development: Biological and psychological consequences. Development and Psychopathology. 2001;13:413–418. [PubMed] [Google Scholar]
- Dawson G, Ashman SB. On the origins of a vulnerability to depression: The influence of the early social environment o the development of psychobiological systems related to risk for affective disorder. In: Nelson C, editor. Minnesota Symposia on Child Psychology: Vol 31. The effects of early adversity on neurobehavioral development. Mahway, NJ: Erlbaum; 2000. pp. 245–280. [Google Scholar]
- De Bellis MD. Neurotoxic effects of childhood trauma: Magnetic resonance imaging studies of pediatric maltreatment-related posttraumatic stress disorder versus nontraumatized children with generalized anxiety disorder. In: Gorman JM, editor. Fear and anxiety: The benefits of translational research. Washington, DC: American Psychiatric Publishing, Inc.; 2004. pp. 151–170. [Google Scholar]
- De Groote I, Roeyers H, Warreyn P. Social-communicative abilities in young high-risk preterm children. Journal of Developmental and Physical Disabilities. 2006;18:183–200. [Google Scholar]
- Donovan WL, Leavitt LA, Walsh RO. Conflict and depression predict maternal sensitivity to infant cries. Infant Behavior and Development. 1998;21:505–517. [Google Scholar]
- Eckerman CO, Oehler JM, Medvin MB, Hannan TE. Premature newborns as social partners before term age. Infant Behavior and Development. 1994;17:55–70. [Google Scholar]
- Field T. Infants of depressed mothers. In: Johnson SL, Hayes AM, Field TM, Schneiderman N, McCabe PM, editors. Stress, coping, and depression. Mahwah, NJ: Erlbaum; 2000. pp. 3–22. [Google Scholar]
- Field T. Maternal depression effects on infants and early interventions. Preventive Medicine: An International Journal Devoted to Practice and Theory. 1998;27:200–203. doi: 10.1006/pmed.1998.0293. [DOI] [PubMed] [Google Scholar]
- Field T. The effects of mother's physical and emotional unavailability on emotion regulation. Monographs of the Society for Research on Child Development. 1994;59(23):208–227. 250–283. [PubMed] [Google Scholar]
- Field T. Games parents play with normal and high-risk infants. Child Psychiatry and Human Development. 1979;10:41–48. doi: 10.1007/BF01433636. [DOI] [PubMed] [Google Scholar]
- Feldman R. From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Developmental Psychology. 2006;42:175–188. doi: 10.1037/0012-1649.42.1.175. [DOI] [PubMed] [Google Scholar]
- Garmezy N. Vulnerability and resilience. In: Funder DC, Parke RD, editors. Studying lives through time: Personality and development. Washington, DC: American Psychological Association; 1993. pp. 377–398. [Google Scholar]
- Gianino A, Tronick EZ. The mutual regulation model: The infant's self and interactive regulation and coping and defensive capacities. In: Field TM, McCabe PM, Schneiderman N, editors. Stress and coping across development. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. pp. 47–68. [Google Scholar]
- Gunnar MR. Early adversity and the development of stress reactivity and regulation. The effects of early adversity on neurobehavioral development. In: Nelson CA, editor. Minnesota Symposia on Child Development. Vol. 31. Mahwah, NJ: Erlbaum; 2000. pp. 163–200. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology: Special Issue: Stress and development: Biological and psychological consequences. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Schlotz W, Stone AA, Pirke KM, Hellhammer D. Allostatic load, perceived stress, and health: A prospective study in two age groups. In: Yehuda R, McEwen B, editors. Biobehavioral response: Protective and damaging effects. Annals of the New York Academy of Sciences. Vol. 1032. New York, NY: New York Academy of Sciences; 2004. pp. 8–13. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Multiple pathways to conscience for children with different temperaments: From toddlerhood to age 5. Developmental Psychology. 1997;33:228–240. doi: 10.1037//0012-1649.33.2.228. [DOI] [PubMed] [Google Scholar]
- Lester BM, Boukydis CFZ, LaGasse L. Cardiorespiratory reactivity during the Brazelton Scale in term and preterm infants. Journal of Pediatric Psychology. 1996;21:771–783. doi: 10.1093/jpepsy/21.6.771. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Platsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children form low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D. The construct of resilience: Implications for interventions and social policies. Development and Psychopathology. 2000;12:857–885. doi: 10.1017/s0954579400004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants' vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Minnesota Symposia on Child Development. Vol. 31. Mahwah, NJ: Erlbaum; 2000. The effects of early adversity on neurobehavioral development. [Google Scholar]
- Pickens J, Field T, Nawrocki T, Martinez A. Full-term and preterm infants' perception of face-voice synchrony. Infant Behavior and Development. 1994;17:447–455. [Google Scholar]
- Post RM, Weiss SRB, Li HL, Smith MA, Zhang LX, Xing G, Osuch EA, McCann UD. Neural plasticity and emotional memory. Development and Psychopathology. 1998;10:829–855. doi: 10.1017/s0954579498001898. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:230–266. [PubMed] [Google Scholar]
- Ruff HA. Attention and organization of behavior in high-risk infants. Journal of Developmental and Behavioral Pediatrics. 1986;7:298–301. doi: 10.1097/00004703-198610000-00004. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience in the face of adversity: Protective factors and resistance to psychiatric disorder. British Journal of Psychiatry. 1985;147:598–611. doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucocorticoids, and damage to the NS: The current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Schwartz EP, Granger DA, Susman EJ, Gunar R, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss MA, Klein MH, Davidso RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Sohr-Preston SL, Scaramella LV. Implications of timing of maternal depressive symptoms for early cognitive and language development. Clinical Child and Family Psychology Review. 2006;9:65–83. doi: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- Tessler R, Nadeau L, Boivin M, Tremblay R. The social behavior of 11- to 12-year old children born as low birthweight and/or premature infants. International Journal of Behavioral Development. Special Issue: Close Relationships Across the Lifespan. 1997;21:795–811. [Google Scholar]
- Weinberg MK, Olson KL, Beeghly M, Tronick EZ. Making up is hard to do, especially for mothers with high levels of depressive symptoms and their infant sons. Journal of Child Psychology and Psychiatry. 2006;47:670–683. doi: 10.1111/j.1469-7610.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- Zlochower AJ, Cohn JF. Vocal timing in face-to-face interaction of clinically depressed and nondepressed mothers and their 4-month-old infants. Infant Behavior and Development. 1996;19:371–374. [Google Scholar]


