Abstract
DNA preparations from two strains of Bacillus megaterium, one which produces megacin and another that is megacin sensitive, were analyzed by dye-buoyant density centrifugation, cesium chloride gradient equilibrium centrifugation in the absence of dye, zone sedimentation in sucrose, and electron microscopy. Both strains were found to contain a substantial proportion of their total DNA as circular, covalently closed, double helical molecules. These DNA elements were heterogeneous in size and appeared slightly less dense than chromosomal DNA. The physiological role and origin of these elements is unknown at present.
Full text
PDF

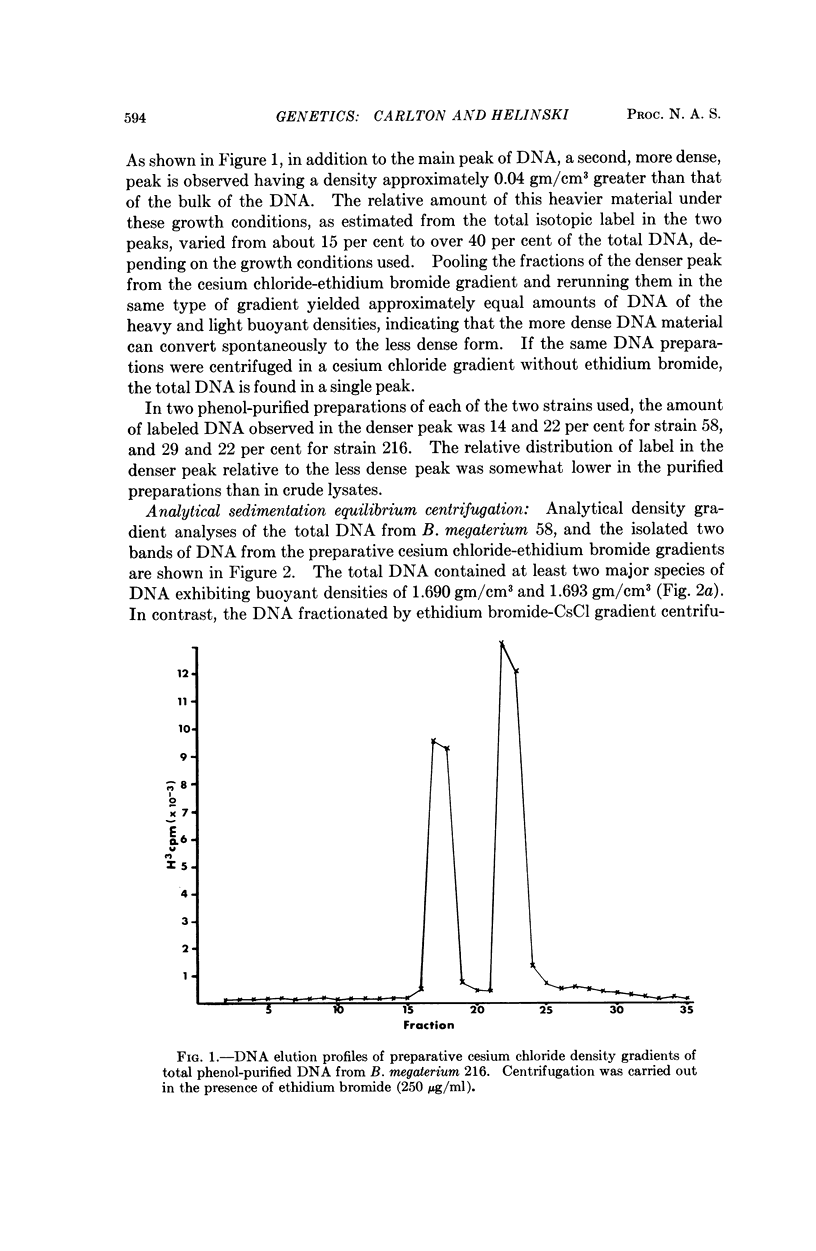
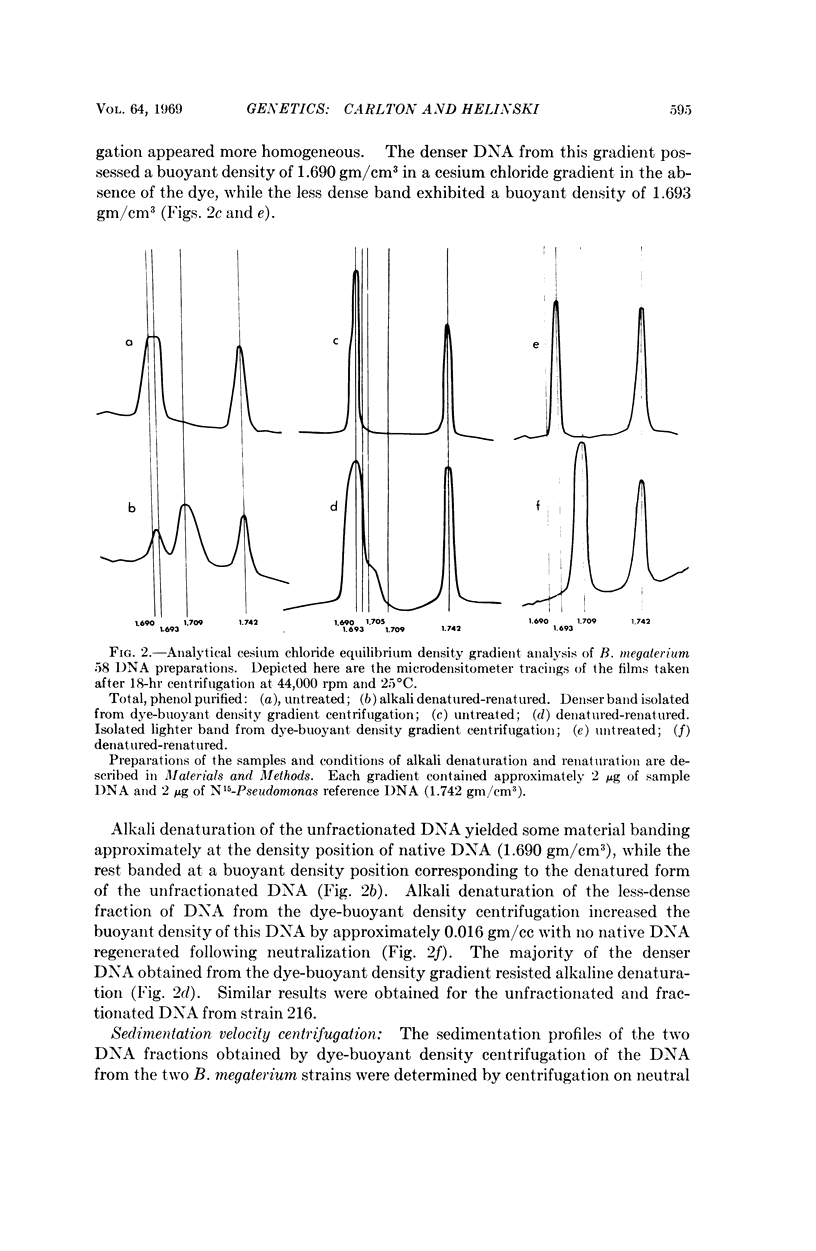
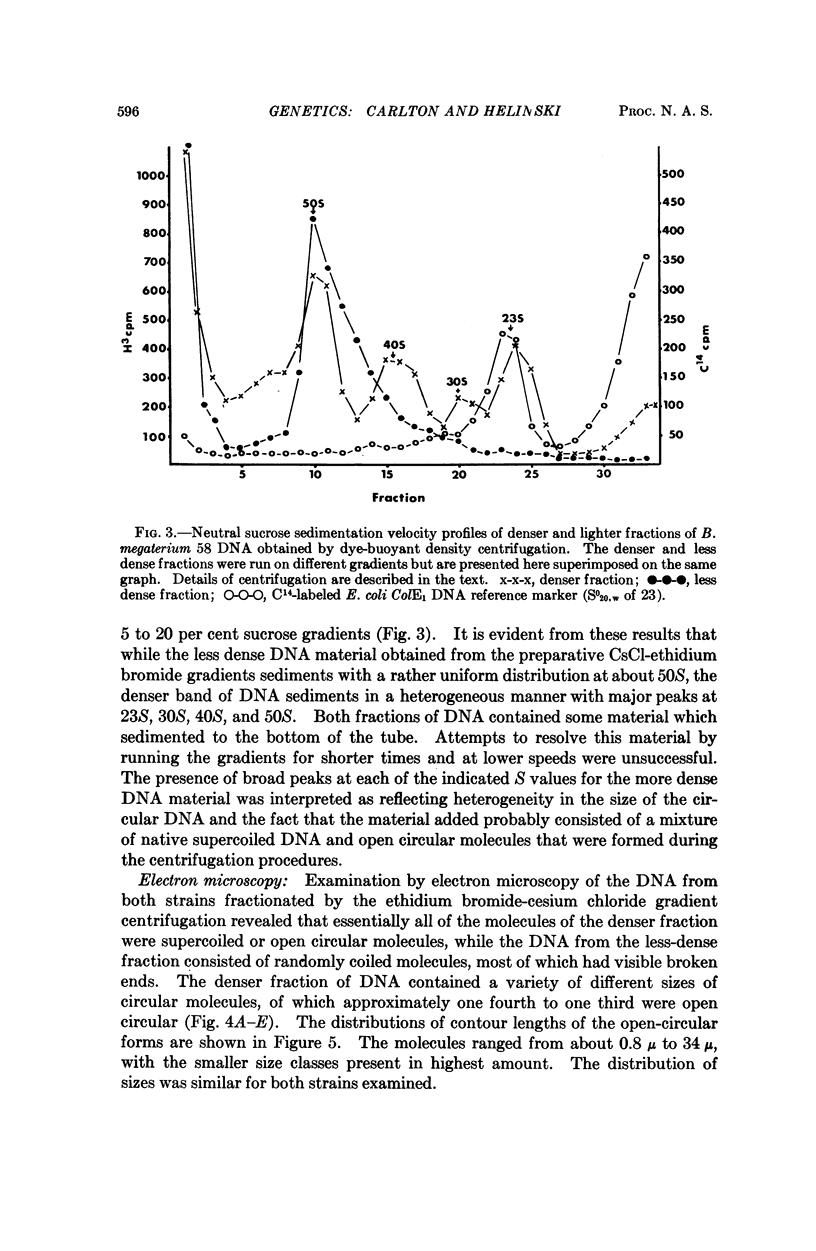



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967 Nov 18;216(5116):652–657. doi: 10.1038/216652a0. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Helinski D. R. Generation of higher multiple circular DNA forms in bacteria. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1406–1413. doi: 10.1073/pnas.61.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Vinograd J. Catenated circular DNA molecules in HeLa cell mitochondria. Nature. 1967 Nov 18;216(5116):647–652. doi: 10.1038/216647a0. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davidson N. Covalently closed minicircular DNA in Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1968 Sep 6;32(5):757–762. doi: 10.1016/0006-291x(68)90304-5. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels P. H., Knijnenburg C. M., van Rotterdam J., Cohen J. A. Structure of the replicative form of bacteriphage phi X174. VI. Studies on alkali-denatured double-stranded phi X DNA. J Mol Biol. 1968 Mar 14;32(2):169–182. doi: 10.1016/0022-2836(68)90002-8. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. F., Helinski D. R. Evidence for circular DNA forms of a bacterial plasmid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):650–657. doi: 10.1073/pnas.58.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Kleinschmidt A. K., Hellmann W., Warner R. C. Multiple-length rings in preparations of phi-X174 replicative form. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1676–1683. doi: 10.1073/pnas.58.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]




