Abstract
The role of group III metabotropic glutamate receptors (mGluRs) in photoreceptor-H1 horizontal cell (HC) synaptic transmission was investigated by analyzing the rate of occurrence and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) in H1 HCs uncoupled by dopamine in carp retinal slices. Red light steps or the application of 100 μM cobalt reduced the sEPSC rate without affecting their peak amplitude, which is consistent with hyperpolarization or the suppression of Ca2+ entry into cone synaptic terminals reducing vesicular transmitter release. Conversely, postsynaptic blockade of H1 HC AMPA receptors by 500 nM CNQX reduced the amplitude of sEPSCs without affecting their rate. This analysis of sEPSCs represents a novel methodology for distinguishing between presynaptic and postsynaptic sites of action. The selective agonist for group III mGluRs, l-2-amino-4-phosphonobutyrate (L-APB or L-AP4; 20 μM), reduced the sEPSC rate with a slight reduction in amplitude, which is consistent with a presynaptic action on cone synaptic terminals to reduce transmitter release. During L-APB application, recovery of sEPSC rate occurred with 500 μM (s)-2-methyl-2-amino-4-phosphonobutyrate (MAP4), a selective antagonist of group III mGluR, and with 200 μM 4-aminopyridine (4-AP), a blocker of voltage-dependent potassium channels. Whole-cell recordings from cones in the retinal slice showed no effect of L-APB on voltage-activated Ca2+ conductance. These results suggest that the activation of group III mGluRs suppresses transmitter release from cone presynaptic terminals via a 4-AP–sensitive pathway. Negative feedback, operating via mGluR autoreceptors, may limit excessive glutamate release from cone synaptic terminals.
Keywords: EPSC, L-APB, horizontal cell, mGluR, photoreceptor cell
INTRODUCTION
Glutamate is the neurotransmitter released by photoreceptors in the dark, mediating synaptic transmission to horizontal and bipolar cells (Copenhagen and Jahr 1988). Light hyperpolarizes cone photoreceptors, reducing the vesicular release rate of glutamate, leading to a reduction in the spontaneous excitatory postsynaptic current (sEPSC) rate and hyperpolarization of postsynaptic H1 horizontal cells (HCs; Hirasawa et al. 2001a). HCs in carp retina receive synaptic input from rods and cones via separate pathways (Kaneko and Yamada 1972) and H1 HCs receive synaptic input mainly from red-sensitive cones (Stell et al. 1975; Djamgoz 1984; Yamada and Yasui 1988; Yamada et al. 1999). We recently have shown that sEPSCs in H1 HCs are mediated by AMPA receptors, which is consistent with previous studies on isolated cells (Lu et al. 1998) that these ionotropic glutamate receptors mediate fast synaptic transmission from cones (Hirasawa et al. 2001a,Hirasawa et al. 2001b). The synaptic input to On bipolar cells from photoreceptors, however, is mediated by a G protein–coupled metabotropic glutamate receptor (mGluR6), which is selectively activated by (l)-2-amino-4-phosphonobutyric acid (L-APB or L-AP4; Shiells et al. 1981; Nawy and Jahr 1990; Shiells and Falk 1990, Shiells and Falk 1995; Nakajima et al. 1993; de la Villa et al. 1995). This is the only known synapse where the total excitatory postsynaptic input is mediated exclusively by a mGluR (Shiells 1994).
However, mGluRs play a role not only in mediating postsynaptic actions, but also are expressed in presynaptic terminals, functioning to modulate transmitter release in the central nervous system (Anwyl 1999; Cartmell and Schoepp 2000). Three mechanisms for the downregulation of transmitter release by mGluRs have been proposed: (1) suppressing presynaptic Ca2+ conductance (Takahashi et al. 1996; Koulen et al. 1999), (2) activating K+ conductance (Sladeczek et al. 1993; Cochilla and Alford 1998), or (3) directly inhibiting transmitter release independently from reducing calcium influx (Scanziani et al. 1995). L-APB, an agonist of group III mGluRs (mGluR4, 6, 7, and 8), hyperpolarized HCs in isolated retina (Nawy et al. 1989; Yasui et al. 1990; Hare and Owen 1992; Takahashi and Copenhagen 1992). Two distinct hypotheses have been proposed to account for this action involving either a presynaptic or a postsynaptic mechanism. Nawy et al. 1989 proposed a presynaptic effect that APB regulates transmitter release from photoreceptor synaptic terminals since postsynaptic HC responses to kainate were unaffected by APB. Supporting this, Koulen et al. 1999 recently reported that photoreceptor terminals in mammalian retina showed mGluR8 immunoreactivity and a reduction in intracellular calcium concentration in response to APB. Group III mGluRs are considered to function as autoreceptors, regulating synaptic transmission from photoreceptor terminals by negative feedback. Takahashi and Copenhagen 1992, however, have proposed a postsynaptic action since glutamate responses of isolated HCs showed suppression by APB. Furthermore, this postsynaptic action was shown to involve the activation of guanylate cyclase, increasing the level of cGMP in HCs, which in turn activated a K+ conductance via a cGMP-dependent kinase (Dixon and Copenhagen 1997). This action is distinctly different from the mGluR6-mediated action of APB in On bipolar cells, which may be either linked via a phosphodiesterase to reduce a cGMP-activated conductance (Nawy and Jahr 1990; Shiells and Falk 1990, Shiells and Falk 2000), or linked to direct channel closure by the G-protein Go (Nawy 1999). A further postsynaptic action of APB in HCs is to activate voltage-dependent calcium currents (Linn and Gafka 1999).
We now show that group III mGluRs regulate synaptic transmission from cone presynaptic terminals, which is consistent with the expression of mGluR8 (Koulen et al. 1999). A novel methodology applying dopamine to uncouple and, thus, electrically isolate H1 HCs in carp retinal slices was used, allowing the recording of discrete sEPSCs (Hirasawa et al. 2001a). The analysis of sEPSC rates of occurrence and amplitudes distinguishes a presynaptic action of L-APB. The results indicate presynaptic expression of mGluRs functioning to reduce transmitter release via a 4-AP–sensitive pathway, which is consistent with the known actions of mGluR8 or mGluR4.
MATERIALS AND METHODS
Retinal Slice Preparation and Superfusion
Slices of retina were prepared as described by Werblin 1978, Shiells and Falk 1990, and Hirasawa et al. 2001a from carp (Cyprinus carpio) kept under natural daylight conditions. Light-adapted fish (200–250 g) were pithed, and the eyes were enucleated and hemi-sected. To remove the vitreous humor, the inside of eyecups were gently washed for 3–4 min with Ringer's solution containing 120 U/ml collagenase and 465 U/ml hyaluronidase (Sigma-Aldrich). The eyecup was cut into four pieces. The retina was detached from the piece of eyecup onto filter paper (pore size 0.45 μm; Millipore Corp.), and was sectioned into 200-μm-thick slices. These were superfused with Ringer's solution containing the following (in mM): 102 NaCl, 2.6 KCl, 1 CaCl2, 1 MgCl2, 28 NaHCO3, 5 glucose, and 5 HEPES, adjusted to pH 7.5 with NaOH when bubbled with 95% O2 and 5% CO2. In all experiments, the control Ringer's solution contained 15–20 μM dopamine to block the gap junctions of H1 cells (Hirasawa et al. 2001a), with 75–100 μM ascorbic acid added to reduce oxidation. 6-Cyano-7-nitroquinoxaline (CNQX, an antagonist of non-NMDA receptors; RBI Ltd.) was dissolved in DMSO and then added to the superfusate. 2-Amino-2-methyl-4-phosphonobutyric acid (MAP4, an antagonist of group III mGluRs; Tocris Cookson) was dissolved with 1 N NaOH and then added to the superfusate. L-APB or L-AP4 (a selective agonist of group III mGluRs; Tocris) or 4-aminopyridine (4-AP) was added directly to the superfusate. Perfusion speed was 1.4 ml/min, and the volume of the recording chamber was 0.5 ml. All experiments were conducted at room temperature (21–23°C). It took 25–30 min to make the slice preparations under room light. The slices were viewed using an upright microscope (model BX50WI; Olympus) with infrared illumination (>850 nm) and monitored on a CRT display with a CCD camera (model C5985; Hamamatsu Photonics).
Light Stimulation
Retinal slices were illuminated by red and blue diffuse light-emitting diodes (peak wavelengths: 650 nm and 450 nm, respectively; models DHR6610 and GNB4510; Iwasaki Electric Ltd.). The blue light was filtered with a band pass filter (transmission peak at 410 nm; model V42; Toshiba Electric Ltd.), and the red light filtered with a sharp cut filter (cut-off wavelength at 650 nm; model R65; Hoya Optics Ltd.). The red light was filtered to selectively stimulate the red cones. HCs were identified by their characteristic morphology, spreading laterally in the distal part of the outer nuclear layer. Subtypes were identified by their characteristic spectral responses to steps of red and blue light. H1 HCs (L-type HCs) responded with outward currents to red and blue light, whereas C-type HCs responded with small inward or no currents to red and outward currents to blue light. The intensity of red light was between 6 and 21 × 105 quanta/μm2/s.
Whole-cell Recording from H1 HCs
Pipettes for whole-cell patch-clamp recording were fabricated from standard-walled borosilicate glass (Clark Electromedical). Intracellular Cs+-based pipette solution contained the following (in mM): 70 cesium methanesulfonate, 30 CsCl, 1 MgCl2, 5 EGTA, 1 Mg-ATP, 1 Na-GTP, and 10 HEPES adjusted to pH 7.2 with 3 N CsOH. H1 HCs were voltage-clamped to their initial dark membrane potentials (corrected for the tip potential) measured just after going to the whole-cell mode. To obtain discrete recordings of sEPSCs under voltage clamp, dopamine was applied to uncouple gap junctions and thus electrically isolate H1 HCs (Hirasawa et al. 2001a). Their input resistance was very low (∼15 MΩ) in the absence of dopamine and, under such poor space-clamp conditions, prominent sEPSC events were not observed (Hirasawa et al. 2001b). sEPSCs were usually recorded from cells with high input resistance (>70 MΩ) using patch electrodes with low series resistance (<12 MΩ) in dopamine containing Ringer's solution. Whole-cell voltage-clamp recordings were obtained using an amplifier (Axopatch 1-D; Axon Instruments).
L-APB modulates membrane currents in HCs by the activation of cGMP-dependent protein kinase (PKG) (Dixon and Copenhagen 1997) and by increasing voltage-dependent Ca+ conductance (Linn and Gafka 1999). To block these effects, 1–5-isoquinolinesulphonyl-2-methylpiperazine (H-7) a nonselective protein kinase inhibitor (Sigma-Aldrich) was included to the internal pipette solution, and HCs were voltage-clamped to potentials more negative than −50 mV. No series resistance compensation was used. Signals were low-pass filtered at 2–5 kHz and digitized at 8–10 kHz. Data were stored on DAT tape and on hard disk.
Whole-cell Recording from Cones
To eliminate the possibility of a direct modulation of voltage-activated Ca2+ conductance in cones, whole-cell voltage-clamp recordings were obtained from cones in carp retinal slices. The same patch-clamp technique was used as for recording from H1 HCs, with one modification. To clearly observe the voltage-activated Ca2+ conductance, we blocked the K+ conductance in cones using Cs+ and TEA in the external solution. The external solution contained the following (in mM): 55 NaCl, 15 CsCl, 30 TEA-Cl, 1 CaCl2, 1 MgCl2, 28 NaHCO3, 5 glucose, and 5 HEPES.
Analysis
sEPSCs in HCs were analyzed by setting a threshold detection level between 4–10 pA, defined as twice the standard deviation (σ) of the baseline noise in darkness (Hirasawa et al. 2001a). Probability density histograms of outward current noise were fitted with Gaussian functions, allowing calculation of the standard deviation of the baseline noise. The selected threshold level remained constant for the entire analysis. All events larger than this level were included if they had a rise time <1 ms, and if they did not arise from the decaying phase of a previous event. The peak of sEPSCs was detected semiautomatically with a moving rectangle (Origin 6.0 software; Microcal Ltd.). The height of rectangle was set at ∼3 pA, and the width was set at 1–3 ms for interval measurement or 10–20 ms for peak amplitude measurement not to detect overlapped peaks. Average sEPSC time courses were obtained by averaging individual sEPSCs aligned to their peak points. sEPSCs with distinct overlapped peaks were not included in the averaging. The statistical data were shown as means ± SEM. Error bars in the graphs indicate SEM.
RESULTS
Presynaptic Suppression of sEPSCs and Baseline Noise by Light or Cobalt
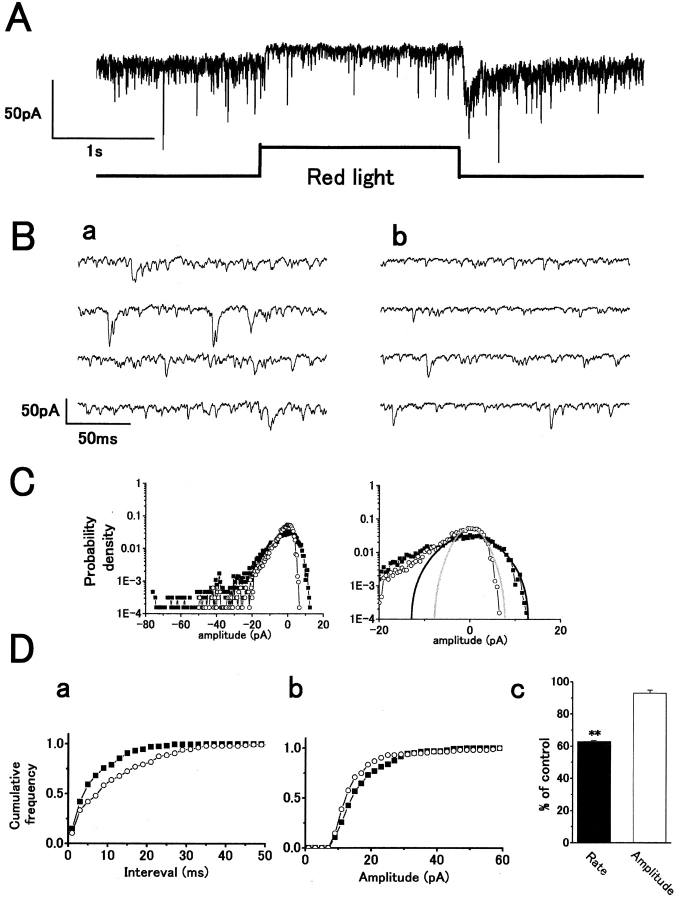
Fig. 1 A shows the postsynaptic current response of an H1 HC, recorded under whole-cell voltage clamp, to a step of red light. Time-expanded traces show that red light stimulation reduced the rate of sEPSCs compared with that in darkness (Fig. 1 B), which is consistent with light hyperpolarizing cone synaptic terminals, leading to a reduction in vesicular glutamate release. The distribution of sEPSC amplitudes was analyzed by constructing probability density histograms of the postsynaptic current. The reduction in sEPSC rate by light resulted in a reduction in the inward current density (Fig. 1 C, left), whereas a reduction of baseline noise variance was associated with a decrease in outward current density (Fig. 1 C, right). sEPSC events have a solely inward current polarity, whereas baseline noise has both inward and outward noise components fluctuating about zero current level. We could predict the distribution of the inward components of baseline noise, obscured by inward sEPSC events in the dark when the quantal release rate is high, by assuming a similar Gaussian distribution to the measurable outward component of baseline noise (Hirasawa et al. 2001a). The cumulative interval histogram shows the reduction in sEPSC rate induced by red light (Fig. 1 D, a, from 141 to 87 s−1) but no significant difference was observed in mean peak amplitude (Fig. 1 D, b, from 17.7 ± 0.6 pA to 15.7 ± 0.8 pA; n = 3, P > 0.06). Although the rate was significantly reduced by 37.4 ± 0.1% (paired t test, P < 0.001) in three cells, the amplitude was not changed (P > 0.05; Fig. 1 D, c).
Figure 1.
Red light illumination reduces the rate of occurrence of spontaneous excitatory postsynaptic currents (sEPSCs) without significant reduction of mean peak amplitude in an H1 horizontal cell (HC), recorded from a carp retinal slice. (A) Whole-cell voltage-clamp recording from an H1 HC showing an outward current response to a step of red light with a reduction in sEPSC rate. 20 μM dopamine was used throughout to block gap junctions to allow recording of sEPSCs (inward current events; Hirasawa et al. 2001a). The intensity of the red light was 6 × 105 quanta/μm2/s. Holding potential V h = −52 mV. (B) Fast sweep recordings in darkness (a) and with red light (b). (C) Probability density distribution histograms of postsynaptic current in darkness (closed squares) and red light (open circles). (left) The maxima of the probability density distributions were set to zero current. The same histograms on an expanded amplitude scale to show the baseline noise distribution (right). Gaussian functions fitted the outward current density in darkness (black line, SD: σ = 5.1 pA) and in red light (gray line, σ = 2.6 pA). (D, a) Cumulative interval histograms showing sEPSC interval distribution in darkness (closed squares, number of events: n = 168) and in red light (open circles, n = 116). The mean sEPSC intervals: 7.1 ms (dark) and 11.5 ms (red light). (b) Cumulative peak amplitude histograms of sEPSCs in darkness (closed squares, n = 282) and in red light (open circles, n = 171). Mean amplitude in red light (15.7 pA) was reduced slightly compared with that in darkness (17.7 pA). (c) Bar chart summarizing the changes in mean rate (a) and peak amplitude (b) from three cells. The red light reduced the rate of sEPSCs by 37.4 ± 0.1% (P < 0.001) but did not change the mean amplitude (7.0 ± 1.9%; P > 0.05). Level of significance: (**) P < 0.01.
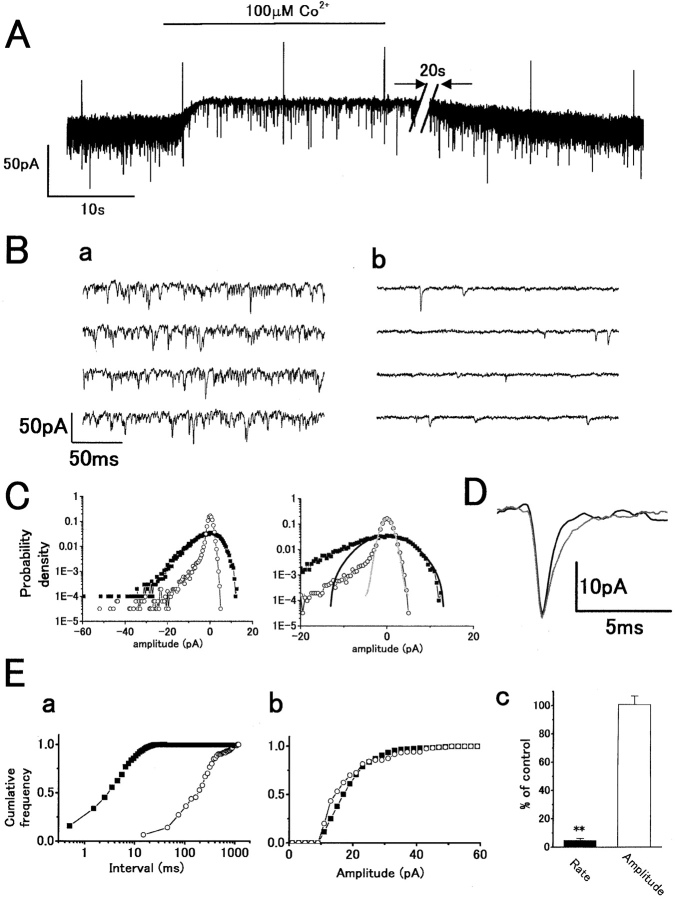
Similar, but more profound, effects to red light stimulation were obtained on superfusion with 100 μM cobalt (Fig. 2 A), which suppresses transmitter release by blocking presynaptic Ca2+ conductance (Dowling and Ripps 1973; Kaneko and Shimazaki 1975). 100 μM cobalt induced an outward current accompanied by a reduction in sEPSC rate. On washout, there was recovery of sEPSC rate and whole-cell current. Time-expanded recordings show that cobalt suppressed the sEPSCs, with a significant reduction in baseline noise (Fig. 2 B). Higher concentrations of cobalt (1–2 mM) suppressed sEPSC rate to <1 s−1 (unpublished data). Analysis of sEPSC amplitude distribution and baseline noise showed a dramatic reduction in the inward current component of the probability density histogram (Fig. 2 C, left), corresponding to the large reduction in sEPSC rate. Reduction of the outward current density reflects an accompanying reduction of the baseline noise variance (Fig. 2 C, right). Fig. 2 D shows the time course of averaged sEPSCs in control and with cobalt. The decay phase in cobalt was slightly more prolonged, but there was no change in mean peak amplitude. The cumulative interval histogram shows reduction of the sEPSC rate by cobalt (Fig. 2 E, a, from 204 to 4 s−1), with no significant difference in the mean peak amplitude (Fig. 2 E, b, from 19.4 ± 0.3 to 18.8 ± 0.8 pA). Although the rate was significantly suppressed by 95.3 ± 1.3% (P < 0.001) in eight cells, the amplitude was not significantly changed (0.8 ± 5.9%: P > 0.8; Fig. 2 E, c). These results are consistent with asynchronous transmitter release from cones being Ca2+-dependent (Reike and Schwartz 1996). The decrease in sEPSC rate results from a reduction in vesicular release rate, whereas the reduction of baseline noise, which was also suppressed by light, probably results from a reduction in the open probability of channels due to a fall in free glutamate concentration in the synaptic cleft (Hirasawa et al. 2001a). The rise in baseline noise in darkness or on washout of cobalt, thus, would result from an increase in single-channel fluctuations in response to a background level of free glutamate. This would be consistent with the Gaussian distribution of the baseline noise, which was fitted by using the distribution of the outward current noise components (Fig. 1 C and 2 C; Hirasawa et al. 2001a).
Figure 2.
External application of 100 μM cobalt reduces the sEPSC rate in darkness without any significant reduction in mean amplitude similarly analyzed as in Fig. 1. Symbols: control (closed squares or black traces) and cobalt (open circles or gray traces). (A) Whole-cell voltage-clamp recording from an H1 HC showing an outward current response to application of cobalt with a reduction of sEPSC rate. A voltage command pulse of 5 mV was applied every 12 s to monitor input conductance. For illustration, the trace was shortened by 20 s (slashes). V h = −44 mV. (B) Fast sweep recordings before (a) and during cobalt application (b). (C) Probability density distribution histograms of postsynaptic current. Gaussian functions fitted the outward current density in control (σ = 4.7 pA) and with cobalt (σ = 1.1 pA). (D) Time course of averaged sEPSCs before (n = 95) and during cobalt application (n = 57). (E, a) Cumulative interval histograms in control (n = 817) and in cobalt (n = 116) indicate lower sEPSC event rate in cobalt. The mean sEPSC intervals: 4.9 ms (control) and 246 ms (cobalt). (b) Cumulative peak amplitude histogram in control (n = 413) and in cobalt (n = 120). Mean amplitude in cobalt (18.8 pA) was slightly reduced compared with that in control (19.4 pA). (c) Summarized bar chart (from eight cells). Cobalt reduced the sEPSC rate by 95.3 ± 1.3%: P < 0.001), but did not change the mean amplitude (0.8 ± 5.9%: P > 0.8).
Postsynaptic Suppression of sEPSCs and Baseline Noise by CNQX
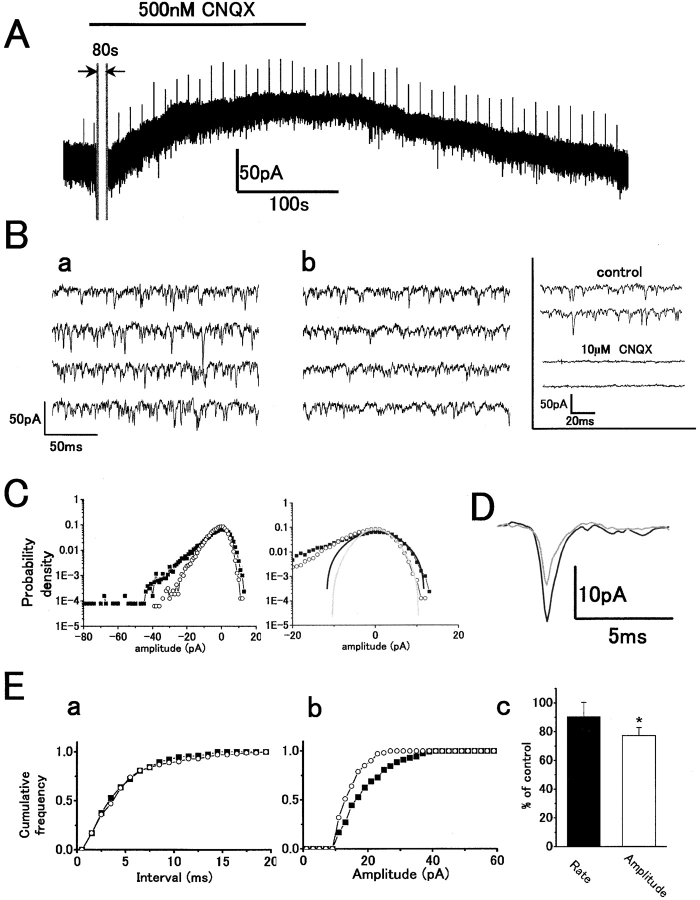
We investigated the postsynaptic suppression of sEPSCs and baseline noise by applying CNQX, an antagonist of non-NMDA receptors. Fig. 3 A shows the effect of 500 nM CNQX. This relatively low concentration of CNQX induced an outward current, which is consistent with blocking postsynaptic AMPA receptors (Hirasawa et al. 2001a,Hirasawa et al. 2001b). Time-expanded traces show that 500 nM CNQX reduced the peak amplitude rather than the rate of sEPSCs (Fig. 3 B). Higher concentrations of CNQX (10 μM) completely suppressed sEPSCs (Fig. 3 B, inset). The probability density histograms (Fig. 3 C, left) show a reduction of inward current events due to reduced sEPSC amplitudes. A corresponding reduction in baseline noise variance reduces the outward current density (Fig. 3 C, right), as would be expected if CNQX antagonized the action of free glutamate in the synaptic cleft. The time course of averaged sEPSCs in control and with CNQX is shown in Fig. 3 D. Although the mean amplitude was reduced by CNQX, there was no change in the sEPSC decay phase. The cumulative interval histogram from this data shows no significant reduction of sEPSC rate by CNQX (Fig. 3 E, a, P > 0.11), but there was a significant reduction of mean amplitude (Fig. 3 E, b, from 15.7 to 10.5 pA). The mean sEPSC rate showed no significant change in four cells (P > 0.4). However, the amplitude was significantly reduced (Fig. 3 E, c).
Figure 3.
500 nM CNQX reduced the sEPSC mean peak amplitude in darkness without any change in rate similarly analyzed as in Fig. 2. Symbols: control (filled squares or black traces) and CNQX (open circles or gray traces). (A) Whole-cell voltage-clamp recording from an H1 HC showing an outward current in response to application of CNQX with a reduction in sEPSC amplitude. For illustration, 80 s of the recording was removed. The initial inward current was probably a solution-switching artifact since this was only observed in one out of four recordings. V h = −60 mV. (B) Fast sweep recordings in darkness before (a) and during (b) CNQX application. (C) Gaussian functions in control (σ = 5.0 pA) and with CNQX (σ = 3.4 pA). (D) Time course of averaged sEPSCs before (n = 139) and during (n = 196) CNQX application. (E, a) Cumulative interval histograms in control (n = 222) and with CNQX (n = 157) indicate no significant change of sEPSC rate with CNQX. The mean sEPSC intervals: 4.3 ms (control) and 4.7 ms (CNQX). (b) Cumulative peak amplitude histograms in control (n = 221) and with CNQX (n = 156). Mean amplitude in CNQX (10.5 pA) was reduced compared with that in control (15.7 pA). (c) Summarized bar chart (from four cells). CNQX reduced the sEPSC amplitude by 22.7 ± 0.1%: P < 0.03) but did not change the mean rate (9.5 ± 9.9%: P > 0.4).
Suppression of sEPSCs, Baseline Noise, and Light Responses by L-APB
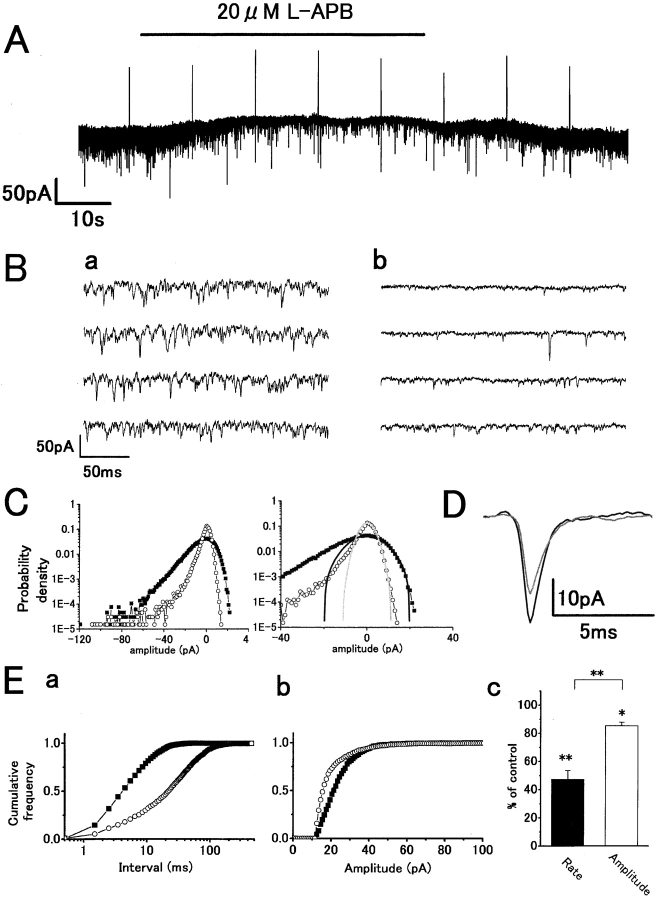
To determine the presynaptic role of group III mGluRs in cone to HC synaptic transmission, we studied the action of L-APB, an agonist of group III mGluRs, on H1 HCs. However, L-APB is known to have postsynaptic actions in modulating the inward-rectifier potassium channel via cyclic GMP-dependent kinase (Dixon and Copenhagen 1997) and in activating voltage-activated Ca2+ channels (Linn and Gafka 1999). To avoid these postsynaptic actions, H-7, a nonselective protein kinase inhibitor, was added to the patch pipette solutions. Whole-cell recordings with H-7 did not show any detectable effect on HC whole-cell currents, sEPSCs, or light responses (unpublished data). Furthermore, the holding potential of HCs was kept more negative to −55 mV to avoid large changes in voltage-activated Ca2+ conductance. Fig. 4 A shows the effect of applying 20 μM L-APB to the HC equilibrated with H-7 in the patch pipette solution on the whole-cell current in the dark. APB induced an outward current with a clear reduction in sEPSC rate (Fig. 4 B, a and b), which is similar to the effect of cobalt or light. The probability density histograms show a reduction of inward current events due to the suppression of sEPSCs (Fig. 4 C, left) and a reduction in outward probability density, indicating a reduction of baseline noise variance (Fig. 4 C, right). Fig. 4 D shows the time course of averaged sEPSCs before and during application of APB. There was a slight reduction in amplitude, but little change in decay phase. The cumulative interval histograms show a marked reduction in the sEPSC rate induced by L-APB from 147 to 25 s−1 (Fig. 4 E, a) and a slight suppression of mean peak amplitude from 28.2 to 20.2 pA (Fig. 4 E, b). This slight reduction in amplitude may be due to incomplete blockade, by H-7, of the known postsynaptic action of APB on HCs (Dixon and Copenhagen 1997). L-APB significantly suppressed the mean rate by 52.7 ± 6.0% (P < 0.001) in 19 cells and also significantly suppressed the mean amplitude by 15.5 ± 2.1% (P < 0.001; Fig. 4 E, c). Therefore, the major effect of 20 μM L-APB under these conditions is to suppress the transmitter release process, having a similar action to red light or cobalt on sEPSC rate and amplitude. Lower doses of APB were also effective, 2 μM L-APB suppressed the rate of sEPSCs by 42.5 ± 22.3% (n = 3).
Figure 4.
L-APB reduces sEPSC rate in darkness with slight reduction of mean peak amplitude similarly analyzed as in Fig. 2. Symbols: control (closed squares or black traces) and L-APB (open circles or gray traces). (A) Whole-cell voltage-clamp recording from an H1 HC showing an outward current response to application of 20 μM L-APB with a reduction in sEPSC rate. The patch-pipette solution contained 100 μM H-7 to block the post-synaptic action of L-APB, mediated by phosphorylation. V h = −57 mV. (B) Fast sweep recordings before (a) and during (b) L-APB application. (C) Gaussian functions (σ = 7.5 pA) and with L-APB (σ = 3.1 pA). (D) Time course of averaged sEPSCs before (n = 84) and during L-APB application (n = 104). (E, a) Cumulative interval histograms in control (n = 2,935) and with L-APB (n = 1,112) indicate reduction of sEPSC rate with L-APB. Mean sEPSC intervals: 6.8 ms (control) and 34.0 ms (L-APB). (b) Cumulative peak amplitude histograms in control (n = 1,249) and with L-APB (n = 885). Mean amplitude in L-APB (20.2 pA) reduced compared with that in control (28.2 pA). (c) Summarized bar chart (19 cells). L-APB reduced the sEPSC rate by 52.7 ± 6.0% (P < 0.001) and slightly reduced the mean amplitude by 15.5 ± 2.1% (P < 0.001). The rate reduction was significantly greater than that of peak amplitude (P < 0.001).
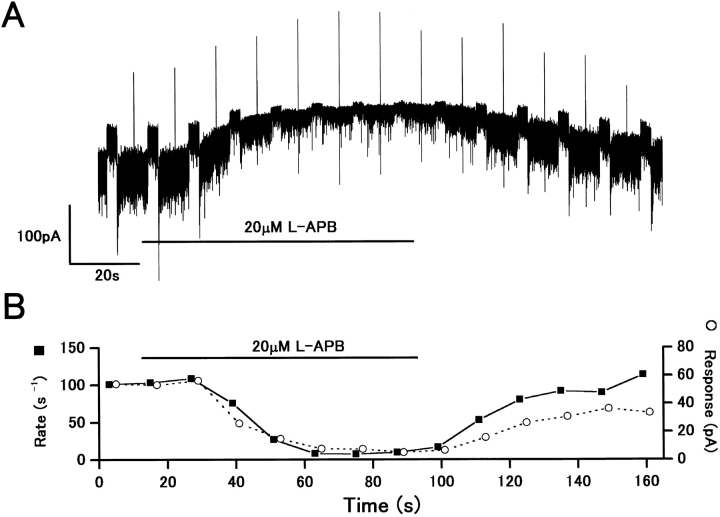
Fig. 5 A shows the effect of 20 μM L-APB on H1 HC light–induced outward current responses. L-APB suppressed the red light responses, accompanied by a reduction in sEPSC rate. The time course of light-induced current responses followed the sEPSC rate changes (Fig. 5 B). L-APB reduced the rate of sEPSCs by 93% (from 101 to 7.5 s−1; Fig. 5 B, closed squares) and reduced the amplitude of light responses by 83.3% (from 54.1 pA to 9.6 pA; Fig. 5 B, open circles). The reduction of sEPSC rate and light responses showed a clear correlation (correlation coefficient: 0.90), suggesting that L-APB limits the amplitude of light responses by suppressing the transmitter release process from cone synaptic terminals.
Figure 5.
The light-induced outward current responses were reduced by L-APB, accompanied by a reduction of sEPSC rate. (A) Whole-cell voltage-clamp recording from an H1 HC showing outward current responses to red light steps (2 × 106 quanta/μm2/s). An application of 20 μM L-APB induced an outward shift in the whole-cell current. A voltage command pulse of 5 mV was applied every 12 s. V h = −60 mV. (B) Light responses (open circles) and sEPSC rate (closed squares) showed a reduction over a similar time course in response to L-APB. The sEPSC rate was determined in the period of 2048 ms just before the onset of light stimulus.
The Effect of APB Is Antagonized by MAP4
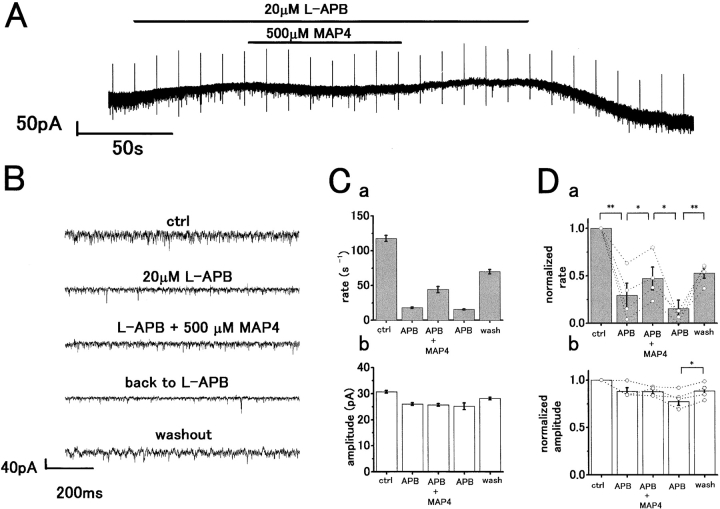
To confirm that L-APB was acting via a group III mGluR, we examined whether MAP4, a selective antagonist of group III mGluRs, antagonized the effects of L-APB. Fig. 6 A shows an application of 500 μM MAP4 made during superfusion of an H1 HC with 20 μM L-APB. The fast sweep recordings in each condition are shown in Fig. 6 B. The initial sEPSC rate in control was 117.6 ± 4.3 s−1 and was reduced to 17.9 ± 1.1 s−1 by L-APB. Coapplication of 500 μM MAP4 partially restored the frequency to 44.1 ± 4.4 s−1, and subsequent washout of MAP4 to 20 μM L-APB alone reduced the frequency to 15.5 ± 0.8 s−1. Some recovery to 69.9 ± 3.2 s−1 was observed on washing out APB (Fig. 6 C, a). The mean peak amplitude was not significantly changed by MAP4, which is consistent with a presynaptic site of action (Fig. 6 C, b). Averaged results from four cells are shown in Fig. 6 D. The sEPSC rate, reduced by L-APB (mean suppression by 71 ± 13% compared with control, P < 0.02) recovered with 500 μM MAP4 (increase to 169.2 ± 102.9% compared with L-APB, P < 0.01) and the effect of MAP4 was reversible (mean suppression by 85 ± 7.3% compared with MAP4, P < 0.05). The mean peak amplitude was not significantly changed by L-APB and MAP4 in four cells (P > 0.05), although on recovery from L-APB there was a small increase in the mean peak amplitude (P > 0.04). Application of MAP4 alone induced an inward current accompanied by a slight, but insignificant, increase in sEPSC rate (unpublished data).
Figure 6.
The action of L-APB in reducing sEPSC rate is antagonized by MAP4. (A) Whole-cell voltage-clamp recording from an H1 HC in the dark. 20 μM L-APB induced an outward current accompanied by a reduction in sEPSC rate. Coapplication of 500 μM MAP4 induced an inward current with some recovery of sEPSC rate. A voltage command pulse of 5 mV was applied every 12 s. V h = −57 mV. (B) Fast sweep recording of current traces in each pharmacological condition shown in A. (C, a) Bar chart representation of sEPSC rate under the different pharmacological conditions. MAP4 application partially restored the L-APB induced reduction in sEPSC rate. (b) A similar plot showing little change in sEPSC peak amplitudes. (D) Bar charts summarizing the mean changes in sEPSC rate (a) and peak amplitude (b) from four cells. Open circles with dotted lines indicate individual data from single cells. Levels of significance calculated by paired t test were as follows: (**) P < 0.01; (*) P < 0.05.
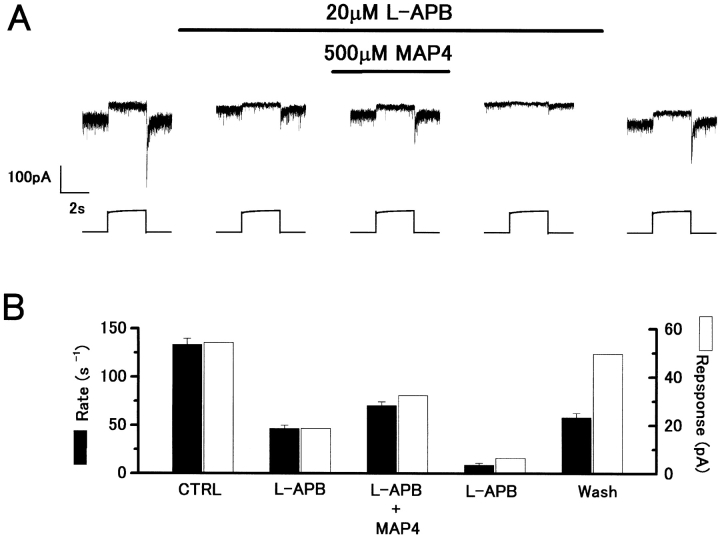
500 μM MAP4 also antagonized the L-APB induced reduction of red light responses and sEPSC rate (Fig. 7 A). Light responses were reduced by APB in proportion to the reduction in sEPSC rate (Fig. 7 B) and both showed partial recovery on application of MAP4. On washing out MAP4, there was a more profound reduction in light responses and sEPSC rate, which showed some recovery on returning to control Ringer. Similar effects were observed in two cells.
Figure 7.
MAP4 antagonizes the presynaptic action of L-APB. (A) Outward current responses to red light (2 × 106 quanta/μm2/s) recorded under whole-cell voltage clamp from an H1 HC. Application of 20 μM L-APB induced an outward current and reduction of the light responses. Coapplication of 500 μM MAP4 induced an inward current, accompanied by some recovery of the light responses. V h = −57 mV. (B) The L-APB–induced reduction in light response amplitude (open bars) and sEPSC rate (filled bars) was restored by MAP4. The sEPSC rate was similarly determined as in Fig. 5 B.
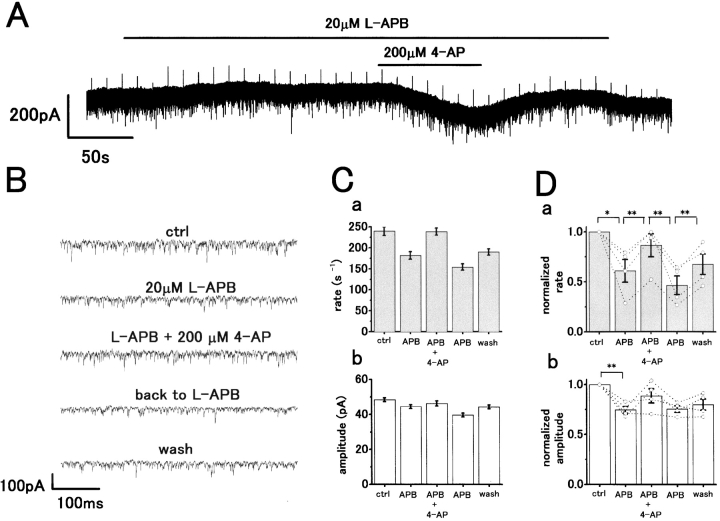
4-AP Reduces the Presynaptic Effect of L-APB
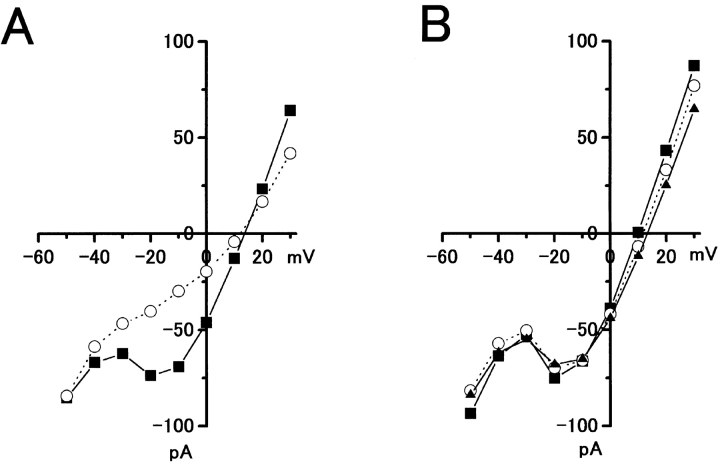
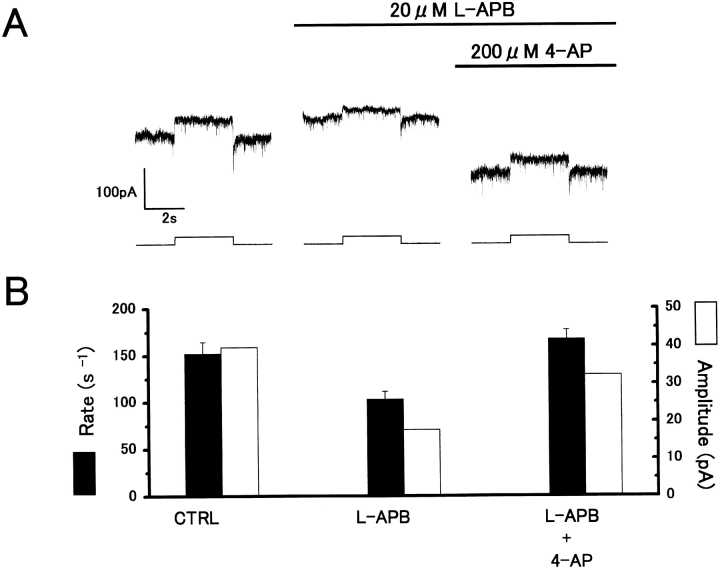
Two principal mechanisms have been proposed to account for the L-APB induced regulation of transmitter release: (1) suppression of a presynaptic Ca2+ conductance (Takahashi et al. 1996), and (2) activation of a presynaptic 4-AP–sensitive K+ channels (Sladeczek et al. 1993; Cochilla and Alford 1998). To determine whether L-APB reduces voltage-dependent Ca2+ conductance in cones, whole-cell voltage-clamp recordings were obtained from cones in the carp retinal slice. The current-voltage (I-V) relation was measured before and during cobalt or APB applications (Fig. 8). Whereas cobalt was effective in reducing the Ca2+ conductance (Fig. 8 A), 20 μM APB did not reduce the voltage-dependent Ca2+ current in cones (Fig. 8 B).
Figure 8.
20 μM APB does not reduce voltage-activated Ca2+ conductance in cones. (A) Current-voltage (I-V) relations obtained from a whole-cell voltage-clamped cone photoreceptor in the carp retinal slice before (circles) and during (squares) 2 μM cobalt application, showing a reduction of voltage-sensitive Ca2+ conductance by cobalt. (B) I-V relationships from the same cone photoreceptor before (circles), during (squares), and after washout (triangles) of a 20-μM APB application to the retinal slice showing no effect of APB on Ca2+ conductance. In both cases, K+ conductance was suppressed by external application of Cs+ and TEA.
Therefore, we examined whether APB was acting to suppress a 4-AP–sensitive K+ current in the presynaptic terminals. Fig. 9 A shows an application of 200 μM 4-AP to an H1 HC made during superfusion with 20 μM APB. This induced an inward current, which is consistent with 4-AP acting to reduce the presynaptic inhibition induced by mGluR activation. Fast sweep recordings in each condition are shown in Fig. 9 B. The sEPSC rate (reduced by APB) showed a significant recovery close to the control level with 200 μM 4-AP. In the presence of APB, the rate increased from 182 ± 9 to 239 ± 9 s−1 on application of 4-AP (Fig. 9 C, a). On washing out 4-AP, this was reduced to 154 ± 8 s−1, and on returning to control, the Ringer's solution recovered to 190 ± 8 s−1. However, the mean peak amplitude showed no significant change with 4-AP (Fig. 9 C, b), suggesting a presynaptic action. Results averaged from four cells are shown in Fig. 9 D. The reduction in mean sEPSC rate induced by L-APB (mean suppression by 39.0 ± 11.4%, P < 0.05) showed a significant recovery with 200 μM 4-AP (P < 0.01; Fig. 9 D). Washout of 4-AP significantly reduced the mean rate (P < 0.01). The mean peak amplitude was significantly decreased by the initial application of APB (P < 0.01, n = 4; Fig. 9 D, b), but showed no further significant changes on application or after washout of 4-AP (P > 0.07, n = 4).
Figure 9.
Blocking effect of K+ conductance with 4-AP induces an inward current and recovery of H1 HC sEPSC rate reduced by L-APB. (A) Whole-cell voltage-clamp recording from an H1 HC in the dark. 20 μM L-APB induced an outward current accompanied by a reduction in sEPSC rate. Coapplication of 200 μM 4-AP induced an inward current. A voltage command pulse of 5 mV was applied every 12 s. V h = −71 mV. (B) Time-expanded sections of the recording in A showing reduction in sEPSC rate with APB, and then some recovery with 4-AP accompanying the inward current. (C) Bar scales show mean sEPSC rate (a) and mean peak amplitude (b) changes from four cells in each pharmacological condition.
The APB-induced reduction of H1 HC red light responses also was reversed by 200 μM 4-AP (Fig. 10 A). The outward current response to light initially was reduced by APB, and then showed some recovery, accompanied by an inward current, on application of 4AP. Mean reduction of light responses and sEPSC rate recorded from two cells on application of APB are shown in Fig. 10 B. A significant recovery resulted on application of 4-AP, which is consistent with APB activation of presynaptic mGluRs reducing transmitter release by increasing a 4-AP–sensitive K+ conductance.
Figure 10.
Blocking effect of K+ conductance with 4-AP reverses the APB-induced reduction of H1 HC light responses. (A) Outward current responses to red light (2 × 106 quanta/μm2/s) recorded under whole-cell voltage clamp from an H1 HC. Application of 20 μM L-APB induced an outward current and reduction of light responses. Coapplication of 200 μM 4-AP induced an inward current, accompanied by some recovery of the light responses. V h = −60 mV. (B) The L-APB–induced reduction in light response amplitude (open bars) and sEPSC rate (filled bars) showed some recovery with 4-AP. The sEPSC rate was similarly determined as in Fig. 5 B.
DISCUSSION
A Novel Analysis for the Determination of Presynaptic versus Postsynaptic Modulation of Synaptic Transmission
The recording of discrete sEPSCs from H1 HCs, suppressed by light, provides direct evidence for asynchronous vesicular transmitter release from cones following a Poisson interval distribution (Hirasawa et al. 2001a). Graded transmitter release events from cones previously were estimated from the power spectrum of voltage noise in bipolar cells (Ashmore and Copenhagen 1983). The analysis of sEPSC rate, time course, and amplitude, however, provides a novel and direct method for determining whether synaptic transmission from cones to H1 HCs is modulated presynaptically or postsynaptically. Presynaptic modulation, involving a change in the rate of quantal release of transmitter from cone synaptic terminals, results in a change in sEPSC rate without any change in mean peak amplitude. Thus, we detected a reduction in the rate, due to hyperpolarization of the synaptic terminals or suppression of Ca2+ influx with red light or cobalt, respectively, with no corresponding reduction in mean amplitude. On the other hand, postsynaptic modulation involves no change in the rate, but does effect a change in amplitude. Accordingly, with CNQX, which blocks H1 HC AMPA receptors (Yang et al. 1998; Hirasawa et al. 2001a,Hirasawa et al. 2001b), we detected a reduction in sEPSC amplitude without any corresponding change in rate. Therefore, monitoring sEPSC rates and amplitudes allows us to distinguish clearly between agents that act postsynaptically, such as CNQX, and could be used to study agents which are thought to act presynaptically, such as nitric oxide (Savchenko et al. 1997) or somatostatin (Akopian et al. 2000) that are assumed to modulate transmitter release.
APB Reduces Transmitter Release from Cone Presynaptic Terminals
Application of APB consistently reduced the sEPSC rate, suggesting a presynaptic action of APB on the cone terminals to reduce transmitter release. This action would be consistent with the activation of a presynaptic mGluR leading to hyperpolarization of cone terminals. However, in the carp retinal slice, other synaptic interactions are possible. Two other possible pathways mediated by negative feedback from horizontal cells to reduce transmitter release from cones can be considered: (1) a GABA-ergic negative feedback pathway or (2) a GABA-independent feedback pathway (Verweij et al. 1996). For example, in the accessory olfactory bulb, the granule cell mGluR2 can regulate GABA-ergic transmission to the mitral cell (Hayashi et al. 1993). In our preliminary experiments, both picrotoxin and bicuculline (antagonists of GABA receptors) failed to restore the sEPSC rate suppressed by L-APB (unpublished data). Therefore, the GABA-ergic feedback from horizontal cells is unlikely to mediate the action of APB to reduce transmitter release from cone presynaptic terminals. The GABA-independent negative feedback pathway also is unlikely since this involves a shift in the voltage range of the Ca2+ current of cones to suppress transmitter release (Verweij et al. 1996). However, L-APB had no effect on the voltage-dependent Ca2+ current of cones recorded from the retinal slice. The recovery of sEPSC rate on 4-AP application suggests that L-APB suppressed transmitter release directly by increasing a 4-AP–sensitive K+ conductance mediated by mGluR activation in cone presynaptic terminals.
In the inner retina, presynaptic group III mGluRs at bipolar cell terminals have been proposed to regulate synaptic transmission between bipolar and ganglion cells (Awatramani and Slaughter 2001). At this site, as in H1 HCs, activation of the presynaptic mGluR by L-APB reduced the sEPSC rate in ganglion cells with little change in sEPSC amplitude.
Identity of Group III mGluR on Cone Presynaptic Terminals
The presynaptic action of L-APB is consistent with the expression of group III mGluRs on cone presynaptic terminals, functioning by negative feedback to reduce transmitter release. The IC50 value of L-APB is in the range of 0.06–1 μM for mGluR4, 6, and 8, but >100 μM for mGluR7 (Cartmell and Schoepp 2000). Since we found that concentrations of 2–20 μM L-APB were effective in reducing sEPSC rate by 47.3 ± 6.0%, this suggests that the presynaptic action of APB may be mediated by mGluR4, 6, or 8 but not by mGluR7. MAP4 failed to antagonize the effect of L-APB in retinal On bipolar cells expressing mGluR6 (Thoreson et al. 1997), and there is no immunocytochemical evidence for the expression of mGluR6 on cone presynaptic terminals (Vardi et al. 2000), excluding mGluR6. MAP4 has been shown to be an antagonist at mGluR4 with an IC50 > 500 μM (Knöpfel et al. 1995), whereas for mGluR8 the IC50 is >25 μM (Saugstad et al. 1997) in expression systems. As yet, we cannot distinguish between mGluR4 or 8 from the pharmacology, but the immunocytochemical evidence would suggest mGluR8 (Koulen et al. 1999).
Presynaptic Suppression Mechanism of Cone Transmitter Release
Koulen et al. 1999 demonstrated the expression of mGluR8 on mammalian cone presynaptic terminals, and that activation by L-APB induced a fall in Ca2+ concentration. Group III mGluRs suppress transmitter release in two principal ways: (1) by suppressing the presynaptic voltage-activated Ca2+ conductance by direct G-protein–channel interaction (Takahashi et al. 1996; Dolphin 1998); or (2) by activation of presynaptic 4-AP–sensitive K+ channels by a cAMP-mediated second messenger pathway (Cartmell and Schoepp 2000). The present results would be consistent with the action of L-APB being mediated by mGluR8 or mGluR4 in cone presynaptic terminals linked to a 4-AP–sensitive pathway. This would hyperpolarize the terminals, which are relatively depolarized in the dark, resulting in a reduction in quantal transmitter release. We found that L-APB had no direct effect on cone voltage-activated Ca2+ conductance, so the fall in Ca2+ concentration observed on exposure of cones to APB (Koulen et al. 1999) may result from a secondary effect due to hyperpolarization (reducing voltage-activated Ca2+ conductance) of the presynaptic terminals.
Glutamate also is known to activate a Cl−-dependent transporter current in cones, which is insensitive to APB (Grant and Werblin 1996) and mediates a presynaptic action of glutamate at the cone output synapse (Sarantis et al. 1988; Tachibana and Kaneko 1988). Since the reversal potential of Cl− in cones is more positive to their membrane potential in the dark, glutamate depolarizes cones by this mechanism. This constitutes a positive feedback mechanism, proposed to increase the gain for the conversion of changes in light intensity into changes in glutamate release (Sarantis et al. 1988). The mGluR-mediated suppression of vesicular glutamate release, reported here, constitutes a negative feedback mechanism acting to limit the release rate of glutamate. Perhaps the mGluR-mediated negative feedback pathway is required to prevent the potential development of regenerative or excessive glutamate release by the positive feedback pathway, which is mediated by the glutamate transporter current.
Negative Feedback Mediated by Group III mGluRs Functioning as Autoreceptors
The present results suggest the possibility of autoreception, such that glutamate released from cones acts via mGluR8 or mGluR4 to reduce the transmitter release process in red cone presynaptic terminals mediated by the 4-AP–sensitive pathway. This negative feedback via group III mGluRs might regulate the tonic release of glutamate in the dark. With light, the rate of glutamate release is reduced by hyperpolarization of the presynaptic terminals, so the negative feedback would accordingly decrease as the level of free glutamate, and hence mGluR activation, is reduced. What precise function this may have in regulating postsynaptic light responses of both HCs and bipolar cells remains to be determined. The mGluR antagonist, MAP4, induced only a small, but insignificant, increase in the sEPSC rate when applied in the absence of APB. This suggests that the presynaptic mGluR is not highly activated in light-adapted retinal slices when the vesicular release rate, and hence free glutamate, is low. Whether mGluR activation is higher in the dark-adapted condition, when vesicular glutamate release is high, remains to be determined.
Acknowledgments
We thank Professor Gertrude Falk for valuable discussions.
We also thank the Wellcome Trust and the Science and Technology Agency of Japan for financial support (Project: Studies on the controlling factors for regeneration of the retinal circuitry and the optic nerve).
Footnotes
The present address of Dr. H. Hirasawa is Department of Physiology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku, Tokyo 160-8582, Japan.
Abbreviations used in this paper: 4-AP, 4-aminopyridine; CNQX, 6-cyano-7-nitroquinoxaline; HC, horizontal cell; L-APB or L-AP4, (l)-2-amino-4-phosphonobutyric acid; mGluR, metabotropic glutamate receptor; sEPSC, spontaneous excitatory postsynaptic current.
References
- Akopian A., Johnson J., Gabriel R., Brecha N., Witkovsky P. Somatostatin modulated voltage-gated K+ and Ca2+ currents in rod and cone photoreceptors of the salamander retina. J. Neurosci. 2000;20:929–936. doi: 10.1523/JNEUROSCI.20-03-00929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptorselectrophysiological properties and role in plasticity. Brain Res. Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Ashmore J.F., Copenhagen D.R. An analysis of transmission from cones to hyperpolarizing bipolar cells in the retina of the turtle. J. Physiol. 1983;340:569–597. doi: 10.1113/jphysiol.1983.sp014781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani G.B., Slaughter M.M. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J. Neurosci. 2001;21:741–749. doi: 10.1523/JNEUROSCI.21-02-00741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J., Schoepp D.D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cochilla A.J., Alford S. Metabotropic glutamate receptor-mediated control of transmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Copenhagen D.R., Jahr C.E. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1988;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- de la Villa P., Kurahashi T., Kaneko A. l-Glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. J. Neurosci. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D.B., Copenhagen D.R. Metabotropic glutamate receptor-mediated suppression of an inward rectifier current is linked via a cGMP cascade. J. Neurosci. 1997;17:8945–8954. doi: 10.1523/JNEUROSCI.17-23-08945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamgoz M.B.A. Electrophysiological characterization of the spectral sensitivities of horizontal cells in cyprinid fish retina. Vision Res. 1984;24:1677–1687. doi: 10.1016/0042-6989(84)90326-2. [DOI] [PubMed] [Google Scholar]
- Dolphin A.C. Mechanisms of modulation of voltage-dependent calcium channels by G-proteins. J. Physiol. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J.E., Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973;292:101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- Grant G.B., Werblin F.S. A glutamate-elicited chloride current with transporter-like properties in rod photoreceptors of the tiger salamander. Visual Neurosci. 1996;13:135–144. doi: 10.1017/s0952523800007185. [DOI] [PubMed] [Google Scholar]
- Hare W.A., Owen W.G. Effects of 2-amino-4-phosphonobutyric acid on cells in the distal layers of the tiger salamander's retina. J. Physiol. 1992;445:741–757. doi: 10.1113/jphysiol.1992.sp018948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Momiyama A., Takahashi T., Oishi H., Ogawa-Meguro R., Shigemoto R., Mizuno N., Nakanishi S. Role of metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- Hirasawa H., Shiells R.A., Yamada M. Analysis of spontaneous EPSCs in retinal horizontal cells of the carp Neurosci. Res. 40 2001. 47 86a [DOI] [PubMed] [Google Scholar]
- Hirasawa H., Shiells R.A., Yamada M. Blocking AMPA receptor desensitization prolongs spontaneous EPSC decay times and depolarizes H1 horizontal cells in carp retinal slices Neurosci. Res. 40 2001. 217 225b [DOI] [PubMed] [Google Scholar]
- Kaneko A., Yamada M. S-potentials in the dark-adapted retina of the carp. J. Physiol. 1972;227:261–273. doi: 10.1113/jphysiol.1972.sp010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Effects of external ions on the synaptic transmission from photoreceptors to horizontal cells in the carp retina. J. Physiol. 1975;252:509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöpfel T., Lukic S., Leonardt T., Flour P.J., Kuhn R., Gasparini F. Pharmaco-logical characterization of MCCG and MAP4 at the mGluR1b, mGluR2 and mGluR4a human metabotropic glutamate receptor subtypes. Neuropharmacology. 1995;34:1099–1102. doi: 10.1016/0028-3908(95)00111-i. [DOI] [PubMed] [Google Scholar]
- Koulen P., Kuhn R., Wässele H., Brandstätter J.H. Modulation of intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc. Natl. Acad. Sci. USA. 1999;96:9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn C.L., Gafka A.C. Activation of metabotropic glutamate receptors modulates the voltage-gated sustained calcium current in a teleost horizontal cell. J. Neurophysiol. 1999;81:425–434. doi: 10.1152/jn.1999.81.2.425. [DOI] [PubMed] [Google Scholar]
- Lu T., Shen Y., Yang X.-L. Desensitization of AMPA receptors on horizontal cells isolated from crucian carp retina. Neurosci. Res. 1998;31:123–135. doi: 10.1016/s0168-0102(98)00031-5. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Iwakabe H., Akazawa C., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for l-2-amino-4-phosphonobutyrate. J. Biol. Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J. Neurosci. 1999;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S., Jahr C.E. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;325:967–972. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nawy S., Sie A., Copenhagen D.R. The glutamate analog 2-amino-4-phosphonobutyrate antagonizes synaptic transmission from cones to horizontal cells in the goldfish retina. Proc. Natl. Acad. Sci. USA. 1989;86:1726–1730. doi: 10.1073/pnas.86.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reike F., Schwartz E.A. Asynchronous transmitter releasecontrol of exocytosis and endocytosis at the salamander rod synapse. J. Physiol. 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis M., Everett K., Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature. 1988;332:451–453. doi: 10.1038/332451a0. [DOI] [PubMed] [Google Scholar]
- Saugstad J.A., Kinzie J.M., Shinohara M.M., Segerson T.P., Westbrook G.L. Cloning and expression of rat metabotropic glutamate receptor 8 reveals an distinct pharmacological profile. Mol. Pharmacol. 1997;51:119–125. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- Savchenko A., Barnes S., Kraner R. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390:694–698. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M., Gahwiler B.H., Thompson S.M. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampusare Ca2+ channels involved? Neuropharmacology. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Shiells R.A. Glutamate receptors for signal amplification. Curr. Biol. 1994;4:917–918. doi: 10.1016/s0960-9822(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Shiells R.A., Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc. R. Soc. Lond. Ser. B. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells R.A., Falk G. Signal transduction in retinal bipolar cells Progress in Retinal and Eye Res. N.N.Osborne and J. Chader, editors. Pergamon Press. 141995. 223 247 [Google Scholar]
- Shiells R.A., Falk G. Activation of Ca2+-calmodulin kinase II induces desensitization by background light in dogfish retinal ‘on’ bipolar cells. J. Physiol. 2000;528:327–338. doi: 10.1111/j.1469-7793.2000.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells R.A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294:592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Momiyama A., Takahashi T. Presynaptic inhibitory action of a metabotropic glutamate receptor agonist on excitatory transmission in visual cortical neurons. Proc. R. Soc. Lond. Ser. B. 1993;242:297–303. doi: 10.1098/rspb.1993.0117. [DOI] [PubMed] [Google Scholar]
- Stell W.K., Lightfoot D.O., Wheeler T.G, Leeper H.F. Goldfish retina; functional polarization of cone horizontal cell dendrites and synapses. Science. 1975;190:989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. l-glutamate-induced depolarization in solitary photoreceptorsa process that may contribute to the interaction between photoreceptors in situ. Proc. Natl. Acad. Sci. USA. 1988;85:5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K.-I., Copenhagen D.R. APB suppresses synaptic input to retinal horizontal cells in fisha direct action on horizontal cells modulated by intracellular pH. J. Neurophysiol. 1992;67:1633–1642. doi: 10.1152/jn.1992.67.6.1633. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Forsythe I.D., Tsujimoto T., Barnes-Davies M., Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Thoreson W.B., Gottesman J., Jane D.E., Tse H.-W., Watkins J.C., Miller R.F. Two phenylglycine derivatives antagonize responses to L-AP4 in On bipolar cells of the amphibian retina. Neuropharmacology. 1997;36:13–20. doi: 10.1016/s0028-3908(96)00164-5. [DOI] [PubMed] [Google Scholar]
- Vardi N., Duvoisin R., Wu G., Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J. Comp. Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Verweij J., Kamermans M., Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Werblin F.S. Transmission along and between rods in the tiger salamander retina. J. Physiol. 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Yasui S. Measurement of DC and AC spectral sensitivities of retinal horizontal cells by ‘voltage clamp by light’. J. Neurosci. Methods. 1988;24:65–72. doi: 10.1016/0165-0270(88)90034-9. [DOI] [PubMed] [Google Scholar]
- Yamada M., Fraser S.P., Furukawa T., Hirasawa H., Katano K., Djamgoz M.B.A., Yasui S. Effects of nitric oxide, light adaptation and APB on spectral characteristics of H1 horizontal cells in carp retina. Neurosci. Res. 1999;35:309–319. doi: 10.1016/s0168-0102(99)00094-2. [DOI] [PubMed] [Google Scholar]
- Yang J.H., Maple B., Gao F., Maguire G., Wu S. Postsynaptic responses of horizontal cells in the tiger salamander retina are mediated by AMPA-preferring receptors. Brain Res. 1998;797:125–134. doi: 10.1016/s0006-8993(98)00373-4. [DOI] [PubMed] [Google Scholar]
- Yasui S., Yamada M., Djamgoz M.B.A. Dopamine and 2-amino-4-phosphonobutyrate differentially affect spectral responses of H1 horizontal cells in carp retina. Exp. Brain Res. 1990;83:79–84. doi: 10.1007/BF00232195. [DOI] [PubMed] [Google Scholar]