Abstract
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is an enzyme of phagocytes that produces bactericidal superoxide anion (O2 −) via an electrogenic process. Proton efflux compensates for the charge movement across the cell membrane. The proton channel responsible for the H+ efflux was thought to be contained within the gp91phox subunit of NADPH oxidase, but recent data do not support this idea (DeCoursey, T.E., V.V. Cherny, D. Morgan, B.Z. Katz, and M.C. Dinauer. 2001. J. Biol. Chem. 276:36063–36066). In this study, we investigated electrophysiological properties and superoxide production of COS-7 cells transfected with all NADPH oxidase components required for enzyme function (COSphox). The 7D5 antibody, which detects an extracellular epitope of the gp91phox protein, labeled 96–98% of COSphox cells. NADPH oxidase was functional because COSphox (but not COSWT) cells stimulated by phorbol myristate acetate (PMA) or arachidonic acid (AA) produced superoxide anion. No proton currents were detected in either wild-type COS-7 cells (COSWT) or COSphox cells studied at pHo 7.0 and pHi 5.5 or 7.0. Anion currents that decayed at voltages positive to 40 mV were the only currents observed. PMA or AA did not elicit detectable H+ current in COSWT or COSphox cells. Therefore, gp91phox does not function as a proton channel in unstimulated cells or in activated cells with a demonstrably functional oxidase.
Keywords: phagocytes, gp91phox, H+ channels, superoxide, respiratory burst
INTRODUCTION
Nicotinamide adenine dinucleotide phosphate (NADPH)* oxidase is an important antibacterial enzyme of granulocytes that produces superoxide anion (Babior, 1999). This multicomponent complex comprises several cytosolic subunits and the membrane-bound cytochrome b 558, which is composed of two subunits (gp91phox and p22phox) that coordinate FAD and two heme moieties (Yu et al., 1998). Upon stimulation, the cytosolic components, p67phox, p47phox, and Rac, a small GTP binding protein, translocate to the membrane and interact with cytochrome b 558 (DeLeo and Quinn, 1996). The assembled complex catalyses the oxidation of NADPH and the transfer of electrons across the membrane to extracellular oxygen, which is an electrogenic process (Henderson et al., 1987). The enzyme can be activated by the phorbol ester PMA and by arachidonic acid (Babior, 1999). Voltage-gated proton currents are also greatly enhanced by these stimuli (DeCoursey et al., 2000, 2001a; Cherny et al., 2001). H+ efflux is thought to compensate electrically for electron movement through NADPH oxidase (Henderson et al., 1987, 1988).
It has been proposed that a proton channel exists within the gp91phox subunit of the NADPH oxidase. This conclusion was based originally on studies of CHO cells transfected with gp91phox in which H+ channel function was deduced from pH changes monitored by fluorescent pH dyes (Henderson et al., 1995, 1997; Henderson, 1998). Using only indirect measurements it is difficult to assess the existence and properties of H+ channels and to rule out the involvement of other mechanisms that transport H+ equivalents. Two recent studies provide direct voltage-clamp evidence of H+ currents in CHO, HEK-293, and COS-7 cells heterologously transfected with gp91phox (Henderson and Meech, 1999; Maturana et al., 2001). However, neither study is convincing. Transfection of gp91phox into HEK-293 cells simply increased the endogenous H+ currents fourfold (Maturana et al., 2001). The putative H+ currents in COS-7 cells transiently transfected with gp91phox and studied in whole-cell configuration (Maturana et al., 2001) appear to reverse >100 mV positive to the Nernst potential for H+, and thus were not proton selective. The currents in CHO cells reported by Henderson and Meech (1999) differ profoundly from H+ currents in all phagocytes studied to date: they are much less sensitive to inhibition by Zn2+, Zn2+ fails to slow τact, no H+ current was detectable at pHi 7.5 even though the currents at pHi 6.9 were larger than native voltage-gated proton currents reported in any cell, and activation was at least an order-of-magnitude more rapid.
Nanda et al. (1994) found normal voltage-gated proton currents in human monocytes from chronic granulomatous disease (CGD) patients who lacked cytochrome b 558, strong evidence that neither gp91phox nor p22phox is a proton channel in unstimulated phagocytes. We demonstrated recently that gp91phox does not mediate voltage-gated proton currents in the myelocytic PLB-985 cell line (DeCoursey et al., 2001b). Upon induction by dimethylformamide, PLB-985 cells express functional NADPH oxidase and are capable of producing superoxide anions (Zhen et al., 1993). PLB-985 cells in which gp91phox was knocked out by gene targeting had large voltage-gated proton currents that were normal in every respect. Furthermore, PMA stimulation increased the H+ conductance to a similar extent in wild-type (WT) and knockout cells. Similar results were obtained in neutrophils from CGD patients lacking gp91phox expression. Therefore, gp91phox does not mediate H+ currents in resting or activated cells. However, these results do not completely exclude the possibility that gp91phox might function as a proton channel. Because of the endogenous H+ currents in PLB-985 cells and CGD neutrophils, a small contribution by gp91phox might have been missed. Furthermore, one might propose that some other molecule might be up-regulated to compensate for the loss of gp91phox, as frequently occurs in genetic knockout studies (Yang et al., 2001). Therefore we performed the converse of the knockout experiment by introducing gp91phox into COS-7 cells, which have no endogenous H+ currents (Maturana et al., 2001). Although the goal of this study was to search for proton currents, there were large anion currents in most COS-7 cells and these are described briefly. We report here that neither WT COS-7 cells (COSWT) nor COS-7 cells transfected with the four main NADPH oxidase components, including gp91phox (COSphox), have detectable voltage-gated proton currents, nor were any induced by PMA stimulation. We conclude that under conditions in which NADPH oxidase activity can be demonstrated, gp91phox does not function as a proton channel.
MATERIALS AND METHODS
Cell Preparation and Maintenance
Green monkey kidney cells (COSWT) were obtained from American Type Culture Collection (CRL-1651). COS-7 cells stably transfected with the four main subunits of NADPH oxidase: gp91phox, p22phox, p47phox, and p67phox(COSphox cells) were developed by Price and colleagues (Price et al., 2002). First, COS-7 cells were stably transfected with p22phox and gp91phox. These cells were then further modified by stable transfection with p47phox, and p67phox to produce COSphox cells. Cells were maintained in Dulbecco's MEM (Sigma-Aldrich) as described previously (Yu et al., 1997), with the exception that media for COSphox cells also contained three antibiotics to ensure selection of transformed cells: 2 μg ml−1 puromycin (Sigma-Aldrich), 0.8 mg ml−1 G418 (Sigma-Aldrich), and 175 μg ml−1 hygromycin (Sigma-Aldrich). COSphox cells were split when 70% confluent. COS-7 cells are adherent and were harvested by incubation with 0.25% trypsin in 1 mM EDTA (GIBCO-BRL) for 5 min at 37°C. For electrophysiologic recordings, cells were allowed to attach to small glass coverslips. The cells were kept in suspension for the cytochrome c assays.
Electrophysiology
Whole cell and permeabilized patch recordings were performed as described previously (Cherny and DeCoursey, 1999; DeCoursey et al., 2000) using micropipettes pulled from 7052 glass (Garner Glass). Although COS cells tend to flatten in culture, we selected rounded cells for recording, because voltage-clamp control is better and because the superoxide assays were done with nonadherent (and hence rounded) cells. Some cells were nearly spherical and others were irregular in shape; we did not attempt to record from flat cells. The cell diameter was ∼15–25 μm, and the capacity in whole-cell measurements was 14.4 ± 4.5 pF (mean ± SD) in 20 sequentially studied COSphox cells. Whole-cell solutions (pipette and bath solutions) contained 100 mM buffer, 1 mM EGTA, 70 mM tetramethylammonium (TMA+) methanesulfonate (MeSO3 −), and 2 mM MgCl2 or 3 mM CaCl2, with an osmolarity of ∼300 mOsm. Buffers were Mes for pH 5.5 and BES (N,N-bis[2-Hydroxyethyl]-2-aminoethanesulfonic acid) for pH 7.0 solutions. In some experiments the Cl− concentration was increased by replacing TMAMeSO3 with TMACl or with Ringer's solution (in mM: 160 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, and 5 HEPES). Permeabilized patch recordings were performed in solutions containing 100 mM TMAMeSO3, 25 mM (NH4)2SO4, 2 mM MgCl2, 5 mM BES, 1 mM EGTA titrated to pH 7.0 with TMAOH. The purpose of the 50 mM NH4 + in the bath and pipette solutions was to clamp pHi near pHo (pH 7.0) (Grinstein et al., 1994; DeCoursey et al., 2000). Pipette solutions also contained 0.5–1 mg ml−1 amphotericin B (Sigma-Aldrich). Pipettes were filled after dipping the tip in amphotericin free solution. Experiments were done at 21°C or at room temperature (21–24°C). Currents were amplified using either Axopatch 200B (Axon Instruments, Inc.) or List EPC-7 (List Electronic) amplifiers and filtered at 100 or 200 Hz. PClamp8 (Axon Instruments, Inc.) or lab-generated software and an Indec Laboratory acquisition and display system (Indec Corporation) were used for data acquisition and manipulation.
Superoxide Anion (O2 −) Production
Cells were harvested by incubating the adherent cells in trypsin/EGTA at 5°C for 10 min. They were centrifuged for 5 min at 2,000 g and resuspended in Hanks' Balanced Salt Solution (HBSS) without phenol red at 5 × 106 cells ml−1. The assays were performed by incubating cells (2.5 × 105 cells well−1) at 37°C in 250 μl HBSS containing 75 μM cytochrome c. Kinetic assays were performed on a Ceres UV900 plate reader (Biotek Instruments) and superoxide production was quantified using an extinction coefficient of 21.1 mM cm−1 for cytochrome c. The superoxide assays were performed on the same batches of cells and during the same time period that the electrophysiological studies were done.
7D5 Antibody Staining
Cells were harvested by brief trypsinization, pelleted (1,200 rpm for 6 min at 4°C), resuspended in PBS as a wash, and pelleted. Cells were resuspended in PBS containing 10% normal goat serum and incubated for 30 min on ice to block nonspecific binding sites. Cells were pelleted again then resuspended in PBS at 107 cells/ml. One aliquot of cells (100 μl) was incubated with 100 μl 7D5 supernatant for 30 min on ice while a second aliquot was incubated with 2 μl of isotype control CD-117 antibody. PBS containing 0.1% BSA (1 ml) was added as a wash and the cells were pelleted. Cells were resuspended in PBS and incubated for 30 min on ice with FITC conjugated goat-anti-mouse secondary antibody (1:100 dilution). PBS containing 0.1% BSA (1 ml) was added as a wash, and the cells were pelleted and then resuspended in 500 μl PBS for fluorescence-activated cell sorter analysis using a FACSCalibur machine (Becton Dickinson) equipped with CellQuest software. A total of 10,000 cells were counted for each condition. Cells with very low fluorescence (comprising ≤7% of the total, and falling below the lower limit of the graph) were considered dead and were gated out. Staining with 7D5 was compared with staining with isotype control. Also, levels of 7D5 staining were compared in COSphox cells and COSWT cells.
RESULTS
COS-7 Cells Have Cl− Currents
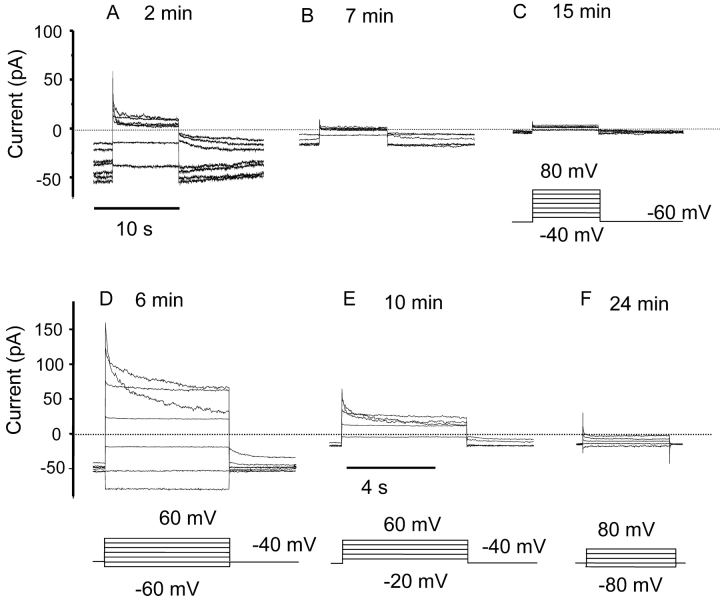
COSWT and COSphox cells were studied in whole-cell configuration in solutions lacking most permeant ions at pHi 5.5 and pHo 7, conditions favorable for detecting proton currents. Many cells appeared to become “leaky” within a few seconds of breaking the membrane patch. This “leak” reflected the appearance of a large time-independent conductance at the holding potential (–60 mV) and at all potentials negative to 40 mV. The properties of this conductance were similar in COSWT (Fig. 1, A–C) and COSphox cells (Fig. 1, D–F). During large depolarizing pulses the currents turned off with time. This decay reflected a genuine decrease in conductance because it was reversed upon repolarization; the inward currents were initially small and turned on with time. As evident in Fig. 1, the conductance decreased progressively after establishing whole-cell configuration in both COSWT and COSphox cells. The rundown produces a progressive decrease in holding current that is evident in some of the families. Eventually, only very small time-independent currents remained (Fig. 1, C and F).
Figure 1.
Rundown of whole-cell currents in COSWT (A–C) and COSphox (D–F) cells. Families of currents were recorded at various times after establishing whole cell configuration at pHi 5.5 and pHo 7.0 in TMAMeSO3 solutions. Insets illustrate the voltage pulses, which were applied at an interval of 15 s. Results are representative of 6 COSWT cells and 22 COSphox cells. The current at the holding potential decreased progressively during most of the families of pulses illustrated.
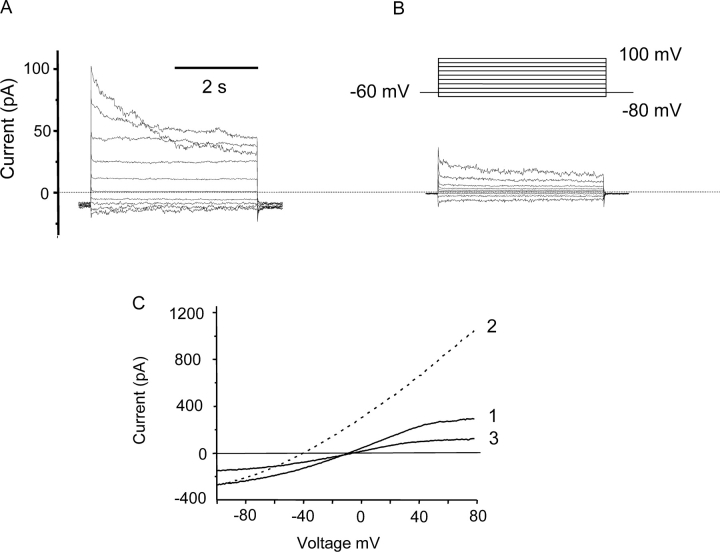
The behavior of the currents in COSWT and COSphox cells, including the rundown phenomenon, was reminiscent of Cl− currents in Chinese hamster ovary cells studied in similar solutions (Cherny et al., 1997). These currents resemble volume-regulated anion currents in several other epithelial cell types (McCann et al., 1989; Solc and Wine, 1991; Arreola et al., 1995). Fig. 2 shows the inhibition of whole-cell currents in a COSphox cell by the traditional Cl− channel blocker 4-acetamido-4'-isothiocyanostilbene-2,2'-disulfonic acid (SITS). The conductance decreased substantially at all potentials upon addition of SITS (Fig. 2 B), and was at least partially restored after wash out (unpublished data). To confirm the Cl− selectivity of the conductance we ramped the voltage from −100 to 80 mV in solutions with different Cl− concentrations (Fig. 2 C). Trace 1 shows ramp currents in symmetrical 5 mM Cl− where the reversal potential (Vrev) was near 0 mV. Substituting the bath solution with 166 mM Cl− increased the outward current at depolarizing voltages and shifted Vrev negative by 31 mV. Upon returning to the original solution, Vrev shifted back and the currents were reduced below the original levels, probably due to rundown (compare Fig. 1). The shift in Vrev is consistent with a relative permeability of ∼0.5 for MeSO3 − compared with Cl−, calculated by the Goldman-Hodgkin-Katz voltage equation after correction for liquid junction potentials. The outward current increased consistently in high [Cl−]. The shift in Vrev was often less pronounced than in Fig. 2 C, but the shift in Vrev was larger when the anion conductance was large (i.e., shortly after establishing whole cell configuration). The predominant conductance in COS-7 cells was anion selective.
Figure 2.
Characterization of the anion currents in COSphox cells. Whole-cell current families are shown in a COSphox cell in the absence (A) or in the presence (B) of the Cl− channel blocker SITS (1 mM). Identical voltage pulse families were applied (inset), with an interval of 12 s at V hold between pulses. (C) Currents recorded during voltage ramps from –100 to 80 mV in a COSphox cell at 5 mM Cl− (solid curves) or 166 mM Cl− (dashed curve), labeled in the order recorded. Records 1, 2, and 3 were obtained ∼9, 11, and 12 min after establishing whole-cell configuration, respectively.
COS-7 Cells Lack H+ Currents
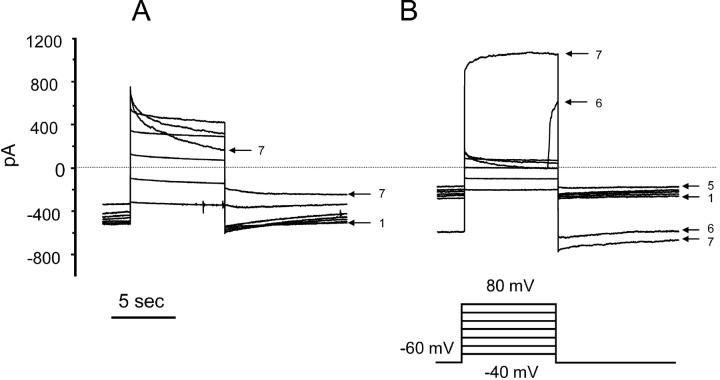
To identify putative voltage-gated proton currents, we waited for the anion conductance to subside and then looked for currents that increased with time during depolarizing pulses. In some cells time-dependent outward currents were seen, although typically this behavior was observed only after the input resistance had decreased markedly. An example is shown in Fig. 3. Normal behavior was observed early in this experiment on a COSWT cell. The family of currents in Fig. 3 A was recorded starting 4 min after whole-cell configuration was established. Anion currents that are time independent except at large positive voltages were observed. The current at V hold = −60 mV decreased progressively, in part because of progression of the rundown of anion currents during the 3.5 min required to record this family of long pulses. After the pulses to 60 and 80 mV that elicited decaying outward currents, the inward current upon repolarization was greatly reduced, reflecting the channel closing at positive voltages that is responsible for the current decay. The family of currents in Fig. 3 B was recorded in the same cell immediately after the previous family. By this time, the anion conductance had run down substantially and the currents at all voltages were scaled down, but otherwise exhibited similar time and voltage dependence. Near the end of the pulse to 60 mV (pulse 6), the current suddenly increased. The current at V hold increased as well, and no recovery was observed before the next pulse, to 80 mV. During this last pulse (pulse 7), the current was much larger and there was a time-dependent increase in outward current during the pulse. Under normal circumstances, we would interpret the sudden increase in conductance to indicate damage to the cell membrane or loss of the seal. Such cells are usually considered to be “dead” and are discarded. However, because the time-dependent increasing outward currents superficially resemble voltage-gated proton currents, we continued recording. In cells with currents like these, changing pHo did not change either V rev or the threshold voltage for activating time-dependent outward current. These currents thus were not H+ selective, nor did they display the pronounced pHo sensitivity of gating of all voltage-gated proton channels (Eder and DeCoursey, 2001). Addition of 100 μM ZnCl2 had no effect on the amplitude or kinetics of these currents. In summary, there was no credible evidence of voltage-gated proton currents in any COSWT or COSphox cell studied.
Figure 3.
Sudden appearance of time-dependent outward current in a COSWT cell, studied at pHo 7.0 and pHi 5.5. (A) A family of currents recorded starting 4 min after establishing whole-cell configuration displays behavior typical of the anion conductance. The pulses were applied in 20-mV increments up to 80 mV, at 30-s intervals. The entire family required ∼3.5 min to complete. The first and last pulses are numbered, showing the progressive decrease in current at −60 mV. (B) A family recorded in the same cell shortly after the end of the previous family. The currents at all voltages including V hold are scaled down, reflecting the rundown typically observed in these cells. In this cell during the pulse to 60 mV (pulse 6), there was a sudden catastrophic increase in leak current. The inward current upon repolarization was greatly increased. Interpolation of the current at the end of the pulse to 60 mV and that immediately after repolarization to −60 mV gives an estimated V rev of 4 mV, suggesting a nonselective leak conductance. The subsequent pulse to 80 mV elicited an outward current that increased with time. Subsequent pulse families evoked time-independent leak current at all voltages, with a time-dependent increasing current at large positive voltages. Changing pHo from 7.0 to 5.5 did not shift V threshold or elicit changes in gating kinetics normally seen in cells with voltage-gated H+ currents. Addition of 100 μM ZnCl2 did not inhibit the time-dependent outward currents. Although such currents bear a superficial resemblance to voltage-gated proton currents, they are artifacts.
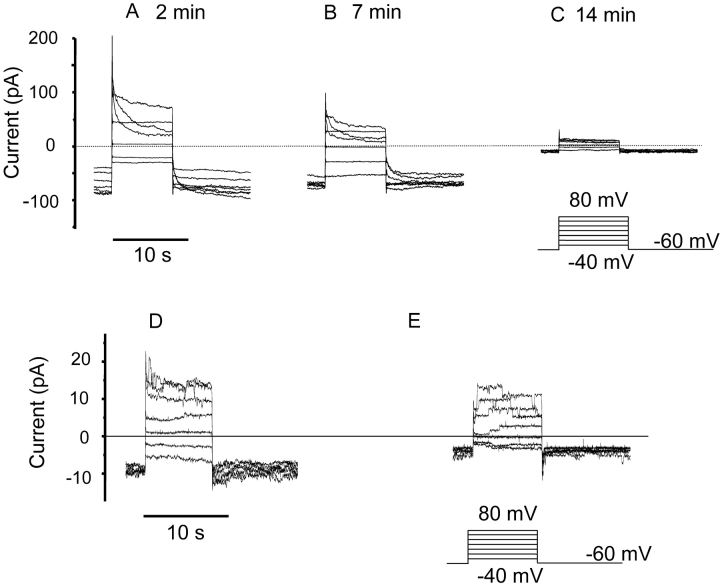
To investigate whether stimuli that activate NADPH oxidase might induce or enhance proton currents, as has been observed in human neutrophils and eosinophils (DeCoursey et al., 2000, 2001a; Cherny et al., 2001) we used the permeabilized patch configuration, which preserves NADPH oxidase function (DeCoursey et al., 2000). Many unstimulated COSphox cells in permeabilized patch configuration exhibited Cl− currents that disappeared with time (Fig. 4, A–C), similar to the currents in whole-cell studies. Increasing [Cl−]o increased the outward current and tended to shift Vrev to more negative voltages (unpublished data). No time-dependent currents were seen in COSphox cells after the Cl− currents ran down. In about one-third of COSphox cells the anion conductance was small or absent throughout the experiment, and the currents were similar to those in Fig. 4 C.
Figure 4.
Lack of effect of PMA on membrane currents COSphox cells in permeabilized patch configuration. (A–C) Families of voltage-clamp currents in a COSphox cell in permeabilized patch configuration at the indicated times after beginning recording. The bath solution contained TMAMeSO3 at pH 7.0, and both pipette and bath contained 50 mM NH4 + to clamp pHi near pHo. Currents at high gain in a COSphox cell before (D) and 10 min after (E) addition of 60 nM PMA. No voltage-gated conductance was activated by PMA or AA in 45 cells studied in permeabilized patch configuration.
Fig. 4, D and E, respectively, show families of currents at high gain before and 10 min after the addition of 60 nM PMA. No time-dependent currents appeared at any voltage after the addition of PMA in this cell. In Fig. 4, D and E, discrete current steps consistent with a single channel conductance of ∼30 pS can be seen at some voltages. These currents are most likely conducted through anion channels similar to volume-sensitive anion channels reported previously (Solc and Wine, 1991). Single voltage-gated proton channel currents are too small to record directly (Byerly and Suen, 1989; DeCoursey and Cherny, 1993). Furthermore, 100 μM Zn2+ did not inhibit any component of the currents in PMA-treated COSphox cells. Thus, both unstimulated (31 cells studied in whole-cell or permeabilized-patch configuration) and PMA- or AA-stimulated COSphox cells (n = 45) lack detectable proton currents.
COSphox Cells Express gp91phox and Produce Superoxide Anion (O2 −)
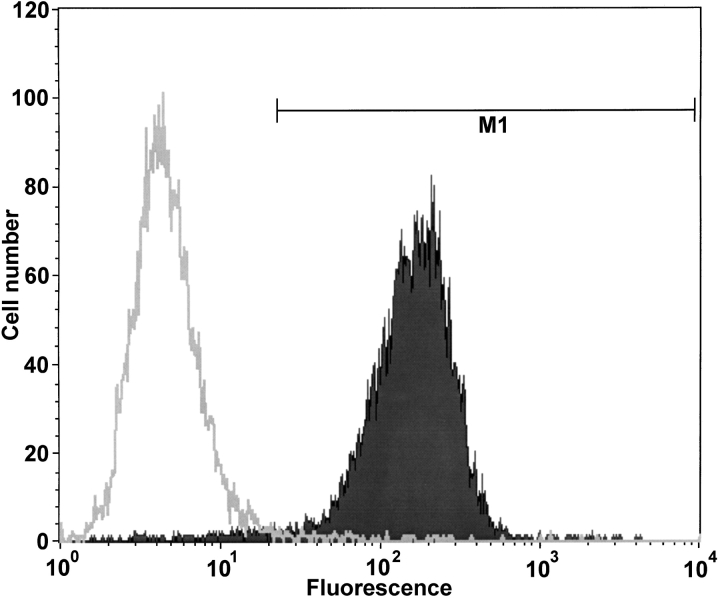
To assess the efficiency of gp91phox expression in COSphox cells, we used the 7D5 antibody, which is directed against an extracellular epitope of the gp91phox molecule (Nakamura et al., 1988; Yu et al., 1998). Expression was tested in freshly thawed cells and passaged cells, in COSWT and COSphox cells, and with the CD-117 antibody as an isotype control. Fig. 5 illustrates histograms of the fluorescence intensity in COSWT (open histogram) and COSphox cells (solid histogram). 98% of the freshly thawed COSphox cells (Fig. 5) and 96% of COSphox cells maintained in culture for several weeks (unpublished data), as during the course of these experiments, stained positively for the 7D5 antibody. If cells with very low fluorescence (which presumably are dead cells) are included, then the 7D5-positive fraction is 95% of the freshly thawed COSphox cells and 93% of COSphox cells maintained in culture. Thus, the vast majority of COSphox cells express gp91phox at the plasma membrane.
Figure 5.
7D5 antibody staining of COSphox cells compared with COSWT cells. Cells harvested by brief trypsinization were incubated with 7D5 monoclonal antibody, which recognizes an extracellular epitope of gp91phox. Following incubation with a FITC-conjugated goat anti–mouse secondary antibody, cells were analyzed by flow cytometry. The marker M1 was set to exclude 99% of the COSWT cells (open histogram), and indicates the range defined as positively stained. By this criterion, 98.3% of the COSphox cells (solid histogram) were positively stained for gp91phox expression.
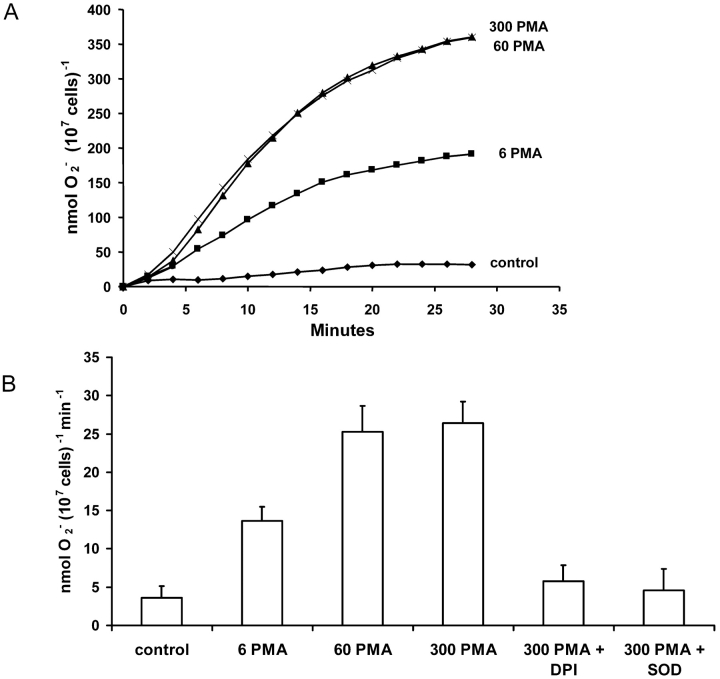
Superoxide anion (O2 −) production by COSphox cells was assessed by their ability to reduce ferricytochrome c. Fig. 6 A shows the average time courses of cumulative O2 − release by four batches of COSphox cells challenged with different concentrations of PMA. O2 − production above control levels was seen at 6 nM PMA. PMA at 60 and 300 nM, respectively, produced similar levels of O2 −. The O2 − release time course was characterized by a delay of several minutes after the addition of PMA, followed by a rapid increase between 5 and 20 min, after which the rate of O2 − production diminished. Fig. 6 B summarizes the average maximal rate of O2 − production in these experiments. The highest maximal rate, observed at 300 nM PMA, was 26 ± 6 nmol min−1 (107 cells)−1. The response was almost completely inhibited by 100 μg/ml superoxide dismutase (SOD), showing that ferricytochrome c reduction was due to the release of O2 − and not peroxides or other oxidizing agents. Diphenylene iodonium (DPI) at 6 μM also inhibited O2 − production, implicating a flavoenzyme (O'Donnell et al., 1994), most likely NADPH oxidase (Cross and Jones, 1986). As reported previously (Price et al., 2002), COSWT cells studied under identical conditions did not produce detectable O2 − (unpublished data).
Figure 6.
Superoxide production by COSphox cells. (A) The average time course of PMA-stimulated superoxide anion production detected as the reduction of cytochrome c is plotted. Cells (106 cells/ml) were incubated at 37°C in HBSS before the addition of PMA (concentrations of PMA are in nM). Control cells were not stimulated. Data are mean values from four experiments with the error bars removed for clarity. (B) The average maximum rate of O2 − production (± SE, n = 4) determined in each experiment in A. Data were collected in 2-min intervals and the rate is given per minute. DPI (6 μM) and 100 μg/ml SOD were added simultaneously with PMA to establish that the reduction of cytochrome c was due to NADPH oxidase and O2 −, respectively.
Arachidonic acid (AA) can stimulate COSphox cells to produce O2 − at a higher rate than that stimulated by PMA (Price et al., 2002). We examined the time course of AA-stimulated O2 − production in six assays (unpublished data). The onset of O2 − production stimulated by 10–100 μM AA was faster than when PMA was used. However, the AA-stimulated respiratory burst was more transient, with little additional O2 − released after 10–15 min, whereas PMA-stimulated O2 − release continued over 30 min.
Because NADPH oxidase is electrogenic (Henderson et al., 1987), its activity can be detected electrically as a small inward current under favorable conditions (Schrenzel et al., 1998; DeCoursey et al., 2000). Electron currents were difficult to detect in COSphox cells, probably because the activity of NADPH oxidase is lower than in human neutrophils (Price et al., 2002) and the baseline currents were larger and less stable. These measurements were made at 0 to −20 mV to minimize the leak (anion) current amplitude. No convincing electron currents were seen with PMA as a stimulus. Because the maximal rate of O2 − production in COSphox cells is larger with AA than PMA as a stimulus (Price et al., 2002) and the onset is more rapid (present study), we also used AA as an agonist. Addition of 5–10 μM AA led to an increase in inward current of 1–2 pA in about half the cells tested, but only a fraction of this current was inhibited by 6–12 μM DPI. Because AA can induce nonspecific membrane leak (Meves, 1994; Cherny et al., 2001), we consider only inward current inhibitable by DPI in the presence of AA to indicate electron current. The mean DPI-inhibited inward current in COSphox cells was −0.26 ± 0.10 pA (mean ± SE, n = 21). Although it is possible that these DPI-sensitive currents reflect genuine electron current, we conclude that the electron currents in COSphox cells are too small to detect reliably.
DISCUSSION
Although the goal of this study was to search for voltage-gated proton currents in cells expressing gp91phox, the only conductance found in COSphox cells was anion selective. Cl−-selective currents similar to volume-sensitive Cl− currents in many other epithelial cells (McCann et al., 1989; Solc and Wine 1991; Arreola et al., 1995; Cherny et al., 1997) were present shortly after establishing whole-cell configuration in most COSWT and COSphox cells. In an earlier study of CHO cells, small voltage-gated proton currents were revealed after the anion conductance ran down (Cherny et al., 1997), but in COSWT and COSphox cells no proton currents were ever observed. These results are consistent with the observation of Maturana et al. (2001) that COSWT cells lack endogenous proton channels.
We saw no H+ currents in COS-7 cells stably transfected with all four major components of the NADPH oxidase, including gp91phox (COSphox cells). In some COSphox and COSWT cells we saw outward currents that increased during large depolarizing pulses. These currents, seen mainly in “leaky” cells, were not inhibited by 100 μM Zn2+, a potent, classical inhibitor of voltage-gated proton channels (Cherny and DeCoursey, 1999), and neither V rev nor the threshold potential was sensitive to pHo, contrary to the properties of voltage-gated proton channels in all cells studied to date (Eder and DeCoursey, 2001). Thus, no credible evidence of voltage-gated proton currents was seen in any COSWT or COSphox cell. The maximum proton current in most phagocytes is ∼100 times greater than that required to fully compensate for the electron flux through NADPH oxidase (Eder and DeCoursey, 2001). If a comparable relationship existed in COSphox cells, one would predict ∼50 pA of proton current. Even a few picoamperes of H+ current would have been evident in COSphox cells after rundown of the anion conductance.
In previous studies, evidence of proton flux was reported when gp91phox was expressed without any other NADPH oxidase components in CHO, HEK-293, and COS-7 cells (Henderson et al., 1995, 1997; Henderson 1998; Henderson and Meech, 1999; Maturana et al., 2001). However, the putative proton currents reported in patch-clamp studies of gp91phox expressed in the CHO and COS-7 heterologous expression systems (Henderson and Meech, 1999; Maturana et al., 2001) differ significantly from proton currents endogenous to phagocytes (see introduction). It is conceivable that gp91phox expressed alone might function as an ion channel, and that this function is absent when it is coexpressed with p22phox. If so, then gp91phox does not function as an ion channel under physiological conditions, because gp91phox is detectable in phagocyte membranes only together with p22phox in the flavocytochrome b 558 heterodimer (Babior, 1999). The expression of gp91phox and p22phox are closely linked in neutrophils, where stable expression of either appears to be favored strongly by heterodimer formation, i.e., assembly to form flavocytochrome b 558 (Roos et al., 1996; Yu et al., 1997; DeLeo et al., 2000).
The COSphox cells in this study were transfected with all the components necessary for a fully functional oxidase. COSphox cells released significant O2 −, confirming that the oxidase was present in the plasma membrane and was functional. The maximum rate of PMA-stimulated O2 − production at 37°C was 26 nmol min−1(107 cells)−1, comparable with the rate we reported previously in these cells (Price et al., 2002). The minimum efficiency of simultaneous expression of all four NADPH oxidase components was 69%, because this fraction was nitroblue tetrazolium (NBT)-positive in COSphox cells (Price et al., 2002). NBT reduction indicates O2 − production, and all four NADPH oxidase components are required for this function (Babior, 1999). Expression of gp91phox in COSphox cells in the plasma membrane was confirmed by 7D5 antibody staining. The 7D5 antibody is directed against an extracellular epitope of gp91phox (Nakamura et al., 1988; Yu et al., 1998). That 96–98% of COSphox cells were stained positively by the 7D5 antibody indicates that essentially all cells expressed gp91phox at the surface membrane.
Although COSphox cells express a functional NADPH oxidase complex, attempts to detect electron currents that reflect the function of this enzyme were frustrated by the presence of background (leak) currents, and by the tiny amplitude of these currents. The maximum rate of O2 − production in COSphox cells is less than one-fourth that in human neutrophils stimulated with PMA (Price et al., 2002). The electron current averages only −2.3 pA in human neutrophils (DeCoursey et al., 2000) and can be detected only because the input resistance in neutrophils is very high (usually >70 GΩ), and the background leak current was small (usually −0.6 pA or less) and stable. If electron currents in COSphox were proportionately smaller they would be approximately −0.5 pA and consequently very difficult to detect; we did not detect electron currents in PMA-stimulated COSphox cells. AA also stimulates O2 − production in COSphox cells (Price et al., 2002). Although AA induced small inward currents in some COSphox cells, DPI reversed only a fraction of the current and thus the identification of these currents as genuine electron currents remains uncertain.
Bánfi et al. (1999) proposed that there were two types of H+ channels in granulocytes; one in the resting cells and another that is active only when NADPH oxidase is active. Using the permeabilized patch configuration allowed us to investigate whether activating the oxidase might induce the appearance of H+ currents in COSphox cells. We have shown previously (DeCoursey et al., 2000, 2001a,b) that PMA stimulation of human neutrophils or eosinophils or PLB-985 cells in these conditions results in H+ currents that closely resemble the NADPH oxidase–related H+ currents described by Bánfi et al. (1999). AA also greatly enhances voltage-gated proton currents (Cherny et al., 2001). No time-dependent currents appeared after addition of PMA or AA to COSphox cells. Evidently the enhancement of H+ currents during the respiratory burst occurs only in cells that express voltage-gated proton channels in the resting state. This pattern is consistent with the idea that respiratory burst agonists modify the properties of preexisting H+ channels (DeCoursey et al., 2000), rather than inducing the appearance of a new type of channel (Bánfi et al., 1999).
A curious point is that COSphox cells devoid of proton channels are still capable of producing substantial O2 −. The main function of voltage-gated proton channels in phagocytes is thought to be to provide a mechanism of charge compensation for the electron efflux through NADPH oxidase (Henderson et al., 1987; Eder and DeCoursey, 2001). Inhibiting H+ currents reduces superoxide production in human neutrophils (Henderson et al., 1988) and eosinophils (Bankers-Fulbright et al., 2001) and in PLB cells (Lowenthal and Levy, 1999). Because anion currents were prominent in COS-7 cells, it is tempting to speculate that Cl− influx may serve this function in lieu of H+ efflux. In preliminary experiments SITS did not inhibit O2 − release, but SITS did not completely inhibit Cl− current (Fig. 2 A). The anion channel inhibitor 4,4-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS) partially reduced O2 − production in eosinophils (Schwingshackl et al., 2000). Alternatively, COSphox cells may have some other mechanism of charge compensation.
Acknowledgments
The authors appreciate the able technical assistance of Tatiana Iastrebova.
This work was supported in part by the Heart, Lung, and Blood Institute of the National Institutes of Health (HL52671 and HL61437 to Dr. DeCoursey and HL45635 to Dr. Dinauer).
Portions of this work were previously published in abstract form (Morgan, D., V.V. Cherny, M.C. Dinauer, M.O. Price, and T.E. DeCoursey. 2002. Biophys. J. 82:625a).
Footnotes
Abbreviations used in this paper: AA, arachidonic acid; CGD, chronic granulomatous disease; DPI, diphenylene iodonium chloride; NADPH, nicotinamide adenine dinucleotide phosphate; pHi, intracellular pH; pHo, extracellular pH; PMA, phorbol 12-myristate 13-acetate; SITS, 4-acetamido-4-isothiocyanostilbene-2,2'-disulphonic acid; SOD, superoxide dismutase; Vrev, reversal potential; WT, wild-type.
References
- Arreola, J., J.E. Melvin, and T. Begenisich. 1995. Volume-activated chloride channels in rat parotid acinar cells. J. Physiol. 484:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior, B.M. 1999. NADPH oxidase: an update. Blood. 93:1464–1476. [PubMed] [Google Scholar]
- Bánfi, B., J. Schrenzel, O. Nüsse, D.P. Lew, E. Ligeti, K.-H. Krause, and N. Demaurex. 1999. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J. Exp. Med. 190:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankers-Fulbright, J.L., H. Kita, G.J. Gleich, and S.M. O'Grady. 2001. Regulation of human eosinophil NADPH oxidase activity: a central role for PKCδ. J. Cell. Physiol. 189:306–315. [DOI] [PubMed] [Google Scholar]
- Byerly, L., and Y. Suen. 1989. Characterization of proton currents in neurones of the snail, Lymnaea stagnalis. J. Physiol. 413:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, V.V., and T.E. DeCoursey. 1999. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J. Gen. Physiol. 114:819–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, V.V., L.M. Henderson, and T.E. DeCoursey. 1997. Proton and chloride currents in Chinese hamster ovary cells. Membr. Cell Biol. 11:337–347. [PubMed] [Google Scholar]
- Cherny, V.V., L.M. Henderson, W. Xu, L.L. Thomas, and T.E. DeCoursey. 2001. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J. Physiol. 535:783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, A.R., and O.T.G. Jones. 1986. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., and V.V. Cherny. 1993. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 65:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., V.V. Cherny, W. Zhou, and L.L. Thomas. 2000. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc. Natl. Acad. Sci. USA. 97:6885–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., V.V. Cherny, A.G. DeCoursey, W. Xu, and L.L. Thomas. 2001. a. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J. Physiol. 535:767–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., V.V. Cherny, D. Morgan, B.Z. Katz, and M.C. Dinauer. 2001. b. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J. Biol. Chem. 276:36063–36066. [DOI] [PubMed] [Google Scholar]
- DeLeo, F.R., and M.T. Quinn. 1996. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase components. J. Leukoc. Biol. 60:677–691. [DOI] [PubMed] [Google Scholar]
- DeLeo, F.R., J.B. Burritt, L. Yu, A.J. Jesaitis, M.C. Dinauer, and W.M. Nauseef. 2000. Processing and maturation of flavocytochrome b 558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 275:13986–13993. [DOI] [PubMed] [Google Scholar]
- Eder, C., and T.E. DeCoursey. 2001. Voltage-gated proton channels in microglia. Prog. Neurobiol. 64:277–305. [DOI] [PubMed] [Google Scholar]
- Grinstein, S., R. Romanek, and O.D. Rotstein. 1994. Method for manipulation of cytosolic pH in cells clamped in the whole cell or perforated-patch configurations. Am. J. Physiol. 267:C1152-C1159. [DOI] [PubMed] [Google Scholar]
- Henderson, L.M. 1998. Role of histidines identified by mutagenesis in the NADPH oxidase-associated H+ channel. J. Biol. Chem. 273:33216–33223. [DOI] [PubMed] [Google Scholar]
- Henderson, L.M., G. Banting, and J.B. Chappell. 1995. The arachidonate-activatable, NADPH oxidase-associated H+ channel: evidence that gp91-phox functions as an essential part of the channel. J. Biol. Chem. 270:5909–5916. [PubMed] [Google Scholar]
- Henderson, L.M., J.B. Chappell, and O.T.G. Jones. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., J.B. Chappell, and O.T.G. Jones. 1988. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 255:285–290. [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., and R.W. Meech. 1999. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 114:771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., S. Thomas, G. Banting, and J.B. Chappell. 1997. The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91-phox. Biochem. J. 325:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal, A., and R. Levy. 1999. Essential requirement of cytosolic phospholipase A2 for activation of the H+ channel in phagocyte-like cells. J. Biol. Chem. 274:21603–21608. [DOI] [PubMed] [Google Scholar]
- Maturana, A., S. Arnaudeau, S. Ryser, B. Bánfi, J.P. Hossle, W. Schlegel, K.-H. Krause, and N. Demaurex. 2001. Heme histidine ligands within gp91phox modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 276:30277–30284. [DOI] [PubMed] [Google Scholar]
- McCann, J.D., M. Li, and M.J. Welsh. 1989. Identification and regulation of whole-cell chloride currents in airway epithelium. J. Gen. Physiol. 94:1015–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves, H. 1994. Modulation of ion channels by arachidonic acid. Prog. Neurobiol. 43:175–186. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., S. Kobayashi, S. Sendo, T. Koga, and S. Kanegasaki. 1988. Deficiency of cytochrome b558 in chronic granulomatous disease demonstrated by monoclonal antibody 7D5. Acta Paediatr. Hung. 29:179–183. [PubMed] [Google Scholar]
- Nanda, A., R. Romanek, J.T. Curnutte, and S. Grinstein. 1994. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J. Biol. Chem. 269:27280–27285. [PubMed] [Google Scholar]
- O'Donnell, V.B., G.C.M. Smith, and O.T.G. Jones. 1994. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol. Pharmacol. 46:778–785. [PubMed] [Google Scholar]
- Price, M.O., L.C. McPhail, J.D. Lambeth, C.-H. Han, U.G. Knaus, and M.C. Dinauer. 2002. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 99:2653–2661. [DOI] [PubMed] [Google Scholar]
- Roos, D., M. de Boer, F. Kuribayashi, C. Meischl, R.S. Weening, A.W. Segal, A. Åhlin, K. Nemet, J.P. Hossle, E. Bernatowska-Matuszkiewicz, and H. Middleton-Price. 1996. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 87:1663–1681. [PubMed] [Google Scholar]
- Schrenzel, J., L. Serrander, B. Bánfi, O. Nüsse, R. Fouyouzi, D.P. Lew, N. Demaurex, and K.-H. Krause. 1998. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 392:734–737. [DOI] [PubMed] [Google Scholar]
- Schwingshackl, A., R. Moqbel, and M. Duszyk. 2000. Involvement of ion channels in human eosinophil respiratory burst. J. Allergy Clin. Immunol. 106:272–279. [DOI] [PubMed] [Google Scholar]
- Solc, C.K., and J.J. Wine. 1991. Swelling-induced and depolarization-induced Cl− channels in normal and cystic fibrosis epithelial cells. Am. J. Physiol. 261:C658-C674. [DOI] [PubMed] [Google Scholar]
- Yang, S., P. Madyastha, S. Bingel, W. Ries, and L. Key. 2001. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 276:5452–5458. [DOI] [PubMed] [Google Scholar]
- Yu, L., L. Zhen, and M.C. Dinauer. 1997. Biosynthesis of the phagocyte NADPH oxidase cytochrome b 558: role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J. Biol. Chem. 272:27288–27294. [DOI] [PubMed] [Google Scholar]
- Yu, L., M.T. Quinn, A.R. Cross, and M.C. Dinauer. 1998. Gp91phox is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. USA. 95:7993–7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, L., A.A. King, Y. Xiao, S.J. Chanock, S.H. Orkin, and M.C. Dinauer. 1993. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc. Natl. Acad. Sci. USA. 90:9832–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]