Abstract
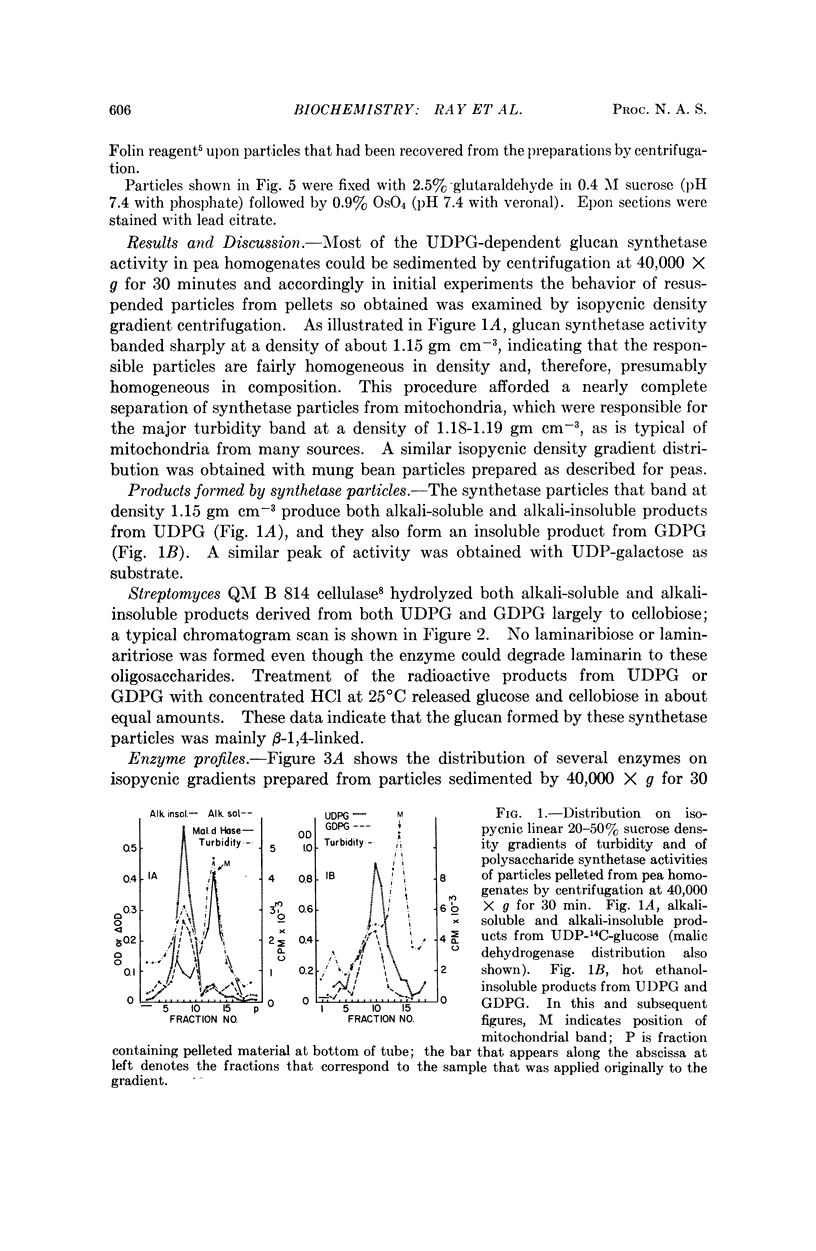
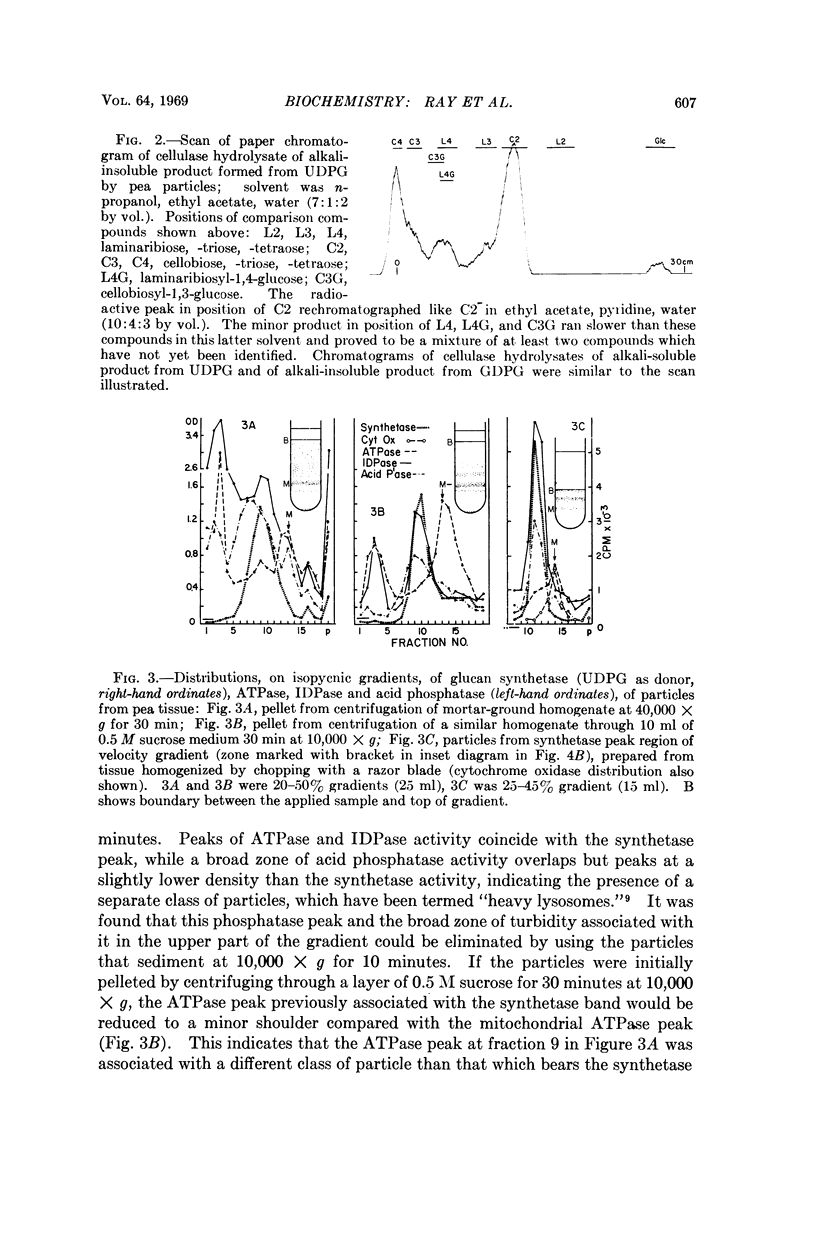
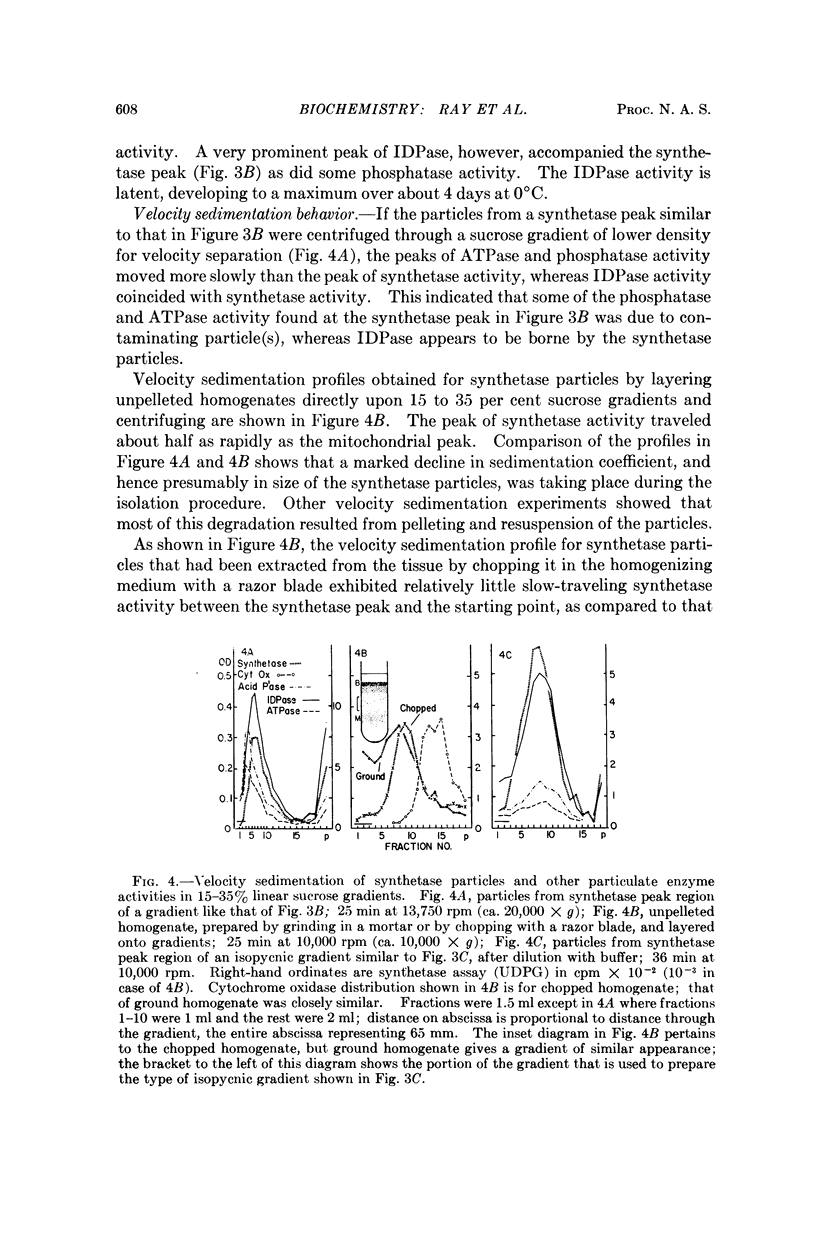
A variety of particle-bound synthetases that use sugar nucleotides as glycosyl donors for the formation of polysaccharides similar to those of the cell wall have been demonstrated in mung beans and other plant tissues,1 but the particles in question have not been previously identified.2, 3 The polysaccharide synthetase particles from peas that form mainly β-1,4-glucan from UDPG and GDPG have now been separated from other cell particles by combinations of velocity and isopycnic density gradient centrifugation. The particles have an effective density of about 1.15 gm cm-3, exhibit latent nucleoside diphosphatase activity upon IDP, UDP, GDP, and to a lesser extent upon ADP, and also possess acid phosphatase and weak ATPase activity. The isolated synthetase particles consist of somewhat condensed Golgi dictyosomes and free dictyosomal membranes bearing vesicles. It is concluded that the synthetase particles are Golgi membranes. The nucleoside diphosphatase activity of these particles may represent inactivated polysaccharide synthetase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dauwalder M., Whaley W. G., Kephart J. E. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969 Mar;4(2):455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Biosynthesis of a cell wall glucomannan in mung bean seedlings. J Biol Chem. 1969 Mar 25;244(6):1608–1616. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H., CHAMBERS J. E. GLUTARALDEHYDE STABILIZATION AS AN AID TO GOLGI APPARATUS ISOLATION. Exp Cell Res. 1965 Jun;38:672–675. doi: 10.1016/0014-4827(65)90392-7. [DOI] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H. ISOLATION OF THE GOLGI APPARATUS FROM PLANT CELLS. J Cell Biol. 1964 Nov;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps J. D. Further observations on the Golgi apparatus and its functions in cells of the wheat seedling. J Ultrastruct Res. 1967 May;18(3):287–303. doi: 10.1016/s0022-5320(67)80119-9. [DOI] [PubMed] [Google Scholar]
- REESE E. T., SMAKULA E., PERLIN A. S. Enzymic production of cellotriose from cellulose. Arch Biochem Biophys. 1959 Nov;85:171–175. doi: 10.1016/0003-9861(59)90460-6. [DOI] [PubMed] [Google Scholar]




