Abstract
Cell-surface interactions play a crucial role for biomaterial application in orthopaedics. It is evident that not only the chemical composition of solid substances influence cellular adherence, migration, proliferation and differentiation but also the surface topography of a biomaterial. The progressive application of nanostructured surfaces in medicine has gained increasing interest to improve the cytocompatibility and osteointegration of orthopaedic implants. Therefore, the understanding of cell-surface interactions is of major interest for these substances. In this review, we elucidate the principle mechanisms of nano- and microscale cell-surface interactions in vitro for different cell types onto typical orthopaedic biomaterials such as titanium (Ti), cobalt-chrome-molybdenum (CoCrMo) alloys, stainless steel (SS), as well as synthetic polymers (UHMWPE, XLPE, PEEK, PLLA). In addition, effects of nano- and microscaled particles and their significance in orthopaedics were reviewed. The significance for the cytocompatibility of nanobiomaterials is discussed critically.
1. INTRODUCTION
Nanobiomaterials are characterized by constituent particles and/or surface features less than 100 nm in at least one dimension [1]. Starting with photolithography and dry etching in the 1980's to high-resolution electron beam lithography and other technologies in the 1990's, nanotechnology allows for making surface structures for cell engineering and has led to an increasing application in healthcare over the last decades.
Nanolayers are used to enhance the surface biocompatibility of polymeric drug delivery systems, control the release of substances such as antibiotics or growth factors [2], act as gene-delivery vehicles, or serve as robust light emitters for cellular labeling and tracking [semiconductor nanocrystals, quantum dots (QDs)] [3]. Nanotechnology is also applied to modify and improve the surface structure in orthopaedic implants to promote their osseous integration.
However, there are also side effects of nano- and microparticles in vivo. Micro- and nanoparticles released by friction of articulating partners from artificial joints are a major reason for aseptic implant loosening in orthopaedic surgery and may lead to severe peri-implant osteolysis (particle disease) [4]. In addition, nanoparticles can induce or promote allergic or inflammatory reactions or influence hemolysis and blood coagulation [5–7].
Although the cytocompatibility of a biomaterial is strongly influenced by its chemical composition, surface topography plays a crucial role for cell-surface interactions [8]. Material surface properties have been studied intensively, but still lack from reliable data about cytocompatibility. Especially, the superordinate principles of cellular responses to surfaces with a defined topography are not well known and poorly understood. Because many variables influence cellular interactions to surface structures, it is difficult to draw conclusions and formulate general principles for nano- and microstructured surfaces.
This review summarizes recent data of effects by nano- and microstructured biomaterials and particles in vitro designed for orthopaedic application to get a solid framework outlining the critical interactions that govern the cytocompatibility. Because biomaterials in orthopaedics are predominantly applied on bone, this review is focussed on the interactions of osteoblasts and bone-marrow-derived cells with structured biomaterials.
2. BONE CELLS
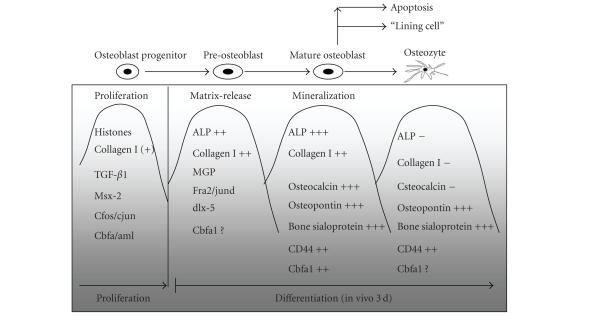
Osteoblasts and osteoclasts are mainly responsible for the osteointegration of nanostructured biomaterials in orthopaedics. Osteoblasts derive from mesenchymal progenitor cells which are localized mainly in the bone marrow and periosteum. They are characterized by cuboidal and flat morphology (diameter about 20 μm), present a large amount of rough endoplasmatic reticlum and a large Golgi apparatus, and are potent to produce osteoid, a collagen I rich matrix [9]. In addition, these mononuclear cells are also responsible for osteoid calcification (hydroxyapatite). Typical marker proteins for osteoblasts are Cbfa1/Runx2, osteocalcin, osteopontin, osteonectin, bone sialoprotein (BSP), osteoprotegerin (OPG), collagen I, and alkaline phosphates (ALP). Figure 1 gives a brief summary of the expression of several markers during osteoblast differentiation.
Figure 1.
The differentiation of osteoblast is characterized by different stages and lasts in vivo about 3 days. 50 to 70% of all osteoblasts undergo programed cell death (apoptosis) whereas the rest differentiate into osteocytes or persistist as resting or bone-lining cells [10].
When trapped into the mineralized bone, osteoblasts differentiate into osteocytes. Osteocytes act in a paracrine and mechanosensory manner, and can activate osetoblasts and osteoclasts. The latter cell type derived from the hematopoietic line, has multiple nuclei and is responsible for bone resorption. Its ruffled border is flanked by a sealing zone which facilitates local acidification and removal of bony matrix such as Ca2+, H3PO4, and H2CO3 by endocytosis. Osteoclasts express high levels of tartrate-resistant acid phosphatase (TRAP) and cathepsin K. The interaction between osteoblasts and osteoclasts is complex. During differentiation, the ostoblast progenitors express receptor activator of nuclear factor κβ ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) which are strong stimuli for osteoclastogenesis. In contrast, osteoprotegerin (OPG) is a potent inhibitor of osteoclasts. Moreover, the interactions between osteoblasts and osteoclasts in vivo are regulated by several hormones and cytokines, including parathyroid hormone (PTH), calcitonin, and IL-6.
3. CYTOCOMPATIBILITY OF MICRO- AND NANOSTRUCTURED SURFACES
3.1. Principles and problems
It is generally accepted that the three-dimensional surface topography (size, shape, surface texture) is one of the most important parameters that influence cellular reactions [2, 11–19]. Although many studies have investigated cellular reaction to different surface pattern, the significance of macro structure studies on bone cell behavior is questionable since in vivo adhesion structures (e.g., cell membranes, basement membranes) are comprised of much smaller nanometer scale features [20, 21].
The immature bone is characterized by an average inorganic grain size of 10–50 nm whereas mature bone has an average inorganic grain size of 20–50 nm (2–5 nm in diameter) [22]. Considering these parameters, modern implants for bone application have been designed with a smooth surface at the nanometer level. It was surprising that some of these have induced the formation of peri-implant fibrous tissue and implant loosening in vivo, while other implants with a higher degree of roughness showed significant better osteoconductive properties [23–25].
There are various methods to modify the degree of roughness as well as surface energy and topography in orthopaedic implants. Typically applied techniques to enhance the degree of roughness and promote the osteointegrative properties of biometals (e.g., Ti, CoCrMo, SS) are chemical etching or anodization and also sand-blasting, sputter-coating, and machine-tooling.
The lack of knowledge in cellular reaction to nanostructered biomaterials is based to a great extent on the difficulty in varying surface chemistry and topography independently. Moreover, the use of different cell lineages and culture conditions makes it difficult to compare results from different investigators [26–31] (Table 1). There is also a lack of consensus concerning the proper representation of implant surface topography [32]. One major misunderstanding is the practice of defining a surface by its manufacturing process instead of concisely defining the topographic measurements [17, 33]. Considering these limitations for interpretation, the following review gives an overview of cellular reactions to surface structures of different orthopaedic biomaterials.
Table 1.
Major parameters which influence the outcome in cytocompatibility testing of a biomaterial.
| Biomaterial parameter (measuring instruments, techniques) | Cell | Culture conditions |
|---|---|---|
| Manufacturing process | Cell type | Temperature |
| Chemical composition (EDX) | Source | Saturation |
| Degree of roughness (profilometer) | Differentiation stage | Vol% CO2 and O2 |
| Geometry/topography of surfaces | Monolayer culture | Culture medium |
| Hydrophobicity (wettability) | Passage | Material of culture dishes |
| Surface energy, Zeta potential | Intervals of medium exchange | |
| Ability to release ions/H changes | Soluble stimuli: cytokines, growth factors | |
| (concentration) | ||
| Cytomechanical forces (e.g., ultrasound load transfer) | ||
| Cultivation period |
Cellular attachment and adherence —
The first step after exposure of any biomaterial to a biological environment results in the rapid adsorption of proteins to its surface [34]. The composition, type, amount, and conformation of adsorbed proteins regulate the secondary phenomena such as cellular adherence and protein exchange [35–37] and also following cellular reactions such as migration, proliferation, and differentiation. The potency for biomaterials to adsorb proteins is influenced by its physiochemical characteristics such as surface energy or hydrophobicity, and is also dependent on the local environment (H, concentration of ions, composition and functional groups of proteins, strength of solution, temperature) (Vroman effect) [38] (Figures 2, and 3).
For inorganic nanocrystals and microstructured surfaces there are at least two approaches to change their hydrophobic surfaces: a ligand exchange reaction can replace the original hydrophobic surface with bifunctional coupling molecules or an inorganic coating such as silica (1) or an encapsulation of nanocrystals in an amphiphile organic coating (2).
The first phase of protein adsorption onto a biomaterial's surface is characterized by the attachment of small rapidly diffusing proteins, followed by a progressive replacement by larger proteins with a high affinity to the substrate. Here, especially proteins with Arg-Gly-Asp (RGD) containing sequences such as fibronectin or vitronectin act as cell receptors and have chemotactic or adhesive properties to bone cells. In addition, these RGD-peptides also have a strong effect on matrix maturation and biomineralization [46–48].
After conditioning of a naked biomaterial by protein adsorption, cells attach rapidly on the protein-coated surface [49]. Besides the influence of proteins, the cellular attachement to a nanostructed surface is also influenced by its physiochemical properties, especially by the outer functional groups [30, 50, 51].
Schweikl et al. [52] showed on self-assembly monolayers that the osteoblast proliferation on hydrocarbon chains, terminated by −CH3, was as high as on amino groups (−NH2) and hydrophilic oxidized surfaces, but significantly lower on fluorocarbon (−CF3) groups. Möller et al . [53] showed that 3-aminopropyl triethoxysilane (APTS) presents amine functional groups which allow for grafting RGD tripeptides and that the RGD-APTS hybrid promotes cell adhesion, spreading, and cytoskeletal organization.
Here, the zetal potential (differences in potentials between the surface of a tightly bounded layer and a diffuse layer) and the interfacial tension (wettability) of a surface is crucial [54, 55].
It was demonstrated for cpTi surfaces that the contact angle (CA), parameter for wettability, increases linearly with the average roughness when the angles were higher than 45° , but decreases linearly with roughness when the angle was less than 45° [56]. Recent data examining osteoblast response to controlled surface chemistries indicate that hydrophilic surfaces (high number of polar components) improve cell attachment and matrix synthesis and also the osteogenic potency compared to hydrophobic surfaces [57–59]. Stock et al. [60] compared Ti alloys and CoCr alloys towards protein absorptive properties and cell attachment with an osteoblast precursor cell line. They found no significant differences between Ti alloys and CoCr, but significantly greater cell adhesion rates for the Ti implants and concluded that cell adhesion is a result of higher hydrophilicity of Ti alloys. In contrast, other data showed that a low degree of wettability promotes protein adhesion and also cellular attachment to a biomaterial [61], and Möller et al. [55] found no direct correlation between the wettability of the material surface and the osteoblast attachment and proliferation rate. Also Qu et al. [62] found no significant differences of cell attachement on various titanium surfaces with different degrees of wettabilities (hydrophobic acid-etched, coarse-blasted large grit acid-etched, hydrophilic modified acid-etched, and modified coarse-blasted large grit, acid-etched ) on MG68 cells.
Heating (oxygen/atm) or peroxide treatment of biometals result in a thicker oxide layer and a more hydrophilic surface. Kern et al. [63] showed that heat-treated titanium surfaces changed the wettability (more hydrophilic) but does not significantly affect the fibronectin and albumin adsorption as well as the initial osteoblast precursor cell attachment in vitro. Based on data from their in vitro experiments, MacDonald et al. [64] emphasized that the rate of protein correlates more with changes in chemical composition than with changes in wettability in metal surfaces. They showed that a preheating of Ti6Al4V specimen does not only lead to a thicker oxide layer but also results in an enrichment of V and Al within the surface oxide. In contrast, post-treatment with butanol after preheating reduces the content of V, but not in Al, and significantly increases the rate of fibronectin adsorption up to 20–40% [64].
Compared to the cellular attachment phase, the following adhesion phase lasts longer and involves various proteins and molecules (Figure 2). As a link between cell and biomaterial, the interactions of a surface topography and serum proteins are crucial for the cytocompatibility of a biomaterial. Especially, the adsorption of adhesion proteins, such as fibronectin and vitronectin, from serum containing solutions and integrin-mediated signaling has been demonstrated to mediate cell adhesion and spreading [65].
It has been shown that nanotube or nanoparticle surfaces created by anodization have promoted osteoblast adhesion up to three times compared to unanodized Ti [66]. These results were confirmed by the group of Webster [67] and other investigators [68–71] who demonstrated that the initial attachment of osteoblasts onto the surface of biometals such as cpTi, Ti6Al4V, and CoCrMo is enhanced by submicron to nanometer consistent particles compared to metals composed of respective micron particles. One possible explanation of this phenomenon is the higher amount of particle binding sites for osteoblast adhesion at the surfaces of nanophase metals compared to micron particle size metals. The theory of enhanced protein and cell binding capacities by larger surface areas/roughness degrees was also confirmed for porous HA materials [72].
Another example of the significance of surface structures for protein binding and osteoblast attachment is the helical rosette nanotubes (HRN) which can build self-assembly surface structures. It was demonstrated that a significant change of HRN coverage by heating correlated with the protein-binding and osteoblast adhesion potency in titanium surfaces [73, 74].
It is evident that not only the surface topography influences protein deposition and cell adherence but also proteins and cells modify the surface properties of a defined surface. Based on a surface analysis of the different biometal specimen before and after cell cultivation, we showed previously [57] that a cell attachment and/or protein precipitation increase the roughness in polished biomaterials (steel, Ti6Al4V, and CoCr). For porous coated CoCr surfaces, we found only slight and no relevant changes in roughness whereas cell cultivation onto sandblasted Ti6Al4V lead to a strong decrease in specimen roughness. Both, the increase in roughness after cell culturing in the different biometals and the decrease in roughness of sandblasted Ti6Al4V could be explained by the dense cellular growth and accumulation of debris in depth of the structured surfaces and/or protein deposition as shown by other investigators [75, 76].
In addition, not only the amount but also the type of protein adsorption by a surface is crucial for cellular adherence and following reactions such as migration and differentiation. As an example, Ti surfaces (Ra: 0.37–0.01 μm) adsorp fibronectin in higher concentrations compared to albumin, and fibronectin-coated Ti surface promoted more osteoblast attachments in comparison to albumin-coated Ti surfaces [77]. These results correspond to the data of other authors who showed excellent osteoconductive properties after fibronectin adsorption onto a biomaterials' surface [78–80].
Based on IRM and TEM analysis, the closest distance of cells to a surface (glass) was found to be approximately 10 nm [81, 82]. Historically, results from chicken fibroblasts have lead to a classification of three different types of separation.
(1) Focal contacts (FC): approximately 10–15 nm separation from the substrate under the peripheral regions of the leading lamellae (appearing black in TEM). FC act as an interface between intra and extracellular components and occur linearly beneath the associated cytoplasmic stress fibres [83, 84]. They are tenacious adhesion sites that remain attached to the substratum even when cells are forcibly detached, indicating their function as anchorage structures [85].
(2) Close contacts: corresponding to approximately 30 nm separation (broader grey areas in TEM).
(3) Greater separation: corresponding to approximately 100–140 nm (white regions in TEM).
It is evident that not only FC appear soon after cellular attachment but also that (β-catenin-positive) adherence junctions occur within 1–4 hours for grooved Ti-based substrates [20]. These observations underline the high significance of an early intercellular communication soon after adherence to a surface. The mechanisms of initial cellular adherence to a surface are different from long-term adherence as shown by a lack of statistical correlation between short-term adhesion (strength of cell attachment and early adhesion) and long-term adhesion (strength of cell-matrix interface) forces [14, 15, 86]. Based on a progressive trypsine-detachment method, Bigerelle et al. [86] showed that the cultivation time has an influence on the long-term adhesion in biometal surfaces according to (t) = , a being independent of b (: time-dependend adhesion index, a: surface-dependent parameter, b: substrat-independent exponent, ).
For polylactides (PLLA), it was shown on OCT-1 osteoblast-like cells that cell adhesion but not the proliferation could be enhanced by nanoscale and microscale roughness compared to smooth surfaces [87]. In addition, there is evidence that FC show a dynamic behavior which allows for cellular migration and motility. Linear PLLA fibres with length scales of 0.5–2 μm, constructed by electrospinning, have shown cellular contact guidance and enhanced osteoblastic differentiation. Here, cell morphology revealed that cells grown on fibres had smaller projected areas than those on planar surfaces [88]. These results were confirmed by other authors [89–92]. Also other polymers such as PLGA have been shown to be effective in enhancing osteoblast differentiation in vitro [93].
Diener et al. [94] demonstrated on MG-63 osteoblastic cells that FC adhesion was smaller on Ti and SS than on collagen-coated glass coverslips and that all FC showed a mobility of focal adhesions. However, Anselme et al. [13] found higher adhesions on Ti6Al4V substrates than on noncollagen-covered glass samples, and emphasized that substrates with various surface compositions but with the same surface topography did not induce significant differences of adhesion.
Based on the knowledge of protein adsorption and its effects on cellular attachment and adherence, a selective surface coating of nanostructured surfaces with RGD or collagen proteins offer a promising solution to improve the number of osteoblasts adhered on artificial surfaces [53, 95–102]. Imprinting surfaces technology with deposition of specific protein-recognition sites can help to promote osteoblastic growth and differentiation [103–106].
Protein-recognition can be based on a protein-ligand binding and/or electron donor-acceptor interactions or other types of binding forces. One example is the binding of different integrin subunits to fibronectin. Integrin α5β1 and α5 vβ3 subunits competitively bind to RGD-sites of fibronectin [107, 108]. Dependent on the surface topography and chemistry of the biomaterial, fibronectin undergoes changes in structure including modulation in functional activity and shift in integrin binding capacity.
Based on the data of self-assembled monolayers, it was shown that integrin subunits show selective binding capacities to different terminal groups. Integrin α5β1 shows a strong affinity to −OH and −NH2 surfaces, whereas α5β1 and α5vβ3 bind also to −COOH but show poor binding capacities on −CH3 surfaces [109–113].
Furthermore, some data show that −OH and −NH2 surfaces can up-regulate osteoblast-specific gene expression but also matrix mineralization compared with −COOH and −CH3 functional groups [47, 112].
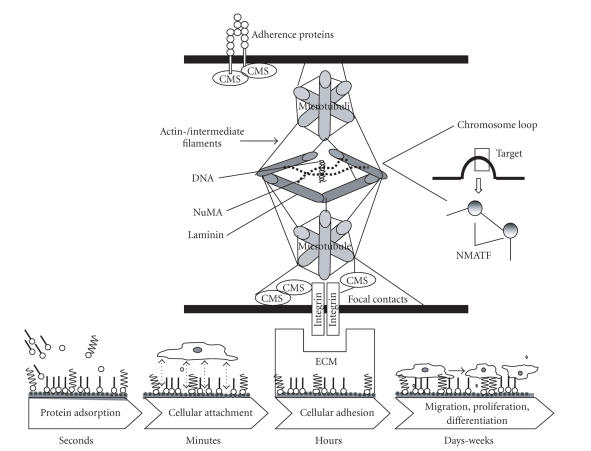
Figure 2.
The scheme shows principal interactions of extracellular matrix (ECM) proteins and adjacent cells [39–41]. Interlinking proteins, focal adhesion proteins (predominantly integrins), adherence junctions, the cytoskeleton (microtubuli, actin- and intermediate filaments), and the nuclear matrix, characterized by laminin and NuMA are involved to connect the cyto- and the nucleoskeleton with the ECM [42]. Here, especially the heterodimeric integrins can act as molecular bridges between adsorbed ECM proteins of a biomaterial and interacting cells [43–45]. Several proteins of the connective membrane skeleton (CMS) such as p, zyxin, moesin, paxiliin, fembrin, VASP are connected to the nucleus by focal adhesion proteins and act as signal transducers. These proteins are potent to transfer information from the cell membrane to the intracellular space and control the conformation and activity of gene promotors via nuclear matrix architectural transcription factors (NMATF). Integrins also play a crucial role in transduction of cytomechanical forces from ECM proteins to the cytoskeleton. In addition, cells are connected via N-cadherin, which is strongly expressed by osteoblasts.
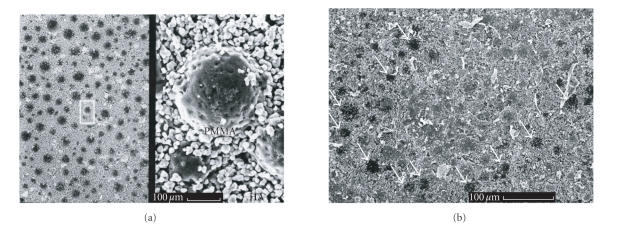
Figure 3.
Surfaces of a polymethylenemethacrylate (PMMA)-hydroxyapatite(HA) composit (bone cement, Osteopal) which were incubated in PBS without FCS (a) and DMEM culture solution supplemented by 20% FCS (b) for 4 weeks. The latter probe showed a protein adsorption in SEM whereas the sample which was exposed in serum-free PBS showed no protein layer on its surface. Figure 2b demonstrates the different protein-adsorbing potency between PMMA and HA. All HA granules were covered by protein deposition whereas some PMMA “balls” (arrows) were uncovered.
3.2. Cellular migration and proliferation
Cell migration and proliferation is the attachment following phase between the cell and the material surface. It is evident for designing nanostructured implants that cells use the nanotopography of a substrate for orientation and migration [117–119]. Although it is known that bone cells align along defined substrate morphologies (contact guidance), the detailed relation between ordered nanotopography and cell behavior remains unknown in detail [120]. For the first time, in 1964 it was shown that convex surfaces enhance cellular overlap, while grooves minimize cellular overlap [82].
As pre-requisite to reach a defined cell colonization during directed tissue formation, structured nanophase surfaces lead to a predictable osteoblast orientation and migration on these surfaces [17, 121, 122]. Interaction between the ECM and associated changes in the orientation of the cytoskeleton are crucial for cell metabolism of cells and morphology due to actin-myosin tension structures [123]. Anisotropic topographies (e.g., topographical grooves, chemically patterned stripes, or curved surfaces of a fibre) are potent to exert morphological as well as physiochemical features on cells at the same time, indicative for the complex environmental influence on cells.
Focal contacts are important structures for cellular adherence onto a surface but may also delay migration and mobility of the cells. It was shown that bone-derived cells (MG63 cells) respond to a nanoscale roughness by a higher cell thickness and a delayed appearance of focal contacts [20]. Especially, nanoporous Ti-oxide surfaces promote cellular spreading and induce numerous filopods and osteoblastic differentiation [124, 125]. On electrochemically microstructured hexagonal pattern, MG63-cells go inside 30–100 μm but not in 10 μm cavities [20]. Most authors report a parallel orientation of cells cultured on polished (smooth) surfaces [57, 114, 126] (Figure 4).
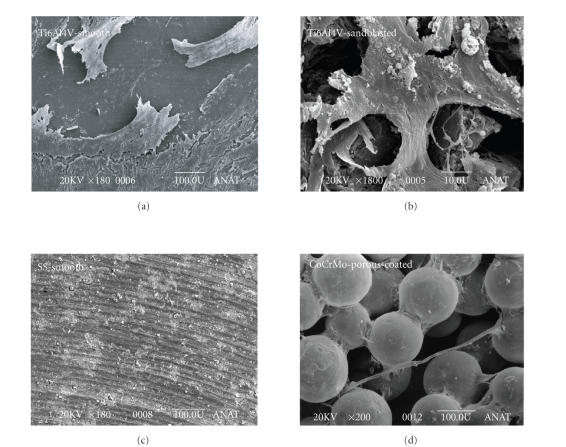
Figure 4.
Typical view of human bone marrow cells onto different surfaces with an endoprosthesis equivalent topography. (a) Polished Ti surface with flat adherent cells after 21 days in vitro. Smooth (polished) surfaces tend to induce a flat cells with a spindle-shape morphology as shown above and also confirmed by other investigators for different orthopaedic biometals such as Ti, SS, CoCoMo [9, 19, 114–116]. (b) The cells adhered onto a sandblasted Ti surface showed a more inhomogenous star-like morphology. (c) A polished stainless steel (SS) surface showed potential cytotoxic effects on human bone marrow cells which were characterized by a small and round body. (d) A porocoated CoCrMo surface induced various cellular shapes. The high flexibility of the cells is demonstrated by an interconnecting filopode which crosses two metal balls.
Another method to not only enhance cellular adherence but also to promote osteoblastic differentiation and biomineralization of biometals is a surface anodization, for example, by β-glycerophosphate sodium and calcium acetate [66–71].
Cellular adhesion via FC may strengthen the linkage between cell and ECM and also impair the ability to dynamically remodelling the ECM and influence the migration rate [94]. For collagen-coated coverslips, focal adhesion of MG-63 osteoblastic cells moved with a speed of 60 nm/min, whereas the speed was reduced in Ti and more in SS surfaces [94]. Another study on Nb2O5-coated polished cpTi samples showed that MC3T3-E1-osteoblast migration was fastest on smooth surfaces (Ra = 7 nm), whereas adhesion strength, spreading area, and collagen-I synthesis were promoted by intermediate roughness (Ra = 15 nm). However, it was surprising that higher degrees of roughness (Ra = 40 nm) were rather peaked and reduced the speed of adhesion process in the same study [127].
Besides the surface properties of a biomaterial, the cellular migration rate is dependent on the cell type and its differentiation stage. A higher migration rate is associated with a lower level of osteoblast differentiation. Cells with a low motility are characterized by a strong formation of FC while motile cells form less adhesive structures. It was found that mature osteoblasts spread out and form a greater number of FC when settled on smoother surfaces [28]. Although cellular spreading is higher on smoother surfaces, some data indicate that the ALP-expression is higher for rough isotropic surfaces (electro-erosion, acid-etching, sandblasted) compared to smoother substrates (machine tooling, polishing) [11].
Considering recent publications, there is no or only week statistical significance that there is a difference between the initial number of adherent cells and following proliferation of cells cultured onto a biometal or ceramic nano-/microscale surface in vitro [50]. However, some authors emphasize that the influence of functional chemical groups for cellular migration and proliferation are stronger than general surface properties such as wettability [51]. Especially a TiO2-layer seems to promote cellular growth and proliferation on nanostructured biometals [128, 129].
Other examples for a promotion of cell-to-bone contact in vitro and also in vivo are machine-etched Ti-surfaces (e.g., Osteotite™) [130], defined sand-blasted implants [124, 125, 131], and hydroxyapatite (HA) coatings, for example, by plama-spray techniques [132–134].
3.3. Cellular differentiation, gene expression, and protein synthesis
Recent studies investigating the response of adherent cells to nanography surfaces indicate that different cell phenotypes have different levels of sensitivities [117, 135–137]. Here, osteoblasts react to features as low as to the 10 nm dimensions, which is comparable in size to a single collagen fibre [138].
Moreover, the qualitative and quantitative kinetics in gene and protein expression is strongly influenced by topography and physiochemistry of a defined surface. Microporous HA surfaces seem to promote a high number of FC and increased levels of ALP but short actin stress fibres compared to nonmicroporous HA surfaces [72, 139]. There is also evidence that Ti and HA surfaces can activate early intracellular signalling pathways as shown by expression of relevant molecules such as α- and β1-integrin, FAK, ERK followed by c-jun and c-fos genes for proliferation and ALP for differentiation [139, 140]. However, Hallgren et al. [141] found no significant histomorphometric and biomechanical differences between nanopatterned and control implants. Hamilton et al. [142] showed that microfabricated discontinuous-edge surfaces (DES), repeated open square boxes with a depth of 10 μm, alter osteoblast adherence and migration but enhance cell multilayering, matrix deposition and mineralization when compared to smooth controls.
In contrast to our data [57], Anselme et al. [13] found higher proliferation rates on SS compared to Ti6Al4V. However, Bigerelle et al. [14] demonstrated that neither material composition nor surface roughness amplitude influence cell proliferation, whereas they found a very significant influence on manufacturing process and surface topography for long-term adherence and proliferation in vitro.
Our in vitro results [57] confirm the well known osteogenic in vivo properties of Ti implants, which may be based on surface factors observed on its outer TiO2-layer [143–146]. Müller et al. [147] demonstrated the ability of osteoblasts to grow into an open-porous Ti implant (metal foam) and Li et al. [148] also demonstrated that MC3T3-E1 cells attach to and are able to divide well in the inner surface of a highly porous trabecular Ti6Al4V implant.
Some in vitro studies demonstrated an enhanced total protein and collagen production, as well as increased ALP activity of osteoblasts cultured on nanoparticulate metals (cpTi, Ti6Al4V, and CoCrMo) indicating advantages for nanostructured surfaces for osteointegration [1, 149, 150]. Based on the data of Redey et al. [58], it can be concluded that the low attachment and collagen production rates are related to a low wettability of a nanosurface. Nanotextured surfaces of Ti surfaces prepared by chemical etching have upregulated the expression of BSP and OP [66]. As demonstrated by Qu et al. [62], the expression of the bone-associated genes such as ALP, OC, type-I-collagen, osteoprotegerin, and glyceraldehyde-3-phosphate-dehydrogenase is promoted by modSLA Ti surfaces. Some data also suggest that fluoride-modified Ti surfaces can stimulate osteoblastic differentiation compared to unmodified titanium surfaces [151, 152].
Ward et al. [1] showed in their in vitro experiments that nanophase biometals induce significantly greater calcium and phosphorus deposition by osteoblasts and also allow for calcium and phosphorous precipitation from culture media without osteoblasts in contrast to microphase Ti6Al4V and CoCrMo. Furthermore, the authors found advantages in mineral precipitation without osteoblast for TiAl4V but no differences in dependency to the type of Ti (wrought, microphase, or nanophase). It was evident that the increased calcium and phosphorus mineral content correlated to greater amounts of underlying aluminium content on Ti6Al4V surfaces. Although some data indicate that nanostructured Ti alloys promote non-cell-mediated Ca/PO4-mineral deposition from culture media compared to CoCrMo substrates, the greatest cell-dependend calcium and phosphorus mineral deposition occurred on nanophase CoCrMo [1].
It is evident that micropattern collagen films or scaffolds promote not only cellular adhesion but also allow for an osteoblastic differentiation and biocalcification in vitro [153–155]. For HA- and DCPP-coated, Ti surfaces the Ca/P ratio influence the biomineralization rate in vitro [156].
Besides the osteoblast-promoting effects of defined substrates and surface topographies, some data also allocate an inflammatory response induced by nano- or microstructured biomaterials. It was shown in many studies that cell-biomaterial interactions can activate macrophages which results in the synthesis of proinflammatory agents such as TNFα, IFNγ, IL-1 and -6, RANKL and NO [157–159]. Some data have shown proinflammatory effects of different biomaterials which increase with the degree of surface roughness. Here, macrophage inflammatory protein-1, TNFα, monocyte chemoattractant protein-1, and members of the interleukine and leukotriene family play a crucial role in biometal-induced inflammations [160–164]. Most studies report about an enhanced expression of pro-inflammatory cytokines and chemokines by cells attached to rougher surfaces [164].
Some data also indicate that anionic and neutral hydrophilic surfaces increase macrophage-monocyte apoptosis and reduce macrophage fusion to modulate inflammatory responses to implanted materials [165].
However, adverse cellular effects seen with metallic implants may also be attributed to corrosion products or to the separation of metal ions (Fe, Cr, Ni) which may have a major impact on cellular survival and differentiation [166–168]. Those studies which suggest that a cell-mediated metal ion release by biometals that did not affect the cell viability or proliferation are characterized by short cultivation periods or other conditions which limit the reliability of data [169–171].
Up to date, only few authors report about no significant influence of the cellular adherence and expression of osteoblast proteins by different biometals and surfaces such as ALP expression [172, 173].
3.4. Cytocompatibility of micro- and nanoscaled particles
In contrast to the great opportunity enhancing biocompatibility and osteogenic potency of surfaces applied on bone by nanotechnology, micro- and nanoscaled particles released by friction of artificial joints can induce severe inflammation and may lead to osteolysis and implant failure [174, 175] (Figure 5, Table 2).
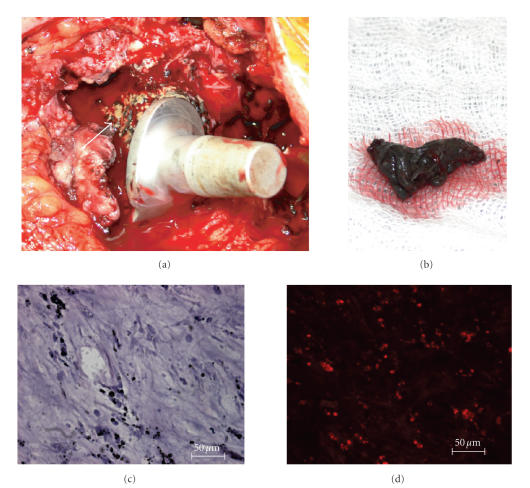
Figure 5.
Although traditional biocompatibility focuses on the implant-host interface the movement of particles within the human body should be considered. As shown above micro- and nanoparticles derived from the acetabular component of a failed artificial total hip joint were transported by diffusion and/or cell migration to the proximal femur and induce severe peri-implant osteolysis. (a) The bone around the proximal femur is resorbed (arrow) and substituted by layers of fibrous soft tissue. (b) The black colour of peri-implant tissue presented on a lab sponge results from metal wear debris (metallosis). (c) The tissue section of the fibrous layer showed small intra- and pericellular particles in different sizes (estimated size range 0.1–10 μm) in H.E. staining. (d) The immunfluorescent CD68 staining showed the high number of activated macrophages (red) within the fibrous tissue.
Table 2.
Results of in vitro cytocompatiblity of different nano- and microparticles.
| Author | Chemical composition | Particle size | Cell type | Result | |
|---|---|---|---|---|---|
| Yao et al. (1995) [182] | Ti | <3 μm | fibroblasts, osteoblast -like MG-63 cells | Periprosthetic osteolysis by release of MMPs and mediators that result in suppression of collagen synthesis in osteoblasts. | |
| Manlapaz et al. (1996) [183] | Ti6Al4V | μm | fibroblasts | Activation and release of proinflammatory mediators after exposition to Ti alloy wear particles (IL-6, TNFα, collagenases, bFGF). | |
| Shanbhag et al. (1997) [184] | Ti6A4lV, UHMWPE (wear debris) | 77.5 μm | human peripheral monocytes | Stimulation of fibrogenesis, fibroblast proliferation and fibroplasia. | |
| Santavirta et al. (1998) [185] | SiC | 5 μm | JCRB0603 cells | Inhibition of colony outgrowth by one-third in contrast to SiC-coated pins. | |
| Green et al. (1998) [181] | PE particles (Ceridust 3615, GUR 120) | 0.21 μm versus 0.49 μm versus 4.3 μm versus 7.2 μm (Centridust), 88 μm (GUR) | C3H murine peritoneal macrophages | Particles in the phagocytosable size range (0.3–10 μm) are the most biologically active. | |
| Dean et al. (1999) [179] | UHMWPE | 0.6 μm (95% <1.5 μm), particles/g tissue. | MG63 osteoblast-like osteosarcoma cells | Decrease of ALP, OC, and collagen expression and proteoglycan sulfation ind increase of PGE2 expression. | |
| Sun et al. (1999) [186] | HA | 0.5–3.0 μm versus 37–53 μm versus 177–205 μm versus, 420–841 μm | primary osteoclasts/osteoblasts | Depending on particle size, activation of osteoclasts and decrease of osteoblasts, inhibition of cellular growth, degrease of TGFβ1, increase of PGE2 and LDH. | |
| Nakashima et al. (1999) [178] | Ti | 0.7 μm | mononuclear leukocytes/macrophages | Induction of macrophage release of TNFαand IL-6 without phagocytosis in presence of tyrosine and serine/threonine kinase activity. | |
| Green et al. (2000) [187] | UHMWPE (wear debris) | GUR 1120 (0.24 to 7.62 μm), GUR 1120 PE (88 μm) | C3H murine peritoneal macrophages | Osteolytic response of macrophages in vitro dependent on size and dose of polyethylene particles. | |
| Akisue et al. (2002) [188] | Ti | <10 μm | human monocyte/macrophage cell line (THP-1) | No initiation of inflammatory cellular response in differentiated THP-cells. | |
| Wilke et al. (2002) [189] | Ti6Al4V | <0.1 μm | human bone marrow cell | Induction of proinflammatory and osteolytic mediators (IL-6, IL-1β, TNFα), high dose toxicity. | |
| Germain et al. (2003) [190] | CoCr, Al2O3 | CoCr: nm, range 5–200 nm, Al2O3: 5–20 nm in size (98%) | U937 histiocytes and L929 fibroblasts | Higher toxicity of CoCr particles then Al2O3 particles. Nature, size and volume are important in assessing biological effects of wear debris on cellsin vitro. | |
| Howling et al. (2003) [191] | carbon-based composite materials: HMU-CVD, SMS-CVD, P25-CVD, and CFRPEEK | 24.2 (P25) 71.8 (HMU) | L929 fibroblasts and U937 monocytic cells | Lesser cytotoxity of P25-CVD than CoCr. | |
| Miyanishi et al. (2003) [177] | Ti (non-spherical) | 1–3 mm | human monocyte/macrophages | Particle-induced release of VEGF, upregulation of p44/42, MAPK and AP-1. | |
| Granchi et al. (2004) [192] | Al2O3, UHMWPE, CrCo | 1.5 μm | osteoblasts, osteoclasts | Less activitiy in promotion of osteoclastogenesis of Al2O3. | |
| Howling et al. (2004) [193] | carbon-carbon composite materials: HMU-PP(s), HMU-RCP(s), and SMS-RC-P(s) | <100 nm | L929 fibroblasts | SMS-RC-P(s) particles showedgood biocompatibility and low cytotoxicity compared to metal wear particles. SMS-RCP(s) did not significantly stimulate TNFα production at a particle volume to cell number ratio of 80 : 1. | |
| O'Connor et al. (2004) [176] | Ti | osteoblasts | 1.5–4 μm Ti particles have the greatest effect on osteoblast proliferation and viability in vitro. | ||
| Barrias et al. (2005) [194] | Ca‐Ti‐PO4‐ microspheres | 205 μm | bone marrow stromal cells | ALP activity decreases after an initial peak which occurs usually during the first 10 days in vitro. | |
| Petit et al. (2006) [195] | Al2O3, UHMWPE | 1.3 μm | J774 mouse macrophages incubated | The effect of bisphosphonates on particle-stimulated macrophages is particle composition dependent. | |
| Tan et al. (2007) [196] | CdSe/ZnS (encapsulated in chitosan) | 60 nm | primary myoblasts | Reduction of cytotoxicity of the QDs after chitosan encapsulation. Nanoparticles can be internalized into myoblast cells. | |
There is a wide range in particles size and morphology produced by simulators for artificial joints. Particles released from metal-metal (CrCoMo alloys) are predominantly chromium oxide particles or CoCrMo with varying ratios of Co and Cr. They show a round to oval morphology and also a substantial number of needle-shaped particles were found during the first circles. O'Connor et al. [176] emphasize the importance of particle size as a critical factor in osteoblasts proliferation and viability in vitro. They showed that 1.5–4 μm Ti particles have the greatest effect. Some data indicate that in contrast to Ti-surfaces nano- and mircoparticles induce an inflammatory response although titanium is one of the biometals with the highest degree of cytocompatibility. As shown by Miyanishi et al. [177], the release of VEGF may play a crucial role in the pathogenesis of Ti-induced osteolysis. Some data indicate that phagocytosis of Ti particles is not a precondition for an inflammatory response such as a release of TNFα or IL-6 in cultured macrophages [178]. It is evident that a binding of the macrophage CD11b/CD18 (macrophage Mac-1 receptors/receptor of complement CR3bi, can also bind to ICAM-1 and ICAM-2) by integrin-specific antibodies also increased the release of TNFα and IL-6 in macrophages. This finding also suggests that the complement system plays a role in the pathogenesis of particle-induced inflammation, too. Especially, UHMWPE particles with a size range of 0.1–1.0 μm have been shown to be most reactive for macrophage activation and cytokine secretion in bone marrow cells [179, 180].
However, not only the particle size but also the particle volume (number) is a critical factor for particle-mediated release of cytokines by macrophages. Green et al. [181] demonstrated for PE that the cell-particle ratios of 1 : 100 (size 0.49–7.2 μm) and 1 : 10 (size: 0.49–4.2 μm) induced significant stronger release of TNFα and IL-1β in macrophages. The authors conclude that especially particles in the phagocytosable size range of 0.3–10 μm appear to be the most biologically active ones.
The latter statement was also confirmed for silicon carbide (SiC) particles and biometals such as cpTi, Ti6Al4V and UHMWPE [184, 185].
Granchi et al. [192] investigated the in vitro effects of Al2O3 and UHMWPE particles in an osteoblast-osteoclast co-culture system. Both particles did not affect either cell viability or TNF and GM-CSF release, whereas IL6 release was dependent on the particle concentration. UHMWPE particles increased the release of RANKL from osteoblasts and induced large amounts of multinucleated TRAP-positive giant cells in an osteoblast-osteoclast co-culture system. In contrast, Al2O3 wear debris was less active. Also, carbon-based particles with low wear factors such as P25-CVD showed a high degree of cytocompatibility in vitro. Howling et al. [191] demonstrated on fibroblasts and monocytes that P25-CVD particles <100 nm were significantly less cytotoxic to both cell types than CoCr metal wear particles. While the classical water-suspendable nano−C60 nanocrystal is apparently cytotoxic to various cell lines, the closely related fully hydroxylated, C60(OH)24 , is nontoxic, thus producing no cellular response [197]. Also, functionalized single-walled carbon nanotubes are nontoxic to cells in culture [198–200].
There is evidence that not only particle size and chemical content but also the concentration strongly influence cellular reactions in vitro. Wilke et al. [189] showed a positive correlation between the release of proinflammatory cytokines (IL-6, -1β, and TNFα) and amounts of Ti6Al4V-particles (109, 108, 107, and 106 particles/ml) by human bone marrow cells over 2 weeks.
Some in vitro data also indicate that Ti particles induce a stronger fibroblastic differentiation signal than UHMWPE in monocytes and other cells [182–184].
Warashina et al. [201] showed that particles of high-density polyethylene (HDP) and Ti6Al4V induced significantly more proinflammatory mediators (IL-1β, IL-6, TNFα) and bone resorption compared to Al2O3 and ZrO2 in vivo. Based on these data, it can be assumed that ceramics show a high degree of cytocompatibiltiy.
For HA especially, particles with a size <53 μm inhibit cellular proliferation, especially in osteoblasts and lead to a decrease in TGFβ1 and a significant increase in PGE2 and LDH concentration, but did not influence the TNFα or ALP titer in vitro [186]. It could be concluded that larger HA particles may be compatible with bone cells while smaller-sized HA particles can both activate the osteoclasts and decrease the cell population of the osteoblasts in vitro.
3.5. Summary and conclusions
Numerous variables influence the biocompatibility and osteogenic potency of nanostructured biomaterials in vitro and in vivo. Besides the locotypical environment in vivo or in vitro, the surface structure and the composition of a biomaterial affects cellular attachment, adherence, proliferation and migration, and also differentiation and survival of defined cell types. Here, information about typical parameters such as chemical composition, surface structure (topography, geometry, roughness, particle size), surface energy, hydrophobicity, and the degree of solubility in aqueous solutions of a biomaterial will help to value and grade a defined implant concerning its osteblast promoting potency.
Considering recent publications, we could assume some general principles of cytocompatiblity and cell-surface interactions in nano- and microstructured surfaces.
(1) Wettability of a nanosurface influences significantly protein adsorption, which is a prerequisite of cellular adherence in serum containing solutions.
(2) Nanostructured surfaces enhance the surface area of biomaterials and promote cellular adherence.
(3) The chemical outer functional groups of a nanosurface significantly influence cellular migration, proliferation, and differentiation but direct correlations between distinct parameters and cell functions are not entirely cleared.
(4) The formation of FC underly a dynamic process and influence the motility and migration of cells.
(5) A higher degree of differentiation is corresponding to a decreased cellular motility.
(6) Phagocytable particles with a size <10 μm induce the strongest cellular response with regard to releasing inflammatory cytokines.
(7) Although Ti has a high degree of cytocompatibility in vitro, phagocytable Ti particles can induce a fibroblastic differentiation.
lIST OF ABBREVIATIONS
- ADP:
adenosine-diphosphate
- AFM:
Atomic forced microscopy
- Al:
aluminium
- ALP:
alkaline phosphatase
- cp:
commercialy pure
- C:
carbon
- Ca:
calcium
- DCPP:
dicalcium pyrophosphate
- Cd:
cadmium
- CD:
cluster of differentiation
- DES:
discontinuous-edge surfaces
- CMS:
connective membrane skeleton
- Co:
cobalt
- CR:
complement receptor
- CSF:
colony stimulating factor
- CVD:
chemical vapour deposition
- DES:
microfabricated discontinuous-edge surface
- DMEM:
Dulbeccos modified eagles medium
- ECM:
extracellular matrix
- ERK:
extracellular signal-regulated kinase
- FC:
focal contacts
- FAC:
focal adhesion kinase
- FCS:
fetal calf serum
- Fe:
ferrum
- GM-CSF:
granulocyte-macrophage colony-Stimulating Factor
- HA:
hydroxyapatite
- HDP:
high-density polyethylene
- H.E.:
hematoxilin exosin
- HOB:
human osteoblasts
- IL:
interleukin
- IFN:
interferone
- ICAM:
intercellular adhesion molecule
- IRM:
interference reflection microscopy
- LDH:
lvctic acid dehydrogenase
- modSLA:
modified coarse-blasted large-gritand acid-etched
- Mo:
molybdenum
- NMATF:
nuclear matrix architectural transcription factors
- NuMA:
nuclear mitotic apparatus
- O:
oxygen
- PARP:
poly(ADP-ribose)polymerase
- PBS:
phosphate buffer saline
- PEEK:
polyaryletherketone
- N:
nitrogen
- Nb:
niobium
- Ni:
nickel
- PE:
polyethylene
- PG:
prostaglandin
- PLGA:
poly-DL-lactic-co-glycolic acid
- PMMA:
poly-methyl-methacrylate
- PLLA:
poly-L,L-lacide acid
- QDs:
quantum dots
- RANKL:
receptor activator of NF-kappaB ligand
- S:
Sulphur
- Se:
selenium
- Si:
silicon
- SS:
stainless steel
- TEM:
transmission electron microscopy
- Ti:
titanium
- TNF:
Tumor necrosis factor
- TRAP:
Tatrate-resistant acid phosphatase
- UHMWPE:
Ultra high molecular weight polyethylene
- V:
vanadium
- VASP:
vasodilator-stimulated phosphoprotein
- VEGF:
vascular endothelial growth factor
- XLPE:
Highly cross-linked polyethylene
- Zn:
zinc
References
- 1.Ward BC, Webster TJ. The effect of nanotopography on calcium and phosphorus deposition on metallic materials in vitro. Biomaterials. 2006;27(16):3064–3074. doi: 10.1016/j.biomaterials.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Lai C-Y, Trewyn BG, Jeftinija DM, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. Journal of the American Chemical Society. 2003;125(15):4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 3.Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annual Review of Biomedical Engineering. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 4.Gul RM, McGarry FJ, Bragdon CR, Muratoglu OK, Harris WH. Effect of consolidation on adhesive and abrasive wear of ultra high molecular weight polyethylene. Biomaterials. 2003;24(19):3193–3199. doi: 10.1016/s0142-9612(03)00165-0. [DOI] [PubMed] [Google Scholar]
- 5.Di Iorio D, Traini T, Degidi M, Caputi S, Neugebauer J, Piattelli A. Quantitative evaluation of the fibrin clot extension on diffferent implant surfaces: an in vitro study. Journal of Biomedical Materials Research Part B. 2005;74(1):636–642. doi: 10.1002/jbm.b.30251. [DOI] [PubMed] [Google Scholar]
- 6.Inoue K-I, Takano H, Yanagisawa R, et al. Effects of nano particles on antigen-related airway inflammation in mice. Respiratory Research. 2005;6:106. doi: 10.1186/1465-9921-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, El-Shall H, Dennis D, Morey T. Interaction of PLGA nanoparticles with human blood constituents. Colloids and Surfaces B. 2005;40(2):83–91. doi: 10.1016/j.colsurfb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Meredith DO, Eschbach L, Riehle MO, Curtis ASG, Richards RG. Microtopography of metal surfaces influence fibroblast growth by modifying cell shape, cytoskeleton, and adhesion. Journal of Orthopaedic Research. 2007 doi: 10.1002/jor.20430. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt C, Kaspar D, Sarkar MR, Claes LE, Ignatius AA. A scanning electron microscopy study of human osteoblast morphology on five orthopedic metals. Journal of Biomedical Materials Research. 2002;63(3):252–261. doi: 10.1002/jbm.10185. [DOI] [PubMed] [Google Scholar]
- 10.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. Journal of Bone and Mineral Research. 1998;13(5):793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 11.Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomaterialia. 2005;1(2):211–222. doi: 10.1016/j.actbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Anselme K, Bigerelle M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short-term adhesion. Journal of Materials Science: Materials in Medicine. 2006;17(5):471–479. doi: 10.1007/s10856-006-8475-8. [DOI] [PubMed] [Google Scholar]
- 13.Anselme K, Noël B, Hardouin P. Human osteoblast adhesion on titanium alloy, stainless steel, glass and plastic substrates with same surface topography. Journal of Materials Science: Materials in Medicine. 1999;10(12):815–819. doi: 10.1023/a:1008992109670. [DOI] [PubMed] [Google Scholar]
- 14.Bigerelle M, Anselme K. Statistical correlation between cell adhesion and proliferation on biocompatible metallic materials. Journal of Biomedical Materials Research Part A. 2005;72(1):36–46. doi: 10.1002/jbm.a.30212. [DOI] [PubMed] [Google Scholar]
- 15.Bigerelle M, Anselme K. A kinetic approach to osteoblast adhesion on biomaterial surface. Journal of Biomedical Materials Research Part A. 2005;75(3):530–540. doi: 10.1002/jbm.a.30473. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Willard F, Wolde-Giorgis D, Al-Kassab T, et al. Focused ion beam preparation of atom probe specimens containing a single crystallographically well-defined grain boundary. doi: 10.1016/j.micron.2007.01.001. to appear in Micron . [DOI] [PubMed] [Google Scholar]
- 17.Meyer U, Büchter A, Wiesmann HP, Joos U, Jones DB. Basic reactions of osteoblasts on structured material surfaces. European Cells & Materials. 2005;9:39–49. doi: 10.22203/ecm.v009a06. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira S, Monteiro FJ, Ferraz MP, Vilar R, Eugeénio S. Laser surface treatment of hydroxyapatite for enhanced tissue integration: surface characterization and osteoblastic interaction studies. Journal of Biomedical Materials Research Part A. 2007;81(4):920–929. doi: 10.1002/jbm.a.31073. [DOI] [PubMed] [Google Scholar]
- 19.Bigerelle M, Anselme K, Dufresne E, Hardouin P, Iost A. An unscaled parameter to measure the order of surfaces: a new surface elaboration to increase cells adhesion. Biomolecular Engineering. 2002;19(2–6):79–83. doi: 10.1016/s1389-0344(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 20.Anselme K, Bigerelle M, Loison I, Noël B, Hardouin P. Kinetic study of the expression of -catenin, actin and vinculin during osteoblastic adhesion on grooved titanium substrates. Bio-Medical Materials and Engineering. 2004;14(4):545–556. [PubMed] [Google Scholar]
- 21.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803–1810. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan F, Hayes W, Keaveny T, Boskey A, Einhorn T, Iannotti J. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. In: Sp S, editor. Orthopedic Basic Science. American Academy of Orthopedic Surgeons; 1994. pp. 460–478. [Google Scholar]
- 23.Grizon F, Aguado E, Huré G, Baslé MF, Chappard D. Enhanced bone integration of implants with increased surface roughness: a long term study in the sheep. Journal of Dentistry. 2002;30(5-6):195–203. doi: 10.1016/s0300-5712(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 24.Phillips TW, Messieh SS. Cementless hip replacement for arthritis. Problems with a smooth surface Moore stem. Journal of Bone and Joint Surgery, British. 1988;70(5):750–755. doi: 10.1302/0301-620X.70B5.3192573. [DOI] [PubMed] [Google Scholar]
- 25.Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NHJ. Implant surface roughness and bone healing: a systematic review. Journal of Dental Research. 2006;85(6):496–500. doi: 10.1177/154405910608500603. [DOI] [PubMed] [Google Scholar]
- 26.Jäger M, Fischer T, Schultheis A, Lensing-Höhn S, Krauspe R. Extensive release by bone substitutes affects biocompatibility in vitro testing. Journal of Biomedical Materials Research Part A. 2006;76(2):310–322. doi: 10.1002/jbm.a.30515. [DOI] [PubMed] [Google Scholar]
- 27.Jäger M, Wilke A. Comprehensive biocompatibility testing of a new PMMA-HA bone cement versus conventional PMMA cement in vitro. Journal of Biomaterials Science, Polymer Edition. 2003;14(11):1283–1298. doi: 10.1163/156856203322553491. [DOI] [PubMed] [Google Scholar]
- 28.Jayaraman M, Meyer U, Bühner M, Joos U, Wiesmann H-P. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials. 2004;25(4):625–631. doi: 10.1016/s0142-9612(03)00571-4. [DOI] [PubMed] [Google Scholar]
- 29.Martin JY, Schwartz Z, Hummert TW, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) Journal of Biomedical Materials Research. 1995;29(3):389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz Z, Boyan BD. Underlying mechanisms at the bone-biomaterial interface. Journal of Cellular Biochemistry. 1994;56(3):340–347. doi: 10.1002/jcb.240560310. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz Z, Kieswetter K, Dean DD, Boyan BD. Underlying mechanisms at the bone-surface interface during regeneration. Journal of Periodontal Research. 1997;32(1, part 2):166–171. doi: 10.1111/j.1600-0765.1997.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 32.Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. Journal of Prosthetic Dentistry. 2000;84(5):522–534. doi: 10.1067/mpr.2000.111966. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald W, Campbell P, Fisher J, Wennerberg A. Variation in surface texture measurements. Journal of Biomedical Materials Research Part B. 2004;70(2):262–269. doi: 10.1002/jbm.b.30036. [DOI] [PubMed] [Google Scholar]
- 34.Andrade JD, Hlady V. Plasma protein adsorption: the big twelve. Annals of the New York Academy of Sciences. 1987;516:158–172. doi: 10.1111/j.1749-6632.1987.tb33038.x. [DOI] [PubMed] [Google Scholar]
- 35.Hakansson M, Linse S. Protein reconstitution and 3D domain swapping. Current Protein and Peptide Science. 2002;3(6):629–642. doi: 10.2174/1389203023380459. [DOI] [PubMed] [Google Scholar]
- 36.Iuliano DJ, Saavedra SS, Truskey GA. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. Journal of Biomedical Materials Research. 1993;27(8):1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- 37.Steele JG, Dalton BA, Johnson G, Underwood PA. Adsorption of fibronectin and vitronectin onto and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomaterials. 1995;16(14):1057–1067. doi: 10.1016/0142-9612(95)98901-p. [DOI] [PubMed] [Google Scholar]
- 38.Vroman L. The importance of surfaces in contact phase reactions. Seminars in Thrombosis and Hemostasis. 1987;13(1):79–85. doi: 10.1055/s-2007-1003477. [DOI] [PubMed] [Google Scholar]
- 39.Balcerzak M, Hamade E, Zhang L, et al. The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochimica Polonica. 2003;50(4):1019–1038. [PubMed] [Google Scholar]
- 40.Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. Journal of Cellular Biochemistry. 2003;88(1):104–112. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- 41.Weyts FA, Bosmans B, Niesing R, van Leeuwen JP, Weinans H. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcified Tissue International. 2003;72(4):505–512. doi: 10.1007/s00223-002-2027-0. [DOI] [PubMed] [Google Scholar]
- 42.Ingber DE. Mechanobiology and diseases of mechanotransduction. Annals of Medicine. 2003;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 43.Coppolino MG, Dedhar S. Bi-directional signal transduction by integrin receptors. The International Journal of Biochemistry and Cell Biology. 2000;32(2):171–188. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- 44.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 45.Howe A, Aplin AE, Alahari SK, Juliano R. Integrin signaling and cell growth control. Current Opinion in Cell Biology. 1998;10(2):220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 46.Meyer U, Meyer T, Jones DB. Attachment kinetics, proliferation rates and vinculin assembly of bovine osteoblasts cultured on different pre-coated artificial substrates. Journal of Materials Science: Materials in Medicine. 1998;9(6):301–307. doi: 10.1023/a:1008894612021. [DOI] [PubMed] [Google Scholar]
- 47.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. Journal of Cell Science. 1997;110(18):2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 48.Rezania A, Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. Journal of Biomedical Materials Research. 2000;52(4):595–600. doi: 10.1002/1097-4636(20001215)52:4<595::aid-jbm3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 50.Rouahi M, Champion E, Gallet O, Jada A, Anselme K. Physico-chemical characteristics and protein adsorption potential of hydroxyapatite particles: influence on in vitro biocompatibility of ceramics after sintering. Colloids and Surfaces B. 2006;47(1):10–19. doi: 10.1016/j.colsurfb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Webb K, Hlady V, Tresco PA. Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces. Journal of Biomedical Materials Research. 2000;49(3):362–368. doi: 10.1002/(sici)1097-4636(20000305)49:3<362::aid-jbm9>3.0.co;2-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schweikl H, Müller R, Englert C, et al. Proliferation of osteoblasts and fibroblasts on model surfaces of varying roughness and surface chemistry. Journal of Materials Science: Materials in Medicine. 2007 doi: 10.1007/s10856-007-3092-8. [DOI] [PubMed] [Google Scholar]
- 53.El-Ghannam AR, Ducheyne P, Risbud M, et al. Model surfaces engineered with nanoscale roughness and RGD tripeptides promote osteoblast activity. Journal of Biomedical Materials Research Part A. 2004;68(4):615–627. doi: 10.1002/jbm.a.20051. [DOI] [PubMed] [Google Scholar]
- 54.Andrade JD. Interfacial phenomena and biomaterials. Medical Instrumentation. 1973;7(2):110–119. [PubMed] [Google Scholar]
- 55.Heide H, Jones DB, Meyer U, Möller K, Priessnitz B, Szulczewski DH. The influence of zeta-potential and interfacial-tension on osteoblast-like cells. Cells and Materials. 1994;4(3):263–274. [Google Scholar]
- 56.Lim YJ, Oshida Y. Initial contact angle measurements on variously treated dental/medical titanium materials. Bio-Medical Materials and Engineering. 2001;11(4):325–341. [PubMed] [Google Scholar]
- 57.Jäger M, Urselmann F, Witte F, et al. Osteoblast differentiation onto different biometals with a endoprosthetic surface geometry in vitro. doi: 10.1002/jbm.a.31552. to appear in Journal of Biomedical Materials Research Part A . [DOI] [PubMed] [Google Scholar]
- 58.Redey SA, Nardin M, Bernache-Assolant D, et al. Behavior of human osteoblastic cells on stoichiometric hydroxyapatite and type A carbonate apatite: role of surface energy. Journal of Biomedical Materials Research. 2000;50(3):353–364. doi: 10.1002/(sici)1097-4636(20000605)50:3<353::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 59.Zhao G, Schwartz Z, Wieland M, et al. High surface energy enhances cell response to titanium substrate microstructure. Journal of Biomedical Materials Research Part A. 2005;74(1):49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 60.Stock C, Yang Y, Ong J. Protein adsorption and cell adhesion on different implant metals. The IADR/AADR/CADR 83rd General Session; 2005 March; Baltimore, Md, USA. [Google Scholar]
- 61.Bumgardner JD, Wiser R, Elder SH, Jouett R, Yang Y, Ong JL. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. Journal of Biomaterials Science, Polymer Edition. 2003;14(12):1401–1409. doi: 10.1163/156856203322599734. [DOI] [PubMed] [Google Scholar]
- 62.Qu Z, Rausch-Fan X, Wieland M, Matejka M, Schedle A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. Journal of Biomedical Materials Research Part A. 2007;82(3):658–668. doi: 10.1002/jbm.a.31023. [DOI] [PubMed] [Google Scholar]
- 63.Kern T, Yang Y, Glover R, Ong JL. Effect of heat-treated titanium surfaces on protein adsorption and osteoblast precursor cell initial attachment. Implant Dentistry. 2005;14(1):70–76. doi: 10.1097/01.id.0000154795.93155.ee. [DOI] [PubMed] [Google Scholar]
- 64.MacDonald DE, Rapuano BE, Deo N, Stranick M, Somasundaran P, Boskey AL. Thermal and chemical modification of titanium-aluminum-vanadium implant materials: effects on surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials. 2004;25(16):3135–3146. doi: 10.1016/j.biomaterials.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 65.García AJ, Boettiger D. Integrin-fibronectin interactions at the cell-material interface: initial integrin binding and signaling. Biomaterials. 1999;20(23-24):2427–2433. doi: 10.1016/s0142-9612(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 66.de Oliveira PT, Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials. 2004;25(3):403–413. doi: 10.1016/s0142-9612(03)00539-8. [DOI] [PubMed] [Google Scholar]
- 67.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731–4739. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Das K, Bose S, Bandyopadhyay A. Surface modifications and cell-materials interactions with anodized Ti. Acta Biomaterialia. 2007;3(4):573–585. doi: 10.1016/j.actbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Kim KH, Kwon TY, Kim SY, et al. Preparation and characterization of anodized titanium surfaces and their effect on osteoblast responses. The Journal of Oral Implantology. 2006;32(1):8–13. doi: 10.1563/741.1. [DOI] [PubMed] [Google Scholar]
- 70.Park J-M, Koak J-Y, Jang J-H, Han C-H, Kim S-K, Heo S-J. Osseointegration of anodized titanium implants coated with fibroblast growth factor-fibronectin (FGF-FN) fusion protein. International Journal of Oral and Maxillofacial Implants. 2006;21(6):859–866. [PubMed] [Google Scholar]
- 71.Sohn S-H, Jun H-K, Kim C-S, et al. Biological responses in osteoblast-like cell line according to thin layer hydroxyapatite coatings on anodized titanium. Journal of Oral Rehabilitation. 2006;33(12):898–911. doi: 10.1111/j.1365-2842.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 72.Rouahi M, Gallet O, Champion E, Dentzer J, Hardouin P, Anselme K. Influence of hydroxyapatite microstructure on human bone cell response. Journal of Biomedical Materials Research Part A. 2006;78(2):222–235. doi: 10.1002/jbm.a.30682. [DOI] [PubMed] [Google Scholar]
- 73.Chun AL, Moralez JG, Webster TJ, Fenniri H. Helical rosette nanotubes: a biomimetic coating for orthopedics? Biomaterials. 2005;26(35):7304–7309. doi: 10.1016/j.biomaterials.2005.05.080. [DOI] [PubMed] [Google Scholar]
- 74.Fenniri H, Mathivanan P, Vidale KL, et al. Helical rosette nanotubes: design, self-assembly, and characterization. Journal of the American Chemical Society. 2001;123(16):3854–3855. doi: 10.1021/ja005886l. [DOI] [PubMed] [Google Scholar]
- 75.Toworfe GK, Composto RJ, Adams CS, Shapiro IM, Ducheyne P. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. Journal of Biomedical Materials Research Part A. 2004;71(3):449–461. doi: 10.1002/jbm.a.30164. [DOI] [PubMed] [Google Scholar]
- 76.von Recum AF, van Kooten TG. The influence of micro-topography on cellular response and the implications for silicone implants. Journal of Biomaterials Science. 1995;7(2):181–198. doi: 10.1163/156856295x00698. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Cavin R, Ong JL. Protein adsorption on titanium surfaces and their effect on osteoblast attachment. Journal of Biomedical Materials Research Part A. 2003;67(1):344–349. doi: 10.1002/jbm.a.10578. [DOI] [PubMed] [Google Scholar]
- 78.Bierbaum S, Beutner R, Hanke T, Scharnweber D, Hempel U, Worch H. Modification of Ti6Al4V surfaces using collagen I, III, and fibronectin—I: biochemical and morphological characteristics of the adsorbed matrix. Journal of Biomedical Materials Research Part A. 2003;67(2):421–430. doi: 10.1002/jbm.a.10080. [DOI] [PubMed] [Google Scholar]
- 79.Goldstein AS, DiMilla PA. Examination of membrane rupture as a mechanism for mammalian cell detachment from fibronectin-coated biomaterials. Journal of Biomedical Materials Research Part A. 2003;67(2):658–666. doi: 10.1002/jbm.a.10125. [DOI] [PubMed] [Google Scholar]
- 80.van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PHM, Mikos AG, Jansen JA. Effect of fibronectin- and collagen I-coated titanium fiber mesh on proliferation and differentiation of osteogenic cells. Tissue Engineering. 2003;9(3):505–515. doi: 10.1089/107632703322066688. [DOI] [PubMed] [Google Scholar]
- 81.Cornell R. Cell-substrate adhesion during cell culture. An ultrastructural study. Experimental Cell Research. 1969;58(2-3):289–295. doi: 10.1016/0014-4827(69)90507-2. [DOI] [PubMed] [Google Scholar]
- 82.Curtis ASG, Varde M. Control of cell behavior: topological factors. Journal of the National Cancer Institute. 1964;33:15–26. [PubMed] [Google Scholar]
- 83.Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture—IV: electron microscopy of the leading lamella. Experimental Cell Research. 1971;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- 84.Izzard CS, Lochner LR. Cell to substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. Journal of Cell Science. 1976;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- 85.Balaban NQ, Schwartz US, Riveline D, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 86.Bigerelle M, Anselme K. Bootstrap analysis of the relation between initial adhesive events and long-term cellular functions of human osteoblasts cultured on biocompatible metallic substrates. Acta Biomaterialia. 2005;1(5):499–510. doi: 10.1016/j.actbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Wan Y, Wang Y, Liu Z, et al. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(L-lactide) Biomaterials. 2005;26(21):4453–4459. doi: 10.1016/j.biomaterials.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 88.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27(4):596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 89.Ikarashi Y, Tsuchiya T, Kaniwa M, Nakamura A. Activation of osteoblast-like MC3T3-E1 cell responses by poly(lactide) Biological and Pharmaceutical Bulletin. 2000;23(12):1470–1476. doi: 10.1248/bpb.23.1470. [DOI] [PubMed] [Google Scholar]
- 90.Isama K, Tsuchiya T. Enhancing effect of poly(L-lactide) on the differentiation of mouse osteoblast-like MC3T3-E1 cells. Biomaterials. 2003;24(19):3303–3309. doi: 10.1016/s0142-9612(03)00216-3. [DOI] [PubMed] [Google Scholar]
- 91.Liu H, Slamovich EB, Webster TJ. Increased osteoblast functions among nanophase titania/poly(lactide-co-glycolide) composites of the highest nanometer surface roughness. Journal of Biomedical Materials Research Part A. 2006;78(4):798–807. doi: 10.1002/jbm.a.30734. [DOI] [PubMed] [Google Scholar]
- 92.Woo KM, Jun J-H, Chen VJ, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Webster TJ, Smith TA. Increased osteoblast function on PLGA composites containing nanophase titania. Journal of Biomedical Materials Research Part A. 2005;74(4):677–686. doi: 10.1002/jbm.a.30358. [DOI] [PubMed] [Google Scholar]
- 94.Diener A, Nebe B, Lüthen F, et al. Control of focal adhesion dynamics by material surface characteristics. Biomaterials. 2005;26(4):383–392. doi: 10.1016/j.biomaterials.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 95.Benoit DSW, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26(25):5209–5220. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 96.Biebuyck HA, Whitesides GM. Self-organization of organic liquids on patterned self-assembled monolayers of alkanethiolates on gold. Langmuir. 1994;10(8):2790–2793. [Google Scholar]
- 97.Gau H, Herminghaus S, Lenz P, Lipowsky R. Liquid morphologies on structured surfaces: from microchannels to microchips. Science. 1999;283(5398):46–49. doi: 10.1126/science.283.5398.46. [DOI] [PubMed] [Google Scholar]
- 98.Kim E, Xia Y, Whitesides GM. Polymer microstructures formed by moulding in capillaries. Nature. 1995;376(6541):581–584. [Google Scholar]
- 99.Morra M, Cassinelli C, Cascardo G, et al. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials. 2003;24(25):4639–4654. doi: 10.1016/s0142-9612(03)00360-0. [DOI] [PubMed] [Google Scholar]
- 100.Sarikaya M, Tamerler C, Jen AK-Y, Schulten K, Baneyx F. Molecular biomimetics: nanotechnology through biology. Nature Materials. 2003;2(9):577–585. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 101.Xia Y, Whitesides GM. Extending microcontact printing as a microlithographic technique. Langmuir. 1997;13(7):2059–2067. [Google Scholar]
- 102.Zhang S, Marini DM, Hwang W, Santoso S. Design of nanostructured biological materials through self-assembly of peptides and proteins. Current Opinion in Chemical Biology. 2002;6(6):865–871. doi: 10.1016/s1367-5931(02)00391-5. [DOI] [PubMed] [Google Scholar]
- 103.Cutler SM, García AJ. Engineering cell adhesive surfaces that direct integrin binding using a recombinant fragment of fibronectin. Biomaterials. 2003;24(10):1759–1770. doi: 10.1016/s0142-9612(02)00570-7. [DOI] [PubMed] [Google Scholar]
- 104.Nagai M, Hayakawa T, Fukatsu A, et al. In vitro study of collagen coating of titanium implants for initial cell attachment. Dental Materials Journal. 2002;21(3):250–260. doi: 10.4012/dmj.21.250. [DOI] [PubMed] [Google Scholar]
- 105.Schliephake H, Scharnweber D, Dard M, Rößler S, Sewing A, Hüttmann C. Biological performance of biomimetic calcium phosphate coating of titanium implants in the dog mandible. Journal of Biomedical Materials Research Part A. 2003;64(2):225–234. doi: 10.1002/jbm.a.10363. [DOI] [PubMed] [Google Scholar]
- 106.Shi H, Tsai W-B, Garrison MD, Ferrari S, Ratner BD. Template-imprinted nanostructured surfaces for protein recognition. Nature. 1999;398(6728):593–597. doi: 10.1038/19267. [DOI] [PubMed] [Google Scholar]
- 107.Danen EHJ, Aota S-I, van Kraats AA, Yamada KM, Ruiter DJ, van Muijen GNP. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin . Journal of Biological Chemistry. 1995;270(37):21612–21618. doi: 10.1074/jbc.270.37.21612. [DOI] [PubMed] [Google Scholar]
- 108.Huveneers S, van den Bout I, Sonneveld P, Sancho A, Sonnenberg A, Danen EHJ. Integrin controls activity and oncogenic potential of primed c-Src. Cancer Research. 2007;67(6):2693–2700. doi: 10.1158/0008-5472.CAN-06-3654. [DOI] [PubMed] [Google Scholar]
- 109.Keselowsky BG, Bridges AW, Burns KL, et al. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials. 2007;28(25):3626–3631. doi: 10.1016/j.biomaterials.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. Journal of Biomedical Materials Research Part A. 2003;66(2):247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 111.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25(28):5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 112.Keselowsky BG, Collard DM, García AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Keselowsky BG, Wang L, Schwartz Z, García AJ, Boyan BD. Integrin controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. Journal of Biomedical Materials Research Part A. 2007;80(3):700–710. doi: 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- 114.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21(7):667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 115.Kononen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. Journal of Biomedical Materials Research. 1992;26(10):1325–1341. doi: 10.1002/jbm.820261006. [DOI] [PubMed] [Google Scholar]
- 116.Li D, Liu B, Wu J, Chen J. Bone interface of dental implants cytologically influenced by a modified sandblasted surface: a preliminary in vitro study. Implant Dentistry. 2001;10(2):132–138. doi: 10.1097/00008505-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 117.Curtis ASG, Wilkinson C. Nantotechniques and approaches in biotechnology. Trends in Biotechnology. 2001;19(3):97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 118.Dalby MJ, Di Silvio L, Davies GW, Bonfield W. Surface topography and HA filler volume effect on primary human osteoblasts in vitro. Journal of Materials Science: Materials in Medicine. 2000;11(12):805–810. doi: 10.1023/a:1008957630020. [DOI] [PubMed] [Google Scholar]
- 119.Wang J-H, Yao C-H, Chuang W-Y, Young T-H. Development of biodegradable polyesterurethane membranes with different surface morphologies for the culture of osteoblasts. Journal of Biomedical Materials Research. 2000;51(4):761–770. doi: 10.1002/1097-4636(20000915)51:4<761::aid-jbm26>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 120.Domke J, Dannöhl S, Parak WJ, Müller O, Aicher WK, Radmacher M. Substrate dependent differences in morphology and elasticity of living osteoblasts investigated by atomic force microscopy. Colloids and Surfaces B. 2000;19(4):367–379. doi: 10.1016/s0927-7765(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 121.Gleiche M, Chi LF, Fuchs H. Nanoscopic channel lattices with controlled anisotropic wetting. Nature. 2000;403(6766):173–175. doi: 10.1038/35003149. [DOI] [PubMed] [Google Scholar]
- 122.Lenhert S, Meier M-B, Meyer U, Chi L, Wiesmann HP. Osteoblast alignment, elongation and migration on grooved polystyrene surfaces patterned by Langmuir-Blodgett lithography. Biomaterials. 2005;26(5):563–570. doi: 10.1016/j.biomaterials.2004.02.068. [DOI] [PubMed] [Google Scholar]
- 123.Martines E, McGhee K, Wilkinson C, Curtis ASG. A parallel-plate flow chamber to study initial cell adhesion on a nano featured surface. IEEE Transactions on Nanobioscience. 2004;3(2):90–95. doi: 10.1109/tnb.2004.828268. [DOI] [PubMed] [Google Scholar]
- 124.Mustafa K, Wennerberg A, Wroblewski J, Hultenby K, Lopez BS, Arvidson K. Determining optimal surface roughness of blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clinical Oral Implants Research. 2001;12(5):515–525. doi: 10.1034/j.1600-0501.2001.120513.x. [DOI] [PubMed] [Google Scholar]
- 125.Mustafa K, Wroblewski J, Hultenby K, Lopez BS, Arvidson K. Effects of titanium surfaces blasted with particles on the initial attachment of cells derived from human mandibular bone: a scanning electron microscopic and histomorphometric analysis. Clinical Oral Implants Research. 2000;11(2):116–128. [PubMed] [Google Scholar]
- 126.Anselme K, Bigerelle M, Noel B, et al. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. Journal of Biomedical Materials Research. 1999;49(2):155–166. doi: 10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 127.Eisenbarth E, Velten D, Müller M, Thull R, Breme J. Nanostructured niobium oxide coatings influence osteoblast adhesion. Journal of Biomedical Materials Research Part A. 2006;79(1):166–175. doi: 10.1002/jbm.a.30823. [DOI] [PubMed] [Google Scholar]
- 128.Kim H-W, Kim H-E, Knowles JC. Fluor-hydroxyapatite sol-gel coating on titanium substrate for hard tissue implants. Biomaterials. 2004;25(17):3351–3358. doi: 10.1016/j.biomaterials.2003.09.104. [DOI] [PubMed] [Google Scholar]
- 129.Oh S, Daraio C, Chen L-H, Pisanic TR, Fiñones RR, Jin S. Significantly accelerated osteoblast cell growth on aligned nanotubes. Journal of Biomedical Materials Research Part A. 2006;78(1):97–103. doi: 10.1002/jbm.a.30722. [DOI] [PubMed] [Google Scholar]
- 130.Veis AA, Papadimitriou S, Trisi P, Tsirlis AT, Parissis NA, Kenealy JN. Osseointegration of and machined-surfaced titanium implants in membrane-covered critical-sized defects: a histologic and histometric study in dogs. Clinical Oral Implants Research. 2007;18(2):153–160. doi: 10.1111/j.1600-0501.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- 131.Persson LG, Mouhyi J, Berglundh T, Sennerby L, Lindhe J. Carbon dioxide laser and hydrogen peroxide conditioning in the treatment of periimplantitis: an experimental study in the dog. Clinical Implant Dentistry and Related Research. 2004;6(4):230–238. doi: 10.1111/j.1708-8208.2004.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 132.Zhu L, Ye X, Tang G, et al. Biomimetic coating of compound titania and hydroxyapatite on titanium. Journal of Biomedical Materials Research Part A. 2007 doi: 10.1002/jbm.a.31401. [DOI] [PubMed] [Google Scholar]
- 133.Mancini L, Tamma R, Settanni MP, et al. Osteoblasts cultured on three-dimensional synthetic hydroxyapatite implanted on a chick Allantochorial membrane induce ectopic bone marrow differentiation. Annals of the New York Academy of Sciences. 2007 doi: 10.1196/annals.1402.008. [DOI] [PubMed] [Google Scholar]
- 134.Yang CY, Lee TM, Yang CW, Chen LR, Wu MC, Lui TS. In vitro and in vivo biological responses of plasma-sprayed hydroxyapatite coatings with posthydrothermal treatment. Journal of Biomedical Materials Research Part A. 2007 doi: 10.1002/jbm.a.31246. [DOI] [PubMed] [Google Scholar]
- 135.Curtis ASG, Wilkinson C. New depths in cell behaviour: reactions of cells to nanotopography. Biochemical Society Symposium. 1999;65:15–26. [PubMed] [Google Scholar]
- 136.Dalby MJ, Yarwood SJ, Riehle MO, Johnstone HJH, Affrossman S, Curtis ASG. Increasing fibroblast response to materials using nanotopography: morphological and genetic measurements of cell response to 13-nm-high polymer demixed islands. Experimental Cell Research. 2002;276(1):1–9. doi: 10.1006/excr.2002.5498. [DOI] [PubMed] [Google Scholar]
- 137.Scotchford CA, Ball M, Winkelmann M, et al. Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein- and cell-interactions—II: protein adsorption and early cell interactions. Biomaterials. 2003;24(7):1147–1158. doi: 10.1016/s0142-9612(02)00488-x. [DOI] [PubMed] [Google Scholar]
- 138.Rice JM, Hunt JA, Gallagher JA, Hanarp P, Sutherland DS, Gold J. Quantitative assessment of the response of primary derived human osteoblasts and macrophages to a range of nanotopography surfaces in a single culture model in vitro. Biomaterials. 2003;24(26):4799–4818. doi: 10.1016/s0142-9612(03)00381-8. [DOI] [PubMed] [Google Scholar]
- 139.Rouahi M, Champion E, Hardouin P, Anselme K. Quantitative kinetic analysis of gene expression during human osteoblastic adhesion on orthopaedic materials. Biomaterials. 2006;27(14):2829–2844. doi: 10.1016/j.biomaterials.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 140.Hendrich C, Nöth U, Stahl U, et al. Testing of skeletal implant surfaces with human fetal osteoblasts. Clinical Orthopaedics & Related Research. 2002;(394):278–289. doi: 10.1097/00003086-200201000-00033. [DOI] [PubMed] [Google Scholar]
- 141.Hallgren C, Reimers H, Gold J, Wennerberg A. The importance of surface texture for bone integration of screw shaped implants: an in vivo study of implants patterned by photolithography. Journal of Biomedical Materials Research. 2001;57(4):485–496. doi: 10.1002/1097-4636(20011215)57:4<485::aid-jbm1194>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 142.Hamilton DW, Wong KS, Brunette DM. Microfabricated discontinuous-edge surface topographies influence osteoblast adhesion, migration, cytoskeletal organization, and proliferation and enhance matrix and mineral deposition in vitro. Calcified Tissue International. 2006;78(5):314–325. doi: 10.1007/s00223-005-0238-x. [DOI] [PubMed] [Google Scholar]
- 143.Germanier Y, Tosatti S, Broggini N, Textor M, Buser D. Enhanced bone apposition around biofunctionalized sandblasted and acid-etched titanium implant surfaces: a histomorphometric study in miniature pigs. Clinical Oral Implants Research. 2006;17(3):251–257. doi: 10.1111/j.1600-0501.2005.01222.x. [DOI] [PubMed] [Google Scholar]
- 144.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27(32):5561–5571. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 145.Slaets E, Carmeliet G, Naert I, Duyck J. Early cellular responses in cortical bone healing around unloaded titanium implants: an animal study. Journal of Periodontology. 2006;77(6):1015–1024. doi: 10.1902/jop.2006.050196. [DOI] [PubMed] [Google Scholar]
- 146.Vallés G, González-Melendi P, González-Carrasco JL, et al. Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials. 2006;27(30):5199–5211. doi: 10.1016/j.biomaterials.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 147.Müller U, Imwinkelried T, Horst M, Sievers M, Graf-Hausner U. Do human osteoblasts grow into open-porous titanium? European Cells and Materials. 2006;11:8–15. doi: 10.22203/ecm.v011a02. [DOI] [PubMed] [Google Scholar]
- 148.Li JP, Li SH, Van Blitterswijk CA, de Groot K. A novel porous Ti6A14V: characterization and cell attachment. Journal of Biomedical Materials Research Part A. 2005;73(2):223–233. doi: 10.1002/jbm.a.30278. [DOI] [PubMed] [Google Scholar]
- 149.Ward BC, Webster T. Effect of metal substrate nanometer topography on osteoblast metabolic activities. Biological and Bioinspired Materials and Devices; Materials Research Society; 2004. Apr, pp. 249–254. [Google Scholar]
- 150.Ward BC, Webster T. Effect of metal substrate nanometer topography on osteoblast metabolic activities. TMS Conference Proceedings; 2005. pp. 13–17. [Google Scholar]
- 151.Bibby JK, Bubb NL, Wood DJ, Mummery PM. Fluorapatite-mullite glass sputter coated Ti6Al4V for biomedical applications. Journal of Materials Science. 2005;16(5):379–385. doi: 10.1007/s10856-005-6975-6. [DOI] [PubMed] [Google Scholar]
- 152.Isa ZM, Schneider GB, Zaharias R, Seabold D, Stanford CM. Effects of fluoride-modified titanium surfaces on osteoblast proliferation and gene expression. The International Journal of Oral & Maxillofacial Implants. 2006;21(2):203–211. [PubMed] [Google Scholar]
- 153.Ber S, Torun Köse G, Hasirci V. Bone tissue engineering on patterned collagen films: an in vitro study. Biomaterials. 2005;26(14):1977–1986. doi: 10.1016/j.biomaterials.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 154.García AJ, Reyes CD. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. Journal of Dental Research. 2005;84(5):407–413. doi: 10.1177/154405910508400502. [DOI] [PubMed] [Google Scholar]
- 155.Jäger M, Feser T, Denck H, Krauspe R. Proliferation and osteogenic differentiation of mesenchymal stem cells cultured onto three different polymers in vitro. Annals of Biomedical Engineering. 2005;33(10):1319–1332. doi: 10.1007/s10439-005-5889-2. [DOI] [PubMed] [Google Scholar]
- 156.Yonggang Y, Wolke JG, Yubao L, Jansen JA. In vitro evaluation of different heat-treated radio frequency magnetron sputtered calcium phosphate coatings. Clinical Oral Implants Research. 2007;18(3):345–353. doi: 10.1111/j.1600-0501.2006.01332.x. [DOI] [PubMed] [Google Scholar]
- 157.Baumann B, Rader CP, Seufert J, et al. Effects of polyethylene and TiAIV wear particles on expression of RANK, RANKL and OPG mRNA. Acta Orthopaedica Scandinavica. 2004;75(3):295–302. doi: 10.1080/00016470410001222. [DOI] [PubMed] [Google Scholar]
- 158.Huang J, Best SM, Bonfield W, et al. In vitro assessment of the biological response to nano-sized hydroxyapatite. Journal of Materials Science. 2004;15(4):441–445. doi: 10.1023/b:jmsm.0000021117.67205.cf. [DOI] [PubMed] [Google Scholar]
- 159.Peters K, Unger RE, Kirkpatrick CJ, Gatti AM, Monari E. Effects of nano-scaled particles on endothelial cell function in vitro: studies on viability, proliferation and inflammation. Journal of Materials Science. 2004;15(4):321–325. doi: 10.1023/b:jmsm.0000021095.36878.1b. [DOI] [PubMed] [Google Scholar]
- 160.Bruni S, Martinesi M, Stio M, Treves C, Bacci T, Borgioli F. Effects of surface treatment of Ti-6AI-4V titanium alloy on biocompatibility in cultured human umbilical vein endothelial cells. Acta Biomaterialia. 2005;1(2):223–234. doi: 10.1016/j.actbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 161.Eriksson AS, Thomsen P. Leukotriene , interleukin 1 and leucocyte accumulation in titanium and PTFE chambers after implantation in the rat abdominal wall. Biomaterials. 1991;12(9):827–830. doi: 10.1016/0142-9612(91)90069-m. [DOI] [PubMed] [Google Scholar]
- 162.Giudiceandrea F, Iacona A, Cervelli G, et al. Mechanisms of bone resorption: analysis of proinflammatory cytokines in peritoneal macrophages from titanium implant—an experimental design. Journal of Craniofacial Surgery. 1998;9(3):254–259. [PubMed] [Google Scholar]
- 163.Martinesi M, Bruni S, Stio M, Treves C, Borgioli F. In vitro interaction between surface-treated Ti-6Al-4V titanium alloy and human peripheral blood mononuclear cells. Journal of Biomedical Materials Research Part A. 2005;74(2):197–207. doi: 10.1002/jbm.a.30366. [DOI] [PubMed] [Google Scholar]
- 164.Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. Journal of Biomedical Materials Research Part A. 2004;70(2):194–205. doi: 10.1002/jbm.a.30075. [DOI] [PubMed] [Google Scholar]
- 165.Brodbeck WG, Patel J, Voskerician G, et al. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morais S, Dias N, Sousa JP, Fernandes MH, Carvalho GS. In vitro osteoblastic differentiation of human bone marrow cells in the presence of metal ions. Journal of Biomedical Materials Research. 1999;44(2):176–190. doi: 10.1002/(sici)1097-4636(199902)44:2<176::aid-jbm8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 167.Morais S, Sousa JP, Fernandes MH, Carvalho GS, de Bruijn JD, van Blitterswijk CA. Effects of AISI 316L corrosion products in in vitro bone formation. Biomaterials. 1998;19(11-12):999–1007. doi: 10.1016/s0142-9612(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 168.Puleo DA, Huh WW. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells. Journal of Applied Biomaterials. 1995;6(2):109–116. doi: 10.1002/jab.770060205. [DOI] [PubMed] [Google Scholar]
- 169.Lin H-Y, Bumgardner JD. Changes in the surface oxide composition of Co-Cr-Mo implant alloy by macrophage cells and their released reactive chemical species. Biomaterials. 2004;25(7-8):1233–1238. doi: 10.1016/j.biomaterials.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 170.Lin H-Y, Bumgardner JD. In vitro biocorrosion of Ti-6AI-4V implant alloy by a mouse macrophage cell line. Journal of Biomedical Materials Research Part A. 2004;68(4):717–724. doi: 10.1002/jbm.a.20092. [DOI] [PubMed] [Google Scholar]
- 171.Lin H-Y, Bumgardner JD. In vitro biocorrosion of Co-Cr-Mo implant alloy by macrophage cells. Journal of Orthopaedic Research. 2004;22(6):1231–1236. doi: 10.1016/j.orthres.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 172.Hunter A, Archer CW, Walker PS, Blunn GW. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials. 1995;16(4):287–295. doi: 10.1016/0142-9612(95)93256-d. [DOI] [PubMed] [Google Scholar]
- 173.Kuroda S, Takeda S, Nakamura M. Effects of six particulate metals on osteoblast-like MG-63 and HOS cells in vitro. Dental Materials Journal. 2003;22(4):507–520. doi: 10.4012/dmj.22.507. [DOI] [PubMed] [Google Scholar]
- 174.Granchi D, Amato I, Battistelli L, et al. Molecular basis of osteoclastogenesis induced by osteoblasts exposed to wear particles. Biomaterials. 2005;26(15):2371–2379. doi: 10.1016/j.biomaterials.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 175.Rader CP, Baumann B, Rolf O, et al. Detection of differentially expressed genes in particle disease using array-filter analysis. Biomedizinische Technik. 2002;47(5):111–116. doi: 10.1515/bmte.2002.47.5.111. [DOI] [PubMed] [Google Scholar]
- 176.O'Connor DT, Choi MG, Kwon SY, Paul Sung K-L. New insight into the mechanism of hip prosthesis loosening: effect of titanium debris size on osteoblast function. Journal of Orthopaedic Research. 2004;22(2):229–236. doi: 10.1016/S0736-0266(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 177.Miyanishi K, Trindade MC, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. Journal of Bone and Mineral Research. 2003;18(9):1573–1583. doi: 10.1359/jbmr.2003.18.9.1573. [DOI] [PubMed] [Google Scholar]
- 178.Nakashima Y, Sun D-H, Trindade MC, et al. Signaling pathways for tumor necrosis factor- and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. Journal of Bone and Joint Surgery, American. 1999;81(5):603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 179.Dean DD, Schwartz Z, Liu Y, et al. The effect of ultra-high molecular weight polyethylene wear debris on MG-63 osteosarcoma cells in vitro. Journal of Bone and Joint Surgery, American. 1999;81(4):452–461. doi: 10.2106/00004623-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 180.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 181.Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials. 1998;19(24):2297–2302. doi: 10.1016/s0142-9612(98)00140-9. [DOI] [PubMed] [Google Scholar]
- 182.Yao J, Glant TT, Lark MW, et al. The potential role of fibroblasts in periprosthetic osteolysis: fibroblast response to titanium particles. Journal of Bone and Mineral Research. 1995;10(9):1417–1427. doi: 10.1002/jbmr.5650100920. [DOI] [PubMed] [Google Scholar]
- 183.Manlapaz M, Maloney WJ, Smith RL. In vitro activation of human fibroblasts by retrieved titanium alloy wear debris. Journal of Orthopaedic Research. 1996;14(3):465–472. doi: 10.1002/jor.1100140317. [DOI] [PubMed] [Google Scholar]
- 184.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Giant TT. Effects of particles on fibroblast proliferation and bone resorption in vitro. Clinical Orthopaedics & Related Research. 1997;(342):205–217. [PubMed] [Google Scholar]
- 185.Santavirta S, Takagi M, Nordsletten L, Anttila A, Lappalainen R, Konttinen YT. Biocompatibility of silicon carbide in colony formation test in vitro: a promising new ceramic THR implant coating material. Archives of Orthopaedic and Trauma Surgery. 1998;118(1-2):89–91. doi: 10.1007/s004020050319. [DOI] [PubMed] [Google Scholar]
- 186.Sun J-S, Lin F-H, Hung T-Y, Tsuang Y-H, Chang WH-S, Liu H-C. The influence of hydroxyapatite particles on osteoclast cell activities. Journal of Biomedical Materials Research. 1999;45(4):311–321. doi: 10.1002/(sici)1097-4636(19990615)45:4<311::aid-jbm5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 187.Green TR, Fisher J, Matthews JB, Stone MH, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. Journal of Biomedical Materials Research. 2000;53(5):490–497. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 188.Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. Journal of Biomedical Materials Research. 2002;59(3):507–515. doi: 10.1002/jbm.1264. [DOI] [PubMed] [Google Scholar]
- 189.Wilke A, Endres S, Griss P, Herz U. Cytokine profile of a human bone marrow cell culture on exposure to titanium-aluminium-vanadium particles. Zeitschrift für Orthopädie und Ihre Grenzgebiete. 2002;140(1):83–89. doi: 10.1055/s-2002-22096. [DOI] [PubMed] [Google Scholar]
- 190.Germain MA, Hatton A, Williams S, et al. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials. 2003;24(3):469–479. doi: 10.1016/s0142-9612(02)00360-5. [DOI] [PubMed] [Google Scholar]
- 191.Howling GI, Sakoda H, Antonarulrajah A, et al. Biological response to wear debris generated in carbon based composites as potential bearing surfaces for artificial hip joints. Journal of Biomedical Materials Research Part B. 2003;67(2):758–764. doi: 10.1002/jbm.b.10068. [DOI] [PubMed] [Google Scholar]
- 192.Granchi D, Ciapetti G, Amato I, et al. The influence of alumina and ultra-high molecular weight polyethylene particles on osteoblast-osteoclast cooperation. Biomaterials. 2004;25(18):4037–4045. doi: 10.1016/j.biomaterials.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 193.Howling GI, Ingham E, Sakoda H, et al. Carbon-carbon composite bearing materials in hip arthroplasty: analysis of wear and biological response to wear debris. Journal of Materials Science. 2004;15(1):91–98. doi: 10.1023/b:jmsm.0000010102.26218.d1. [DOI] [PubMed] [Google Scholar]
- 194.Barrias CC, Ribeiro CC, Lamghari M, Miranda CS, Barbosa MA. Proliferation, activity, and osteogenic differentiation of bone marrow stromal cells cultured on calcium titanium phosphate microspheres. Journal of Biomedical Materials Research Part A. 2005;72(1):57–66. doi: 10.1002/jbm.a.30217. [DOI] [PubMed] [Google Scholar]
- 195.Petit A, Mwale F, Antoniou J, Zukor DJ, Huk OL. Effect of bisphosphonates on the stimulation of macrophages by alumina ceramic particles: a comparison with ultra-high-molecular-weight polyethylene. Journal of Materials Science. 2006;17(7):667–673. doi: 10.1007/s10856-006-9230-x. [DOI] [PubMed] [Google Scholar]
- 196.Tan WB, Huang N, Zhang Y. Ultrafine biocompatible chitosan nanoparticles encapsulating multi-coloured quantum dots for bioapplications. Journal of Colloid and Interface Science. 2007;310(2):464–470. doi: 10.1016/j.jcis.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 197.Sayes CM, Liang F, Hudson JL, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicology Letters. 2006;161(2):135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 198.Koyama S, Haniu H, Osaka K, et al. Medical application of carbon-nanotube-filled nanocomposites: the microcatheter. Small. 2006;2(12):1406–1411. doi: 10.1002/smll.200500416. [DOI] [PubMed] [Google Scholar]
- 199.Liopo AV, Stewart MP, Hudson J, Tour JM, Pappas TC. Biocompatibility of native and functionalized single-walled carbon nanotubes for neuronal interface. Journal of Nanoscience and Nanotechnology. 2006;6(5):1365–1374. doi: 10.1166/jnn.2006.155. [DOI] [PubMed] [Google Scholar]
- 200.Nahar M, Dutta T, Murugesan S, et al. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Critical Reviews in Therapeutic Drug Carrier Systems. 2006;23(4):259–318. doi: 10.1615/critrevtherdrugcarriersyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 201.Warashina H, Sakano S, Kitamura S, et al. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials. 2003;24(21):3655–3661. doi: 10.1016/s0142-9612(03)00120-0. [DOI] [PubMed] [Google Scholar]