Abstract
Indoor air quality has become increasingly important as we live in a society where the majority of our time is spent indoors. Specific attention has been drawn to airborne fungal spores as a factor affecting indoor air quality. This study targeted shortcomings of other studies by utilizing long-term air sampling and total fungal spore enumeration to determine associations between health outcomes and fungal spore concentrations. Infants (n = 144) were clinically evaluated and had skin prick tests (SPT) for 17 allergens. Airborne fungal spores were collected using a Button Personal Inhalable Sampler (SKC Inc.) for 48 h at a flow rate of 4 l/min. Sampling was conducted in the spring (March–May) or fall (August–October) in 2003–2004. Fungal spores were analyzed using microscopy-based total counting and identified to the genus/group level. Total spore and individual genus concentrations were analyzed for associations with rhinitis and positive SPT results. Overall, concentrations varied widely, between <2 and 2294 spores/m3. While no relationship was observed between SPT(+) and total fungal counts, several significant associations were found when analysis was conducted on the various fungal genera and health outcomes. Positive associations were obtained between: Basidiospores and rhinitis (p < 0.01), Penicillium/Aspergillus and SPT(+) to any allergen (p < 0.01), and Alternaria and SPT(+) to any allergen (p < 0.01). Inverse associations were found between: Cladosporium and SPT(+) to any allergen (p < 0.05), and Cladosporium and SPT(+) to aeroallergens (p < 0.05). This study indicates that health outcome may vary by fungal genera; some fungal types may have sensitizing effects while others may have a beneficial role.
Keywords: fungal spores, inhalation exposure, air microbiology, allergic sensitization, sampling, child, rhinitis
In a society, where we spend the majority of our time inside, the effect of indoor air contaminants on human health has become increasingly important. Of particular interest is the health outcome that may result from exposure to fungi, as well as other microbial contaminants. This interest is largely based on research, which has determined that exposures to biological agents in occupational and residential indoor environments are associated with adverse health effects that have broad public health implications (1). There is increasing evidence that fungal growth indoors is a risk factor for the development of childhood asthma and allergies (2). Small children have become the focus of many studies on asthma and allergies because they have a higher incidence of allergies than adults, and they spend the majority of their time in the home (3). Early childhood exposure to fungi may be important for many reasons. A natural stimulation of the immune development of children is required for proper immune system development. However, exposure to fungi at a young age may perturb this system and increase the risk for reactions to inhaled antigens and irritants found in the environment (4).
According to published data, the majority of identified airborne fungal species have been characterized as potential allergens, and exposure to airborne fungi may provoke specific immune responses. Previous studies have found associations between skin sensitivity to Alternaria allergen and exposure to fungal mycelial fragments and spores of Alternaria fungi using skin prick testing (SPT) (5). Bobbit et al. (6) reported a strong correlation among atopy, fungal sensitization, and sensitization to specific fungi that were identified in the patient’s environmental report. Furthermore, an increase in the relative risk of fungi sensitization in symptomatic children with allergic rhinitis has been observed utilizing percutaneous skin testing. It has also been determined that patients who are sensitized to fungi may display a SPT(+) to more than one fungal species. It has also been found that children who were sensitized to fungi were also more likely to be sensitized to other aeroallergens (7).
Several studies have reported an association between moisture and/or visible fungal growth with the reporting of rhinitis symptoms (8). The mechanisms by which fungi and home dampness are associated with rhinitis and respiratory symptoms are not well defined (9).
In addition to the reports on health effects commonly associated with fungal exposures (i.e. asthma, allergies, and wheezing), some studies have shown that exposure to fungal spores may be associated with a decreased risk of developing atopic asthma in adults (10). Similar trends have been observed among children living in rural areas with high exposure to bacterial endotoxins (11). This inverse relationship of microbial exposures and health outcomes is consistent with the ‘hygiene hypothesis’ (12), which states that microbial exposures (particularly endotoxin exposure) early in life may actually protect from the development of atopy and allergic asthma, although the mechanisms for such beneficial effects are not yet well elaborated (13).
While most studies have utilized the practice of culture-based sampling and analysis, there are some shortcomings in using this method. Culturable count is only an indicator of the number of viable, or ‘live’ fungal spores that are present within the space. Although this method is useful when identifying spores at the species level, it does not account for the airborne fungal spores that are present, but not culturable, and may grossly underestimate actual airborne fungal spore concentrations (14). Furthermore, the type of agar media, the time required for incubation, and the growth conditions may be more favorable for certain fungal genera, and may therefore, inhibit the growth of others.
While several authors have reported a positive association between visible fungal growth and health outcome, previous investigations have found little or no association between airborne fungal spore concentrations and health outcomes (8). This may be because of the limitations of the sampling and analytical methods that were used in the previous studies, given that culture-based analytical techniques were utilized and that the sampling duration was too short to account for concentration variability. In this study, the relationship between airborne fungal spore concentrations and rhinitis and SPT(+) were investigated by utilizing a long-term sampling and total fungal spore enumeration.
Materials and methods
Cohort
This study is part of the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) (15). All families were enrolled in the CCAAPS study from early 2002 through 2003. For a child to be enrolled one parent had to have tested positive to one of 15 aeroallergens. The CCAAPS cohort study design includes a subgroup of children in a nested case–control study (atopic children and controls matched on birth date within 1 month). Overall, 37.5% of the cohort reported rhinitis. Within the case group 38.5% had rhinitis vs. 36.1% of the controls. Biannual air sampling was attempted in all of the homes of cases and controls. However, not all parents agreed to have the indoor air sampling conducted in their homes. In an effort to use all of the data, all homes that had indoor air sampling were included in the unmatched statistical analysis. For this study, all subjects were between the ages of one and three (mean = 1.7 yr) at the time of the home visit. Parents signed an informed consent form approved by the Institutional Review Board at the University of Cincinnati.
Air sample collection
Airborne fungal spores were collected using a Button Personal Inhalable Aerosol Sampler (SKC Inc., PA, USA). The sampler collects inhalable particles onto a 25 mm polycarbonate membrane filter of 3 μm pore size (GE Osmonics Inc., Minnetonka, MN, USA), and has 99% collection efficiency for 1 μm diameter particles. Samples were collected in 144 homes at a flow rate of 4 l/min for a period of 48 h. Samples were collected in the spring and in the fall. A child’s home was evaluated on average within 8 months of the clinic visit identifying the child as skin prick test positive (SPT+) or negative (SPT−). This study includes the first 2 yr of sampling data and thus, the samples were collected in 2003 and 2004.
Indoor stationary samples were collected in the room that the child’s parent reported as the primary activity room. Areas selected were most typically located in the living/family room of the home (55%). The parent or caregiver was asked to relocate the sampler to the child’s bedroom while the child slept. The sampler was placed in a small noise-insulated carrying box (approximately 11″ × 8″ × 10″). A questionnaire was utilized at the conclusion of the sampling session in order to determine the approximate length of time that the sampler was in the same room as the child. It was determined that the average time the child spent in the home during the 48 h sampling session was 39 h (81% of time), and during those 39 h, the sampler was in the same room as the child for 35 h (73% of time).
Outdoor samples were collected with the Button sampler at the CCAAPS monitoring station established to monitor outdoor aeroallergens (16). All outdoor air samples were collected at a flow rate of 4 l/min for a period of 24 h. All air sampling pumps were calibrated before and after the sampling period using a Bios DryCal® DC-Lite Calibrator (SKC Inc.).
Sample preparation and analysis
Fungal spore samples were analyzed by microscopic counting. Microscopy-based enumeration of fungal spores is an alternative exposure assessment method in which all spores are counted under a microscope regardless of their culturability. As it allows for the determination of the total fungal spore count, it is believed to better represent the airborne fungal spore concentrations to which children might be exposed. Additionally, the sample analysis can be preformed immediately after collection, in contrast to the culture-based method that requires incubation.
Following the sampling period, filters were removed from the filter holders. The collected airborne particles were then extracted into 2 ml of phosphate buffer. An 800 μl aliquot of the suspension was made available for the analysis of total fungal spore counts. Samples were prepared for microscopic analysis by filtering 800 μl of the suspension through a 13 mm diameter membrane of cellulose ester (MCE) filter. Each filter was placed onto a slide and allowed to completely dry. During the drying process the filter was isolated in a covered petri plate. Once the filter was dry, the filter was made clear by treating the filter with acetone vapor as described by Adhikari et al. (16).
Fungal spores in all air samples were counted using a bright light microscope (Olympus CX31; Olympus America Inc., Melville, NY or Labophotz, Nikon Corp., Japan) at a magnification of 600× or 400×. Samples were analyzed in two separate laboratories, and quality control procedures showed no difference in the analysis of samples between the laboratories. The counting was conducted by examining 40 randomly selected fields of view. Fungal spores were identified morphologically as described in Adhikari et al. (16). All fungal spores were identified to the genus or group level when possible. If the identification of the fungal spore was unknown, the spore was listed as an unidentified spore. Background debris on the sample was identified and recorded. Undercounting of small and hyaline spores may occur in samples containing high amounts of background debris. Therefore, phase contrast was used to identify hyaline spores. Fungal spore concentration was calculated according to Adhikari et al. (16). The limit of detection (LOD) was two spores per cubic meter of air.
Health outcomes
Airborne fungal spore concentrations were examined to determine if there was an association between the concentrations and health outcomes of rhinitis and allergen sensitization determined by SPT. Skin prick tests were administered into the superficial epidermis on the child’s back and observed for local reactions to two food allergens (milk and egg) and 15 aeroallergens (Meadow grass, Timothy grass, White Oak, Maple Mix, American Elm, Red Cedar, Short Ragweed, Alternaria, Aspergillus fumigatus, Penicillium mix, Cladosporium, cat, dog, German cockroach, and house dust mite). Children who showed a positive reaction (>3 mm wheel, greater than saline control) to any of the antigens were classified as sensitized.
Information on rhinitis within the cohort was obtained by a healthcare professional administering a symptom questionnaire to the parent of the child. Parents were asked if the infant had a history of sneezing, runny nose, or stuffy nose when the child did not have a cold or flu. The questionnaire was administered to the parent during the child’s first physician’s office visit, at approximately 13 months of age. Rhinitis symptoms were divided into three categories: (i) any rhinitis (sensitized or non-sensitized without consideration of original case–control status), (ii) rhinitis combined with sensitization to any allergen (food or aeroallergen), and (iii) rhinitis combined with sensitization to at least one of the 15 aeroallergens.
Statistical analysis
All statistical analyzes were conducted using SPSS software, Version 12.0, for Windows.
The focus of this study was to evaluate the unadjusted effects of fungal exposure on childhood allergy and sensitization. Descriptive statistics of each fungal genera and total fungal spore concentrations were obtained after the log transformation was applied to approximate normality as judged by Kolmogorov–Smirnov tests. These included the geometric mean (GM) and 95% confidence intervals (95% CI) for characterizing averages and expected ranges of mean values of fungal spore concentrations. Many of the values for the individual fungal genera were below the LOD and were recorded as zero. Between 10% and 30% of the four most frequent fungal types were observed as having values below the LOD, and between 40% and 80% of the samples for the less frequent fungal types were observed as having values below the LOD. In order to apply the log-transformation a value of one was added to all concentration values before analysis (and deducting one from the result obtained) (17).
Log-transformed data on spore concentrations were tested for seasonal differences using analysis of variance and Student’s t-test. Seasons were combined if the average concentrations were not significantly different in order to determine if the statistical analyzes of health outcomes would need to be controlled for such differences. All analyzes were controlled for season as an association between airborne fungal spore concentration and season was observed. Total concentrations of airborne fungi as well as concentrations of the most common fungal genera were evaluated for associations between concentrations and health outcomes. Fungal concentrations were used as the independent variable in data analyzes and health outcomes (rhinitis symptoms and SPT sensitivity) served as dependent variables.
The total concentration and four most frequent fungal genera (observed in 70% or more of the homes) were modeled continuously as independent variables in separate logistic regression models and adjusted for season. This allowed us to determine if there were any associations between studied parameters and health outcomes. For the fungal genera that occurred in 15–70% of the homes, concentrations were ranked categorically as either below or above the LOD, and the analysis was subsequently conducted using a chi-square (χ2) test to evaluate associations between spore concentrations and health outcomes. Unidentified spores and fungal types that occurred in <15% of the homes were not evaluated. Environmental data closest to the child’s first physician’s visit were utilized for the health outcome analysis (n = 144).
Results
Frequency of fungal genera
Table 1 shows the frequency of fungal genera in the indoor and outdoor environment. The most frequently occurring indoor and outdoor fungal types were Penicillium/Aspergillus type spores, Cladosporium sp., Basidiospores, and Ascospores. These four types of fungi were observed in 70% or more of the homes. Six other fungal types occurred in 15–70% of the homes, and included Smuts/Myxomycetes, Ganoderma, Alternaria sp., Pithomyces sp., Epicoccum sp., and those spores that were unidentified. While unidentified spores occurred in 62% of the homes, it should be noted that concentrations of unidentified spores were very low and contributed only between <1% and 13% (mean = 2%) to the total concentration in their respective samples.
Table 1.
Percentage of air samples containing various fungal genera, identified by microscopic observation
| Frequency of mold genera (% of samples)
|
||
|---|---|---|
| Fungal genera | Indoor data (n = 144) | Outdoor data (n = 147) |
| Penicillium/Aspergillus type | 90 | 100 |
| Cladosporium | 85 | 99 |
| Basidiospores | 74 | 91 |
| Ascospores | 72 | 99 |
| Unknown | 62 | 91 |
| Smuts/myxomycetes | 59 | 80 |
| Alternaria | 27 | 59 |
| Ganoderma | 23 | 47 |
| Epicoccum | 20 | 59 |
| Pithomyces | 17 | 25 |
| Torula | 10 | 16 |
| Nigrospora | 8 | 24 |
| Oidium | 8 | 5 |
| Periconia | 7 | 14 |
| Curvularia | 7 | 12 |
| Cercospora | 4 | 22 |
| Rusts | 3 | 27 |
| Agrocybe | 3 | 12 |
| Peronospora | 3 | 8 |
| Bispora | 3 | 3 |
| Chaetomium | 2 | 1 |
| Drechslera | 2 | 6 |
| Fusarium | 1 | 5 |
| Polythrincium | 1 | 18 |
| Tetracoccosporium | 1 | 5 |
| Botrytis | 0 | 3 |
Seasonal variations
Table 2 shows GM and ranges of seasonal concentrations for the fungal spore data in indoor and outdoor air. Total fungal spore concentrations in 144 indoor air samples ranged between 2 and 2295 spores/m3 (GM = 145 spores/m3). Each sampling session (spring 2003, fall 2003, spring 2004, and fall 2004) was also evaluated to determine if it was different in concentration from the other three sampling sessions. It was discovered that only the spring 2004 sampling period was different from the other sampling sessions (p < 0.001). This difference was most likely caused by the variation in the outdoor air concentration as the outdoor sampling data displayed similar seasonal trends as the indoor sampling data. Therefore, fall 2003 and 2004 data were combined as one season, and the spring 2003 and 2004 periods were kept independent from one another. This divided all of the data into three seasonal categories, which were used to control for season.
Table 2.
Geometric mean (GM) in number of spores per cubic meter for total concentration and four most frequent fungal genera in indoor and outdoor samples by season
| All data
|
Spring 2003
|
Spring 2004
|
Fall 2003 and 2004
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genera | GM | Minimum | Maximum | GM | Minimum | Maximum | GM | Minimum | Maximum | GM | Minimum | Maximum |
| Indoor | ||||||||||||
| n = 144
|
n = 25
|
n = 45
|
n = 74
|
|||||||||
| Total count | 145 | 2 | 2295 | 277 | 10 | 1252 | 54 | 3 | 568 | 229 | 2 | 2295 |
| Cladosporium | 30 | <2 | 920 | 35 | <2 | 333 | 7 | <2 | 92 | 52 | <2 | 920 |
| Penicillium/Aspergillus | 23 | <2 | 1205 | 160 | 10 | 612 | 30 | <2 | 392 | 15 | <2 | 1205 |
| Basidiospores | 16 | <2 | 791 | 15 | <2 | 133 | 3 | <2 | 95 | 36 | <2 | 791 |
| Ascospores | 7 | <2 | 248 | 15 | <2 | 116 | 3 | <2 | 45 | 10 | <2 | 248 |
| Outdoor | ||||||||||||
| n = 147
|
n = 37
|
n = 38
|
n = 72
|
|||||||||
| Total count | 2469 | 438 | 10,417 | 2474 | 1219 | 5829 | 1170 | 438 | 2685 | 3658 | 836 | 10,417 |
| Cladosporium | 365 | 0 | 6565 | 321 | 33 | 2101 | 141 | 0 | 745 | 691 | 11 | 6565 |
| Penicillium/Aspergillus | 1144 | 147 | 3428 | 1258 | 621 | 2868 | 680 | 147 | 1661 | 1433 | 227 | 3428 |
| Basidiospores | 113 | 0 | 1188 | 114 | 3 | 966 | 72 | 0 | 273 | 318 | 0 | 1188 |
| Ascospores | 202 | 0 | 1424 | 492 | 222 | 1424 | 76 | 0 | 632 | 226 | 33 | 1287 |
Fall 2003 and 2004 datasets were combined because no differences were observed in average concentrations.
Health outcomes
Rhinitis
No significant associations were found between rhinitis and the total fungal spore concentration. When statistical analyzes were conducted on the various fungal taxa, however, several associations were observed. An association between Basidiospore concentrations and any rhinitis (regardless of allergic sensitization) was observed after controlling for season (p < 0.01; Fig. 1). It was determined that the fall season was driving this difference in spore concentration [GM of Basidiopores in the fall: GM Rhinitis(−) = 23 spores/m3, GM Rhinitis(+) = 71 spores/m3]. In addition, Ganoderma sp. was found to have a positive borderline relationship with rhinitis (p < 0.10; Table 3).
Fig. 1.
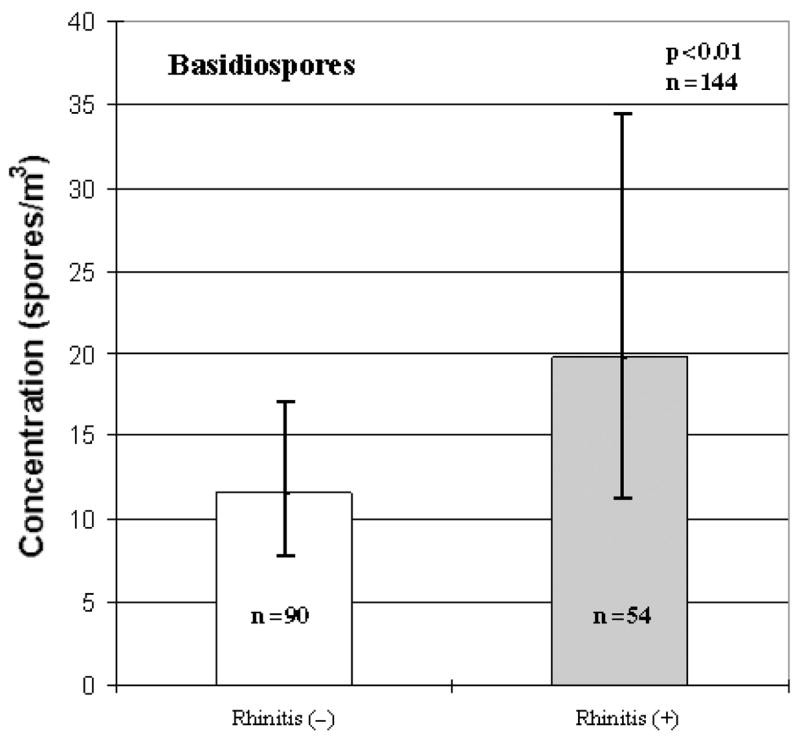
Association between Basidiospore concentrations and rhinitis (any rhinitis). Each bar represents the geometric mean of spore concentration and error bars represent the 95% confidence interval.
Table 3.
Selected results of associations among SPT(+) and rhinitis and fungal spore concentrations*
| p-Values [positive (+) or negative (−) association]
|
||||
|---|---|---|---|---|
| Health outcome
|
||||
| SPT
|
Rhinitis
|
|||
| Genus | Any | Aero-allergen | Any | Combined with SPT(+) to any allergen |
| Total concentration | n.s. | n.s. | 0.10 (+) | n.s. |
| Alternaria† | 0.01 (+) | n.s. | 0.10 (−) | n.s. |
| Ganoderma† | 0.10 (−) | 0.07 (−) | 0.06 (+) | n.s. |
| Pithomyces† | n.s. | n.s. | n.s. | 0.10 (−) |
| Basidiospores‡ | 0.09 (−) | n.s. | 0.01 (+) | n.s. |
| Penicillium/Aspergillus type‡ | 0.01 (+) | 0.10 (+) | n.s. | n.s. |
| Cladosporium‡ | 0.04 (−) | 0.03 (−) | n.s. | n.s. |
n.s., not significant; LOD, limit of detection; SPT, skin prick tests.
p-values ≤ 0.10 are reported only.
Classified as <LOD or ≥LOD and analyzed by chi-square testing.
Modeled continuously and analyzed by logistic regression.
Allergic sensitization
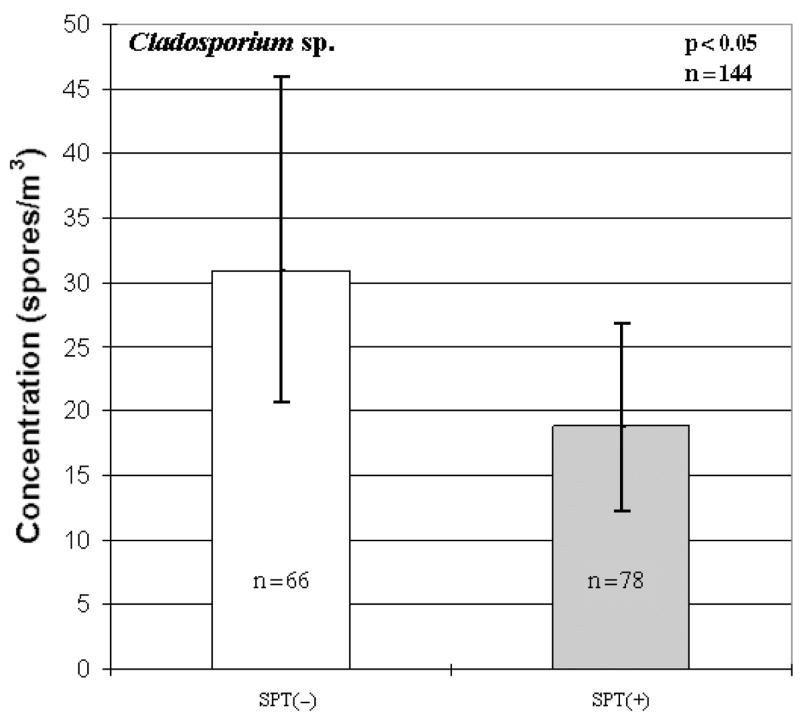
When analyzing the total concentrations with allergic sensitization to any allergen, no differences were observed between SPT(+) and SPT(−) children. However, when analysis was conducted on the various fungal genera, much like the rhinitis data, several significant associations emerged. When determining if there were positive associations between the various fungal taxa and SPT+ to any allergen, it was shown that both Penicillium/Aspergillus type spores (p < 0.01; Fig. 2) and Alternaria sp. (p < 0.01; Table 3) were significant. Conversely, Cladosporium sp. had an inverse association with SPT(+) to any allergen (p < 0.05; Fig. 3). Similarly, an inverse relationship between Cladosporium sp. and SPT(+) to any aeroallergens was observed (p < 0.05; Fig. 4). Furthermore, Ganoderma (p < 0.10; Table 3) showed borderline significant negative association with the child being SPT(+) to aeroallergens.
Fig. 2.
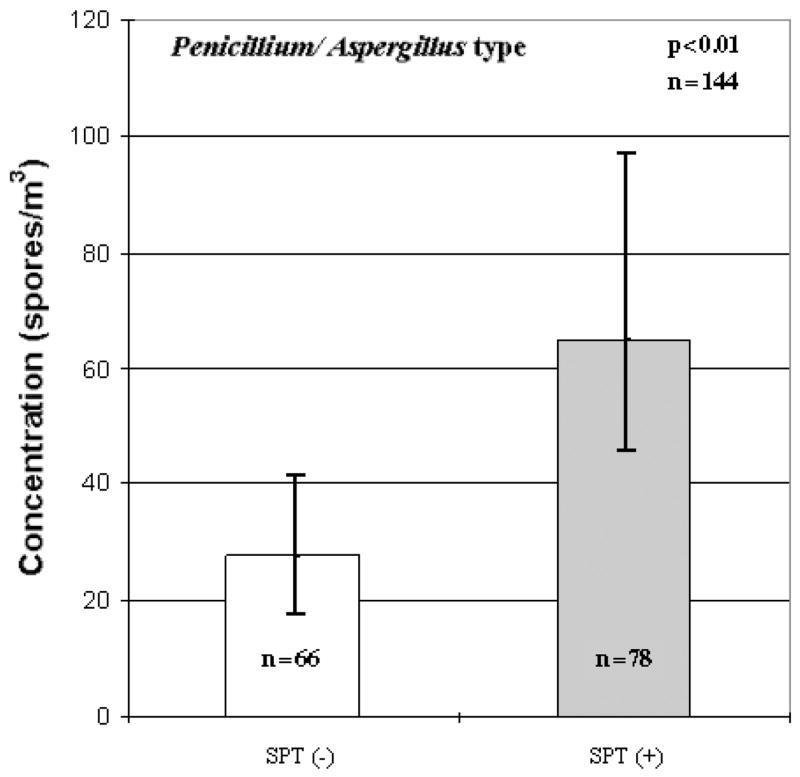
Association between Penicillium/Aspergillus type spores and skin prick tests(+) to any allergen. Each bar represents the geometric mean of spore concentration and error bars represent the 95% confidence interval.
Fig. 3.
Inverse association between Cladosporium sp. and skin prick tests(+) to any allergen. Each bar represents the geometric mean of spore concentration and error bars represent the 95% confidence interval.
Fig. 4.
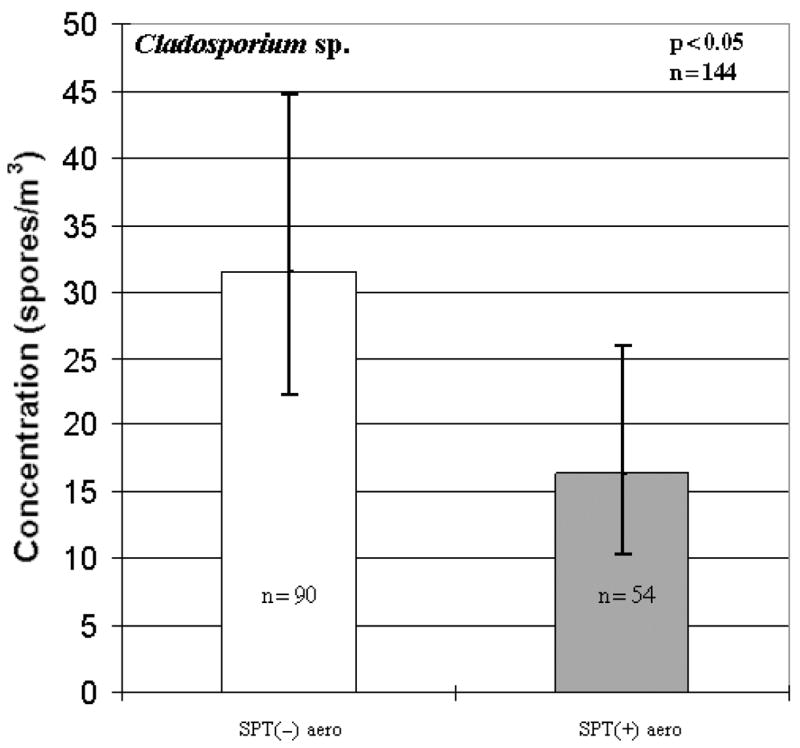
Inverse association between Cladosporium sp. and skin prick tests [SPT(+)] to aeroallergens. SPT(−)aero represents a negative SPT to aeroallergens; SPT(+) aero represents a positive SPT to aeroallergens. Each bar represents the geometric mean of spore concentration and error bars represent the 95% confidence interval.
No significance was observed between SPT(+) to fungi, either total concentrations or concentrations of specific fungal genera. This is likely because of the small number of children that had SPT(+) to fungi: only 24 of the study’s subjects [16% of total subjects, 31% of SPT(+) aero].
Discussion
The unique feature of this study was the long-term air sampling of fungal spores. In most of the previous studies, air samples for fungal spores were collected during a short sampling interval of 5–10 min. Because of this short sampling duration, the peaks and valleys inherent in the fluctuation of airborne fungal spore concentrations are not captured. Therefore, through chance, concentrations captured at one point in time may be significantly different from the concentrations measured in the same location at another time. The aerosol concentration may vary because of varied air currents in the sampling space, fluctuations in humidity, diurnal variations of spore release in different fungal species, and gravitational settling of the spores. We used a sampling time of 48 h to obtain a long-term average concentration, regardless of the peaks and valleys that may occur. This average concentration may be more beneficial in diagnosing or characterizing the indoor air of the space and could provide further insight into the concentrations to which occupants of that space would typically be exposed over an extended period of time.
We did not find associations between total fungal spore concentrations and health outcomes with the exception of a borderline significant relationship between the total concentration and the number of children having rhinitis symptoms. However, when examining the various fungal genera that were detected within the samples, associations between health outcomes and concentrations became apparent. Cladosporium sp., Basidiospores, Penicillium/Aspergillus type spores, and Alternaria were most frequently associated with significant health outcomes. Data indicated that exposure to certain types of fungi may increase childhood sensitization to allergens other than fungi.
An interesting finding of this study is the inverse association that Cladosporium had on the allergen sensitization of infants. A similar inverse relationship has also been observed for bacterial endotoxin exposures (12). In the late 1990s, the possibility of a beneficial role of bacterial endotoxin in protecting against atopic disease was first considered (18). The proposed mechanism for the protective effects measured in endotoxin exposures lies in the Th1/Th2 immune paradigm (19, 20). It has been suggested that these same agents might also inhibit immunoglobulin E production by inhibiting the Th2 immune responses. Although it has not been investigated extensively, fungal exposures may result in an alternative immunologic event, similar to that of bacterial endotoxin exposures, that favors the Th1 directed cytokine responses and may underlie the lack of allergic sensitization in children (20). The interaction between these pathways is not well understood at this time, including whether both pathways are activated by the same concentrations of microbial agents (10). However, it is not an entirely new concept, which suggests that persistent immune challenge may provide an explanation for the observed inverse association of fungal spores and health outcomes (20). The reverse trend was observed for Alternaria and Penicillium/Aspergillus, however, which may indicate species-specific effects or a chance finding.
A potential criticism of this study may be based on previous studies having clearly identified Cladosporium sp. as a contributor to individual sensitization and to symptoms in already sensitized populations (21). However, studies in which these findings have been reported have been conducted in adult populations only. Very little is known about the health effects of fungal exposure during infancy. Although not quite comparable, we found among our entire CCAAPS cohort that if the mother was sensitized to Cladosporium, there was an inverse significant association with the child being atopic (OR 0.6; 95% CI 0.4, 0.9) (15). As endotoxin and fungi often co-exist in the environment, it has been speculated that fungi may contribute to the protective effect mainly associated with endotoxin exposures early in life (11). To our knowledge, this study is the first one to report an inverse association between fungal exposure and health outcome (rhinitis and allergic sensitization) in infants. Eduard, et al. reported similar findings for fungal exposure and atopic asthma in adults (10).
We believe that contrasting relationships among the various fungal genera to the health outcomes investigated in this study might actually cancel the effect that total concentration may have on these outcomes. This finding would help to explain some of the lack of association in the reporting of health effects and total fungal spore concentrations observed in previous studies. However, it should be noted that the indoor environment (specifically the residential indoor environment) is a complicated one, where allergens, pollutants, and toxins coexist, and can have potential for synergistic relationships (19), which were not examined in this study. Based on the data presented in this paper, it appears that clinicians and researchers should be attentive to the composition of the fungal spore profile and the respective concentrations of the fungal genera present rather than total or culturable spore count alone. Furthermore, the present study was conducted in an infant population, and the health outcomes may or may not be transient. Long-term follow-up of this cohort will show how early fungal exposure affects the development of allergy and asthma in children later in life. Furthermore, additional investigation in older children, as well as adult and occupational populations should be performed to determine if age is a factor in the role that fungi play in immune response.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences (NIEHS) Grant No. ES11170. We would like to thank the families that participated in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), field teams, subject recruitment teams, and clinic personnel who have made this all possible.
References
- 1.Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2002;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 2.Etzel R, Rylander R. Indoor mold and children’s health: preface. Environ Health Perspect. 1999;107:463. doi: 10.1289/ehp.107-1566224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornehag CG, Sundell J, Sigsgaard T. Dampness in buildings and health (DBH): report from an ongoing epidemiological investigation on the association between indoor environmental factors and health effects among children in Sweden. Indoor Air. 2004;14(Suppl 7):59–66. doi: 10.1111/j.1600-0668.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- 4.Rylander R, Etzel R. Introduction and summary: workshop on children’s health and indoor mold exposure. Environ Health Perspect. 1999;107:465. doi: 10.1289/ehp.99107s3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadel R, David B, Paris S, Guesdon JL. Alternaria spore and mycelium sensitivity in allergic patients: in vivo and in vitro studies. Ann Allergy. 1992;69:329–35. [PubMed] [Google Scholar]
- 6.Bobbit RC, Jr, Crandall MS, Venkataraman A, Bernstein JA. Characterization of a population presenting with suspected mold-related health effects. Ann Allergy Asthma Immunol. 2005;94:39–44. doi: 10.1016/S1081-1206(10)61283-5. [DOI] [PubMed] [Google Scholar]
- 7.Kidon M, See Y, Goh A, Chay O, Balakrishnan A. Aeroallergen sensitization in pediatric allergic rhinitis in Singapore: is air conditioning a factor in the tropics? Pediatr Allergy Immunol. 2004;15:340–3. doi: 10.1111/j.1399-3038.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 8.IOM (Institute of Medicine) Damp Indoor Spaces and Health. Washington DC: National Academy of Sciences; 2004. [Google Scholar]
- 9.Kilpeläinen M, Terho EO, Helenius H, Koskenvuo M. Home dampness, current allergic diseases, and respiratory infections among young adults. Thorax. 2002;56:462–7. doi: 10.1136/thorax.56.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eduard W, Douwes J, Omenaas F, Heederik D. Do farming exposures cause or prevent asthma? Results from a study of Norwegian farmers. Thorax. 2004;59:381–6. doi: 10.1136/thx.2004.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schram D, Doekes G, Boeve M, et al. Bacterial and fungal components in house dust of farm children, Rudolf Steiner school children, and reference children – PARSIFAL study. Allergy. 2005;60:611–8. doi: 10.1111/j.1398-9995.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD. Maturation of immune responses at the beginning of asthma. J Allergy Clin Immunol. 2005;103:355–61. doi: 10.1016/s0091-6749(99)70456-2. [DOI] [PubMed] [Google Scholar]
- 13.Douwes J, Pearce N, Heederik D. Does environmental endotoxin exposure prevent asthma? Thorax. 2002;57:86–90. doi: 10.1136/thorax.57.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxter DM, Perkins JL, McGhee CR, Seltzer JM. A Regional comparison of mold spore concentrations outdoors and inside ‘clean’ and ‘mold contaminated’ southern California buildings. J Occup Environ Hyg. 2005;2:8–18. doi: 10.1080/15459620590897523. [DOI] [PubMed] [Google Scholar]
- 15.LeMasters GK, Wilson K, Levin L, et al. Infant Aeroallergen Sensitization: higher than previously reported. J Pediatr. 2006 in press. [Google Scholar]
- 16.Adhikari A, Martuzevicius D, Reponen T, et al. Performance of the button personal inhalable sampler for the measurement of outdoor aeroallergens. Atmos Environ. 2003;37:4723–33. [Google Scholar]
- 17.Eudney L, Su HJ, Burge HA. Biostatistics and bioaerosols. In: Burge HA, editor. Bioaerosols. Boca Raton: CRC Press Inc; 1995. pp. 269–307. [Google Scholar]
- 18.Michael O, Kips J, Duchateau J. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;151:1641–46. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 19.Niven R. The endotoxin paradigm: a note of caution. Clin Exp Allergy. 2003;33:273–6. doi: 10.1046/j.1365-2745.2003.01618.x. [DOI] [PubMed] [Google Scholar]
- 20.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 21.Hasnain S, Al-Frayh A, Al-Suwaine A, Gad-El-Rab M, Fatima K, Al-Sedairy S. Cladosporium and respiratory disease allergy: diagnostic implications in Saudi Arabia. Mycopathologia. 2002;157:171–9. doi: 10.1023/b:myco.0000020592.72238.a6. [DOI] [PubMed] [Google Scholar]