Abstract
The objective of our study was to investigate the association between daily pediatric asthma hospital visits and daily concentrations of aeroallergens and their specific species. Records of daily asthma visits in Cincinnati area were retrieved from Cincinnati Children’s Hospital Medical Center and aeroallergen sampling was performed by the Button Inhalable Sampler. The Poisson generalized linear model was carried out in which the log of the number of asthma hospital visits was related to aeroallergen level, treated as a continuous variable with adjustment for seasonal time trend, day of the week, ozone and PM2.5 concentrations, temperature and humidity. The aeroallergens having a significant impact on asthma hospital visits were ragweed, oak/maple and Pinaceae pollen. Their relative risks on asthma hospital visits with respect to a 100 counts/m3 increase in concentration were in the range of 1.23 to 1.54. The effects in causing the asthma exacerbation were delayed by 3 or 5 days.
Keywords: Ambient aeroallergens, Pediatric asthma hospital visit, Poisson generalized linear model
1. Introduction
Approximately 20.3 million people in the United States have asthma, 4500 of whom die annually from the disease. Asthma is now one of the most commonly reported diseases of childhood. An estimated 8.7% of children under age 18 have asthma.
It is usually accepted that about 60% of asthmatics are atopic and it has been known for some time that the aeroallergens, such as pollen and fungal spores, can affect the prevalence and/or severity of asthma. They could act to induce, or augment directly, airway inflammation and hyperresponsiveness, and some of them may have the ability to facilitate allergic sensitization or to enhance the severity of allergic reactions. The skin-prick test and intradermal test using extracts of pollen and/or fungal spore allergens can determine atopic characteristics. Blood tests such as the raido-allergosorbent test (RAST) and multiple allergosorbent chemiluminescent assay (MAST_CLA) can also indicate allergy to pollen and fungal spore allergens (Agata et al., 1993; Kam and Hsieh, 1994). Although the associations have been evidenced in studies in a clinical setting, few studies were conducted to investigate the impact of ambient aeroallergens on the burden of illness in the population and the findings of those studies were not in agreement. Some studies found significant association between daily fungal spore concentration and asthma hospital visits (Rosas and McCartney, 1998; Dales et al., 2000), while others found significant association of the daily pollen concentration, but insignificant association of daily fungal spore concentration, with the number of hospital asthma visits (Lierl and Hornung, 2003). The inconsistencies may have been related to the sampling methods employed in measuring the aeroallergens. The Rotorod and Hirst-Burkard samplers were used in these studies, and they are not the optimal sampling methods for measuring airborne pollen and fungal spores as their sampling efficiency are lower for the particles less than 10 μm and 2.5 μm, respectively (Frenz, 1999; Aizenberg et al., 2000). In the mean time, in order to obtain a better understanding of the association between aeroallergen and asthma, research is needed on the influence of individual aeroallergen species.
The goal of the present study was to examine the relationship of daily number of hospital visits for treatment of acute pediatric asthma attacks and daily concentrations of total outdoor pollen and fungal spores in the air of the greater Cincinnati area. Individual allergenic species were also investigated. Included species were pollen grains of ragweed, grass, oak/maple, Pinaceae and fungal spores of Alternaria, Aspergillus/Penicillium and Cladosporium. The Button Inhalable Aerosol Sampler (SKC, Inc., Eighty Four, PA) was used to monitor the aeroallergens. This sampler is known to be efficient for the sampling of outdoor aeroallergens and especially for small size particles (<5 μm) (Adhikari et al., 2003).
2. Methods
2.1. Hospital asthma visits
Cincinnati Children’s Hospital Medical Center (CCHMC) is the major pediatric hospital serving the Greater Cincinnati Metropolitan area. An “asthma visit” was defined as either an emergency room visit or an out-patient clinic visit for the diagnosis of asthma. Daily records of CCHMC asthma visits from April through October 2002 were retrieved by means of a hospital computer search for all visits with ICD9 codes 493–493.91. The age range of the subjects was 1–18 years. If a child had more than one asthma visit in a single day, only one visit was counted. The daily number of asthma visits was calculated by adding the number of visits occurring on the same day.
2.2. Weather and exposure data for aeroallergens, PM2.5 and ozone
Daily meteorological data from April through October 2002 were extracted from the website of the National Virtual Data System.
The daily aeroallergen, PM2.5 and ozone data during the specified period were obtained from an outdoor monitoring station that was located in the Cincinnati metropolitan area on a rooftop of a two-storied office building about 3 miles north of downtown Cincinnati. The nearby vegetation was sparse and there were no tall buildings in the proximity allowing free movement of wind and spatially uniform intensity of the solar radiation at the sampling location. Aeroallergen sampling was performed by the Button Inhalable Sampler, which has high efficiency for aeroallergens of small particle size and low sensitivity to the wind direction and velocity (Adhikari et al., 2003). Two Button Samplers were fixed onto a sampling tripod about 7.5 cm below a rain shield. The position of the Button Samplers was vertical to ground level and they were placed back to back so that the two inlets were oriented 180° opposite to each other: one towards southwest (SW) direction and the other towards northeast (NE). Both Button Samplers were operated at a flow rate of 4 l/min continuously for 24 h from Sunday morning to Friday morning. The samples were analyzed by microscopic counting as described by Adhikari et al. (2003). Results of paired t-test showed that for fungal spores; no statistically significant difference was observed between the bio-particle concentrations determined from the two Button Samplers facing opposite directions. Thus, daily concentrations of Aspergillus/Penicillium, Cladosporium, Alternaria and total fungal spores were obtained from the Button Sampler facing southwest, for the days when southwest data was not available, the same day concentrations from Button Sampler facing northeast were substituted. For pollen grains, since paired t-test showed that SW Button Sampler had significantly different concentrations from those of NE Button Sampler, daily concentrations of ragweed, grass, oak/maple, Pinaceae and total pollen grains were obtained by taking the average of measurements gained from SW and NE Button Samplers.
PM2.5 was collected on Teflon filters using SASS-sampler (Met One Instruments, Inc., Grants Pass, OR) and analyzed gravimetrically. The sampling was done every sixth day with a flow rate of 16.67 l/min and sampling time of 24 h. Ozone was continuously measured using an ultraviolet photometric ozone analyzer (Model 1008 PC, Dasibi Environmental Corp., Glendale, CA). The 24-h average concentrations of PM2.5 and ozone were used in the data analysis.
2.3. Statistical methods
Counts of rare events, such as daily hospital visits, usually follow a skewed pattern characterized by Poisson distribution. Therefore, Poisson multiple regression was applied to investigate the relationship between asthma hospital visits and ambient aeroallergen concentrations. First, daily asthma visits were fitted to a model without aeroallergen concentration to investigate the independent effects of other variables that were a priori believed to possibly confound the aeroallergen and asthma visit relationship. A model that was linear in parameters describing the effects of season, day of the week, temperature, humidity, ozone and PM2.5 levels on asthma visits was chosen. In this model, the cyclic seasonal trends were modeled by a restricted cubic spline term of time with turning points at quantiles of 0.1, 0.5 and 0.9 of the distribution. Day-of-the-week indicator variables were created to model the variations of daily asthma visits during the week. Dependence of asthma on temperature, humidity, ozone and PM2.5 was integrated by separate indicator variables corresponding to the different ranges. The number of ranges into which these were divided was chosen by starting with five categories, and then incrementally increasing the number of categories until the improvement in model fit from an additional category was not statistically significant. After variable scaling was completed, a Poisson regression of asthma visits on these variables was carried out and diagnostic plots of residuals were graphically reviewed with respect to each independent variable to assess homogeneity of variance and trend. Only after the diagnostic showed a nonsystematic pattern was the daily aeroallergen concentration inserted linearly in the model. Hence, the overall model was summarized as
where HVt is the expected number of hospital asthma visits on a particular day t; α is the intercept term; Xt is the design matrix for the confounding factors; ηc is the corresponding vector of coefficients; β, the exposure parameter, is equal to the coefficient of the linear aeroallergen exposure. The relative risk (RR) on asthma hospital visit associated with a change in exposure level, Δexp, can be calculated as eβΔexp. The percent increase in daily asthma hospital visits associated with this change in exposure is equal to (RR − 1)×100 if RR>1. Standard error of the exposure parameter was corrected for the correlation between daily aeroallergen concentrations and possible influences of variables not included in the model by scaling the standard error by the magnitude of the overdispersion factor (McCullagh and Nelder, 1983).
To examine the robustness of the regression coefficients of aeroallergen concentration to the modeling of covariates (season, temperature, humidity, ozone and PM2.5 concentrations), the analysis was repeated using a generalized additive model (GAM) (Hastie and Tibshirani, 1990). This model allows the trend of a response measurement to be summarized by a sum of parametric and nonparametric functions of covariates. Hence, the daily asthma visits were fitted with smoothing splines of time, temperature, humidity, ozone and PM2.5 and day-of-the-week indicators and aeroallergen concentration were modeled linearly. The smoothing parameter was selected by minimizing the generalized cross validation function.
In order to investigate the sensitivity of the results to outlying values, both modeling approaches were repeated on the dataset excluding the observations that had extremely high aeroallergen concentrations.
3. Results
As shown in Table 1, there were 1254 children between the ages of 1 and 17.9 years in this analysis. Sixty-eight percent of the subjects were age 5 or over. Table 2 provides the descriptive statistics of monthly means (standard deviations) for aeroallergen concentrations, temperature, humidity, ozone, PM2.5 and asthma hospital visits for the months of April through October 2002. Daily asthma hospital visits were highest in the months of May, September and October and lowest in July and August.
Table 1.
Summary of asthma patient population
| ICD9 codes | Mean age (years) (standard deviation) | Age, min–max | Age, median | # Subjects |
|---|---|---|---|---|
| 493–493.11 | 9.3 (4.8) | 1.4–17.9 | 9.2 | 120 |
| 493.9 | 7.2 (4.9) | 1.0–17.9 | 5.9 | 986 |
| 493.91 | 7.8 (4.9) | 1.1–17.7 | 7.4 | 148 |
| All | 7.4 (4.9) | 1.0–17.9 | 6.4 | 1254 |
Table 2.
Monthly means (standard deviation) of daily asthma hospital visits, aeroallergen concentrations, ozone, PM2.5 levels, temperature and humidity of April through October 2002
| Month | April | May | June | July | August | September | October |
|---|---|---|---|---|---|---|---|
| Hospital visits (days) | 9 (3.6) | 12 (4.2) | 7 (3.3) | 6 (3.3) | 7 (2.2) | 12 (4.1) | 11 (3.6) |
| Total pollen (m3) | 946 (1766.2) | 203 (224.5) | 61 (79.7) | 6 (4.5) | 26 (36.9) | 47 (44.7) | 2 (2.9) |
| Ragweed (m3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 24 (36.2) | 42 (43.0) | 1 (1.0) |
| Grass (m3) | 1 (1.3) | 6 (11.2) | 21 (24.1) | 4 (2.6) | 1 (1.0) | 1 (1.2) | 0 (0.5) |
| Oak/maple (m3) | 73 (104.9) | 46 (66.5) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.1) | 0 (0.0) |
| Pinaceae (m3) | 18 (33.1) | 47 (64.1) | 5 (6.6) | 0 (0.4) | 0 (0.0) | 0 (0.1) | 0 (0.0) |
| Total fungal (m3) | 2646 (1642.3) | 2861 (1559.4) | 5487 (1918.6) | 3986 (1227.0) | 7249 (3265.1) | 5401 (3739.6) | 40,149 (26,538.1) |
| Alternaria (m3) | 9 (18.6) | 3 (8.1) | 71 (67.8) | 88 (80.8) | 161 (105.0) | 173 (247.5) | 962 (687.5) |
| Aspergillus/Penicillium | (m3) 1412 (980.5) | 1044 (951.1) | 2467 (1311.1) | 1849 (805.9) | 1515 (796.7) | 1240 (615.1) | 11,049 (8861.5) |
| Cladosporium (m3) | 416 (486.0) | 412 (228.1) | 1339 (1008.5) | 976 (816.3) | 3842 (2566.7) | 2596 (2728.7) | 11,282 (11,134.4) |
| Ozone (ppb) | 28.8 (7.2) | 29.2 (8.6) | 36.0 (7.3) | 42.4 (9.5) | 35.0 (5.9) | 28.5 (11.7) | 11.1 (5.9) |
| PM2.5 (μg/m3) | 12.4 (3.8) | 13.6 (5.8) | 21.6 (9.9) | 25.8 (11.9) | 20.3 (8.7) | 19.5 (11.1) | 12.8 (6.4) |
| Temperature (°F) | 56.2 (11.4) | 60.3 (8.7) | 72.6 (5.6) | 77.9 (3.3) | 75.7 (3.8) | 71.7 (7.4) | 53.4 (10.8) |
| Humidity (%) | 65.8 (12.9) | 72.4 (12.0) | 72.5 (11.2) | 69.5 (10.2) | 70.3 (11.5) | 66.7 (15.0) | 75.8 (10.1) |
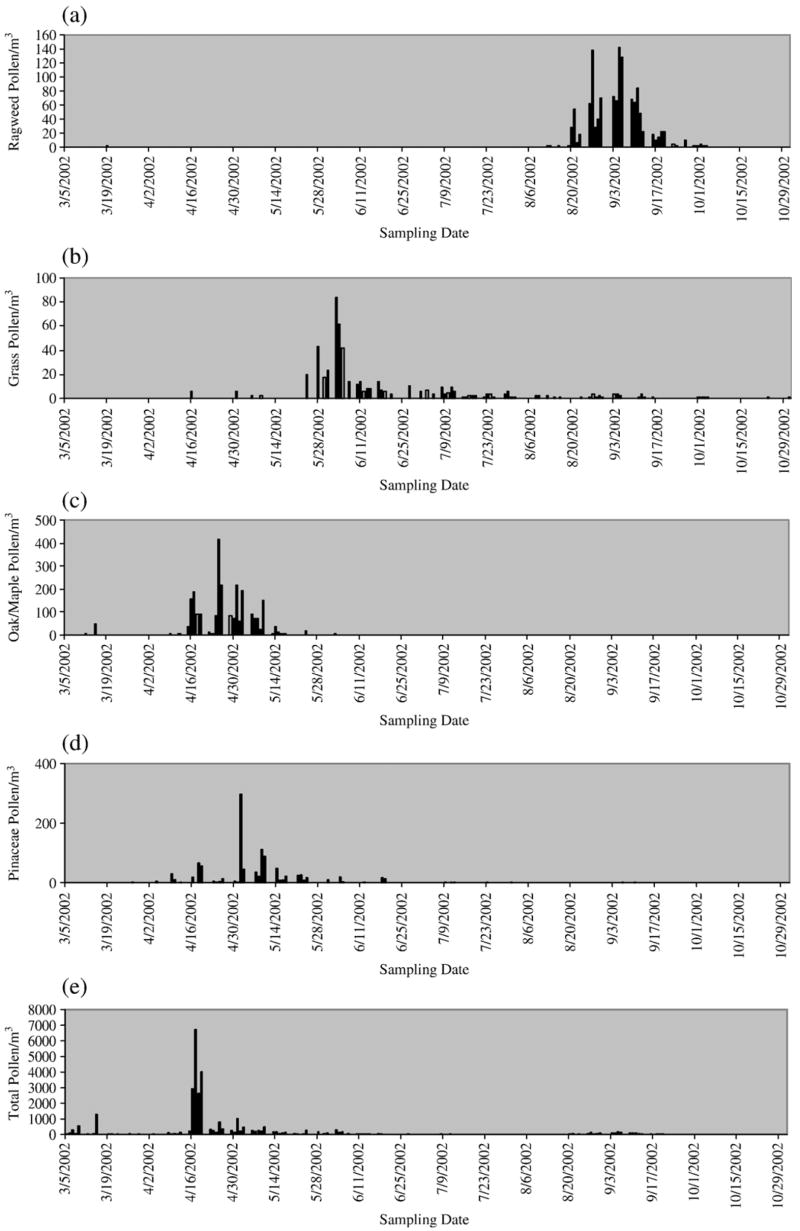
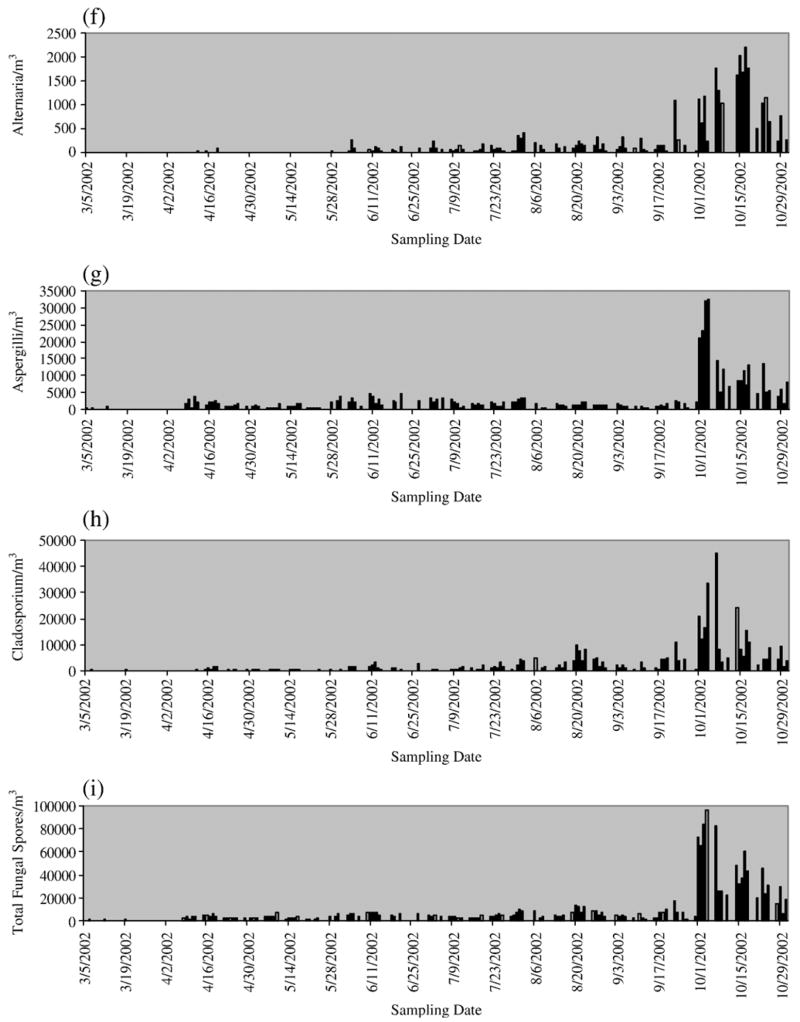
As shown in Fig. 1, seasonal patterns varied considerably among different pollen types. The grass pollen was present throughout the whole study period, while the prevalent month for Pinaceae and oak/maple pollen was from April to June. The ragweed pollen was present only from August to October. In contrast to pollen, large concentrations of fungal spores were found through the whole study period and their peak concentrations were all observed in October. Pairwise correlations were found to be insignificant between aeroallergen concentrations and covariates of season, temperature, humidity, ozone and PM2.5.
Fig. 1.
Daily aeroallergen concentrations: (a) daily ragweed pollen concentration, (b) daily grass pollen concentration, (c) daily oak/maple concentration, (d) daily Pinaceae concentration, (e) daily total pollen concentration, (f) daily Alternaria concentration, (g) daily Aspergillus/Penicillin concentration, (h) daily Cladosporium concentration and (i) daily total fungal spore concentration.
The nonparametric smooth curve fitted to daily asthma hospital visits over study months indicated a time trend characterized by a restricted cubic splines with three knots. The optimal number of categories for temperature was found to be six based on the assessment of model fit and the perfect straight line pattern in the residual diagnostic plot. The optimal number of categories for ozone and PM2.5 were both found to be three. The humidity was excluded from the final model, since the addition of humidity to the model did not lead to significant coefficient changes of other confounders.
Since ragweed, oak/maple and Pinaceae pollen were not present during the whole study period, analyses for these three pollen were performed on the data for their relevant months only. Restricted cubic splines with three knots were used to control for time trend. Temperature was modeled categorically, three categories were selected for ragweed and oak/maple pollen, while six categories were selected for Pinaceae pollen to adjust for the dependence of hospital admissions on temperature. Similarly, the number of categories for ozone and PM2.5 was selected to be two for ragweed and oak/maple pollen, and one for Pinaceae pollen.
Exposure to an aeroallergen on a given day may not result in asthma hospital visit at the same day, thus aeroallergen concentrations at days 1 to 5 before the asthma visit were investigated as well. Results from generalized linear model indicated that aeroallergens having statistically significant and meaningful impact on asthma hospital visits were oak/maple pollen concentration 3 days before the visit and Pinaceae concentration 5 days before the visit. With a 100 counts/m3 increase in the concentrations, their relative risks on the hospital asthma visits equal to 1.27 (95% CI: 1.07–1.51) and 1.34 (95% CI: 1.20–1.49), respectively.
In the generalized additive model, for the aeroallergens that are present during the whole study period, smoothing splines with degrees of freedom equal to 2.456, 4.841, 1.005 and 2.008 were selected respectively for time, temperature, ozone and PM2.5; for aeroallergens whose prevalence was only for certain months, smoothing splines with different degrees of freedom were chosen. Table 3 provides a summary of selected findings from the generalized additive model. Similar results as those obtained from the generalized linear model were observed, however, since the generalized additive model used a more flexible approach in fitting, more significant results were observed. Ragweed concentration 5 days before the visit was found to be significant predictors for asthma hospital visits. With a 100 counts/m3 increase in the concentration, its relative risk on the asthma hospital visit equals to 1.54 (95% CI: 1.02–2.33). Little effects were seen on the association between aeroallergen and asthma hospital visit when the two modeling approaches were repeated excluding 5% of the days with highest aeroallergen concentration.
Table 3.
Selected results from Poisson generalized additive model (GAM)a
| Aeroallergen (lag days)b | Relative riskc (95% CI) | Estimated increase in daily asthma visitsd (95% CI) |
|---|---|---|
| Oak/maple pollen (3) | 1.23 (1.02–1.49) | 23% (2–49%) |
| Pinaceae pollen (5) | 1.34 (1.24–1.45) | 34% (24–45%) |
| Ragweed pollen (5) | 1.54 (1.02–2.33) | 54% (2–133%) |
Concentrations of total pollen, Cladosporium and Alternaria were also found to be statistically significant, but their RRs were too small to be clinically meaningful.
Number of days between asthma hospital visit and high spore levels prior to visit.
The relative risk is for a 100 counts/m3 increase in aeroallergen concentration.
The estimated increase in daily asthma visits is for a 100 counts/m3 increase in aeroallergen concentration.
4. Discussion
The impact of total concentrations of airborne pollen and fungal spore on pediatric asthma hospital visits was investigated as well as the effects of their seven individual allergenic species. Significant predictors for asthma visits were found for the concentrations of oak/maple pollen, Pinaceae pollen and ragweed pollen. Various patterns of delayed effects were also observed among these different types of aeroallergens. The associations found do not necessarily reflect a cause and effect relationship. Based on the analysis methods we have implemented, it is unlikely that the associations are due to the uncontrolled confounding factors. Meanwhile, because daily changes in pollen and fungal spore concentrations were compared to daily changes in asthma visits, the major risk factors for asthma such as socioeconomic and demographic characteristics would not be expected to confound our findings as these do not change day-to-day in concert with aeroallergen levels. Considering the seven-month period of this study, changes in diagnostic practices seem an unlikely explanation as well. The aeroallergen sampling conducted with Button Inhalable Aerosol Sampler also adds more strength to the findings. Previous studies show that for smaller size particles, the collection efficiency of the Button Inhalable Aerosol Sampler is much higher and its microscopic counts are considerably more consistent than those of the Burkard sampler and Rotorod Sampler (Aizenberg et al., 2000; Adhikari et al., 2003). Further, the sampling efficiency of the Button Inhalable Aerosol Sampler is not affected by changes in the wind direction and velocity (Adhikari et al., 2003).
It was hypothesized that unlike fungal spores, pollen grains are deposited in the upper airways. Because of this deposition, pollen related asthma exacerbation may be due to factors other than particle size. Recent studies have shown, however, that air pollutants can influence the way a pollen, once inhaled, is processed. Airway mucosal damage and impaired mucociliary clearance induced by air pollution may facilitate the access of inhaled pollen and the cellular response of the immune systems (D’Amato and Liccardi, 2002). It has also been proposed by Beggs (1998) that particles smaller than intact pollen grains may act as adjuvants and asthma symptoms can be triggered by the penetrating capability of these small particles into the lower airways. All the above factors may contribute to the significant influences on asthma for certain types of pollen and their delayed effects.
In this study, statistically significant associations were found between asthma hospital visits and outdoor concentrations of oak/maple pollen, Pinaceae pollen and ragweed pollen. Exposure-response curve of the aero-allergen influence on asthma can be further investigated via the generalized additive model. Also considering the greater amount of time children spend indoors, air sampling of indoor environment may be pursued in future research. It has been demonstrated that a synergistic interaction exists between exposure to diesel exhaust particles and pollen in enhancing airway immunoglobulin IgE production leading to more severe asthma symptoms (Takafuji and Suzuki, 1989; Diaz-Sanchez et al., 1997; Adhikari et al., 2006; Ryan et al., 2005). Therefore, exposure to diesel exhaust will be considered along with aeroallergens in a future asthma study.
Acknowledgments
This work was supported by National Institute of Environmental Health Science Grant No. R01 Es 11170.
References
- Adhikari A, Martuzevicius D, Reponen T, Cho S, Grinshpun S, Sivasubramania S, et al. Performance of the Button Personal Inhalable Sampler for the measurement of outdoor aeroallergens. Atmos Environ. 2003;37:4723–33. [Google Scholar]
- Adhikari A, Reponen T, Grinshpun S, Martuzevicius D, LeMasters G. Correlation of ambient inhalable bioaerosols with particulate matter and ozone: a two-year study. Environ Pollut. 2006;140:16–28. doi: 10.1016/j.envpol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Agata H, Yomo A, Hanashiro Y, Muraki T, Kondo N, Orii T. Comparison of the MAST chemiluminescent assay system with RASAT and skin tests in allergic children. Ann Allergy. 1993;70:153–7. [PubMed] [Google Scholar]
- Aizenberg V, Reponen T, Grinshpun S, Wileke K. Performance of Air-O-Cell, Burkard, and Button Samplers for total enumeration of airborne spores. AIHAJ. 2000;61:855–64. doi: 10.1080/15298660008984598. [DOI] [PubMed] [Google Scholar]
- Beggs PJ. Pollen and pollen antigen as triggers of asthma–what to measure? Atmos. Environ. 1998;32(10):1777–83. [Google Scholar]
- Dales RE, Cakmak S, Burnett R, Judek S, Coates F, Brook JR. Influence of ambient fungal spores on emergency visits for asthma to a regional children’s hospital. Am J Respir Crit Care Med. 2000;162:2087–90. doi: 10.1164/ajrccm.162.6.2001020. [DOI] [PubMed] [Google Scholar]
- D’Amato G, Liccardi G. The increasing trend of seasonal respiratory allergy in urban areas. Allergy. 2002;57(Suppl 71):35–6. doi: 10.1034/j.1398-9995.2002.057s71035.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–13. [PubMed] [Google Scholar]
- Frenz DA. Comparing pollen and spore counts collected with the Rotorod Sampler and Burkard spore trap. Ann Allergy Asthma & Immun. 1999;83:341–9. doi: 10.1016/S1081-1206(10)62828-1. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Generalized additive models. London: Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- Kam KL, Hsieh KH. Comparison of three in vitro assays for serum IgE with skin testing in asthmatic children. Ann Allergy. 1994;73:329–36. [PubMed] [Google Scholar]
- Lierl MB, Hornung RW. Relationship of outdoor air quality to pediatric asthma exacerbations. Ann Allergy Asthma & Immun. 2003;90:28–33. doi: 10.1016/S1081-1206(10)63610-1. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman and Hall; 1983. [Google Scholar]
- Rosas I, McCartney HA. Analysis of their relationships between environmental factors (aeroallergens, air pollution, and weather) and asthma emergency admissions to a hospital in Mexico City. Allergy. 1998;53:394–401. doi: 10.1111/j.1398-9995.1998.tb03911.x. [DOI] [PubMed] [Google Scholar]
- Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun S, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;16:279–84. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Takafuji S, Suzuki S. Enhancing effect of suspended particulate matter on the IgE antibody production in mice. Int Arch Allergy Appl Immunol. 1989;90:1–7. doi: 10.1159/000234990. [DOI] [PubMed] [Google Scholar]