Abstract
Background
(1–3)-β-D-glucan is a fungal cell wall component, suspected to cause respiratory symptoms in adults. However, very little is known on the possible health effects of (1–3)-β-D-glucan during infancy. We examined the association between (1–3)-β-D-glucan exposure and the prevalence of allergen sensitization and wheezing during the first year of life in a birth cohort of 574 infants born to atopic parents. Endotoxin exposure was included as a possible confounder.
Methods
(1–3)-β-D-glucan and endotoxin exposures were measured in settled dust collected from infants’ primary activity rooms. The primary outcomes at approximately age one included parental reports of recurrent wheezing and allergen sensitization evaluated by skin prick testing to a panel of 15 aeroallergens as well as milk and egg white.
Results
Exposure to high (1–3)-β-D-glucan concentration (within fourth quartile) was associated with reduced likelihood of both recurrent wheezing [adjusted OR (aOR) = 0.39, 95% CI = 0.16–0.93] and recurrent wheezing combined with allergen sensitization (aOR = 0.13, 95% CI = 0.03–0.61). Similar trends were found between (1–3)-β-D-glucan concentrations and allergen sensitization (aOR = 0.57, 95% CI = 0.30–1.10). In contrast, recurrent wheezing with or without allergen sensitization was positively associated with low (1–3)-β-D-glucan exposure within the first quartile (aOR = 3.04, 95% CI = 1.25–7.38; aOR = 4.89, 95% CI = 1.02–23.57). There were no significant associations between endotoxin exposure and the studied health outcomes.
Conclusions
This is the first study to report that indoor exposure to high levels of (1–3)-β-D-glucan (concentration >60 μg/g) is associated with decreased risk for recurrent wheezing among infants born to atopic parents. This effect was more pronounced in the subgroup of allergen-sensitized infants.
Keywords: (1–3)-β-D-glucan, allergen sensitization, allergens, asthma hygiene hypothesis, endotoxin, environment, infants, molds, wheeze
Exposure to indoor molds during infancy has been associated with respiratory symptoms, such as increased risk for persistent cough and wheeze (1, 2). However, evaluation of human health effects of mold exposure has been hampered by lack of simple and reliable measurements of exposure. (1–3)-β-D-glucan is a biologically active polyglucose molecule comprising up to 60% of the cell wall of mold, and some soil bacteria and plants. (1–3)-β-D-glucan levels in samples of airborne or settled dust have been used in several studies as a surrogate measure of mold exposure (3–5).
Experimental exposures of mice, guinea pigs and adult human subjects to particulate (1–3)-β-D-glucan failed to elicit inflammatory responses in the nasal or bronchial airways (6–8). On the other hand, soluble (1–3)-β-D-glucan has been shown to enhance allergen induced airway inflammation by increasing eosinophil infiltration and specific IgE in guinea pigs and mice sensitized to ovalbumin (9, 10).
A positive association between occupational exposure to (1–3)-β-D-glucan and general (tiredness, headache) and respiratory symptoms (nose and throat irritation, cough), airways inflammation and lung function has been found in the following workplaces: organic dusts in woodwork and paper industry (11–13); household waste collection (14, 15), poultry (16), composting (17) and sewage treatment (18, 19). Even though indoor exposures are lower, similar symptoms have been found in adult populations exposed to indoor environments with elevated (1–3)-β-D-glucan (20–23).
Data from adults may not be applicable for young children, as the immune system develops in the first years of life, and immune responses may be modified by exposure to microbial products (24). For example, early exposure to environmental endotoxin during infancy may have a protective effect on subsequent aeroallergen sensitization in childhood (25, 26). Exposure to (1–3)-β-D-glucan may have a similar protective effect (3). Although no previous studies have linked mold exposure to protective effects (2, 27), our recent study has shown an inverse relationship between exposure to Cladosporium and allergen sensitization to any allergen (P < 0.05), as well as to aeroallergens (P < 0.05) in infants (28). There is limited data on health effects of (1–3)-β-D-glucan exposure in children. Rylander et al. (29) reported that exposure to increased airborne (1–3)-β-D-glucan positively correlated with upper airway symptoms in atopic school children (6–13 years old). Two recent European studies found increased dustborne (1–3)-β-D-glucan concentrations to have a slight protective effect on atopic wheeze (1.2-fold decrease, P < 0.10) in school children (5–13 years old) (3), and both asthma (aOR = 0.70, 95% CI = 0.30–1.60) and persistent wheeze (aOR = 0.43, 95% CI = 0.15–1.21) in children at age 1–4 (30).
As described above, existing data on health effects of (1–3)-β-D-glucan are contradictory and few studies to date have assessed (1–3)-β-D-glucan exposure on clinical outcomes in an infant birth cohort. We hypothesized that the prevalence of wheezing and allergen sensitization in a large birth cohort of high-risk infants is inversely related to exposure to high indoor concentrations of both (1–3)-β-D-glucan and endotoxin.
Methods
Recruitment
Infants were identified from birth certificate records in the Greater Cincinnati area from October 2001 through July 2003. Parents were interviewed for allergic symptoms and those reporting one or more symptoms were invited to be skin prick tested (SPT) for 15 aeroallergens. Infants with at least one parent having positive SPT [SPT(+)] were eligible for enrolment in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) as described previously (31–35). One purpose of CCAAPS is to examine gene-environmental interactions; thus, parental atopy was a critical criterion to obtain the most genetically at risk group. There were 1879 families with at least one parent reporting allergy symptoms. Of these, 1152 parents agreed to participate in the SPT, and 881 had at least one parent with a SPT(+). Thus, the infants of the 881 families were eligible and 758 agreed to participate. The participating children (n = 758) were 20.1% African-American, reflecting the greater Cincinnati, Ohio area distribution of 23.4%. The University of Cincinnati Institutional Review Board approved the study.
Exposure assessment
When infants reached an average age of 8 months, families were visited at their homes to administer a detailed questionnaire to the parents on home characteristics, including observations of visible mold/water damage. The homes were classified into three groups: (i) no mold or water damage; (ii) low-visible mold (area <0.2 m2); and (iii) high-visible mold (area ≥0.2 m2) (33). Dust samples were vacuumed from the baby’s primary activity room floor (area of at least 2 m2). The baby’s primary activity room was defined as the one where the infant spends most of his/her time, and in >90% of cases, it was the living room or the family room. Thus bias because of selection of different types of rooms was minimized. For carpeted floors (>90% of homes), a sample was collected from an area of 2 m2 (2 min/m2), while for smooth floors the sample was collected from the entire room at a rate of 1 min/m2 (33). Collected dust was weighed, sieved through a 355-μm sieve, and stored desiccated at −20°C until extraction. Samples were analysed for indoor allergens as described by Cho et al. (34) and for (1–3)-β-D-glucan and endotoxin as described below.
(1–3)-β-D-glucan and endotoxin analysis
As the exposure to (1–3)-β-D-glucan was not included in the CCAAPS study, we have analysed only dust samples with sufficient amount of dust (at least 25 mg), after analyses for all other allergens was completed. Thus from the initially collected 758 samples, only 574 were available for the additional analysis. The (1–3)-β-D-glucan and endotoxin activities in dust samples were determined by the endpoint chromogenic Limulus amebocyte lysate assay (LAL; Associates of Cape Cod, East Falmouth, MA, USA). Two separate modifications of the assay were used, the Glucatell assay for (1–3)-β-D-glucan analysis, and the Pyrochrome assay for endotoxin. Each modification used a unique enzyme: factor G in the Glucatell assay, and factor C in the Pyrochrome assay. Thus false-positive results were avoided. Endotoxin analysis was performed as described by Campo et al. (35). For the (1–3)-β-D-glucan, 50 mg of each dust sample was extracted in 2 ml of 0.6 M NaOH and shaken for 1 h at −4°C. Twenty-five μl of Glucatell reagent was added to each well of serially diluted (1: 100 000 and 1: 1 000 000) dust extract and a control standard (1–3)-β-D-glucan (Pachyman, Associates of Cape Cod, East Falmouth, MA, USA), placed in a 96-well, flat-bottomed microplate. After 30-min incubation at 37°C, diazo-reagents were added to stop the reaction. The optical density was recorded at 540 nm. The median coefficient of variation (CV) was 9% for the intra-plate variability and 27% for the inter-plate variability. All samples of (1–3)-β-D-glucan were above the lower limit of detection (LOD) of the Glucatell assay (5 pg/ml). Thirty-five of the 574 endotoxin samples were below the LOD of 0.0625 EU/ml and were recorded as LOD. LOD values were divided by the square root of two for the data analyses.
As currently it is not clear whether the concentration (μg/g) or loading (μg/m2) unit better represents the actual exposure (36), results were reported in both measures – expressed as μg/g and μg/m2 for (1–3)-β-D-glucan and EU/mg and EU/m2 for endotoxin exposures.
Medical evaluation of infants
The medical evaluation of infants was performed during a clinic visit at the average age of 13 months (range 11–18 months, of these 95% were <15-months old). Sensitization to both food and aeroallergens before age one is an important risk factor for development of persistent wheeze symptoms and asthma in children born to atopic parents (37). Thus, infants were tested for allergen sensitization by SPT to a panel of food (milk, egg) and 15 common indoor and outdoor aeroallergens (seven pollen, four mold, cat, dog, German cockroach, house dust mite). An SPT(+) to at least one allergen was defined as a wheal ≥3 mm larger than the saline control after 15 min (32–35). All SPTs were performed using the Accu-set tips and aeroallergen (provided by ALK-Abelló, Inc., Round Rock, TX, USA) (32). We analysed data on SPT positivity to at least one allergen (regardless whether food or aeroallergen), as well as SPT positivity to at least one aeroallergen. At clinic visits, the parents were interviewed regarding wheezing using questions adapted from the ISAAC questionnaire for 4–5 years old (38). The following outcome variables were used: recurrent wheezing (≥2 episodes in the past 12 months), recurrent wheezing combined with SPT(+), and allergen sensitization (a positive SPT to at least one aeroallergen and/or food antigen). The reference group for recurrent wheezing consisted of infants with ≤1 wheezing episodes in the past 12 months regardless of the SPT status. The reference group for the recurrent wheezing with allergen sensitization consisted of infants with ≤1 wheezing episodes and SPT(−) status.
Data analysis
The associations between (1–3)-β-D-glucan and each health outcome were investigated for 574 infants. Histograms and quantile–quantile plots showed that (1–3)-β-D-glucan levels were approximately lognormally distributed.
Univariate logistic regressions were initially performed to evaluate associations between wheezing outcomes and predictor variables believed, a priori, to be related to wheezing in infants (39). Predictor variables that were significant at the 20% level in the univariate analyses were initially included in the multivariate logistic regression analyses. This significance level was chosen to include covariates that were moderately correlated to wheeze outcomes. Variables, which maintained significance levels approximately equal to 5% in at least one wheeze model, and/or changed the regression coefficient (or SE) of another variable by at least 15% when dropped from the model, were kept in the final model. (1–3)-β-D-glucan remained, a priori, in all models. Predictor variables that were evaluated, but not included in the final model, were breastfeeding duration (<1, 1–24, >25 weeks), dust-mite, and cockroach allergens, and number of dogs and/or cats in the home.
After univariate analyses, allergen sensitization (SPT+), recurrent wheeze, and recurrent wheeze with allergen sensitization were analysed by multiple logistic regression analyses, in which loge (1–3)-β-D-glucan and endotoxin, were continuously modelled. Categorically coded day-care attendance (yes vs no), either parent asthma (yes vs no), gender, race (African-American vs non-African-American), number of siblings in the same household (one vs none, more than one vs none), visible mold in home (low vs none, high vs none), mother’s smoking (≥20 cigarettes a day vs none), lower respiratory condition (at least one of whooping cough, croup, viral infections, bronchitis/bronchiolitis, flu, pneumonia, vs none), and upper respiratory condition (at least one of cold, ear infection, sinus infection, strep throat, tonsillitis, coloured drainage vs none) were also modelled. Covariates were chosen using a backward elimination technique.
(1–3)-β-D-glucan exposure was modelled continuously. ORs and 95% CI for the (1–3)-β-D-glucan variable were obtained to estimate the odds of each health outcome for an infant within each quartile. The reference value was the lower endpoint of each of the quartiles. We also tested for an interaction effect between (1–3)-β-D-glucan and endotoxin, and as such was not found in the wheezing outcomes, we included endotoxin as a confounder in the wheezing analyses. Endotoxin was modelled both in quartiles and continuously. In the latter case, ORs and 95% CI for endotoxin were estimated for an increase from the 25th to 75th percentile of the range of observed values (inter-quartile range). We performed two types of analyses – with and without endotoxin included in the final logistic models.
Graphical interpretations of wheeze prevalence (both wheeze outcomes) vs (1–3)-β-D-glucan showed a nonlinear relationship, which was modelled by dividing the range of (1–3)-β-D-glucan into four nonoverlapping intervals, approximately equal to (1–3)-β-D-glucan quartiles. On each interval, a third degree function of (1–3)-β-D-glucan was fitted to wheeze prevalence. Fitted curves were smooth at the points of connection and were constrained to be linear in the tails. This transformation is known as a restricted cubic spline (RCS) function. It allowed parameter estimates to be obtained to estimate the effect of (1–3)-β-D-glucan on wheeze outcomes over the inter-quartile range of (1–3)-β-D-glucan values.
Other analyses that were performed outside of the regression model included correlations between (1–3)-β-D-glucan and endotoxin by levels of visible mold and analysis of variance testing differences among means of log-transformed (1–3)-β-D-glucan levels in homes with visible mold levels 0, 1 and 2. The latter analysis was followed by a test of linear trend between increasing levels of (1–3)-β-D-glucan levels and visible mold. The analyses were performed using S-PLUS software (Insightful Corp., Seattle, WA, USA, 2000).
Results
Exposure and subject characteristics
The descriptive statistics for (1–3)-β-D-glucan and endotoxin levels are presented in Table 1. (1–3)-β-D-glucan levels in concentration units correlated significantly with those in loading units (Spearman’s correlation: r = 0.69, P < 0.001). The correlation between endotoxin and (1–3)-β-D-glucan was significant in loading units (r = 0.51, P < 0.001), and nonexistent in concentration units (r = 0.08, P = 0.052). The analysis of variance of three categorical levels of visible mold exposure (no, low and high) showed no significant overall differences for the (1–3)-β-D-glucan exposure (respective geometric mean values for concentrations: 53.2, 57.4 and 49.7 μg/g, and for loadings: 17.2, 19.0 and 25.7 μg/m2; results not shown in table). The test of linear trend between increasing levels of (1–3)-β-D-glucan and visible mold was not significant either (both units).
Table 1.
Geometric mean (GM), geometric standard deviation (GSD) and inter-quartile (IQ) range of (1–3)-β-D-glucan and endotoxin concentration (μg/g, EU/mg) and loading (μg/m2, EU/m2), measured in homes of 574 infants
| n = 574 | GM | GSD | IQ* |
|---|---|---|---|
| (1–3)-β-D-glucan | |||
| μg/g | 55.1 | 3.7 | 21.9–133.5 |
| μg/m2 | 18.4 | 5.7 | 5.9–57.9 |
| Endotoxin | |||
| EU/mg | 70.7 | 3.4 | 39.2–171.0 |
| EU/m2 | 23.7 | 5.6 | 9.4–74.5 |
EU, endotoxin units.
Interquartile range = 25th percentile to 75th percentile.
Among the infants, 114 (19.9%) had recurrent wheezing (defined as two or more episodes in the last 12 months) and 41 (7.1%) had recurrent wheezing combined with a SPT(+) to at least one allergen (Table 2). There was a borderline significant difference between groups in the (1–3)-β-D-glucan exposure (P = 0.08) and significant difference between the groups in visible mold exposures (P < 0.01) regarding the recurrent wheeze outcome. Day-care attendance, parents without asthma, African-American race, and no siblings were associated with lower prevalence of recurrent wheeze and/or recurrent wheeze with allergen sensitization. As expected, those who experience lower and/or upper respiratory condition, also tend to wheeze more.
Table 2.
Characteristics of predictor variables and prevalence and per cent of infants (n, % of total) reporting health outcome* by levels of (1–3)-β-D-glucan, endotoxin and demographic characteristics
| Characteristic of predictor variables | Allergen sensitization (SPT+) n = 169 (29.4% of 574)§ | Recurrent wheeze n= 114 (19.9% of 574)§ | Recurrent wheeze with allergen sensitization n = 41 (11.0% of 373)§ | ||||
|---|---|---|---|---|---|---|---|
| (1–3)-β-D-glucan quartiles | |||||||
| μg/g | μg/m2 | μg/g | μg/m2 | μg/g | μg/m2 | μg/g | μg/m2 |
| I: 3–22 (144) | I: 0.2–6 (143) | 44 (26.0) | 40 (23.7) | 22 (19.3) | 27 (23.7) | 9 (22.0) | 8 (19.5) |
| II: 22–60 (149) | II: 6–18 (144) | 45 (26.6) | 51 (30.2) | 34 (29.8) | 24 (21.1) | 16 (39.0) | 11(26.8) |
| III: 61–134 (138) | III: 19–58 (143) | 43 (25.4) | 41 (24.3) | 35 (30.7) | 35 (30.1) | 10 (24.4) | 15(36.6) |
| IV: 134–900(143) | IV: 58–2966 (144) | 37 (21.9) | 37 (21.9) | 23 (20.2) | 28 (24.6) | 6 (14.6) | 7 (17.1) |
| Endotoxin quartiles | |||||||
| EU/mg | EU/m2 | EU/mg | EU/m2 | EU/mg | EU/m2 | EU/mg | EU/m2 |
| I: 3–39 (144) | I: 0.09–9 (144) | 35 (24.3) | 45 (31.3) | 29 (20.1) | 31 (21.5) | 8 (8.6) | 8 (8.6) |
| II: 39–80 (143) | II: 9–25 (143) | 51 (35.7) | 36 (25) | 25 (17.5) | 25 (17.5) | 10 (10.8) | 12 (12.9) |
| III: 80–171 (143) | III: 25–74 (143) | 41 (28.7) | 58 (40.6) | 33 (23.1) | 21 (14.7) | 13 (14) | 6 (6.5) |
| IV: 171–2800 (144) | IV: 74–5120 (144) | 42 (29.2) | 30 (21.0) | 27 (18.8) | 37 (25.7) | 10 (10.6) | 15 (16.0) |
| Visible mold | |||||||
| None (255) | 76 (45.0) | 43 (37.7) | 13 (31.7) | ||||
| Low (<0.2 m2) (296) | 82 (48.5) | 61 (53.5) | 22 (53.7) | ||||
| High (≥0.2 m2) (23) | 11 (6.5) | 10 (8.8) | 6 (14.6) | ||||
| Mother’s smoking (average number of cigarettes per day) | 1.9 | 3.1 | 3.6 | ||||
| Daycare (yes) (50) | 15 (8.9) | 15 (13.2) | 3 (7.3) | ||||
| Breastfeeding duration | |||||||
| None (177) | 53 (31.4) | 42 (36.8) | 18 (43.9) | ||||
| 1–24 weeks (252) | 80 (47.3) | 48 (42.1) | 17 (41.5) | ||||
| 25+ weeks (145) | 36 (21.3) | 24 (21.1) | 6 (14.6) | ||||
| Dog in home (yes) (219) | 72 (42.6) | 41 (36.0) | 17 (41.5) | ||||
| Cat in home (yes) (114) | 38 (22.5) | 23 (20.2) | 11 (26.8) | ||||
| Either parent asthma (yes) (183) | 56 (33.1) | 51 (44.7) | 21 (51.2) | ||||
| Gender (male) (311) | 93 (55.0) | 67 (58.8) | 24 (58.5) | ||||
| Race | |||||||
| Afro-American (96) | 31 (18.3) | 24 (21.0) | 11 (26.8) | ||||
| All other races (478) | 138 (81.7) | 90 (79.0) | 30 (73.2) | ||||
| Siblings | |||||||
| 0 (193) | 65 (38.5) | 28 (24.6) | 9 (22.0) | ||||
| 1 (220) | 57 (33.7) | 43 (37.7) | 16 (39.0) | ||||
| ≥2 (161) | 47 (27.8) | 43 (37.7) | 16 (39.0) | ||||
| Lower respiratory condition† (yes) (208) | 56 (33.1) | 70 (61.4) | 26 (63.4) | ||||
| Upper respiratory condition‡ (yes) (355) | 106 (62.7) | 89 (78.1) | 33 (80.5) | ||||
EU, endotoxin units.
Numbers in brackets for each predictor variable represent the number of infants that fall in each class of that predictor variable. Cell entries in the health outcome columns are number of subjects reporting outcome (% of column total).
Allergen sensitization (SPT+) = positive skin prick test to any of the tested 17 allergens (n = 169). Infants that were SPT(−) were used as the comparison group (n = 405). Total n = 169 + 405 = 574.
Recurrent wheeze = 2 or more wheezing episodes in the last 12 months (n = 114). Infants that had one or no wheezing episodes in the last 12 months were used as the comparison group (n = 460). Total n = 114 + 460 = 574.
Recurrent wheeze with allergen sensitization = two or more wheezing episodes in the last 12 months and SPT(+) (n = 41). Infants that had one or no wheezing episodes in the last 12 months and were SPT(−) were used as the comparison group (n = 332). Total n = 41 + 332 = 373.
Lower respiratory condition includes any of the following: whooping cough, croup, viral infections, bronchitis/bronchiolitis, flu, pneumonia.
Upper respiratory condition includes any of the following: cold, ear infection, sinus infection, Strep throat (positive culture), tonsillitis, colored drainage
Values in parentheses in the column are percentages.
Infantile wheezing
We first investigated the association between the wheezing outcomes and (1–3)-β-D-glucan exposure in concentration unit (μg/g) in quartiles (Table 3). Recurrent wheezing was significantly less likely among infants with very high (1–3)-β-D-glucan exposure levels (>60 μg/g). In contrast, recurrent wheezing with or without allergen sensitization was positively associated with (1–3)-β-D-glucan exposure in the first quartile. The recurrent wheezing combined with allergen sensitization to any allergen was also significantly less likely in infants exposed to high (1–3)-β-D-glucan concentrations (>60 μg/g). Similar trend was observed also for recurrent wheezing combined with sensitization to aeroallergens only, but was not statistically significant (data not shown). After stratification by sensitization group, the inverse association between (1–3)-β-D-glucan exposure and recurrent wheeze with allergen sensitization became stronger in the group of sensitized wheezers compared with sensitized nonwheezers, than those compared with the nonsensitized nonwheezers (Table 3, last column).
Table 3.
Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for recurrent wheeze and recurrent wheeze with allergen sensitization in relation to upper vs lower endpoints of continuously measured (1–3)-β-D-glucan quartiles (μg/g) (reference category is the value of the lower endpoint of each quartile)
| Recurrent wheeze*, aOR (95% CI) | Recurrent wheeze with SPT(+) vs no wheeze, SPT(−)†, aOR (95% CI) | Recurrent wheeze with SPT(+) vs no wheeze, SPT(+)‡, aOR (95% CI) | |
|---|---|---|---|
| (1–3)-β-D-glucan quartile endpoints (μg/g) | |||
| I. 3–22 | 3.04 (1.25–7.38) | 4.89 (1.02–23.57) | 160.51 (4.85–5311.00) |
| II. 23–60 | 1.29 (0.99–1.67) | 1.23 (0.79–1.92) | 2.54 (0.97–6.62) |
| III. 61–133 | 0.82 (0.65–1.05) | 0.59 (0.38–0.92) | 0.17 (0.05–0.57) |
| IV. 134–900 | 0.39 (0.16–0.93) | 0.13 (0.03–0.61) | 0.00 (0.00–0.07) |
| Endotoxin interquartile endpoints (39.19–171.17 EU/g) | 0.99 (0.71–1.37) | 1.17 (0.69–1.98) | 1.60 (0.58–4.41) |
| Visible mold (low vs none) | 1.18 (0.73–1.91) | 1.29 (0.57–2.90) | 2.64 (0.89–7.86) |
| Visible mold (high vs none) | 4.44 (1.63–12.05) | 9.51 (2.34–38.63) | 42.47 (4.70–384.14) |
| Mother’s smoking (≥20 vs 0 cigarettes/day) | 5.16 (2.33–11.44) | 10.17 (2.58–40.09) | 10.17 (2.58–40.09) |
| Parental asthma | 1.87 (1.17–3.00) | 2.22 (1.05–4.71) | 2.09 (0.76–5.77) |
| Race (Afro-American vs other) | 2.08 (1.15–3.73) | 3.93 (1.57–9.84) | 10.04 (2.45–41.14) |
| Siblings (1 vs 0) | 1.38 (0.77–2.47) | 1.84 (0.66–5.09) | 8.87 (1.85–42.51) |
| Siblings (≥2 vs 0) | 1.96 (1.08–3.57) | 2.46 (0.87–6.93) | 7.83 (1.60–38.38) |
| Lower respiratory condition§ | 3.98 (2.47–6.41) | 4.63 (2.05–10.46) | 9.93 (3.06–32.16) |
| Upper respiratory condition¶ | 2.15 (1.26–3.67) | 2.75 (1.08–7.04) | 4.47 (1.24–16.07) |
Recurrent wheeze = 2 or more wheezing episodes in the last 12 months (n = 114). Infants that had one or no wheezing episodes in the last 12 months were used as the comparison group (n = 460).
Recurrent wheeze with allergen sensitization = two or more wheezing episodes in the last 12 months and SPT(+) (n = 41). Infants that had one or no wheezing episodes in the last 12 months and were SPT(−) were used as the comparison group (n = 332).
Recurrent wheeze with allergen sensitization = two or more wheezing episodes in the last 12 months and SPT(+) (n = 41). Infants that had one or no wheezing episodes in the last 12 months and were SPT(+) were used as the comparison group (n = 128).
Lower respiratory condition includes any of the following: whooping cough, croup, viral infections, bronchitis/bronchiolitis, flu, pneumonia.
Upper respiratory condition includes any of the following: cold, ear infection, sinus infection, strep throat (positive culture), tonsillitis, colored drainage.
Text in bold indicates statistical significance.
In addition, as smoking is associated to a number of other risk factors, some of which such as diet were not evaluated in the study, a separate reporting of findings among smoking vs nonsmoking mothers was performed. After analyzing the recurrent wheeze data separately for infants whose mothers smoke (n = 83) and those whose mothers do not smoke (n = 491), the same trends of decrease of occurrence with increase in (1–3)-β-D-glucan exposure was observed. Because of the smaller sample size, however, the significance was lost, except for the strength and significance of the inverse association for infants of smoking mothers, which became greater (within third quartile, aOR = 0.41, 95% CI = 0.17–0.98; within fourth quartile, aOR = 0.01, 95% CI = 0.00–0.45).
Data were also analysed using loading unit (μg/m2) for (1–3)-β-D-glucan exposure. Similar to the data in concentration units, we observed an increase in wheezing outcomes when (1–3)-β-D-glucan exposure levels were low (first and second interquartile range), and a decrease when (1–3)-β-D-glucan exposure levels were high (>19 μg/m2) (third and fourth interquartile range). These associations however, were mostly nonsignificant (data not reported). Only the inverse association between recurrent wheezing combined with allergen sensitization and exposure to high (1–3)-β-D-glucan loading was statistically significant (within the third quartile, aOR = 0.64, 95% CI = 0.42–0.97; within the fourth quartile, aOR = 0.05, 95% CI = 0.00–0.51).
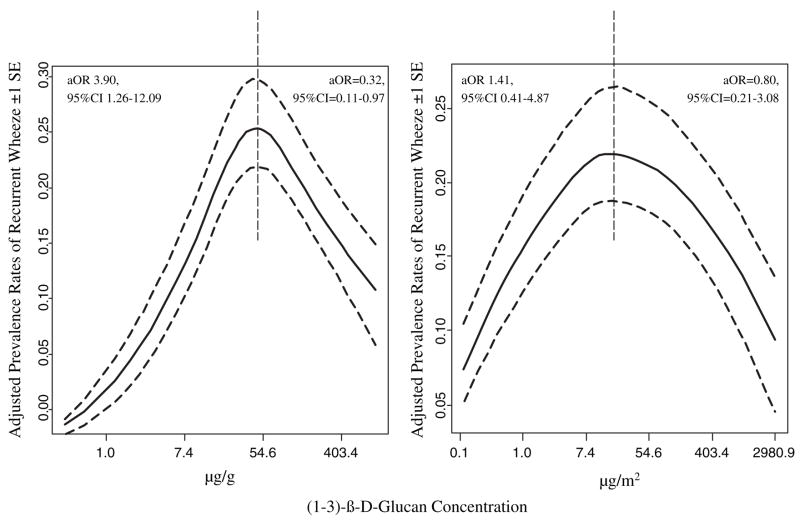
Visually, the association between continuously measured log-transformed beta-glucan and wheezing is presented in Fig. 1. A curvilinear relationship was found which was approximated by fitting a RCS function to the wheezing data and the levels of (1–3)-β-D-glucan. Points where contiguous curves meet are called knots. The spline functions that best fit the data had three knots. The cubic spline function was modelled allowing for three turning points (knots) in the curvilinear relationship between (1–3)-β-D-glucan and log(odds of wheeze). Knots were located at the 5, 50 and 95% percentiles of (1–3)-β-D-glucan.
Figure 1.
Smoothed plot of the adjusted prevalence rates of recurrent wheezing in relation to the log-transformed (1–3)-β-D-glucan concentration (solid lines). Dotted lines represent ± 1 standard error (SE).
The visual presentation of data prompted us to perform an additional analysis of the odds of wheeze when (1–3)-β-D-glucan was equal to the value at which the predicted value of wheeze turns around (approximately the midpoint of the range: 60 μg/g, 19 μg/m2) compared with the minimum value. The same analysis was also performed when (1–3)-β-D-glucan was equal to the maximum value (900 μg/g, 2966 μg/m2) compared with the midpoint. As expected, the logistic regression analyses showed increased odds for recurrent wheezing at (1–3)-β-D-glucan levels below the turning point and decreased odds above the turning point (Fig. 1). Similar trends were observed for recurrent wheeze with allergen sensitization (3–60 μg/g: aOR = 6.05, 95% CI = 0.84–43.79; 0.2–19 μg/m2: aOR = 9.23, 95% CI = 0.85–99.99; 60–900 μg/g: aOR = 0.08, 95% CI = 0.01–0.59; 19–2966 μg/m2: aOR = 0.08, 95% CI = 0.01–0.97). Therefore, the conclusions of inverse association between increase in (1–3)-β-D-glucan levels above the midpoint and wheeze outcomes (Table 3) were confirmed.
Endotoxin showed no effect neither on recurrent wheezing nor allergen sensitization when tested either on continuous scale or quartiles. Because of the scientific interest in endotoxin, we report the data with endotoxin in the model (Table 3).
As literature suggests that (1–3)-β-D-glucan may be related to visible mold (40), we also ran the models with no visible mold as a confounder, to check for over adjustment. We obtained similar results as the trend of significant inverse association was preserved. However, the 95% CI became wider. As in our study we did not find any correlation between (1–3)-β-D-glucan and visible mold, and the role of (1–3)-β-D-glucan as a surrogate of mold exposure is uncertain because of various other sources of the (1–3)-β-D-glucan, such as pollen and plants (41), we are reporting the full models, including visible mold as a confounder.
Among the other covariates, high visible mold, mother’s smoking, parent’s asthma, Afro-American race, siblings, as well as other lower and upper respiratory conditions were risk factors for both recurrent wheezing and recurrent wheezing combined with allergen sensitization (Table 3).
Allergen sensitization
Among the infants, 169 (29.4%) were sensitized to at least one allergen (food and/or aeroallergen), 25 were sensitized to food only and 79 to aeroallergens only (32). Exposure to high (1–3)-β-D-glucan concentrations and loadings had borderline significant inverse association with allergen sensitization assessed by SPT(+) to at least one aeroallergen and/or food antigen (μg/g: within third quartile, aOR = 0.89, 95% CI = 0.74–1.06; within fourth quartile, aOR = 0.57, 95% CI = 0.30–1.10) and (μg/m2: within third quartile, aOR = 0.90, 95% CI = 0.76–1.06; within fourth quartile, aOR = 0.48, 95% CI = 0.18–1.26). We also tested for interaction effect between (1–3)-β-D-glucan and endotoxin, and such was found in the outcome including allergen sensitization to any allergens, but only in loading unit (P = 0.02). Thus, we included the interaction effect in the SPT model when the exposure was in loading unit (within third quartile, aOR = 1.33, 95% CI = 0.83–2.15; within fourth quartile, aOR = 152.62, 95% CI = 6.57–3543.18). The latest result, however, may be due to the strong and significant correlation between (1–3)-β-D-glucan and endotoxin in this unit, and thus whether the result is attributed to (1–3)-β-D-glucan or endotoxin, or both, is unclear. None of the other covariates in the multivariate analysis were significantly associated with SPT(+).
There were no significant associations between (1–3)-β-D-glucan and allergen sensitization assessed by SPT(+) to aeroallergens only [(μg/g: within fourth quartile, aOR = 1.07, 95% CI = 0.71–1.62) and (μg/m2: within fourth quartile, aOR = 1.21, 95% CI = 0.77–1.89)].
Discussion
This study demonstrated a significant inverse association between increasing exposure to high (1–3)-β-D-glucan concentrations (>60 μg/g) and recurrent wheezing (aOR = 0.39) in high-risk infants. Even stronger association was found for recurrent wheezing combined with allergen sensitization (aOR = 0.18). Although others have reported a weak inverse relationship with (1–3)-β-D-βD-glucan and atopic wheeze in children aged 1–4 and 5–15 years (3, 30), this is the first study to demonstrate a statistically significant relationship between high (1–3)-β-D-glucan exposure and reduced wheezing in infants.
We did not find significant differences in (1–3)-β-D-glucan concentrations or loadings between the homes in three visible mold categories although increasing trend was seen in (1–3)-β-D-glucan loadings. This finding is in line with the findings of Gehring et al. (40) who only found difference in (1–3)-β-D-glucan loading, and not in concentration, between homes in two visible mold categories. This indicates that the variance in levels per square metre was largely determined by the amount of dust sampled. Thus after transformation from concentration to loading unit, there was a decrease in the between samples variation.
Previous studies show that (1–3)-β-D-glucan concentrations do not consistently correlate with total culturable mold spore counts (40, 42, 43), and more likely reflect exposure from multiple environmental sources of (1–3)-β-D-glucan, including mold, pollen, plants and their fragments (41, 44). Furthermore, (1–3)-β-D-glucan content varies between mold species (44), and this may lead to variance of health outcomes by fungal genera (28). For example, in this cohort, we have also found inverse association between the concentration of airborne Cladosporium and SPT(+) to any allergen (P < 0.05), and Cladosporium and SPT(+) to aeroallergens (P < 0.05), but positive associations between Penicillium/Aspergillus and SPT(+) to any allergen (P < 0.01) and between Alternaria and SPT(+) to any allergen (P < 0.01) (28). Molds contain (1–3)-β-D-glucan but also number of other agents such as sugars and enzymes. Apart from the fungi themselves mold growth is often associated with growth of other microbes. Thus, no conclusions concerning causal relationships between (1–3)-β-D-glucan and mold can be made. However, it seems (1–3)-β-D-glucan may be an independent measure of biologically active exposure. This may explain why visible mold exposure, unlike (1–3)-β-D-glucan, was a risk factor for recurrent wheezing in this cohort [as reported in detail by Cho et al. (33)].
Our study demonstrated that low levels of (1–3)-β-D-glucan exposure are associated with increase in the prevalence of recurrent wheeze, while the opposite was observed for the exposure to high levels of (1–3)-β-D-glucan. Similar trend was also found for the health outcome including wheeze combined with allergen sensitization. Children are a special group of interest as their immune system is evolving and thus may be more susceptible to environmental exposure factors (24). In fact, research has shown (45) that the effective priming of aeroallergen-specific memory T-cells is initiated during infancy and is consolidated by the end of preschool years in relation to the Th1–Th2 balance. An interesting observation that Th2 (allergic) priming is preferentially favoured by low-dose antigen exposure, whereas higher doses favor Th1 priming, was made by Constant et al. and Rogers et al. (46, 47). Moreover, epidemiological studies on exposure to indoor allergens have revealed a biphasic pattern in which sensitization risk increases with exposure levels until a plateau is reached, above which risk decreases with further increase in exposure (48–50). This same pattern was demonstrated in our study.
To date, knowledge on health effects of (1–3)-β-D-glucan in children is limited. Rylander et al. (29) reported that both nonatopic and atopic wheezing in school children (age 6–13 years) were positively correlated with airborne (1–3)-β-D-glucan concentrations, analysed by the LAL assay. Douwes et al. (36) also showed that increased levels of dustborne (1–3)-β-D-glucan were positively related to variability in peak expiratory flow in nonatopic and atopic children 7–11 years of age. In contrast, Schram-Bijkerk et al. (3) and Douwes et al. (30) reported that dustborne (1–3)-β-D-glucan concentrations were inversely associated with atopic wheeze in 5 to13- years-old children and both asthma and persistent wheeze in 1 to 4-years-old children. All these studies, similarly to ours, showed that the health outcome was more significantly associated with exposure in the subgroup of atopic children. Interestingly, in the latter two studies (3, 30) inclusion of both (1–3)-β-D-glucan and endotoxin in the models lead to loss of significance of either the effect of endotoxin (3) or (1–3)-β-D-glucan (30). In addition, these studies reported a strong and significant correlation between endotoxin and (1–3)-β-D-glucan (3, 30), thus it is uncertain whether the observed effects were driven by endotoxin, (1–3)-β-D-glucan or both. The lack of correlation between (1–3)-β-D-glucan and endotoxin concentrations in our database strengthens the finding that the effect seen for (1–3)-β-D-glucan exposure is not confounded by the endotoxin exposure.
In this cohort, we have also found an inverse association between the concentration of airborne Cladosporium and allergen sensitization (28). Indeed this data is somewhat consistent with the hygiene hypothesis, which postulates that exposure to microbial products (such as endotoxin) early in life favours modification of Th2 directed immune responses (25). Studies on exposure to high endotoxin and increased or decreased frequency of wheezing during infancy have shown conflicting results (25, 26, 51). Possible explanation for this controversy may be that these studies did not concomitantly assess indoor (1–3)-β-D-glucan levels. However, several studies have revealed a strong adjuvant activity of (1–3)-β-D-glucan on the systemic allergic immune response in animal models (9, 10, 52). Therefore, it must be emphasized that the immunologic impact of (1–3)-β-D-glucan exposure on Th2 directed atopic disorders remains uncertain and requires further investigation.
The above findings were further supported by the inverse trend between (1–3)-β-D-glucan exposure and allergen sensitization. This and the stronger inverse association between (1–3)-β-D-glucan and wheezing in allergen sensitized infants compared with wheezing in all infants, even stronger after stratification by sensitization, suggests that (1–3)-β-D-glucan could modify allergic respiratory responses in infants. It must be emphasized that it is uncertain if this early observed effect will impact the risk of later development of childhood asthma among this cohort of sensitized infants with recurrent wheezing as health outcomes associated with (1–3)-β-D-glucan exposure determined in this infant population may be transient.
Previous studies have shown stronger association between dust (1–3)-β-D-glucan exposures and the health outcome in loading units than in concentration units (36), or no association at all in the concentration unit (30). We found similar trends between the health outcomes and (1–3)-β-D-glucan exposures in the two units, but the associations were stronger for the concentration unit. These differences may be due to the different health outcome or different (1–3)-β-D-glucan analysis methods used: Douwes et al. (30, 36) analysed (1–3)-β-D-glucan content by the EIA, while we used the LAL.
We collected dust samples only from the baby’s primary activity room because the main living room allergen levels for dust allergens have shown to be higher than that in the bedding in the infants’ homes (52). Furthermore, despite the fact that infants spend most of their time in bed, a recent European study (30) has shown association only between living area exposure and asthma in 4-years-old children, but none with mattress exposure. This was attributed to low allergen levels in infants’ mattresses as they were newly purchased (use of <3 months). In addition, the collection of dust by trained technicians using a standardized protocol and the same model of vacuum cleaner is strength of the present study as it decreases the collection bias. Further, home exposure assessment was conducted almost concurrently with health outcome assessment.
A potential limitation of the study generalizability is that the cohort consists only of infants born to atopic parents. However, the Third National Health and Nutrition Examination Survey (NHANES III [53]), 1988–94, designed to obtain nationally representative information on the health of the population of the United States, showed that 54.3% of the US population was sensitized to one or more allergens. This finding suggests that the results observed in the current study can be widely generalized to over 50% of the US population. Research has shown that exposure to maternal smoking and aeroallergens (54) impact the development of allergen sensitization as early as in pregnancy. Thus, the absence of antenatal exposure data may be another study limitation. In addition, the low number of recurrent wheezers with SPT(+) also limits the power of the study. Unfortunately, as the CCAAPS study did not initially include the analysis for (1–3)-β-D-glucan, we were able to analyse only samples with sufficient dust amount left after analyses for all other allergens were completed. Although this introduces a selection bias, and may limit the generalizability of the results, we were still able to analyse 76% of the samples. In addition, the amount of dust and wheeze outcomes did not correlate (P = 0.32). Therefore, we do not expect dust amount to bias the results.
In conclusion, we found that the concentration of (1–3)-β-D-glucan is a measure of biological exposure that is independent from observed visible mold. A significant inverse biphasic association, a model of a hormesis doseresponse relationship, was found between high (1–3)-β-D-glucan levels and recurrent wheezing. This association was even stronger in a subgroup of allergen-sensitized infants. It seems that exposure to high (1–3)-β-D-glucan levels (>60 μg/g, >19 μg/m2) may be conducive to reduced wheezing in infants at high risk for developing asthma (as those born to atopic parents). Long-term follow up of this cohort will help determine how early (1–3)-β-D-glucan exposure affects the development of phenotypes such as atopy, allergic rhinitis and asthma in later childhood.
Acknowledgments
This study was supported by National Institute of Environmental Health Sciences (NIEHS) grant ES11170 and the National Institute for Occupational Safety and Health (NIOSH) Training Program of the University of Cincinnati Education and Research Center Grant T42/CCT510420. We would like to thank our participating families, our recruitment team, Sherry Stanford and Stephanie Maier who performed infant assessment.
References
- 1.Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, et al. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect. 2002;110:781–786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, et al. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants, and maternal history of asthma. Am J Epidemiol. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- 3.Schram-Bijkerk D, Doekes G, Douwes J, Boeve M, Riedler J, Ublagger E, et al. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clin Exp Allergy. 2005;35:1272–1278. doi: 10.1111/j.1365-2222.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 4.Schram D, Doekes G, Boeve M, Douwes J, Riedler J, Ublagger E, et al. Bacterial and fungal components in house dust of farm children, Rudolf Steiner school children and reference children-the PARSIFAL Study. Allergy. 2005;60:611–618. doi: 10.1111/j.1398-9995.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- 5.Fogelmark B, Thorn J, Rylander R. Inhalation of (1–>3)-beta-D-glucan causes airway eosinophilia. Mediators Inflamm. 2001;10:13–19. doi: 10.1080/09629350123707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogelmark B, Sjostrand M, Rylander R. Pulmonary inflammation induced by repeated inhalations of beta(1,3)-D-glucan and endotoxin. Int J Exp Pathol. 1994;75:85–90. [PMC free article] [PubMed] [Google Scholar]
- 7.Korpi A, Kasanen JP, Kosma VM, Rylander R, Pasanen AL. Slight respiratory irritation but not inflammation in mice exposed to (1–>3)-beta-D-glucan aerosols. Mediators Inflamm. 2003;12:139–146. doi: 10.1080/0962935031000134851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rylander R. Airway responsiveness and chest symptoms after inhalation of endotoxin or 1,3, beta-d-glucan. Indoor Build Environ. 1996;154:106–111. [Google Scholar]
- 9.Rylander R, Holt PG. (1–>3)-beta-D-glucan and endotoxin modulate immune response to inhaled allergen. Mediators Inflamm. 1998;7:105–110. doi: 10.1080/09629359891252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ormstad H, Groeng EC, Lovik M, Hetland G. The fungal cell wall component beta-1,3-glucan has an adjuvant effect on the allergic response to ovalbumin in mice. J Toxicol Environ Health. 2000;61:55–67. doi: 10.1080/00984100050116780. [DOI] [PubMed] [Google Scholar]
- 11.Alwis K, Mandryk J, Hocking A. Exposure to biohazards in wood dust: bacteria, fungi, endotoxins, and (1-3)-β-D-glucans. Appl Occup Environ Hyg. 1999;14:598–608. doi: 10.1080/104732299302404. [DOI] [PubMed] [Google Scholar]
- 12.Mandryk J, Alwis K, Hocking A. Effects of personal exposure on pulmonary function and work-related symptoms among sawmill workers. Ann Occup Hyg. 2000;44:281–289. [PubMed] [Google Scholar]
- 13.Rylander R, Thorn J, Attefors R. Airways inflammation among workers in a paper industry. Eur Respir J. 1999;13:1151–1157. doi: 10.1034/j.1399-3003.1999.13e35.x. [DOI] [PubMed] [Google Scholar]
- 14.Thorn J. Seasonal variations in exposure to microbial cell wall components among household waste collectors. Ann Occup Hyg. 2001;45:153–156. [PubMed] [Google Scholar]
- 15.Heldal K, Halstensen A, Thorn J, Eduard W, Halstensen T. Airway inflammation in waste handlers exposed to bioaerosols assessed by induced sputum. Eur Respir J. 2003;21:641–645. doi: 10.1183/09031936.03.00059702. [DOI] [PubMed] [Google Scholar]
- 16.Rylander R, Carvalheiro M. Airways inflammation among workers in poultry houses. Int Arch Occup Environ Health. 2006;79:487–490. doi: 10.1007/s00420-005-0072-5. [DOI] [PubMed] [Google Scholar]
- 17.Douwes J, Wouters I, Dubbeld H, van Zwieten L, Steerenberg P, Doekes G, et al. Upper airway inflammation assessed by nasal lavage in compost workers: a relation with bio-aerosol exposure. Am J Ind Med. 2000;37:459–468. doi: 10.1002/(sici)1097-0274(200005)37:5<459::aid-ajim2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Rylander R. Health effects among workers in sewage treatment plants. Occup Environ Med. 1999;56:354–357. doi: 10.1136/oem.56.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladding T, Thorn J, Stott D. Organic dust exposure and work-related effects among recycling workers. Am J Ind Med. 2003;43:584–591. doi: 10.1002/ajim.10220. [DOI] [PubMed] [Google Scholar]
- 20.Rylander R. Airborne (1 →3)-β-glucan and airway disease in a day-care center before and after renovation. Arch Environ Health. 1997;52:281–285. doi: 10.1080/00039899709602199. [DOI] [PubMed] [Google Scholar]
- 21.Wan G-H, Li CS. Indoor endotoxin and glucan in association with airway inflammation and systemic symptoms. Arch Environ Health. 1999;54:172–179. doi: 10.1080/00039899909602256. [DOI] [PubMed] [Google Scholar]
- 22.Wouters I, Hilhorst S, Kleppe P, Doekes G, Douwes J, Perez C, et al. Upper airway inflammation and respiratory symptoms in domestic waste collectors. Occup Environ Med. 2002;59:106–112. doi: 10.1136/oem.59.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorn J, Rylander R. Airways inflammation and glucan in a row house area. Am J Respir Crit Care Med. 1998;157:1798–1803. doi: 10.1164/ajrccm.157.6.9706081. [DOI] [PubMed] [Google Scholar]
- 24.Holt P, Upham J, Sly P. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies Review. J Allergy Clin Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 25.von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, Maisch S, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 26.Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitization in infants at high risk of asthma. Lancet. 2000;355:1680–1683. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 27.Douwes J, Pearce N. Invited commentary: is indoor mold exposure a risk factor for asthma? Am J Epidemiol. 2003;158:203–206. doi: 10.1093/aje/kwg149. [DOI] [PubMed] [Google Scholar]
- 28.Osborne M, Reponen T, Adhikari A, Cho SH, Grinshpun S, Levin L, et al. Specific fungal exposures, allergic sensitisation, and rhinitis in infants. Pediatr Allergy Immunol. 2006;17:450–457. doi: 10.1111/j.1399-3038.2006.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rylander R, Norrhall M, Engdahl U, Tunsater A, Holt P. Airways inflammation, atopy, and (1–> 3)-beta-D-glucan exposures in two schools. Am J Respir Crit Care Med. 1998;158:1685–1687. doi: 10.1164/ajrccm.158.5.9712139. [DOI] [PubMed] [Google Scholar]
- 30.Douwes J, van Strien R, Doekes G, Smit J, Kerkhof M, Gerritsen J, et al. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol. 2006;117:1067–1073. doi: 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Ryan P, LeMasters G, Biagini J, Bernstein D, Grinshpun S, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 32.LeMasters G, Wilson K, Levin L, Biagini J, Ryan P, Lockey J, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–511. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho S-H, Reponen T, LeMasters G, Levin L, Huang J, Meklin T, et al. Mold damage in homes and wheezing in infants. Ann Allergy Asthma Immunol. 2006;97:539–545. doi: 10.1016/S1081-1206(10)60947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho S-H, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, et al. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ. 2006;371:31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campo P, Kalra HK, Levin L, Reponen T, Olds R, Lummus Z, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006;118:1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douwes J, Zuidhof A, Doekes G, van der Zee SC, Wouters I, Boezen M, et al. (1–>3)-beta-D-glucan and endotoxin in house dust and peak flow variability in children. Am J Respir Crit Care Med. 2000;162:1348–1354. doi: 10.1164/ajrccm.162.4.9909118. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes H, Thomas P, Sporik R, Holgate S, Cogswell J. A birth cohort study of subjects at risk of atopy: twenty-twoyear follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165:176–180. doi: 10.1164/ajrccm.165.2.2104032. [DOI] [PubMed] [Google Scholar]
- 38.Beasley R, Keil U, von Mutius E, Pearce N. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 39.Gold D, Burge H, Carey V, Milton D, Platts-Mills T, Weiss S. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 40.Gehring U, Douwes J, Doekes G, Koch A, Bischof W, Fahlbusch B, et al. INGA Study Group. Indoor Factors and Genetics in Asthma. Beta (1–>3)-glucan in house dust of German homes: housing characteristics, occupant behavior, and relations with endotoxins, allergens, and molds. Environ Health Perspect. 2001;109:139–144. doi: 10.1289/ehp.01109139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rylander R, Fogelmark B, McWilliam A, Currie A. (1–>3)-beta-D-glucan may contribute to pollen sensitivity. Clin Exp Immunol. 1999;115:383–384. doi: 10.1046/j.1365-2249.1999.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douwes J, Doekes G, Heinrich J, Koch A, Bischof W, Brunekreef B. Endotoxin and β(1-3)-Glucan in house dust and the relation with home characteristics: a pilot study in 25 German houses. Indoor Air. 1998;8:255–263. [Google Scholar]
- 43.Wan GH, Li CS. Indoor endotoxin and glucan in association with airway inflammation and systemic symptoms. Arch Environ Health. 1999;54:172–179. doi: 10.1080/00039899909602256. [DOI] [PubMed] [Google Scholar]
- 44.Foto M, Plett J, Berghout J, Miller JD. Modification of the Limulus amebocyte lysate assay for the analysis of glucan in indoor environments. Anal Bioanal Chem. 2004;379:156–162. doi: 10.1007/s00216-004-2583-4. [DOI] [PubMed] [Google Scholar]
- 45.Holt P, Macaubas C, Stumbles PA, Sly P. The role of allergy in the development of asthma. Nature. 1999;402:B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 46.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers P, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163:1205–1213. [PubMed] [Google Scholar]
- 48.Platts-Mills T, Vaughan JW, Blumenthal K, Woodfolk J, Sporik R. Decreased prevalence of asthma among children with high exposure to cat allergen: relevance of the modified Th2 response. Mediators Inflamm. 2001;10:288–291. doi: 10.1080/09629350152700902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cullinan P, MacNeill S, Harris JM, Moffat S, White C, Mills P, et al. Early allergen exposure, skin prick responses, and atopic wheeze at age 5 in English children: a cohort study. Thorax. 2004;59:855–861. doi: 10.1136/thx.2003.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holt P, Upham J, Sly P. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163:322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 52.Leaderer B, Belanger K, Triche E, Holford T, Gold D, Kim Y, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arbes S, Gergen P, Elliott L, Zeldin D. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Upham J, Holt P. Environment and development of atopy. Curr Opin Allergy Clin Immunol. 2005;5:167–172. doi: 10.1097/01.all.0000162310.79555.ed. [DOI] [PubMed] [Google Scholar]