Abstract
In an effort to better understand the relationship between different fungal sampling methods in the indoor environment, four methods were used to quantify mold contamination in 13 homes with visible mold. Swab, fungal spore source strength tester (FSSST), and air samples (total of 52 samples) were analyzed using both the microscopic (total spore count) and culture-based (CFU count) enumeration techniques. Settled dust samples were analyzed for culturable fungi only, as the microscopic enumeration was restricted by the masking effect. The relationships between the data obtained with the different sampling methods were examined using correlation analysis. Significant relationships were observed between the data obtained from swab and FSSST samples both by the total counting (r = 0.822, p <0.05) and by the CFU counting (r = 0.935, p <0.01). No relationships were observed between air and FSSST samples or air and settled dust samples. Percentage culturability of spores for each sampling method was also calculated and found to vary greatly for all three methods (swab: 0.03% to 63%, FSSST: 0.1% to >100%, air: 0.7% to 79%). These findings confirm that reliance on one sampling or enumeration method for characterization of an indoor mold source might not provide an accurate estimate of fungal contamination of a microenvironment. Furthermore, FSSST sampling appears to be an effective measurement of a mold source in the field, providing an upper bound estimate of potential mold spore release into the indoor air. Because of the small sample size of this study, however, further research is needed to better understand the observed relationships in this study.
Keywords: air sampling, indoor fungi, settled dust
It has been estimated that 20% to 40% of homes in Northern Europe and Canada have mold contamination.(1) This number is likely to be much higher in tropical and subtropical countries.(2,3) In the United States, as many as 40% of homes have mold problems.(1,4) Various health effects, such as respiratory symptoms, allergic rhinitis, asthma, and hypersensitivity pneumonitis, are associated with mold exposure.(5–12) A case control study conducted in Europe suggested a relationship between increases in symptoms in asthmatic patients and increased mold and moisture problems in the home.(13)
Other studies have shown that exposure to visible mold, or excessive moisture, which promotes mold growth, leads to an increase in allergic symptoms.(6,8,14–20) Toxicity caused by exposure to the metabolites of certain molds have also been linked to health effects.(6,21) However, the relationship between specific health effects and the mold spore concentration has not been well defined.(6) It has been criticized that the methodologies for sampling and analysis are neither standardized nor definitive.(22) Available quantitative methods are used in combination with a comprehensive qualitative assessment.(23–25) Jarvis and Morey(22) have suggested that lack of a standard methodology is a primary cause for the poorly understood relationship between fungal exposures and health outcomes. Therefore, it is important to be able to identify and quantify the mold contamination levels in indoor environments using validated methods for sampling and analysis.
One of two approaches is typically used to assess mold contamination with respect to fungal spore identification and enumeration: culture-based analysis (the colony forming unit [CFU] count) and the microscopic analysis (the total spore count). The culture-based analysis, which is more common, gives the ability to identify colonies to the species level and a large reference database is available for proper identification of colonies.(26) Species-level identification is useful in detecting “indicator fungi” that are commonly found in moldy buildings. For many years, the culture-based methods have tended to be the dominant choice of both the practicing indoor air quality professionals and the research community since the Andersen sampler was used as “the gold standard” for bioaerosol sampling.
However, several disadvantages of the CFU analysis are also apparent. The incubation period is usually long (over 7 days for some fungal species),(27,28) and CFU analysis can overlook fungal species that are not easily culturable. Furthermore, it might underrepresent those fungal types that grow slowly because they are overtaken by faster growing colonies.(26,28–32) Kozak et al.(33) demonstrated that although the level of culturable spores may be below the limit of detection, the total number of spores may be sufficient to cause respiratory symptoms.
Some fungal species, such as the spores from Stachybotrys chartartum, have been found to lose their culturability soon after they become airborne; however, this does not appear to affect their allergenicity or toxicity.(3,33,34) Furthermore, some health effects, especially respiratory allergies, have been shown to be associated with the total spore count rather than with the CFU count.(33,35)
Similar to the CFU count, there are some advantages and disadvantages of the total spore count method. Two advantages are that (1) both viable and nonviable spores can be included, and (2) the total count is less time-consuming than the CFU analysis (can be performed within hours of sample collection). Among disadvantages of this enumeration method, there are (a) masking effect, when the background matrix may mask small spores; (b) high data variability when spore density is low; (c) overestimation of large pigmented spores; and (d) impossibility of performing the species-level identification.(26,36)
Other methods for fungal analysis include the use of surrogate markers that measure quantitative loads of fungal biomass, such as β-glucan and ergosterol. These indicator methods are useful for providing general information about the total amount of fungi in the environment but are often not specific enough to relate to health outcomes because of their surrogate nature.(29) Recently, polymerase chain reaction (PCR) and immunochemical methods have become available for fungal analysis.(34,36–41) There is currently, however, very little reference data available with these techniques.
Currently, there are numerous sampling methods available to measure fungal concentrations in the environment. Source sampling, which includes methods such as swab, tape, bulk, and dust, is commonly used to identify indoor fungi. These source sampling methods have been cited by the American Industrial Hygiene Association (AIHA)(42) as “necessary adjuncts” to air sampling, especially under conditions of low air movement, or when air sampling might result in false-negative findings. However, these surface-based methods cannot identify hidden sources of mold.(43) Swab and tape sampling are common methods of fungal exposure assessment through the source characterization, partially because of ease of collection. They are often used as tools for identification of fungi but do not provide measures of exposure to airborne spores. Bulk samples include pieces of material such as wallboard, carpet, or return air filter, that are collected from the contaminated area to identify and find the relative concentration of mold in the sample.(43)
Fungal spores can also be measured in settled dust sampled from the floor.(44,45) This method is usually attempted to evaluate long-term respiratory exposure to fungi, though the stability of microorganisms over time is questionable.(46–48) Flannigan(49) indicated that dust may not adequately reflect human inhalation exposure, evidenced by his research findings that only a very small amount of reaerosolized dust particles is of respirable size. Furthermore, Chew et al.(46) found that culturable air and dust samples represent differing types of potential mold exposure and, thus, are not related indicators of exposure to mold. Settled dust can be analyzed by various techniques, such as CFU, PCR, and biochemical methods for β-glucan and ergosterol. However, it is difficult to conduct the microscopic enumeration from dust samples, in part, because fungi in dust is masked by other particles.(40,46,50) Some investigators have managed to overcome this problem using a two-phase technique.(51)
Air sampling is one of the most common methods used to assess fungal levels in indoor environments. Many studies have related human health effects, such as increases in allergic and asthmatic respiratory symptoms, to airborne fungal spores.(22,33,52–56) As the health effects of fungal exposure are mainly respiratory, air sampling is believed to be adequate to represent the exposure. However, fungal spores have been found to exhibit varying patterns in their release into the air depending on several environmental factors.(22,46,52–56)
In an effort to link the mold source characterization and assessment of exposure to airborne fungal spores, several recent studies addressed the conditions necessary for fungal spore release from a mold source.(57–58) Two devices have been developed to measure the aerosolization potential of a visible fungal source: (1) the fungal spore source strength tester (FSSST)(59–61) and (2) the particle field and laboratory emission cell (PFLEC).(62) Both of these devices use portable aerosolization chambers in which spores are aerosolized from a fungal source and immediately collected into an air sampler.
The relationship between different fungal assessment methods has not been extensively characterized. Very little information is available on the comparison of the data obtained with the microscopic and culture-based enumeration of samples collected by a specific method, as well as the data collected by different sampling methods. Thus, a pilot study was conducted to compare the data collected using four sampling methods in mold contaminated homes. These methods include swab, FSSST, air, and settled dust sampling, and the first three were used to generate the total spore data and CFU data.
METHODS
Twenty-six homes with self-reported mold contamination were screened for this study in the greater Cincinnati, Ohio, metropolitan area. Thirteen homes were selected for evaluation by a trained indoor air quality researcher based on the size of the visible mold contamination (>144 cm2). Four types of sampling were performed on the selected homes: swab and settled dust (representing the sources of sporulation [the former] and resuspension [the latter]), FSSST (representing the source potential for aerosolization from the growth surface), and air sampling (representing the actual air contamination). Swab, FSSST and air methods were used as outlined by Sivasubramani et al.(60)
Prior to the mold sampling, both relative humidity and indoor temperature were recorded using a traceable humidity/temperature pen (Fisher Scientific Company, Pittsburgh, Pa.). The surface moisture content of the test surface was measured with a Protimeter (BLD 5800, GE Protimeter, Wilmington, Del.) and expressed as a percentage of the mass of water in a given volume of a material [(wet mass – dry mass) × 100/(dry mass)]. For a specific material, this percentage is calculated as a wood-equivalent value.
Swab sampling was performed on a 1-cm2-area of the mold contaminated surface (usually a wall). The 1-cm2-area was chosen to be negligibly small as compared with the FSSST sampling area of 90.25 cm2 (internal cross-section that covered the former). The surface was thoroughly swabbed with a sterile wet swab (Fisher Scientific) to remove as much of the mold as possible and collected in a 0.05% Tween 80 solution (Sigma Chemicals Co., St. Louis, Mo.). The FSSST sampling unit is a closed, two-pump aerosolization chamber that is held tightly on the contaminated surface during sampling. A push-vacuum (air supply) pump (11.5 L/min) produces airflow that first passes through a HEPA filter (1244 HEPA capsule filter, PALL Gelman Laboratory, Ann Arbor, Mich.), then is directed through a 112-hole orifice stage, passing over the mold-contaminated surface.
The air is then drawn through a center orifice into an SKC BioSampler (SKC, Inc., Eighty Four, Pa.) at a rate of 12.5 L/min, using another vacuum pump. Each FSSST sample was collected for 10 min, which was shown to be sufficient to determine the spore aerosolization potential.(59–61) Simultaneously with the FSSST samples, the air samples were collected at least 1 m away from the source into the BioSampler (0.05% Tween 80 solution) using a vacuum-pull (air sampling) pump operating at a flow rate of 12.5 L/min for 10 min (a short-term sampling).
Dust sampling was performed using a canister-type vacuum cleaner (Filterqueen Majestic, HMI Industries Inc., Seven Hills, Ohio) fitted with a nozzle filter bag (HEPA) (Filtration Group Inc., Joliet, Ill.). In every home, the dust sample was collected in the same room where the visible mold contamination was identified. Samples were vacuumed from a 2-m2-area of carpet for 4 min. For noncarpeted floors, the settled dust sample was taken at a rate of 1 m2/min, as described by Meklin et al.(40)
The swab, air, and FSSST samples were analyzed for both culturable and total fungal spores. An aliquot of each sample was cultured on malt extract agar (MEA), supplemented with streptomycin sulfate to inhibit bacterial growth.(63) Each sample was plated in triplicate, incubated for 7 days and identified to the species level, whenever possible, based on the colony morphology. The dust samples were analyzed by the culture-based method only, since the total fungal spore count is restricted by masking of spores by other dust particles. For these samples, dust was suspended in a buffer solution containing 0.0425 g l−1 KH2PO4, 0.25 g MgSO4 × 7 H2O l−1, 0.008 g NaOH, and (0.02% v/v) Tween 80. An aliquot of this solution (0.1 mL) was cultured in triplicate on MEA treated with an antibiotic agent to inhibit bacterial growth. The samples were incubated for 7 days and then identified to genus level.
The procedures used for the total count of fungal spores in the swab, air, and FSSST samples have been fully described by Sivasubramani et al.(60) Briefly, an aliquot of each sample was filtered onto a 13-mm mixed cellulose ester filter (0.8 μm pore size; Fisher Scientific) and then placed on a glass slide. Filters were dried overnight, then cleared by acetone vapor using a modified instant acetone-vaporizing unit (Quickfix, Environmental Monitoring Systems, Charleston, S.C.). A 25 × 25-mm cover glass was mounted on the slide using glycerin jelly (gelatin: 20 g, Phenol crystals: 2.4 g, glycerol: 60 mL, water: 70 mL). A light microscope (model Leitz Laborlux S, Leica Mikroskopie und Systeme GmbH, Wetzler, Germany) at a magnification of 400× was used to identify and enumerate the collected fungal spores. For slides with a relatively high number of spores (>50 spores per microscopic field), spores were enumerated in 20 microscopic fields; for the slides with sparse deposit (<50 spores per field), 40 microscopic fields were counted. The spores were microscopically identified to genus/group level. The limits of detection for the total spore count were 82 spores per cm2 for swab samples, 659 spores per m3 for air samples, and 0.9 spores per cm2 for the FSSST samples.
Correlation analyses were used to relate the number of spores collected by the different methods. Multiple comparisons were made between each collection type for both culturable and total spore counts. Scatterplots were generated using Sigma Plot (SPSS Inc.) and a correlation coefficient was calculated and tested for significance at α = 0.05 for each relationship. The statistical significances of the correlation results were calculated using SPSS. The percentage of culturable spores was determined for swab, FSSST, and air samples. Indoor air concentrations of fungi were compared by utilizing the data on the outdoor levels determined on the same day around the greater Cincinnati metropolitan area through the regional ambient monitoring campaign carried out using an SKC Button Aerosol Sampler (24-hour samples). The latter collected particles on a mixed cellulose ester filter at a flow rate of 4 L/min (the method has been fully described by Adhikari et al.(64)). The sampling efficiencies of the air samplers used for indoor (BioSampler) and outdoor (Button Sampler) fungal spore collection are about the same for the size range of fungal spores.
RESULTS
Three types of surfaces with mold contamination were observed in the 13 homes in the study. Mold contamination on concrete surfaces occurred in five of the homes. Contamination of wood surfaces, including wood paneling and wood joists, occurred in four homes. Contamination of drywall also occurred in four homes. Relative humidity ranged from 23% to 74% among the homes. Only four homes had relative humidity values over 50%. Surface moisture values ranged from 5.0% to 18.4% among the homes. The highest surface moisture (18.4%) occurred in the home that was found to be contaminated with Stachybotrys. Temperatures in the homes ranged from 18.8° to 26.1°C.
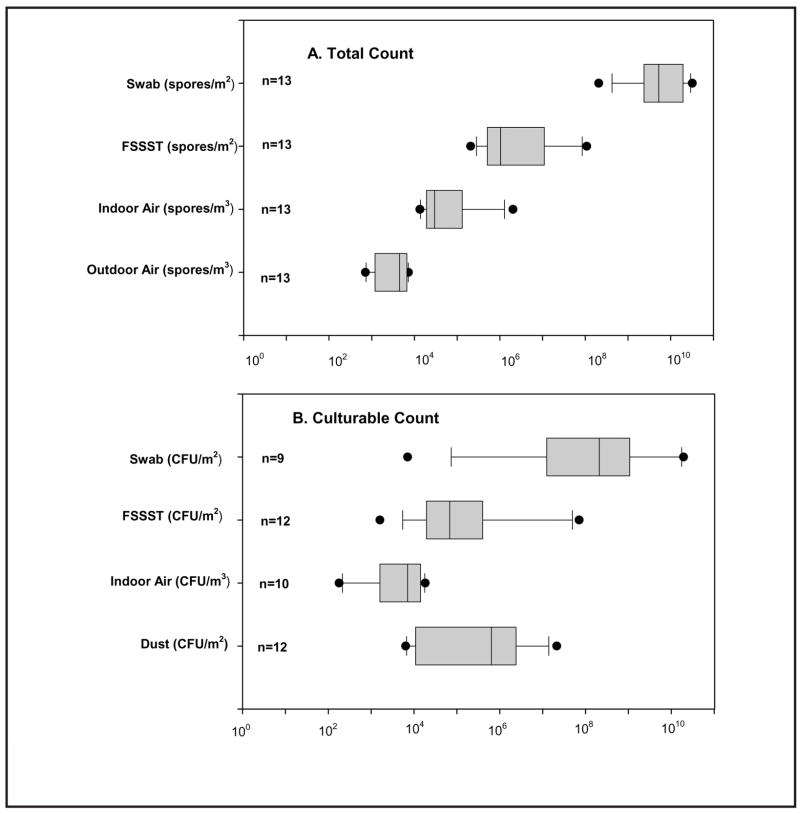
Figure 1 presents boxplots for the concentration of spores with respect to the microscopic and culture-based fungal enumeration obtained from swab, FSSST, air (indoor and outdoor), and dust samples across all 13 homes. For one home, CFU analysis was not performed from any of the sampling methods due to an oversight during the sampling period. In addition, swab samples from two homes and an air sample from one home were contaminated with bacteria, so CFU counts for fungal spores could not be determined. Lastly, culture samples taken from one home did not grow for either the swab or air samples. In all of these homes, the total spore count was still obtained.
FIGURE 1.
Percentile and median values of concentrations of spores and colony forming units across 13 homes. The boxplot shows the following: horizontal lines from left, 5%, 25%, 50%, 75%, 95%, percentiles; symbol • shows the range of data; n = number of homes represented in each sampling method.
Figure 1 shows that the median spore concentrations over all the homes were an order of magnitude higher for both swab and FSSST in the total count method, as compared with the culturable count. The CFU count for these methods, however, had much higher variability. The median spore level for indoor air was also higher for the total count method (by a factor of 4). The variabilities of the indoor air spore count levels were similar for both microscopic and culture-based methods. The median value measured in outdoor air (the sampling methodology utilized in the outdoor monitoring campaign included only the total count) was approximately an order of magnitude lower than the one determined in indoor air. The median culturable dust level fell between the median levels of culturable counts obtained from the FSSST and swab samples and had slightly lower variability.
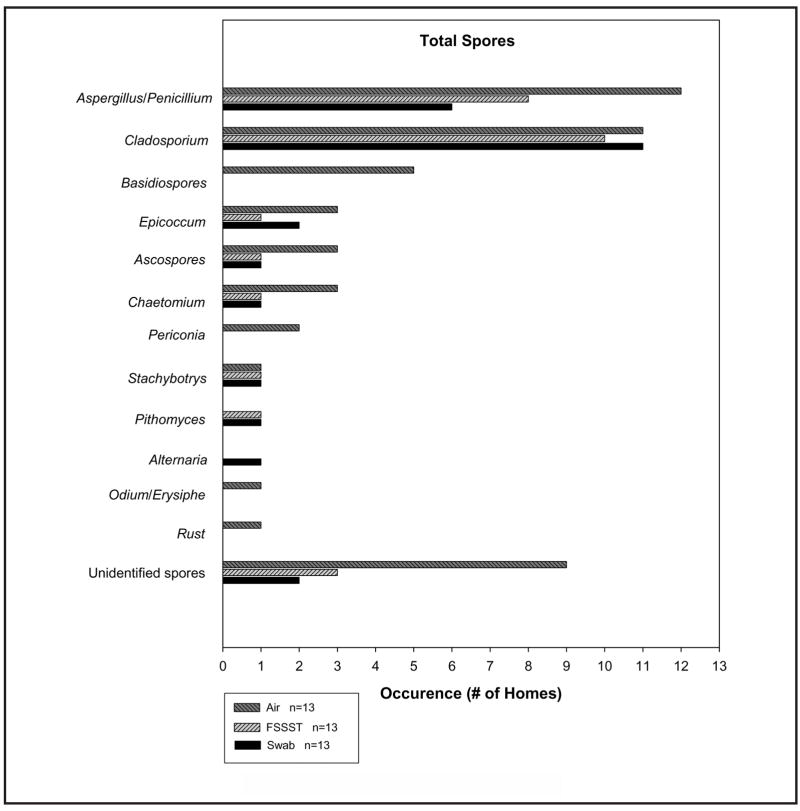
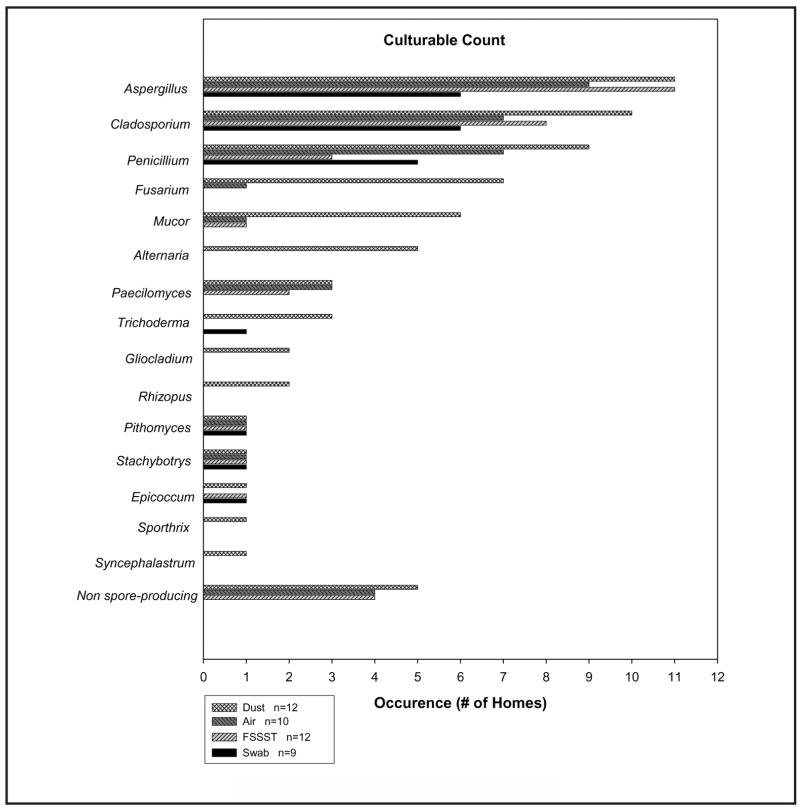
Figures 2 and 3 show the number of homes in which each specific spore type (genus or group) was identified, with the total spore count (Figure 2) and CFU count (Figure 3). The abovementioned number of homes is referred to as “occurrence” in the x-axis of each figure (some investigators would define it as “frequency”). Not all spore types were found for each sampling method. Aspergillus, Penicillium, and Cladosporium were the most common fungal types identified in both the total and culture-based spore counts.
FIGURE 2.
Spore types identified by total spore count
FIGURE 3.
Spore types identified by CFU enumeration
Swab sampling from the visible mold sources (collected from contaminated walls) in 13 homes revealed 8 different types of fungal spores, as well as unidentified spores. For CFU analysis of swab samples taken from 9 homes, 7 spore types were identified. FSSST sampling from the visible mold sources in 13 homes revealed 7 different types of fungal spores present as unidentified spores in the total spore population as well. For CFU analysis of FSSST samples taken from 12 homes, 8 spore types were identified. Short-term air sampling conducted simultaneously with FSSST sampling in each of the 13 homes revealed 10 different types of fungal spores present for the total spore population, as well as unidentified spores. For CFU analysis of the air samples taken from 10 homes, 9 spore types were identified. The settled dust sampling in 12 homes with visible mold contamination revealed 16 different fungal spore types (including nonsporulating colonies) through the CFU enumeration.
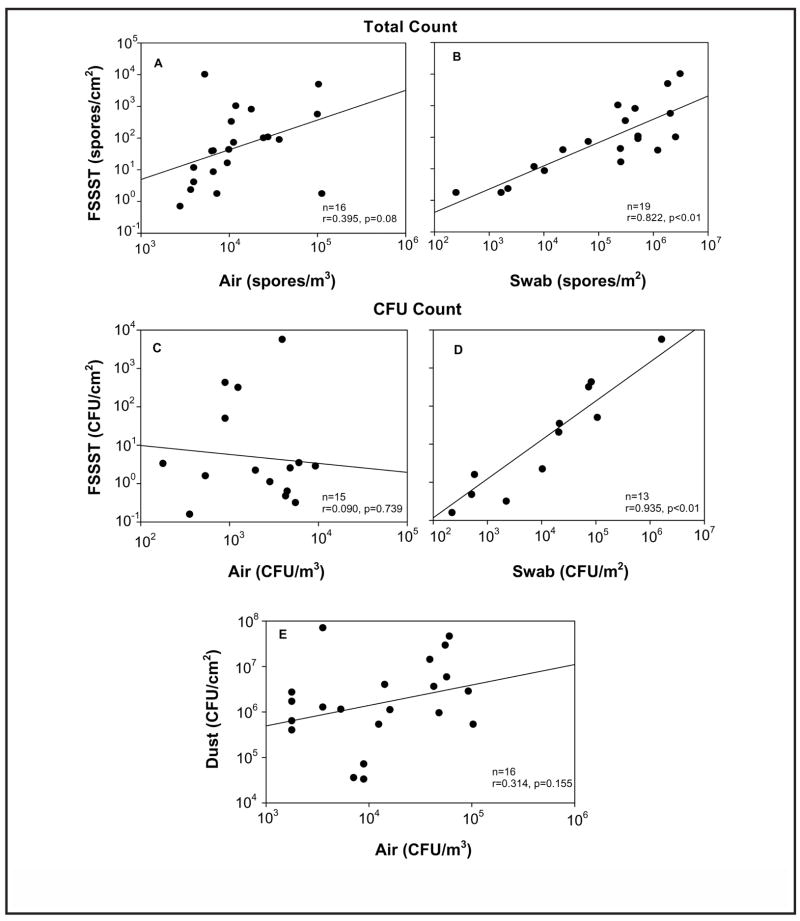
Correlations between different collection methods were calculated for both the total spore count and the CFU count (Figure 4). For total spore counts, the correlation analyses were performed comparing the data obtained with the FSSST technique to both short-term air and swab measures. Since multiple types of fungi were collected in each home, comparisons were made wherever data points could be matched by fungal types. A significant correlation was observed between the FSSST and swab collection techniques (Figure 4B). No significant relationship was observed between the short-term air and FSSST techniques for total spore count (Figure 4A).
FIGURE 4.
Correlations between the data obtained by different measurement methods for both total spore (A,B) and CFU enumerations (C,D,E)
Correlations were also determined wherever data points could be matched by fungal type with respect to the CFU count. Again, the only significant correlation was observed between the FSSST and swab collection techniques (Figure 4D). There was no significant relationship observed between the short-term air and dust techniques nor between air and FSSST techniques for CFU count (Figures 4C and 4E).
The percentage of culturable spores among the total counts obtained with swab, FSSST, and air sampled was calculated for each home. The results of this calculation are presented in Table I. The culturable fungal fraction ranged from 0.03% to 63% in swab samples; FSSST samples revealed the culturability range of 0.1% to over 100%; and short-term air samples showed culturability of 0.7% to 78.7%. No information on the spore culturability in dust samples was obtained because the total count was not available.
TABLE I.
Comparison of Culturability Between Sampling Methods in 12 Homes
| Culturability
|
|||
|---|---|---|---|
| Home ID | Swab (%) | FSSST (%) | Air (%) |
| 1 | N/A | 1.2 | 7.9 |
| 2 | N/A | 2.1 | 78.7 |
| 3 | 21.3 | 6.9 | 8.9 |
| 4 | 4.1 | 36.4 | 0.7 |
| 5 | 0.7 | 12.3 | 48.6 |
| 6 | 1.2 | 3.5 | 9.0 |
| 7 | 0.3 | 0.1 | 1.7 |
| 8 | 60.1 | 65.2 | 8.9 |
| 9 | 0.03 | 0.3 | 1.2 |
| 10 | N/A | 2.8 | N/A |
| 11 | 8.8 | 125 | 15 |
| 12 | 63 | 65 | N/A |
Notes: Culturable count/total count × 100%. N/A = sample was contaminated or no microbial growth was observed.
A comparison was made within homes to determine which type of sampling produced the highest percentage of culturable spores. In 6/11 (55%) homes, the air samples showed the highest spore culturability. The FSSST samples showed the highest culturability in four homes (36%). Swab samples revealed the highest culturablity in only one home (9%).
DISCUSSION
The results comparing the swab and FSSST methods showed that the median levels of spores collected by the swab method (with respect to both the microscopic and culture-based counts) were much greater than those collected by the FSSST. These ranges are similar to those that we observed in our recent study of the FSSST performance.(60) Furthermore, the median indoor total spore counts in this study were about one order of magnitude higher than the outdoor levels. Shelton et al.,(65) who collected both indoor and outdoor mold samples with the Andersen sampler and analyzed the samples using a culture-based analysis, found that the indoor levels were lower than those determined outdoors. Our indoor levels were most likely higher due to the presence of visible mold in housing. Though the indoor and outdoor air samples were collected with different methods (BioSampler and Button Sampler, respectively), both methods have been found to have high collection efficiency in the size range of fungal spores (primarily <5μm).(64,66,67)
The swab samples showed greater variability of the CFU count compared with the total count. This large variation in the enumeration type was seen in neither of the other two methods (FSSST or air). This is possibly due to a limited representativeness of a single swab sample taken in a 1-cm2-area. Perhaps the size of the swab sample led to greater variability of culturable spores determined by this method. In future studies, swab samples should be taken from a larger area when comparing swab with other methods. The surface properties (e.g., the roughness) may also affect the data variability.(58)
Aspergillus, Penicillium, and Cladosporium spores were the most common types of fungi found in this study with both enumeration methods. These spore types are among the most predominant in the United States and are generally considered to have indoor origins.(65,68) In the total spore count method, Cladosporium spores were found in approximately the same number of homes by each sampling method.
Aspergillus/Penicillium spores were identified in 6 of 13 homes for the swab method and in 8 of 13 homes for the FSSST method. In both cases when Aspergillus/Penicillium spores were identified in the FSSST samples but not in the swab, these spores represented a small fraction of the total spore count obtained by the former method. Perhaps these spores were also present in the swab samples, but were masked by the more prominent spore types. Aspergillus/Penicillium spores were also identified in 12 of the 13 homes during air sampling. Furthermore, all of the spore types identified by the total count were found in either equal amounts or, more often, in the air samples as compared with the swab and FSSST samples. Hyvarinen et al.(69) reported similar results, namely, that more fungal species were identified using air sampling than by swab sampling. The investigators suggested that this could be due to the influence of unidentified indoor sources, or outdoor mold sources.
For the culture-based analysis, the FSSST showed a greater number of homes containing both Aspergillus and Cladosporium culturable spores than did the swab or air sampling methods. Aspergillus colonies appeared in the FSSST samples in five more homes, when compared with the swab. For three of these homes, however, the swab CFU samples were either contaminated or did not grow. For the other two homes, Aspergillus spores represented a small fraction of the CFU count in the FSSST samples. It is possible that these spores were present in the swab samples but did not grow due to the much higher concentration of other spore types, which might have overgrown the Aspergillus spores. As previously mentioned, it is also possible that the small size of the swab sample was not fully representative of the source contamination when compared with the FSSST, which samples a much larger area. This explanation is also valid when comparing the samples from two homes, among which the FSSST revealed Cladosporium but the swab method did not.
When comparing the culturable FSSST and air samples, one should remember that the FSSST is designed to assess a “worst-case scenario” for spore aerosolization.(59,60) Thus the FSSST induced culturable spore release in the homes where sporulation was not yet occurring by natural means and therefore was not detected in the air. In the settled dust, culturable spore types were usually found in either equal or greater amounts compared with the other three methods. This supports the hypothesis that dust acts as a long-term sink for fungal spores.(46) Furthermore, three different mold types (Fusarium, Mucor, and Alternaria) were found in five or more homes in the dust but appeared only one time or less in the other sampling methods. Perhaps these fungal types represent the outdoor sources, suggesting the spore penetration and subsequent deposition on the floor.
When investigating the relationship between the different sampling methods, a statistically significant relationship was observed between the FSSST and swab for both the microscopic and culture-based analyses results. This was an expected result, since both techniques measure the fungal source. There was no observed relationship between the FSSST and air level of fungi for either of the enumeration methods. Duchaine and Meriaux(70) reported that the number of mold sources in a home was significantly related to air CFU levels, suggesting an association between air and source samples in homes with visible mold contamination, and recommended that both sampling types are necessary for a complete assessment of molds. Our study went a step further to quantitatively compare mold levels at the source with those found in the air. We did not observe a relationship between these two methods. There are three possible explanations for this. First, spore release from fungal colonies is sporadic, and short-term air sampling might not accurately represent airborne levels.(46,60) Second, fungal spores sampled from the indoor air represent a mixture of spores from other potential indoor sources (other mold contamination, including nonidentified growth on indoor surfaces and inside the ventilation system, as well as from the reaerosolized dust). Outdoor sources may also contribute considerably, as the presence of outdoor spores may lead to underrepresentation of those released from identified sources in indoor air samples. Third, the visibly mold-contaminated areas were different in different homes. Only data points for which fungal types could be matched across sampling types were included in the analysis. It would be expected that correlation values would be less if all fungal types had been taken together.
Results on dust sampled from the carpet (or floor) were also compared with the results on air samples taken in the room with mold contamination. This comparison was made because settled dust is often used as a measure of exposure to fungi, due to the potential reaerosolization of dust particles into the air.(44–46) Again, no relationship was observed between these measures. Similar to the findings reported by Chew et al.,(46) many more types of fungi were identified in the dust, as compared with the indoor air. Although dust has been recognized as a long-term reservoir for fungi, there appears to be little potential for reaerosolization of fungi from indoor dust, as evidenced by this study and other investigations.(46,49,71) One potential factor that has not been considered thus far is the difficulty to analyze dust by the total spore count method. The dust samples analyzed only by CFU method leave the nonculturable fraction unknown. As stated previously, it has been suggested that allergic reaction to fungi is generally independent of culturability of spores.(33) It is then imperative that an appropriate total spore enumeration method for fungal spores in dust be used.
The data collected in this study allowed us to examine the relationship between total and CFU spore counts obtained by each of the three sampling methods (swab, FSSST, and air). Each method resulted in a wide range of culturability of spores both between and within homes (swab: 0.03% to 63%, FSSST: 0.1% to >100%, air: 0.7% to 78.7%). This finding is of particular interest because a number of fungal sampling methods rely solely on the culture-based enumeration technique. The results of this study support previous reports that this reliance might grossly underestimate the number of spores present in a sample, which might potentially lead to an underestimation of the severity of mold contamination.(26) The culturability of spores is dependent on a number of factors, including spore type, temperature, and type of agar. Furthermore, since culturability is not generally linked to allergenic respiratory symptoms, these symptoms may still occur when the culture-based enumeration technique generates results below the limit of detection.(33) In one FSSST sample, the percent culturable spores was found to be greater than 100% (125%). It has been hypothesized, though not tested, that this is due to the release of mycelial fragments, which could potentially grow to form new colonies but would not be counted as spores. The limitations associated with the accuracy and precision of the microscopic spore count might also have contributed to the above discrepancy.
Selecting appropriate methods for sampling fungal spores in indoor environments is crucial in order to link the human exposure and disease caused by fungi. It has been argued that the lack of standardized and definitive methods for mold sampling is a primary cause for the poorly understood relationship between mold exposure and health outcome.(22) Generally, air sampling has been a commonly used method to assess fungal exposure and has also been described as the most representative of human respiratory exposure.(35,72–76) However, this study has demonstrated that short-term air sampling may not be an indicative measure of mold contamination in the indoor environment, as the number of spores released by the source (FSSST) did not relate to the airborne spore concentration. This was the case even though the indoor mold contamination levels were approximately an order of magnitude higher than the outdoor levels. All of the environments chosen in this study had visible mold contamination, and multiple sampling methods were used for its quantification.
It can be argued that in this type of environment, source testing would be the obvious choice for a sampling method. Furthermore, it could be suggested that if the mold source has been identified, there is no reason to sample but, instead, to simply clean the contaminated area. At the same time, if the exposure to fungal spores is to be assessed in the presence of an identified mold source short-term air sampling does not seem to be predictive of the source even when the air sample is taken in the same room where the source was identified.
CONCLUSIONS
The results of this study confirm that reliance on one sampling or enumeration method for characterization of an indoor mold source might not provide an accurate estimate of fungal contamination of a microenvironment. As shown by other investigations, multiple sampling techniques are suggested when attempting to assess indoor mold contamination. The exclusive use of a culture-based enumeration technique must be performed with the understanding that it might drastically underestimate the quantity of mold in the indoor environment. Additionally, culturable spores alone are not responsible for adverse health effects associated with mold exposure.
The relationships between the data obtained with the four different sampling methods were examined using correlation analysis. Significant relationships were observed between the data from swab and FSSST samples both by the microscopic counting and by the CFU counting. No relationships were observed between the data from air and FSSST samples or air and settled dust samples. Percentage culturability of spores for each sampling method was also calculated and found to vary greatly for all three methods (swab: 0.03% to 63%, FSSST: 0.1% to >100%, air: 0.7% to 79%). FSSST sampling appears to be an effective way to assess the mold source in the field, providing an upper bound estimate of potential mold spore release into the indoor air. However, because of the small sample size of this study, further research is needed to better understand the observed relationships in this study.
RECOMMENDATIONS
▪ Multiple sampling methods are recommended to assess indoor mold contamination (which is consistent with the American Conference of Governmental Industrial Hygienists (ACGIH®) and AIHA recommendations).
▪ The total spore count technique is recommended for analysis over CFU technique for assessing indoor mold contamination.
▪ Further research efforts should be pursued to better characterize the relationship between indoor fungal spore concentration and the factors that affect the spore release into the air.
Acknowledgments
Mr. Niemeier was supported in part by the University of Cincinnati through its Education and Research Center from the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. All the above support is deeply appreciated.
The authors are indebted to Taehkee Lee and Sung-Chul Seo for their assistance in collecting the field samples, and to Maureen Niemeier for her help in editing the manuscript.
This study was supported by the U.S. Department of Housing and Urban Development and by the National Institute of Environmental Health Sciences (NIEHS) Center for Environmental Genetics Pilot Project Program.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the institutions that sponsored this study.
References
- 1.Brunekreeef B, Dockery DW, Speizer FE, Ware JH, Spengler JD, Ferris BG. Home dampness and respiratory morbidity in children. Am Rev Respir Dis. 1989;140:1363–1367. doi: 10.1164/ajrccm/140.5.1363. [DOI] [PubMed] [Google Scholar]
- 2.Miller JD. Contamination of food by Fusarium toxin: Studies from Austral-Asia. Proc Jap Assoc Mycotox. 1990;32:17–24. [Google Scholar]
- 3.Miller JD. Fungi as contaminants in indoor air. Atmos Environ. 1992;26A(12):2163–2172. [Google Scholar]
- 4.Spengler J, Neas L, Nakai S, et al. Respiratory symptoms and housing characteristics. Proc Indoor Air. 1993;1:165–168. [Google Scholar]
- 5.Dales RE, Zwanenburg H, Burnett R, Franklin CA. Respiratory health effects of home dampness and molds among Canadian children. Am J Epidemiol. 1991;134:196–203. doi: 10.1093/oxfordjournals.aje.a116072. [DOI] [PubMed] [Google Scholar]
- 6.Fung F, Hughson WG. Health effects of indoor fungal bioaerosols exposure. Appl Occup Environ Hyg. 2003;18:535–544. doi: 10.1080/10473220301451. [DOI] [PubMed] [Google Scholar]
- 7.Peat JK, Dickerson J, Li J. Effects of damp and mould in the home on respiratory health: A review of the literature. Allergy. 1998;53:120–128. doi: 10.1111/j.1398-9995.1998.tb03859.x. [DOI] [PubMed] [Google Scholar]
- 8.Verhoeff AP, Burge HA. Health risk assessment of fungi in home environments. Ann Allergy Asthma Immunol. 1997;78:120–128. doi: 10.1016/S1081-1206(10)63214-0. [DOI] [PubMed] [Google Scholar]
- 9.Bornehag C-G, Blomquist G, Gyntelberg, et al. Dampness in buildings and health. Indoor Air. 2001;11:72–86. doi: 10.1034/j.1600-0668.2001.110202.x. [DOI] [PubMed] [Google Scholar]
- 10.HUD (Department of Housing and Urban Development) Healthy Homes Program. 2003 [online] Available at http://www.hud.gov/offices/lead/hhi/index.cfm.
- 11.Institute of Medicine (IOM) Clearing the Air: Asthma and Indoor Air Exposures. Washington D.C: The National Academies Press; 2000. [PubMed] [Google Scholar]
- 12.Institute of Medicine (IOM) Damp indoor spaces and health. Washington D.C: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 13.Williamson IJ, Martin CJ, McGill G, Monie RD, Fennerty AG. Damp housing and asthma: A case-control study. Thorax. 1997;52:229–234. doi: 10.1136/thx.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB, Persky B, Flay BR, Phil D, Richardson J. An epidemiological study of asthma prevalence and related factors among young adults. J Asthma. 1997;34:67–76. doi: 10.3109/02770909709071205. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen O, Husman T, Hyvarinen A, Reponen T, Nevalainen A. Two moldy day-care centers: A follow-up study of respiratory symptoms and infections. Indoor Air. 1997;7:262–268. [Google Scholar]
- 16.Maier WC, Arrighi HM, Morray B, Llewellyn C, Redding GJ. Indoor risk factors for asthma and wheezing among Seattle school children. Environ Health Perspect. 1997;105:208–214. doi: 10.1289/ehp.97105208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JD. Quantification of health effects of combined exposures: A new beginning. In: Morawska L, editor. Indoor Air-An Integrated Approach. Amsterdam: Elsevier; 1995. pp. 159–168. [Google Scholar]
- 18.Sigsgaard T. Symptoms associated to work in a water-damaged school building. In: Johanning E, editor. Bioaerosols, Fungi, and Mycotoxin: Health Effects, Assessment, Prevention and Control. Albany: Eastern New York Occupational and Environmental Health Center; 1999. pp. 99–105. [Google Scholar]
- 19.Strachen DP, I, Carey M. Home environment and severe asthma in adolescence: A population-based, case-control study. Br Med J. 1995;311:1053–1056. doi: 10.1136/bmj.311.7012.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhoeff AP, van Strien RT, van Wijnen JH, Brunekreef B. Damp housing and childhood respiratory symptoms: The role of sensitization to dust mites and moulds. Am J Epidemiol. 1995;141:103–110. doi: 10.1093/oxfordjournals.aje.a117398. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen KG. Mycotoxin production by indoor molds. Fungal Genetics and Biology. 2003;39:103–117. doi: 10.1016/s1087-1845(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis JQ, Morey PR. Allergenic respiratory disease and fungal remediation in a building in a subtropical climate. Appl Occup Environ Hyg. 2001;16:380–388. doi: 10.1080/10473220117482. [DOI] [PubMed] [Google Scholar]
- 23.Mahooti-Brooks N, Storey E, Yang C, Simcox NJ, Turner W, Hodgson M. Characterization of mold and moisture indicators in the home. J Occup Environ Hyg. 2004;1:826–839. doi: 10.1080/15459620490890332. [DOI] [PubMed] [Google Scholar]
- 24.Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann Occup Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 25.Eduard W, Heederik D. Methods for quantitative assessment of airborne levels of noninfectious microorganisms in highly contaminated work environments. Am Ind Hyg Assoc J. 1998;59:113–127. doi: 10.1080/15428119891010370. [DOI] [PubMed] [Google Scholar]
- 26.Pasanen AL. A review: Fungal exposure assessment in indoor environments. Indoor Air. 2001;11:87–98. doi: 10.1034/j.1600-0668.2001.110203.x. [DOI] [PubMed] [Google Scholar]
- 27.Dillon HK, Miller JD, Sorenson WG, Douwes J, Jacobs RR. Review of methods applicable to the assessment of mold exposure to children. Environ Health Perspect. 1999;107(Suppl 3):473–480. doi: 10.1289/ehp.99107s3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macher JM. Review of methods to collect settled dust and isolate culturable microorganisms. Indoor Air. 2001;11:99–110. doi: 10.1034/j.1600-0668.2001.110204.x. [DOI] [PubMed] [Google Scholar]
- 29.Macher J. Sampling and analysis. In: Macher J, editor. Bioaerosols: Assessment and Control. Cincinnati, Ohio: ACGIH®; 1999. pp. 6–2.pp. 6–3. [Google Scholar]
- 30.MacNeil L, Kauri T, Robertson W. Molecular techniques and their potential application in monitoring the microbiological quality of indoor air. Can J Microbiol. 1995;41:657–675. doi: 10.1139/m95-091. [DOI] [PubMed] [Google Scholar]
- 31.Forgacs J. Stachybotryotoxicosis. In: Kadis S, editor. Microbial Toxins. VIII. New York: Academic Press; 1972. pp. 95–128. [Google Scholar]
- 32.Wu PC, Su HJ, Ho HM. A comparison of sampling media for environmental viable fungi collected in a hospital environment. Environ Res. 2000;82:253–257. doi: 10.1006/enrs.1999.4017. [DOI] [PubMed] [Google Scholar]
- 33.Kozak PP, Gallup J, Cummins LH, Gillman SA. Currently available methods for home mold surveys. II. Examples of problem homes surveyed. Ann Allergy Asthma Immunol. 1979;45:167–176. [PubMed] [Google Scholar]
- 34.Haugland RA, Heckman JL. Identification of putative sequence specific PCR primers for detection of the toxigenic fungal species Stachybotrys chartarum. Mol Cell Probes. 1998;12:387. doi: 10.1006/mcpr.1998.0197. [DOI] [PubMed] [Google Scholar]
- 35.Strachan DP, Flannigan B, McCabe EM, McGarry F. Quantification of airborne moulds in the homes of children with and without wheeze. Thorax. 1990;45:382–387. doi: 10.1136/thx.45.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Blomquist G, Westermark SO, Wang XR. Application of PCR and probe hybridization techniques in detection of airborne fungal spores in environmental samples. J Environ Monit. 2002;4:673–678. doi: 10.1039/b203048a. [DOI] [PubMed] [Google Scholar]
- 37.Haugland RA, Vesper LJ, Wymer LJ. Quantitative measurement of Stachybotrys chartarum conidia using real time detection of PCR products with the Taq ManTM fluorogenic probe system. Mol Cell Probes. 1999;13:329. doi: 10.1006/mcpr.1999.0258. [DOI] [PubMed] [Google Scholar]
- 38.Zhou G, Whong WZ, Ong T, Chen B. Development of a fungus-specific PCR assay for detecting low-level fungi in an indoor environment. Mol Cell Probes. 2000;14:339. doi: 10.1006/mcpr.2000.0324. [DOI] [PubMed] [Google Scholar]
- 39.Williams RH, Ward E, McCartney HA. Methods for integrated air sampling and DNA analysis for detection of airborne fungal spores. Appl Environ Microbiol. 2001;67:2453–2459. doi: 10.1128/AEM.67.6.2453-2459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meklin T, Haugland RA, Reponen T, et al. Quantitative PCR analysis of house dust can reveal abnormal mold conditions. J Environ Monit. 2004;6:1–7. doi: 10.1039/b400250d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmechel D, Gorny RL, Simpson JP, Reponen T, Grinshpun SA, Lewis DM. Limitations of monoclonal antibodies for monitoring of fungal aerosols using Penicillium brevicompactum as a model fungus. J Immunol Methods. 2003;283:235–245. doi: 10.1016/j.jim.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Dillon HK, editor. Field Guide for the Determination of Biological Contaminants in Environmental Samples. Fairfax, Va: American Industrial Hygiene Association; 1996. Viable fungi and bacteria in air, bulk and surface samples; pp. 37–44. [Google Scholar]
- 43.Martyny J. Source sampling. In: Macher J, editor. Bioaerosols: Assessment and Control. Cincinnati, Ohio: ACGIH; 1999. p. 12–1.p. 12–5. [Google Scholar]
- 44.Verhoeff AP, van Wijnen JH, van Reenen-Hoekstra ES, Samson RA, Van Strien RT, Brunekreef B. Fungal propagules in house dust. II. Relation with residential characteristics and respiratory symptoms. Allergy. 1994;49:540–547. doi: 10.1111/j.1398-9995.1994.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffer N, Seidmon EE, Bruskin S. The clinical evaluation of air-borne and house dust fungi in New Jersey. J Allergy. 1953;23:348–354. doi: 10.1016/0021-8707(53)90180-4. [DOI] [PubMed] [Google Scholar]
- 46.Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 47.Takatori K. Comparisons of the house dust mycoflora in Japanese houses. In: Samson RA, editor. Health Implications of Fungi in Indoor Environments. New York: Elsevier; 1994. pp. 99–103. [Google Scholar]
- 48.Verhoeff AP. Fungal propagules in house dust: Comparison of analytical methods. In: Samson RA, editor. Health Implications of Fungi in Indoor Environments. New York: Elsevier; 1994. pp. 49–63. [Google Scholar]
- 49.Flannigan B. Health implications of fungi in indoor environments—An overview. In: Samson RA, editor. Health Implication of Fungi in Indoor Environments. New York: Elsevier; 1994. pp. 3–28. [Google Scholar]
- 50.Chew GL, Douwes J, Doekes G, et al. Fungal extracellular polysaccarides, β (1→3)-glucans and culturable fungi in repeated sampling of house dust. Indoor Air. 2001;11:171–178. doi: 10.1034/j.1600-0668.2001.011003171.x. [DOI] [PubMed] [Google Scholar]
- 51.Pasanen A-l, Kujanpáá L, Pasanen P, Kalliokoski P, Blomquist G. Culturable and total fungi in dust accumulated in air ducts in single-family houses. Indoor Air. 1997;7:121–127. [Google Scholar]
- 52.Bholah R, Subratty AH. Indoor biological contaminants and symptoms of sick building syndrome in office buildings in Mauritius. Int J Environ Health Res. 2002;12:93–98. doi: 10.1080/09603120120110095. [DOI] [PubMed] [Google Scholar]
- 53.Johanning E, Biagini R, Hull DL, Morey P, Jarvis B, Landsbergis P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int Arch Occup Environ Health. 1996;68:206–218. doi: 10.1007/BF00381430. [DOI] [PubMed] [Google Scholar]
- 54.Ross MA, Curtis L, Scheff PA. Association of asthma symptoms and severity with indoor bioaerosols. Allergy. 2000;55:705–711. doi: 10.1034/j.1398-9995.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 55.Sudakin DL. Toxigenic fungi in a water-damaged building: An intervention study. Am J Ind Med. 1998;34:183–190. doi: 10.1002/(sici)1097-0274(199808)34:2<183::aid-ajim12>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 56.Waegemaekers M, van Wageningen N, Brunekreef B, Boleij JS. Respiratory symptoms in damp homes. A pilot study. Allergy. 1989;44:192–198. doi: 10.1111/j.1398-9995.1989.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 57.Foarde KK. Investigating the influence of relative humidity, air velocity, and amplification on the emission rates of fungal spores. In: Raw G, editor. Proceedings of Indoor Air 99 Conference. Vol. 1. London: CRC Ltd; 1999. pp. 507–512. [Google Scholar]
- 58.Gorny RL, Reponen T, Grinshpun SA, Willeke K. Source strength of fungal spore aerosolization from moldy building materials. Atmos Environ. 2001;35:4853–4862. [Google Scholar]
- 59.Sivasubramani SK, Niemeier RT, Reponen T, Grinshpun SA. Fungal spore source strength tester: Laboratory evaluation of a new concept. Sci Total Environ. 2004;329:75–86. doi: 10.1016/j.scitotenv.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Sivasubramani SK, Niemeier RT, Reponen T, Grinshpun SA. Assessment of the aerosolization potential for fungal spores in moldy homes. Indoor Air. 2004;14:405–412. doi: 10.1111/j.1600-0668.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 61.Grinshpun SA, Gorny RL, Reponen T, Willeke K, Trakumas S, Hall P. New method for assessment of potential spore aerosolization from contaminated surfaces. Proceedings of the Sixth International Aerosol Conference; Taipei, Taiwan. September 2002; International Aerosol Research Assembly (IARA); pp. 767–768. [Google Scholar]
- 62.Kildeso J, Wurtz H, Nielsen KF, Kruse P, Wilkin K, Thrane U. Determination of fungal spore release from wet building materials. Indoor Air. 2003;13:148–155. doi: 10.1034/j.1600-0668.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 63.Burge HA, Chatigny M, Feeley J, Kreiss K, Morey P, Otten J. Guidelines for assessment and sampling of saprophytic bioaerosols in the indoor environment. Appl Ind Hyg. 1987;2:R10–R16. [Google Scholar]
- 64.Adhikari A, Martuzevicius D, Reponen T, et al. Performance of the Button Personal Inhalable Sampler for the measurement of outdoor aeroallergens. Atmos Environ. 2003;37:4723–4733. [Google Scholar]
- 65.Shelton BG, Kirkland KH, Flanders WD, Morris GK. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl Environ Microbiol. 2002;68:1743–1753. doi: 10.1128/AEM.68.4.1743-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willeke K, Lin X, Grinshpun SA. Improved aerosol collection by combined impaction and centrifugal motion. Aerosol Sci Technol. 1998;28:439–456. [Google Scholar]
- 67.Aizenberg V, Reponen T, Grinshpun SA, Willeke K. Performance of Air-O-Cell, Burkard, and Button Sampler for total enumeration of spores. Am Ind Hyg J. 2000;61:855–864. doi: 10.1080/15298660008984598. [DOI] [PubMed] [Google Scholar]
- 68.Li DW, Kendrick B. A year-round comparison of fungal spores in indoor and outdoor air. Mycologia. 1995;87:190–195. [Google Scholar]
- 69.Hyvarinen A, Reponen T, Husman T, Ruuskanen J, Nevalainen A. Characterizing mold problem buildings—Concentrations and flora of viable fungi. Indoor Air. 1993;3:337–343. [Google Scholar]
- 70.Duchaine C, Meriaux A. The importance of air sampling and surface analysis when studying problematic houses for mold biodiversity determination. Aerobiologia. 2001;17:121–125. [Google Scholar]
- 71.Kildeso J, Vinzents P, Scheider T, Kloch J. A simple method for measuring the potential resuspension of dust from carpets from the indoor environment. Textile Res J. 1999;69:169–175. [Google Scholar]
- 72.Dales RE, Burnett R, Zwanenburg H. Adverse health effects among adults exposed to home dampness and molds. Am Rev Respir Dis. 1991;143:505–509. doi: 10.1164/ajrccm/143.3.505. [DOI] [PubMed] [Google Scholar]
- 73.Li DW, Kendrick B. Indoor aeromycota in relation to residential characteristics and allergenic symptoms. Mycopathologia. 1995;131:149–157. doi: 10.1007/BF01102894. [DOI] [PubMed] [Google Scholar]
- 74.Koch A, Heilemann KJ, Bichoff W. Indoor viable mold spores—A comparison between two cities, Erfurt (eastern Germany) and Hamburg (western Germany) Allergy. 2000;55:176–180. doi: 10.1034/j.1398-9995.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 75.Su HJ, Rotnitzky A, Burge HA, Spengler JD. Examination of fungi in domestic interiors by using factor analysis: Correlations and associations with home factors. Appl Exp Microbiol. 1992;58:181–186. doi: 10.1128/aem.58.1.181-186.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burge HA. Aerobiology of the indoor environment. Occup Med. 1995;10:27–40. [PubMed] [Google Scholar]