Abstract
The results of a traditional visual mold inspection were compared to a mold evaluation based on the Relative Moldiness Index (RMI). The RMI is calculated from mold-specific quantitative PCR (MSQPCR) measurements of the concentration of 36 species of molds in floor dust samples. These two prospective mold evaluations were used to classify the mold condition in 271 homes of infants. Later, the development of respiratory illness was measured in the infants living in these homes and the predictive value of each classification system was evaluated.
The binary classification of homes as either moldy or non-moldy by on-site visual home inspection was not predictive of the development of respiratory illness (wheeze and/or rhinitis) (P = 0.27). Conversely, a method developed and validated in this paper, using the RMI index fit to a logistic function, can be used to predict the occurrence of illness in homes and allows stake-holders the choice among various levels of risk.
Keywords: mold-specific quantitative PCR, mold, infants, wheezing, relative moldiness index
Introduction
Asthma and allergy afflict an ever-increasing number of children in the US (Mannino et al., 2002). The Institute of Medicine’s report on dampness and health expressed the opinion that there was scientific evidence linking molds and damp environments with asthma symptoms (IOM, 2004). Therefore, predicting which environmental conditions lead to illness may be critical to prevention. In previous research, a relative moldiness index (RMI) was developed, based on the mold-specific quantitative PCR (MSQPCR) (Vesper et al., 2006) analysis of 26 mold species (Group 1) associated with water damage and 10 common species (Group 2) not associated with water damage (Vesper et al., 2004; Meklin et al., 2004).
We are now using these groupings to create an Relative Moldiness Index (RMI) to determine which molds are associated with health problems, especially in children (Vesper et al., 2004). In a study of Cleveland Ohio homes, some of the Group 1 molds were statistically associated with asthma in children but none of the Group 2 molds (Vesper et al., 2006). When the water-damaged homes of these asthmatic children were repaired and remediated, a 10-fold reduction in asthma-related emergency room visits and/or hospital admissions was measured (Kercsmar et al., 2006).
We are currently conducting a prospective birth cohort study (The Cincinnati Childhood Allergy and Air Pollution Study or CCAAPS) aimed at investigating the role of aeroallergens and diesel exhaust particles in the development of atopy and atopic respiratory disorders (Ryan et al., 2005). As a part of the CCAAPS, we conducted a cross-sectional study on the association of exposure to mold/water damage and house dust mite with the prevalence of recurrent wheezing and allergen sensitization in infants at age one (Cho et al., 2006). Another parallel study investigated the association between airborne mold count and rhinitis and allergen sensitization (Osborne et al., 2006). The purpose of this study is to examine the predictive value of the RMI compared to the traditional mold inspection and wheezing and/or rhinitis in infants.
Methods
Study Population
Infants born in Cincinnati, Ohio, and Northern Kentucky between 2001 and 2003 were recruited using birth certificate data. Eligibility for the study required that at least one parent was atopic, defined as having allergic symptoms and a positive reaction in a skin prick test (SPT) to at least one of 15 common aeroallergens (meadow fescue, timothy, white oak, maple, American elm, red cedar, short ragweed, Alternaria spp., Aspergillus fumigatus, Penicillium spp., Cladosporium spp., cat, dog, German cockroach, and house dust mite) (Ryan et al., 2005). The main focus of the overall CCAAPS was proximity to traffic and therefore the primary selection criteria was that the home was within 400 m of a highway (Ryan et al., 2005). Successful recruitment was about 20%. The study was approved by the Institutional Review Board at the University of Cincinnati.
On-site Home Visit and Exposure Assessment
On-site home visits in 777 homes were performed by trained two-person teams when the infants were about 8 months old on average in order to investigate the prevalence of mold damage and to collect floor dust samples for exposure assessment of indoor aeroallergens and mold as described by Cho et al. (2006). In brief, dust samples were collected from flooring materials in the room where the child spent most of his or her daytime. The dust sample was vacuumed at a flow rate of 800 l/min into custom-made cone-shape HEPA filter trap (Midwest Filtration, Cincinnati, OH, USA), which was attached to the nozzle of vacuum cleaner to collect dust samples.
For carpeted floor, samples were collected from the same area of 2 m2 at a vacuuming rate of 2 min/m2 (1 min horizontally, 1 min vertically). For non-carpeted floor (hard wood, linoleum, tile, or sheet floor), only one sample was collected from the entire room at a rate of 1 min/m2. The home dust sample was sieved (355 μm sieve), and the fine dust was divided into sub-samples and stored at −20°C before analyses (Cho et al., 2006). A 5 mg sub-sample of the fine dust was used for the MSQPCR analysis, as previously described (Haugland et al., 2004).
Simultaneously with dust sampling, a home inspection was performed and a questionnaire administered on home characteristics regarding a history of water damage, existence of visible mold, and any known repairs for water damage. Then, an indoor visual observation of the house conditions was conducted (Cho et al., 2006). A “moldy home” (MH) had at least one of the following: water damage history, visible mold/water damage, or moldy odor. A “non-moldy home” (NMH) had none of these. Based on the inspection classification, 154 MHs and 115 NMHs were selected for MSQPCR analysis of the dust. (The fact that a comparison was going to be made using another method was not known to the inspectors at the time of the visual inspection).
Skin Prick Testing and Medical Evaluation
During the infants’ first clinical visit at the average age of 13 months, they underwent skin prick testing for food (milk and egg) and the 15 aeroallergens (Ryan et al., 2005). Infants who showed a positive reaction were classified as sensitized. Meanwhile, a questionnaire on the infants’ respiratory symptoms was administered to the parent. The ISAAC questionnaire for 4–5-year-old children was adapted to develop a wheezing and rhinitis questions for our cohort (ISAAC, 1998). At least two episodes of wheezing, without cold in the previous 12 months, was defined as recurrent wheezing. Rhinitis symptoms included sneezing, runny or stuffy nose when the child did not have a cold or flu. When rhinitis symptoms were combined with allergen sensitization, it was defined as atopic rhinitis. Illness cases included infants who had either persistent wheeze, atopic rhinitis, or both. Controls were non-atopic, both wheeze and rhinitis free.
MSQPCR Analysis of Dust
Methods have been reported previously for preparing conidial suspensions from fungal cultures, extracting DNA, performing MSQPCR analyses, and preparing standard calibration curves for target conidia versus delta cycle threshold values (ΔCT = CT,target − CT,reference), using co-extracted DNA from Geotrichum candidum as an exogenous reference (Haugland et al., 2002; Brinkman et al., 2003; Haugland et al., 2004). All primer and probe sequences, as well as known species comprising the assay groups, were published at the website: http://www.epa.gov/microbes/moldtech.htm. Primers and probes were synthesized commercially (Applied Biosystems, Foster City, CA, USA; Integrated DNA Technologies, Coralville, IA, USA; Sigma Genosys, Woodlands, TX, USA).
Statistical Analyses
Mold concentration data having a minimum detection limit of 1 cell mg−1 dust were treated as left-censored data with appropriate statistical methods applied (Helsel, 2005). Procedurally, non-detections were set at 1/2 the minimum detection limit, and given equal and lowest rank for non-parametric rank-based analyses (Helsel, 2005).
The inspection designated NMH were coded as 0 and MH were coded as 1. This created a cross-classified binary variable with the binary outcome indicating illness absent (0) or present (1). Fisher’s Exact test was performed on the resulting 2 × 2 table. In addition, individual mold species concentrations were compared using the Wilcoxon rank-sum test (Table 1).
Table 1.
Wilcoxon rank-sum analysis mold concentration of homes classified based on inspection classification as either non-moldy homes (NMHs) or moldy homes (MHs).
| Mold species | W-test P-valuesa: n = 269b | GM: MHs n = 154 | GM: NMHs n = 115 |
|---|---|---|---|
| Group 1 | |||
| Aspergillus flavus | 0.986 | 1.9 | 1.9 |
| Aspergillus fumigatus | 0.837 | 7.6 | 8.3 |
| Aspergillus niger | 0.986 | 5.8 | 5.9 |
| Aspergillus ochraceus | 0.531 | 3.3 | 1.9 |
| Aspergillus penicillioides | 0.531 | 43.2 | 31.3 |
| Aspergillus restrictus | 0.986 | 1.4 | 0.9 |
| Aspergillus sclerotiorum | 0.445 | 2.3 | 2.2 |
| Aspergillus sydowii | 0.986 | 1.0 | 1.1 |
| Aspergillus unguis | 0.986 | 1.3 | 1.4 |
| Aspergillus versicolor | 0.553 | 3.3 | 1.9 |
| Aureobasidium pullulans | 0.531 | 4186.9 | 4348.9 |
| Chaetomium globosum | 0.531 | 2.9 | 2.0 |
| Cladosporium sphaerospermum | 0.139 | 197.5 | 95.9 |
| Eurotium chevalieri | 0.553 | 173.9 | 126.3 |
| Paecilomyces variotii | 0.837 | 5.8 | 5.8 |
| Penicillium brevicompactum | 0.553 | 25.8 | 21.3 |
| Penicillium corylophilum | 0.562 | 0.7 | 0.6 |
| Penicillium crustosum | 0.986 | 1.0 | 0.9 |
| Penicillium purpurogenum | 0.986 | 0.6 | 0.6 |
| Penicillium spinulosum | 0.551 | 1.3 | 1.2 |
| Penicillium variabile | 0.986 | 5.1 | 3.8 |
| Scopulariopsis brevicaulis | 0.648 | 3.2 | 2.8 |
| Scopulariopsis chartarum | 0.553 | 1.7 | 1.5 |
| Stachybotrys chartarum | 0.531 | 3.3 | 2.3 |
| Trichoderma viride | 0.553 | 19.5 | 26.3 |
| Wallemia sebi | 0.553 | 69.7 | 44.6 |
| Group 2 | |||
| Acremonium strictum | 0.445 | 2.2 | 3.4 |
| Alternaria alternate | 0.445 | 332.5 | 486.1 |
| Aspergillus ustus | 0.986 | 3.7 | 4.1 |
| Cladosporium cladosporioides (Type 1) | 0.606 | 1686.9 | 1465.6 |
| Cladosporium cladosporioides (Type 2) | 0.226 | 46.4 | 32.3 |
| Cladosporium herbarum | 0.531 | 164.8 | 219.9 |
| Epicoccum nigrum | 0.986 | 266.2 | 267.9 |
| Mucor racemosus | 0.986 | 101.5 | 111.4 |
| Penicillium chrysogenum | 0.783 | 82.9 | 53.3 |
| Rhizopus stolonifer | 0.986 | 2.4 | 2.3 |
P-values adjusted for multiple comparisons.
Only 269 of 271 homes had valid home inspections.
The RMI index was computed for each home by taking the sum of log-transformed Group 1 mold species concentrations minus the sum of log transformed Group 2 mold species concentrations (Vesper et al., 2006). The concentration of the Group 2 species is subtracted from the Group 1 species in order to adjust for variations in cleaning habits (Vesper, 2006). The RMI values allow homes to be ranked on a continuum from lowest to highest burden of mold. A logistic model was developed on the 271 homes that took the RMI value as the independent predictor of the child developing illness. A binary classification of homes using the logistic model allowed for specifying an RMI threshold at which homes are divided into two groups.
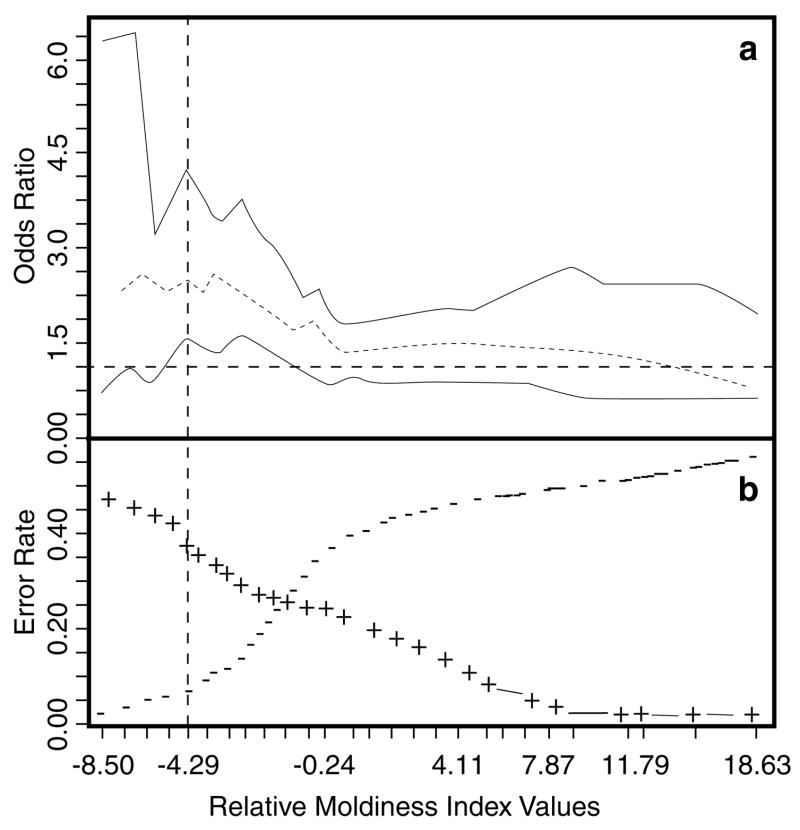
A 10-fold cross-validation procedure was used to assess the accuracy and consistency of this method to predict illness using the 271 study homes (Venables and Ripley, 2000). This procedure first randomized the full set of homes, then divided them into 10 subsets: nine with 27 homes and one with 28. At each of 10 iterations, nine subsets were used as the “training set”, whereas one served as the “test set”. A standardized range of 27 RMI quantiles was determined that spanned from the fifth to the 95th percentile of the empirical distribution of all 271 RMI values (see Figure 1 and Table 2). Model performance metrics including classification error, odds ratio with 95% confidence limits (CL), and significance from Fisher’s Exact test (adjusted for multiple comparisons) were computed for each of these 27 RMI quantiles, that is, thresholds, spanning the middle 90% of the RMI distribution. These combined measures of model performance form a basis for selecting an RMI threshold providing support for a statistical decision analysis. On the basis of this threshold, homes were reclassified using the Wilcoxon rank-sum test applied to the mold concentrations of each of the 36 mold species. The P-values for all Wilcoxon rank-sum and Fisher’s Exact tests were adjusted for multiple comparisons using the Benjamini and Hochberg method (1995).
Figure 1.
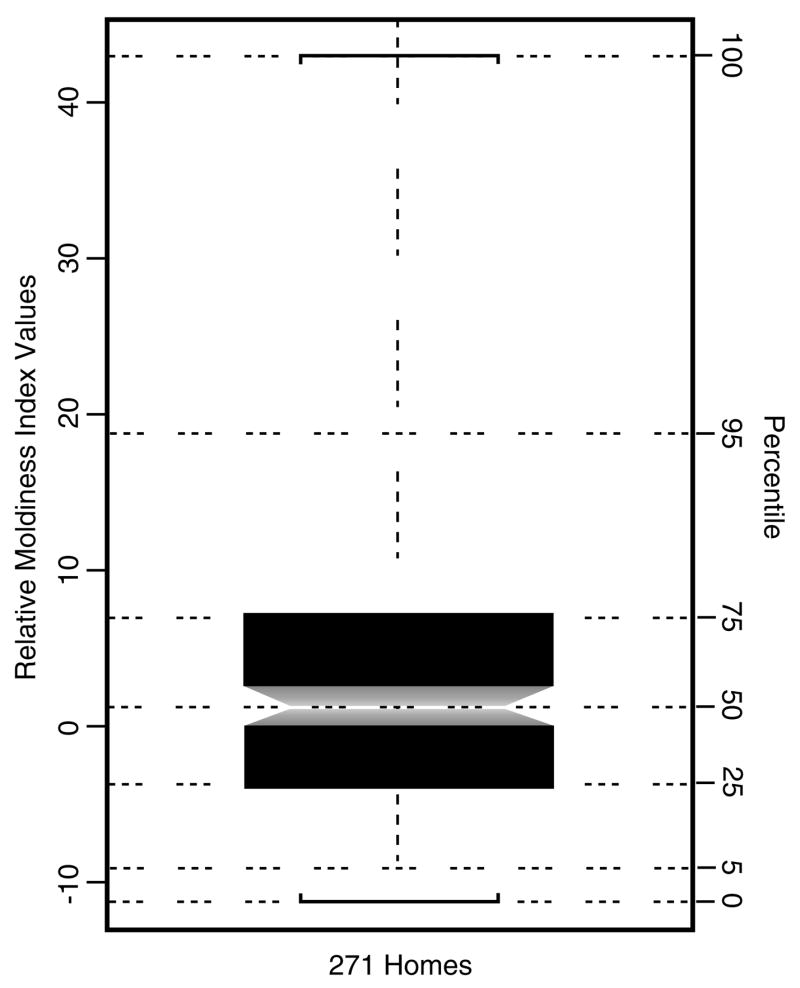
Notched box-plot of RMI values for all 271 study homes. The notch forms an approximate 95% confidence interval around the median. RMI values are shown along the left margin of the plot with corresponding percentiles of the empirical distribution shown along the right margin. This plot shows the overall range and distribution of the RMI values and illustrates the relationship between percentiles and corresponding quantiles.
Table 2.
Results for 10-fold cross-validation of logistic discriminant function using quantiles corresponding to the middle 90% of the RMI distribution: odds ratios with 95% confidence limits (CL), P-values based on Fisher’s exact test and adjusted for multiple comparisons.
| Percentile (%) | RMI quantile | Odds ratio | Lower CL | Upper CL | False positive error | False negative error | P-valuea |
|---|---|---|---|---|---|---|---|
| 5 | −8.5 | 2.099 | 0.687 | 6.455 | 0.44 | 0.02 | 0.52 |
| 8 | −7.46 | 2.593 | 1.025 | 6.604 | 0.42 | 0.03 | 0.15 |
| 12 | −6.29 | 1.618 | 0.776 | 3.385 | 0.40 | 0.05 | 0.52 |
| 15 | −5.53 | 1.941 | 0.986 | 3.838 | 0.38 | 0.06 | 0.19 |
| 19 | −4.29 | 2.530 | 1.351 | 4.773 | 0.34 | 0.07 | 0.03 |
| 22 | −3.81 | 2.139 | 1.192 | 3.861 | 0.33 | 0.09 | 0.06 |
| 26 | −3.06 | 2.097 | 1.210 | 3.655 | 0.31 | 0.10 | 0.05 |
| 29 | −2.46 | 2.359 | 1.381 | 4.053 | 0.29 | 0.11 | 0.03 |
| 33 | −1.79 | 2.192 | 1.312 | 3.684 | 0.27 | 0.13 | 0.03 |
| 36 | −1.09 | 2.020 | 1.224 | 3.352 | 0.26 | 0.15 | 0.05 |
| 40 | −0.24 | 1.769 | 1.084 | 2.898 | 0.25 | 0.18 | 0.10 |
| 43 | 0.14 | 1.524 | 0.940 | 2.477 | 0.24 | 0.20 | 0.30 |
| 47 | 0.84 | 1.368 | 0.847 | 2.214 | 0.23 | 0.23 | 0.50 |
| 50 | 1.39 | 1.431 | 0.887 | 2.315 | 0.21 | 0.24 | 0.36 |
| 53 | 1.86 | 1.215 | 0.752 | 1.964 | 0.21 | 0.27 | 0.57 |
| 57 | 2.64 | 1.099 | 0.678 | 1.782 | 0.19 | 0.30 | 0.77 |
| 60 | 3.2 | 1.086 | 0.666 | 1.771 | 0.18 | 0.32 | 0.83 |
| 64 | 4.11 | 1.185 | 0.719 | 1.953 | 0.16 | 0.33 | 0.62 |
| 67 | 5.02 | 1.297 | 0.779 | 2.165 | 0.14 | 0.34 | 0.56 |
| 71 | 5.79 | 1.307 | 0.769 | 2.224 | 0.12 | 0.36 | 0.56 |
| 74 | 6.94 | 1.357 | 0.783 | 2.355 | 0.11 | 0.38 | 0.56 |
| 78 | 7.87 | 1.272 | 0.711 | 2.279 | 0.09 | 0.40 | 0.57 |
| 81 | 8.57 | 1.339 | 0.723 | 2.485 | 0.08 | 0.42 | 0.57 |
| 85 | 10.7 | 1.397 | 0.706 | 2.771 | 0.06 | 0.44 | 0.56 |
| 88 | 11.79 | 1.162 | 0.553 | 2.444 | 0.05 | 0.46 | 0.77 |
| 92 | 15.27 | 0.975 | 0.400 | 2.379 | 0.04 | 0.49 | 1.00 |
| 95 | 18.63 | 0.540 | 0.172 | 1.692 | 0.03 | 0.51 | 0.56 |
Fisher’s exact test adjusted for multiple comparisons.
Statistical analyses and graphics were performed using SAS (SAS Institute Inc., Cary, NC, USA) and the R Software environment for statistical computing and graphics (http://www.r-project.org/).
Results
The Wilcoxon test performed on the mold concentrations for homes grouped by the inspection process into NMH versus MH (Table 1) showed that no species was significantly different in concentration between these two groups of homes at the 95% CL, when P-values were adjusted for multiple comparisons. Fisher’s exact test on this 2 × 2 table estimated an odds ratio of 1.33 (95% CL 0.80, 2.23) and P-value of 0.27.
Table 2 shows the results for the 10-fold, cross-validation which used a logistic model and the RMI values to predict wheezing and/or rhinitis in children living in the 271 homes. Cross-validation provides more robust estimates of all summary metrics, shown in Table 2, than could be obtained from a single model fit to the data and subsequent classification of homes. By comparing Table 2 with Figure 2a, a range of RMI values between approximately −5.53 and −0.24 show the line depicting the lower 95% CL of the odds ratio that lies above the reference line of one corresponding directly to the same range of RMI values with adjusted P-values showing marginal to strong statistical significance in Table 2.
Figure 2.
(a) Plot showing odds ratios with 95% confidence interval spanning the quantiles corresponding to the middle 90% of the RMI distribution. (b) Prediction error rates from 10-fold cross-validation of logistic discriminant analysis using RMI values to predict illness (wheezing and/or rhinitis). The vertical line shows an example of an RMI threshold of −4.29 subsequently used for both predicting the incidence of illness (Table 2) and for dividing the homes into more moldy homes and less moldy homes (Table 3). False negatives are shown as minus signs and false positives as plus signs.
A useful maximum odds ratio occurred at an RMI value of −4.29 (Table 2). Based on this value, the study homes were reclassified into “more moldy homes” (MMHs) and “less moldy homes” (LMHs). With this reclassification, the mold burden was compared using the Wilcoxon rank-sum test across all 36 species (Table 3). Based on this classification, all Group 1 molds were in significantly higher concentrations in the MMH than the LMH and GM ratios range from 1.24 to 16.92. For the Group 2 molds, only five of 10 were statistically significant at the P < 0.05 level with GM ratios ranging from 0.97 to 3.90. Thus, Group 1 molds were clearly in higher concentrations in homes associated with illness.
Table 3.
Wilcoxon rank-sum analysis of mold concentrations in homes classified based on an RMI value of −4.29 as either “more moldy homes” (MMHs) or “less moldy homes” (LMHs).
| Mold species | W-test P-valuesa | GM: MMHs n = 219 | GM:LMHs n = 52 | GM ratio: MMHs/LMHs |
|---|---|---|---|---|
| Group 1 | ||||
| Aspergillus flavus | 0.0034 | 2.3 | 0.9 | 2.6 |
| Aspergillus fumigatus | <0.0001 | 10.5 | 2.1 | 4.9 |
| Aspergillus niger | <0.0001 | 7.9 | 1.5 | 5.2 |
| Aspergillus ochraceus | <0.0001 | 3.8 | 0.6 | 6.2 |
| Aspergillus penicillioides | <0.0001 | 53.5 | 7.5 | 7.1 |
| Aureobasidium pullulans | 0.0119 | 4832.4 | 2330.2 | 2.1 |
| Aspergillus restrictus | 0.0022 | 1.3 | 0.5 | 2.7 |
| Aspergillus sclerotiorum | 0.0004 | 2.8 | 0.8 | 3.4 |
| Aspergillus sydowii | 0.0029 | 1.2 | 0.5 | 2.5 |
| Aspergillus unguis | 0.0013 | 1.6 | 0.7 | 2.1 |
| Aspergillus versicolor | <0.0001 | 3.8 | 0.5 | 7.5 |
| Chaetomium globosum | 0.0001 | 3.1 | 1.0 | 2.9 |
| Cladosporium sphaerospermum | <0.0001 | 202.4 | 34.7 | 5.8 |
| Eurotium chevalieri | <0.0001 | 215.4 | 33.3 | 6.5 |
| Penicillium brevicompactum | <0.0001 | 40.5 | 2.4 | 16.9 |
| Penicillium corylophilum | 0.0699 | 0.7 | 0.5 | 1.3 |
| Penicillium crustosum | 0.0043 | 1.2 | 0.5 | 2.3 |
| Penicillium purpurogenum | 0.0423 | 0.6 | 0.5 | 1.2 |
| Penicillium spinulosum | 0.0072 | 1.4 | 0.6 | 2.4 |
| Penicillium variabile | <0.0001 | 5.9 | 1.4 | 4.3 |
| Paecilomyces variotii | <0.0001 | 7.6 | 1.7 | 4.5 |
| Scopulariopsis brevicaulis | 0.0001 | 3.6 | 1.4 | 2.6 |
| Scopulariopsis chartarum | 0.0001 | 1.9 | 0.8 | 2.4 |
| Stachybotrys chartarum | <0.0001 | 3.6 | 0.9 | 4.1 |
| Trichoderma viride | <0.0001 | 31.9 | 4.1 | 7.8 |
| Wallemia sebi | <0.0001 | 80.2 | 13.3 | 6.0 |
| Group 2 | ||||
| Alternaria alternate | 0.4484 | 410.5 | 316.1 | 1.3 |
| Acremonium strictum | 0.4484 | 2.8 | 2.1 | 1.3 |
| Aspergillus ustus | 0.0001 | 4.9 | 1.3 | 3.9 |
| Cladosporium cladosporioides (Type 1) | 0.0107 | 1795.3 | 956.4 | 1.9 |
| Cladosporium cladosporioides (Type 2) | 0.1347 | 42.2 | 28.7 | 1.5 |
| Cladosporium herbarum | 0.5166 | 183.4 | 188.2 | 0.9 |
| Epicoccum nigrum | 0.0423 | 296.6 | 165.4 | 1.8 |
| Mucor racemosus | 0.0119 | 121.0 | 60.2 | 2.0 |
| Penicillium chrysogenum | 0.0004 | 89.5 | 22.9 | 3.9 |
| Rhizopus stolonifer | 0.0029 | 2.7 | 1.3 | 2.1 |
The P-values adjusted for multiple comparisons and indicate whether a particular mold species is significantly different in concentration in an MMH versus an LMH. Also, the geometric mean (GM) in each home classification and ratio (MMH/LMH) are given.
Discussion
Reported wheezing has been associated with home dampness in various studies, and the risk of reported wheezing was increased up to five-fold in homes with mold or water damage (Waegemaekers et al., 1989; Zock et al., 2002; Belanger et al., 2003). Our earlier results suggested that infants living in homes with major mold/water damage (>0.2 m2 of visible mold) are at two times greater risk in developing recurrent wheezing compared to infants in non-damaged homes, after controlling for the effects of dust mite exposure and household income (Cho et al., 2006). However, major mold/water damage was observed through home inspection and survey (detailed above) in only 5% of the homes, yet 19.6% of the infants developed wheezing (Cho et al., 2006) and 49% developed rhinitis at age one (Biagini et al., 2006). Therefore, most cases of illness were not predictable on the basis of a home inspection.
These predictions could have been complicated by other exposures, such as smokers living in the home (Biagini et al., 2006). A parallel study did not find any associations between total mold spore count and rhinitis or allergen sensitization. However, several associations emerged when mold species were identified (Osborne et al., 2006). This indicates the importance of assessing mold exposures by a mold species level analysis. However, the methods used in that study (not MSQPCR) are very time consuming and not highly standardized.
MSQPCR provides a highly standardized method to identify and quantify species of molds. The significant differences in spore concentrations and GM ratios in the MMH compared to LMH (Tables 3) suggests that respiratory illnesses in these infants were associated with higher concentrations of Group 1 mold species in their environment. So we next standardized a way to define mold burden with the RMI.
The RMI numeric scale readily lends itself to a logistic discriminant function that takes an independent continuous variable and outputs a probability of occurrence for a binary (0 to 1) dependent variable, like illness. These tools provide a more meaningful interpretation of the mold conditions and may help in home mold remediation decisions. The logistic model provides a sound statistically based platform for identifying homes with potential mold-related problems, and, further, may be adapted to incorporate other indicators for respiratory illness.
As an example to illustrate how an RMI threshold could be selected and used to divide this population of homes into two classes, consider an RMI threshold of −4.29, corresponding to an odds ratio of 2.53 (95% CL 1.35, 4.77). A rationale for this selection could be a risk manager’s desire to maximize the significant odds-ratio values while minimizing the false negative error rate, that is, homes where infants developed respiratory illness that were not predicted to by the model (see Figure 2b).
The example RMI value of −4.29 provided the best predictor of illness with the lowest rate of illnesses missed, that is, false negatives (6.7%). However, the rate of false positives was 34.4%. For the 271 homes in this study, the RMI threshold of −4.29 would recommend remediation on as many as 219 homes. An RMI value of −2.46 gives about the same odds ratio (2.35), but allows a higher rate of false negatives (11.1%) and a lower rate of false positives (28.9%). Using this higher threshold would recommend remediation on as many as 192 or 12% fewer homes. The −4.29 threshold represents a more risk adverse but costlier decision, whereas the −2.46 threshold represents a more risk accepting but lower cost decision (considering only the costs of remediation and excluding the costs of illness).
These examples used the RMI and a logistic model to select a threshold for a binary classification of homes. This was done to facilitate a direct comparison to the home inspection-based classification method. It should be noted however, that the logistic model predicts values on a probability continuum between 0 and 1, offering a far more flexible and sensitive means for identifying homes with potential mold problems. While the RMI concisely and efficiently characterized mold condition in a home and related risk, an immediate and obvious improvement to predicting risk of respiratory illness would be to include additional information to the RMI in the predictive model like smoking in the home, pets, dust mites, or other indicators that could be taken from the home survey. Adding these factors to the logistic model might improve the prediction of illness even more.
The subjects of the study were all atopic and thus very susceptible to respiratory illness. Even relative low RMI values were associated with illness. However, this is only one epidemiological study and to determine the usefulness of the RMI, many additional studies are needed. In another study of water-damaged homes, an RMI value of about 1 increased the likelihood of asthma about five-fold (Vesper et al., 2006). Additional studies are in progress. These will help to define the general applicability of the RMI classification.
Acknowledgments
We are grateful to all parents and children who participated as well as to all home visit teams, subject recruitment teams, and clinic personnel of the CCAAPS. This research was supported by the National Institute of Environmental Health Sciences (NIEHS) Grant No. RO1 ES11170 awarded to the University of Cincinnati. This research was also supported by funding from the EPA Asthma Initiative.
Footnotes
Publisher's Disclaimer: The U.S. Environmental Protection Agency (EPA), through its Office of Research and Development, funded and collaborated in the research described here. It has been subjected to the Agency’s peer review and has been approved as an EPA publication. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use.
MSQPCR technology was patented by the US EPA (# 6 387 652). Thus, the EPA has a financial interest in the commercial use of this technology.
References
- Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, McSharry JE, Gold DR, Platts-Mills TA, Leaderer BP. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants, and maternal history of asthma. Am J Epidemiol. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false-discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- Biagini JM, LeMasters GK, Ryan PH, Levin L, Riponen T, Burkle J, Lockey J. Environmental risk factors in rhinitis in early infancy. Pedi Allergy Immunol. 2006;17:278–284. doi: 10.1111/j.1399-3038.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman NE, Haugland RA, Wymer LJ, Byappanahalli M, Whitman RL, Vesper SJ. Evaluation of a rapid, quantitative real-time PCR method for cellular enumeration of pathogenic Candida species in water. Appl Environ Microbiol. 2003;69:1775–1782. doi: 10.1128/AEM.69.3.1775-1782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-H, Reponen T, LeMasters G, Levin L, Huang J, Meklin T, Villareal M, Berstein DI. Mold damage in homes and wheezing in infants. Ann Allergy Asthma Immunol. 2006 doi: 10.1016/S1081-1206(10)60947-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland RA, Brinkman NE, Vesper SJ. Evaluation of rapid DNA extraction methods for the quantitative detection of fungal cells using real time PCR analysis. J Microbiol Meth. 2002;50:319–323. doi: 10.1016/s0167-7012(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Haugland RA, Varma M, Wymer LJ, Vesper SJ. Quantitative PCR of selected Aspergillus, Penicillium and Paecilomyces species. Sys Appl Microbiol. 2004;27:198–210. doi: 10.1078/072320204322881826. [DOI] [PubMed] [Google Scholar]
- Helsel DR. Environmental Data. Wiley and Sons Inc.; Hoboken, NJ: 2005. Nondetects and Data Analysis, Statistics for Censored Environmental Data, Wiley and Sons, Inc., NY, NY. [Google Scholar]
- Institute of Medicine. National Academies of Science. Damp Indoor Spaces and Health. The National Academies Press; Washington, DC: 2004. p. 355. [PubMed] [Google Scholar]
- International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- Kercsmar CM, Dearborn DG, Schluchter MD, Xue L, Kirchner HL, Sobolewski J, Greenberg SJ, Vesper SJ, Allan TM. Urban mold and moisture project: asthma intervention. Environ Health Perspect. 2006;114:1574–1580. doi: 10.1289/ehp.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma-United States, 1980–1999. MMWR. 2002;51(ss01):1–13. [PubMed] [Google Scholar]
- Meklin T, Haugland RA, Reponen T, Varma M, Lummus Z, Bernstein D, Wymer LJ, Vesper SJ. Quantitative PCR analysis of house dust can reveal abnormal mold conditions. J Environ Monitor. 2004;6:615–620. doi: 10.1039/b400250d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M, Riponen T, Adhikari A, Cho S-H, Grinshpun SA, Levin L, Biagini J, Bernstein DI, LeMasters G. Specific fungal exposures, allergic sensitization, and rhinitis in infants. Pedi Allergy Immunol. 2006;17:450–457. doi: 10.1111/j.1399-3038.2006.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S-Plus. 3. Springer-Verlag Inc.; NY: 2000. [Google Scholar]
- Vesper SJ. Developing the EPA Relative Moldiness Index© based on mold specific quantitative PCR. Synergist. 2006;2:39–42. [Google Scholar]
- Vesper SJ, McKinstry C, Yang C, Haugland RA, Kercsmar CM, Yike I, Schluchter MD, Kirchner HL, Sobolewski J, Allan TM, Dearborn DG. Specific Molds Associated with Asthma. J Occup Environ Med. 2006;48:852–858. doi: 10.1097/01.jom.0000224736.52780.2f. [DOI] [PubMed] [Google Scholar]
- Vesper SJ, Varma M, Wymer LJ, Dearborn DG, Sobolewski J, Haugland RA. Quantitative PCR analysis of fungi in dust from homes of infants who developed idiopathic pulmonary hemorrhaging. J Occup Environ Med. 2004;46:596–601. doi: 10.1097/01.jom.0000128160.17144.6e. [DOI] [PubMed] [Google Scholar]
- Waegemaekers M, Van Wageningen N, Brunekreef B, Boleij JSM. Respiratory symptoms in damp homes. Allergy. 1989;44:192–198. doi: 10.1111/j.1398-9995.1989.tb02261.x. [DOI] [PubMed] [Google Scholar]
- Zock J-P, Jarvis D, Luczynska C, Sunyer J, Burney P. Housing characteristics, reported mold exposure, and asthma in the European Community Respiratory Health Survey. J Allergy Clin Immunol. 2002;110:285–292. doi: 10.1067/mai.2002.126383. [DOI] [PubMed] [Google Scholar]