Abstract
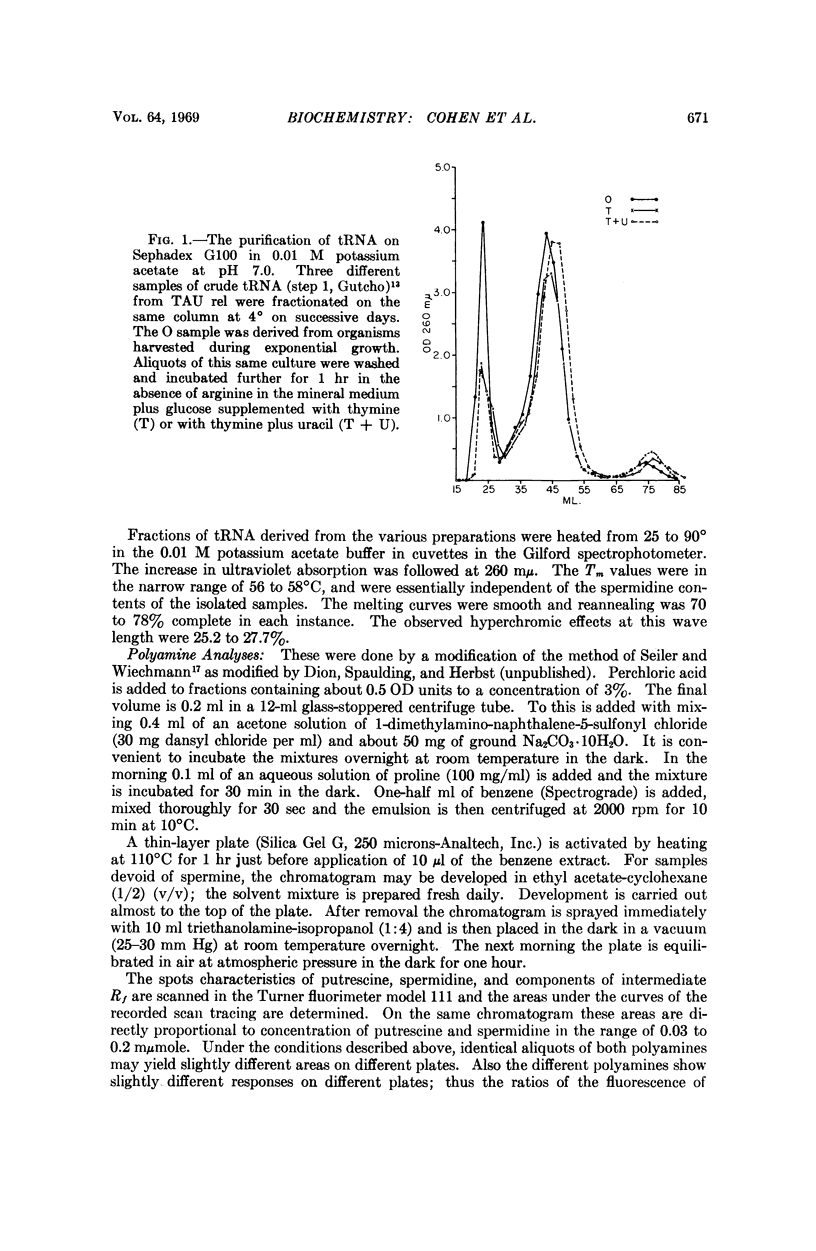
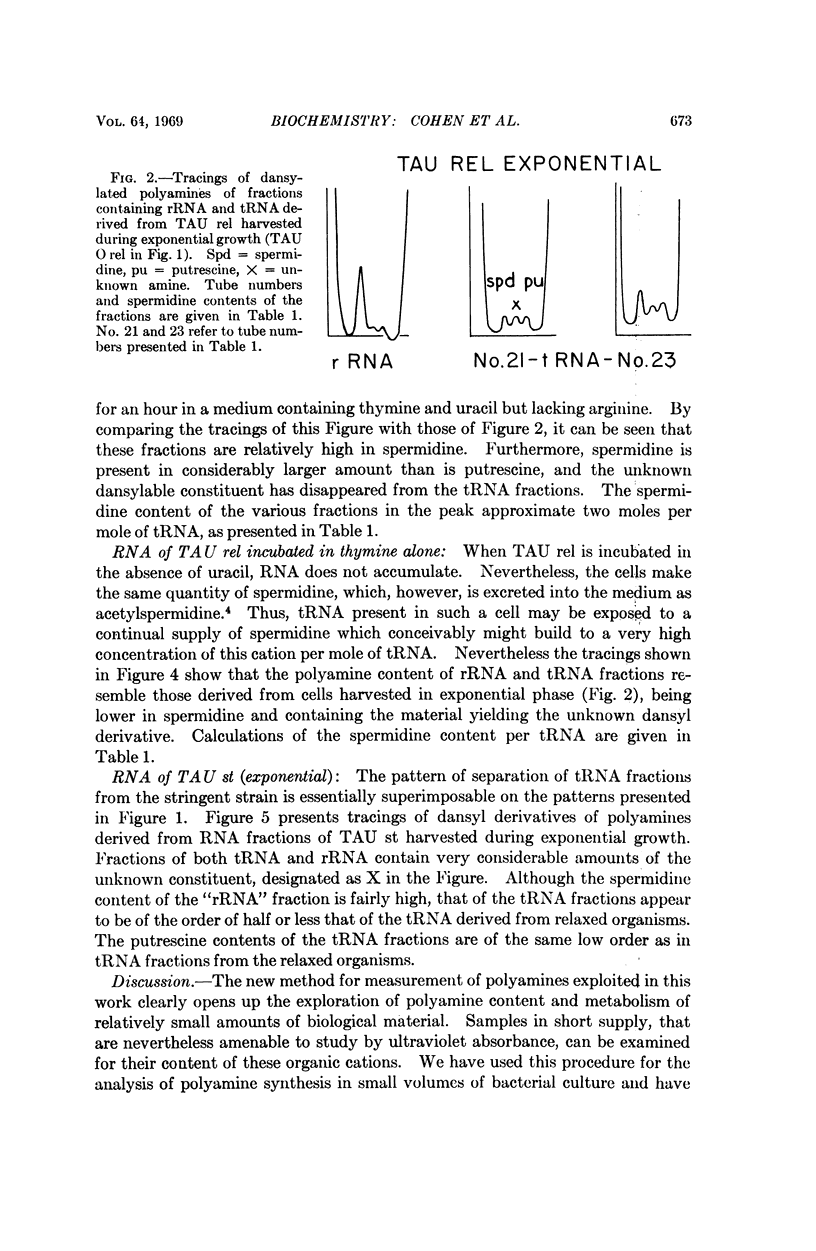
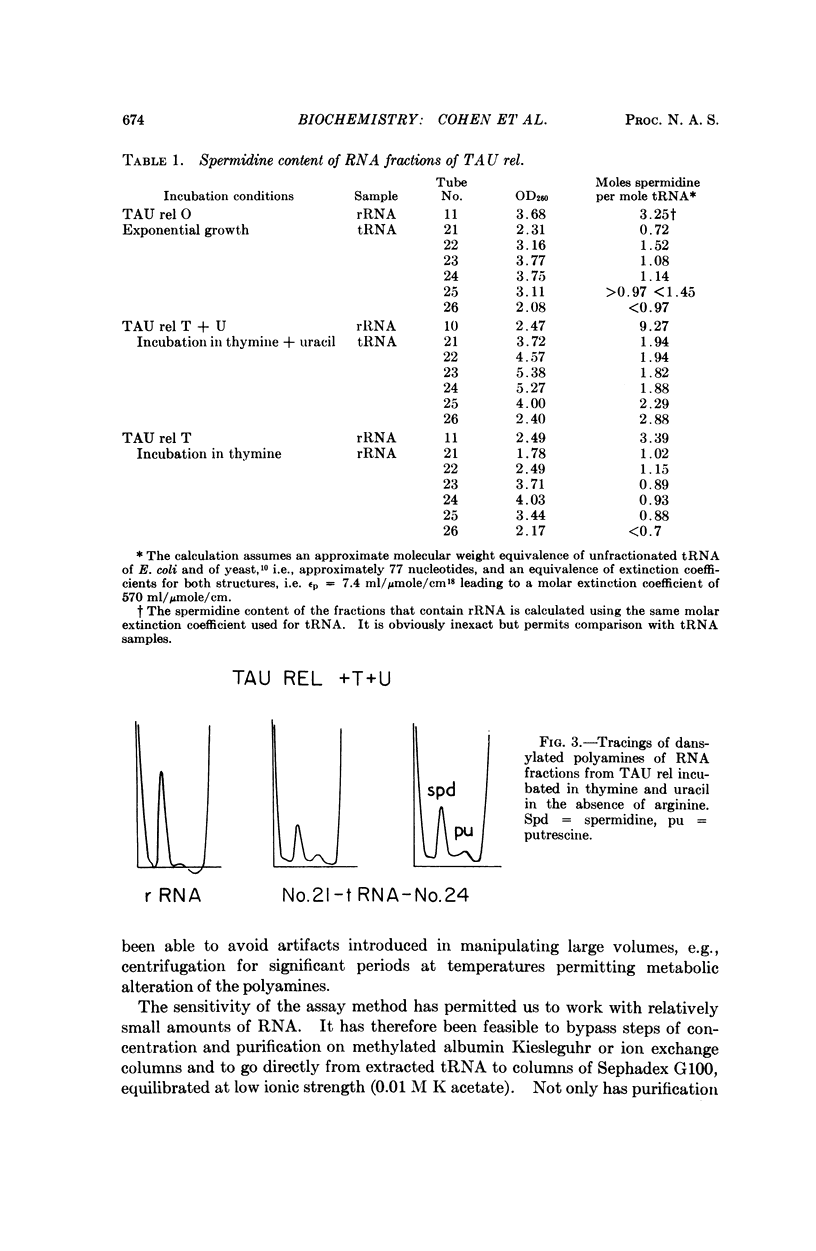
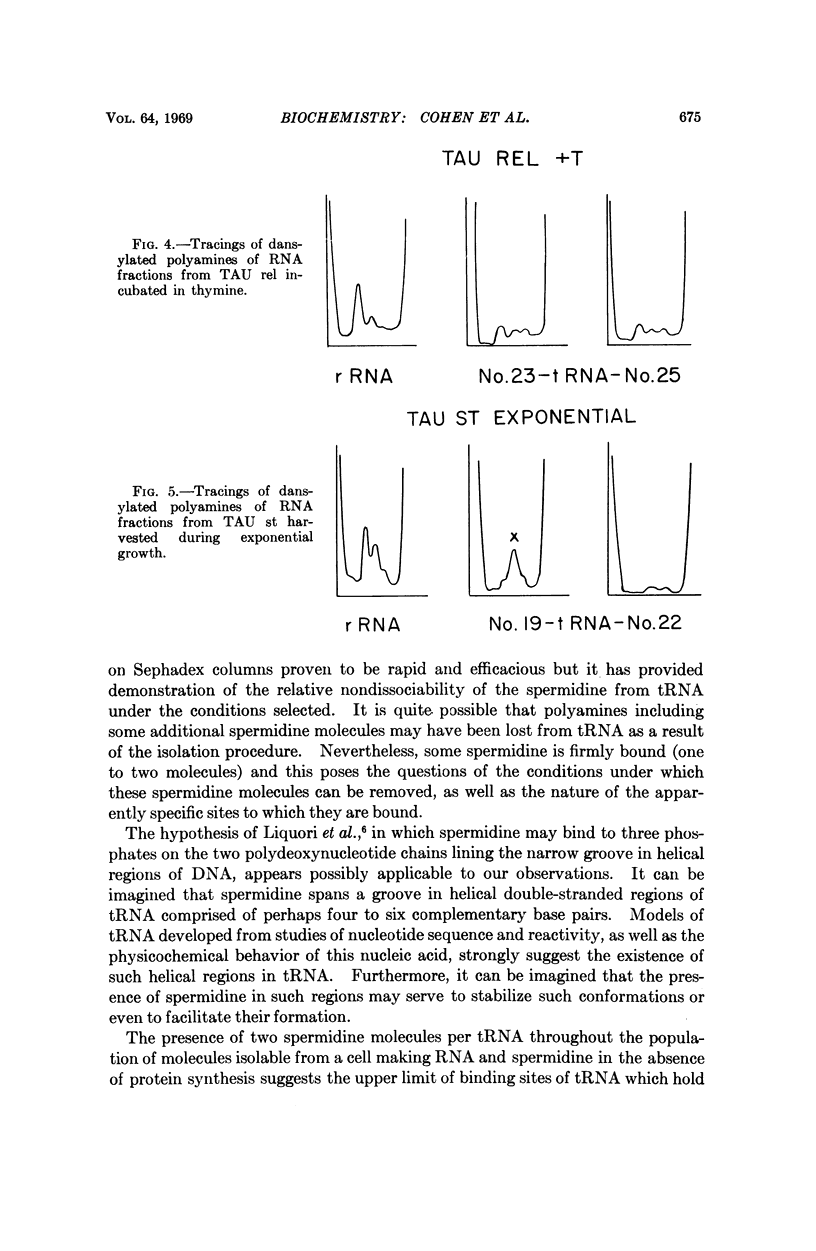
A sensitive method of polyamine estimation has been adapted to the study of the organic cations of small amounts of nucleic acid. A procedure utilizing phenol extraction, alcohol precipitation, and separation on Sephadex G100 has been devised for the isolation of tRNA at low ionic strength. The procedure is applicable to the isolation of tRNA from liter batches of bacterial culture. With these methods we have examined the polyamines of tRNA isolated from polyauxotrophic strains of E. coli incubated under various physiological conditions and have found the following: (1) The tRNA from relaxed bacteria (TAU rel) harvested during exponential growth is heterogeneous with respect to polyamine content. Some portions of the population contain about one mole of spermidine per mole of tRNA. Some putrescine and an unknown amine are also present in low concentration. (2) After exponential TAU rel is incubated with thymine and uracil in the absence of arginine, the tRNA population is far more homogeneous with respect to polyamine content. The various fractions contain two moles of spermidine per mole of tRNA and a small amount of putrescine. (3) After exponential TAU rel is incubated in the absence of both arginine and uracil, the polyamine pattern of the tRNA resembles that isolated from exponential cells. (4) The tRNA from stringent bacterias harvested during exponential growth is heterogeneous with respect to polyamine distribution and some fractions contain relatively high concentrations of the unknown amine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bluestein H. G., Allende C. C., Allende J. E., Cantoni G. L. Seryl transfer ribonucleic acid synthetase from bakers' yeast. 3. The seryladenylate-enzyme complex and its interaction with transfer ribonucleic acids. J Biol Chem. 1968 Sep 25;243(18):4693–4699. [PubMed] [Google Scholar]
- Buck C. A., Nass M. M. Differences between mitochondrial and cytoplasmic transfer RNA and aminoacyl transfer RNA synthetases from rat liver. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1045–1052. doi: 10.1073/pnas.60.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Hoffner N., Jansen M., Moore M., Raina A. POLYAMINES, RNA SYNTHESIS, AND STREPTOMYCIN LETHALITY IN A RELAXED MUTANT OF E. coli STRAIN 15 TAU. Proc Natl Acad Sci U S A. 1967 Mar;57(3):721–728. doi: 10.1073/pnas.57.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco J. R., Adams A., Ascione R., Henley D., Lindahl T. Tertiary structure in transfer ribonucleic acids. Cold Spring Harb Symp Quant Biol. 1966;31:527–537. doi: 10.1101/sqb.1966.031.01.068. [DOI] [PubMed] [Google Scholar]
- Fuchse, Millette R. L., Zillig W., Walter G. Influence of salts on RNA synthesis by DNA-dependent RNA-polymerase from Escherichia coli. Eur J Biochem. 1967 Dec;3(2):183–193. doi: 10.1111/j.1432-1033.1967.tb19514.x. [DOI] [PubMed] [Google Scholar]
- Gutcho S. The preparation of transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1968 Mar 18;157(1):76–82. doi: 10.1016/0005-2787(68)90265-7. [DOI] [PubMed] [Google Scholar]
- Ishida T., Sueoka N. Effect of ambient conditions on conformations of tryptophan transfer ribonucleic acid of Escherichia coli. J Biol Chem. 1968 Oct 25;243(20):5329–5336. [PubMed] [Google Scholar]
- Lindahl T., Henley D. D., Fresco J. R. Molecular weight and molecular weight distribution of unfractionated yeast transfer ribonucleic acid. J Am Chem Soc. 1965 Nov 5;87(21):4961–4963. doi: 10.1021/ja00949a055. [DOI] [PubMed] [Google Scholar]
- Muench K. H., Safille P. A. Transfer ribonucleic acids in Escherichia coli. Multiplicity and variation. Biochemistry. 1968 Aug;7(8):2799–2808. doi: 10.1021/bi00848a015. [DOI] [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jansen M., Cohen S. S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1684–1696. doi: 10.1128/jb.94.5.1684-1696.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Wiechmann M. Die Mikrobestimmung von Spermin und Spermidin als 1-Dimethylamino-naphthalin-5-sulfonsäure-Derivate. Hoppe Seylers Z Physiol Chem. 1967 Oct;348(10):1285–1290. [PubMed] [Google Scholar]
- Szer W. Effect of di- and polyamines on the thermal transition of synthetic polyribonucleotides. Biochem Biophys Res Commun. 1966 Mar 8;22(5):559–564. doi: 10.1016/0006-291x(66)90311-1. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Buck C. A., Granger G. A., Holland J. J. Chromatographic alterations in transfer RNA's accompanying speciation, differentiation and tumor formation. J Mol Biol. 1968 May 14;33(3):809–828. doi: 10.1016/0022-2836(68)90321-5. [DOI] [PubMed] [Google Scholar]


