Abstract
Background
Prompt diagnosis of acute myocardial infarction or acute coronary syndrome is very important.
Aim
A systematic review was conducted to determine the accuracy of 10 important signs and symptoms in selected and non-selected patients.
Design of study
Diagnostic meta-analysis.
Method
Using MEDLINE, CINAHL, EMBASE, tracing references, and by contacting experts, studies were sought out that described one of the 10 signs and symptoms on one or both conditions. Studies were excluded if they were not based on original data. Validity was assessed using QUADAS and all data were pooled using a random effects model.
Results
Sixteen of the 28 included studies were about patients who were non-selected. In this group, absence of chest-wall tenderness on palpation had a pooled sensitivity of 92% (95% confidence interval [CI] = 86 to 96) for acute myocardial infarction and 94% (95% CI = 91 to 96) for acute coronary syndrome. Oppressive pain followed with a pooled sensitivity of 60% (95% CI = 55 to 66) for acute myocardial infarction. Sweating had the highest pooled positive likelihood ratio (LR+), namely 2.92 (95% CI = 1.97 to 4.23) for acute myocardial infarction. The other pooled LR+ fluctuated between 1.05 and 1.49. Negative LRs (LR−) varied between 0.98 and 0.23. Absence of chest-wall tenderness on palpation had a LR− of 0.23 (95% CI = 0.18 to 0.29).
Conclusions
Based on this meta-analysis it was not possible to define an important role for signs and symptoms in the diagnosis of acute myocardial infarction or acute coronary syndrome. Only chest-wall tenderness on palpation largely ruled out acute myocardial infarction or acute coronary syndrome in low-prevalence settings.
Keywords: diagnostic meta-analysis, myocardial ischemia, signs and symptoms
INTRODUCTION
‘Chest pain’ is a symptom of illnesses of different organs (heart, lung, stomach and intestines, muscles, and skeleton) or of psychiatric disorders, all of which require specific treatment. Due to the high mortality and morbidity of coronary disease, in the event of chest pain, a GP will always consider the possibility of an acute myocardial infarction or unstable angina. Moreover, fast treatment — such as thrombolysis, percutaneous coronary intervention, or coronary artery bypass graft — can be life-saving and increase the patient's life expectancy and quality of life.1
The annual incidence of acute myocardial infarction for persons aged 30–69 years is estimated by the British Heart Foundation at 0.6% for men and at 0.1% for women.2 In Belgium the figures are comparable: in the 45–75-year-old age group Bartholomeeussen et al3 found a yearly incidence of acute myocardial infarction of 0.55% for men and 0.19% for women. The incidence of severe heart disease in people complaining of chest pain is highly dependent on the care setting: for Belgium percentages vary between 4.8% when a GP is contacted and 24.2% for patients in the emergency department of a university teaching hospital.4
Severe prolonged chest pain of acute onset is rarely a decision-making problem. Attacks of chest pain that are experienced by the patient and defined as not very severe or prolonged, but distressing enough for them to contact a GP, present a more difficult problem in diagnosis and management.5 In the majority of European countries GPs will perform most of the triage in patients with chest pain and so can only rely on signs and symptoms. The accuracy of these signs and symptoms has already been the subject of systematic reviews. Several authors only used groups consisting of patients with known acute myocardial infarctions in their reviews;6–8 such studies can only determine the sensitivity. The specificity, the positive or negative likelihood ratio (LR+, LR−), the positive predictive value (PPV), and the negative predictive value (NPV) cannot be determined using such samples. Consequently, the accuracy of a test cannot be described fully.
Panju et al only used studies concerning patients included via an emergency department or patients admitted to a hospital.9 Mant et al only used articles dated before 1992 in their review on signs and symptoms,10 while Chun and McGee only used MEDLINE for their search strategy.11 More recent studies were included in this systematic review. Two analyses were made: one of studies of patients who were non-selected (recruited by GPs, paramedics, or emergency departments) and one of studies of patients who were selected (recruited by coronary care units and cardiologists).
Ten signs and symptoms that could be found by history taking or physical examination were investigated. The diagnostic value was examined for acute myocardial infarction or acute coronary syndrome of:
radiating pain (left arm and/or shoulder, right arm and/or shoulder, both arms and/or shoulder, neck, back, epigastric);
oppressive pain;
nausea and/or vomiting;
sweating; and
absence of chest-wall tenderness on palpation (absence of tenderness).
METHOD
Search strategy
MEDLINE, EMBASE and CINAHL were searched. All searches were up to date as of 31 May 2006. In MEDLINE a combination of terms was used involving all possible elements, the target disease and no filters: (“Physicians, Family”[MeSH] OR “Emergency Service, Hospital”[MeSH] OR “Emergency Medical Services”[MeSH] OR “Emergency Medicine”[MeSH]) AND (“Chest Pain”[MeSH] OR “Myocardial Ischemia”[MeSH]).
How this fits in
Most information about signs and symptoms is derived from studies in coronary care units with patients who have 100% acute myocardial infarction. Those data are not similarly accurate in a primary care setting (GP surgery, emergency department, and paramedics). This study was not able to define an important role for signs and symptoms in the diagnosis of acute myocardial infarction or acute coronary syndrome. Only chest-wall tenderness on palpation largely ruled out acute myocardial infarction or acute coronary syndrome in low-prevalence settings.
An adapted version of this search string was used in CINAHL: ((Emergency-Medicine) OR (Emergency-Service) OR (Physicians-Emergency) OR (Emergencies) OR (Emergency-Care) OR (Emergency-Medical-Services) OR (Physicians-Family) OR (Prehospital-Care)) AND ((Angina-Pectoris) OR (Chest-Pain) OR (Myocardial-Infarction) OR (Myocardial-Ischemia)).
The search string used in EMBASE was: (‘emergency health service’ OR ‘general practitioner’ OR ‘emergency health service’ OR ‘emergency ward’ OR ‘emergency medicine’) AND (‘thorax pain’ OR ‘heart muscle ischemia’)
In addition, the reference lists of the retrieved articles were checked. A search for any unpublished study results was limited to contacting known researchers in the field.
Study selection
The study strategy was designed to include all published diagnostic accuracy studies on signs and symptoms for the diagnosis of acute myocardial infarction, unstable angina, or acute coronary syndrome. Studies were excluded if diagnostic tests were not one of the 10 selected signs or symptoms and if they were not based on original data or if the data were insufficient to construct a 2×2 table. Language restrictions were English, French, German, and Dutch. Two independent reviewers screened the titles; a third reviewer resolved any disagreements that occurred between the two. All the selected titles were studied in full text by one reviewer. A list of excluded studies and a log of reasons for exclusion are available from the authors on request. When patients were recruited by GPs, paramedics, or emergency departments, they were considered ‘non-selected’. Patients recruited by coronary care units and cardiologists were considered ‘selected’.
Chosen articles were retrieved in full and further included in the review after they had been assessed for quality using the QUADAS instrument, shown in Appendix 1.12 The selection of participants and the validity of the reference standard were the most important considerations. Studies were excluded from the review if they failed on one of these two items. Studies that failed on other QUADAS questions were not excluded, not even those without blind interpretation of the other tests, as blinding is almost impossible in this case.
Data extraction
The following data were extracted (in duplicate) from the studies:
the design: whether the data were collected prospectively or retrospectively, and whether the participants were included consecutively;
the setting: whether participants were recruited by GPs, cardiologists, paramedics, emergency departments, chest pain observation units, or coronary care units;
age and sex;
index test: pain radiating to left arm and/or shoulder; to right arm and/or shoulder; to both arms and/or shoulders; to neck; to back; epigastric, oppressive pain; nausea and/or vomiting; sweating; absence of tenderness;
the number of patients and the prevalence of the disease in the study group;
the results from the study in absolute numbers (in the absence of the absolute numbers, they were calculated from prevalence, sensitivity and specificity);
the inclusion and exclusion criteria; and
the reference standard.
Statistical analysis and data synthesis
Two groups were analysed separately: the patients who were non-selected and those who were selected. Standard methods recommended for the meta-analysis of diagnostic test evaluations were used.13–15 Analyses were performed using Stata (version 8, Stata Corporation, Texas). Sensitivity, specificity, LR+ (= sensitivity/[1 — specificity]; a positive test result makes the odds of the disease ‘LR+’ times more possible), LR− (= [1 — sensitivity]/specificity; a negative test result makes the odds of the disease ‘LR−’ times less possible) and the odds ratio (OR) were pooled using a random effects model. PPV or NPV were not reported because of the heterogeneity between studies due to differences in setting and prevalence.
Heterogeneity in meta-analysis refers to the degree of variability in results across studies. Forest plots were examined and used, the χ2 and Fisher's Exact tests were used and, in view of the low power of the χ2 test, the I2 statistic was also estimated to detect heterogeneity.16 In order to keep the tables readable, only the I2 data are reported. The potential presence of publication bias using funnel plots and the Egger test was tested for.17–18
RESULTS
Included studies
Figure 1 outlines the study selection process. The great majority of publications concerning acute myocardial infarction and acute coronary syndrome discuss the technical tests and treatments. The number of studies found reporting the selected signs and symptoms was not very extensive — there were 57 in all. A further 29 were excluded because only patients with confirmed acute myocardial infarctions or acute coronary syndromes were included. Twenty-eight articles were included in the meta-analysis:19–46 16 studies were about patients who were non-selected, 11 studies were about patients who were selected, and one study was made in a chest pain observation unit.
Figure 1.
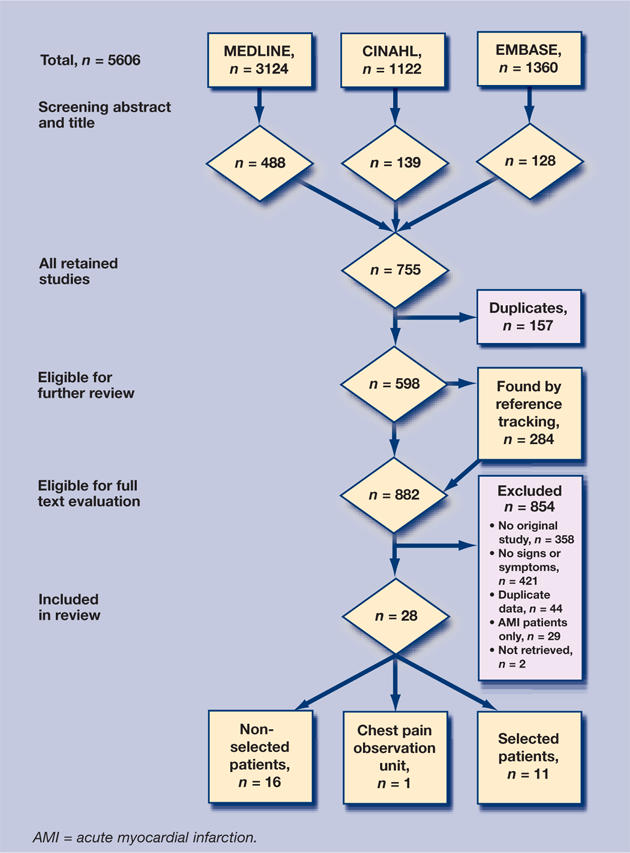
Retrieval of eligible studies: flowchart.
Study characteristics and quality
During the selection process the inter-rater agreement between the two reviewers was very good with a κ of 0.82 (95% confidence interval [CI] = 0.79 to 0.85). There was no disagreement in items of the QUADAS instrument. The results of the studies included on the QUADAS list are outlined in Appendix 2. In an attempt to analyse subgroups for sex and age, 14 authors were contacted by e-mail and additional data were obtained for two studies. Appendices 3 and 4 present the descriptive data from the studies included.
For the final diagnosis of acute myocardial infarction the reference tests used were enzyme rises (n = 23), electrocardiogram (ECG) change (n = 22), history (n = 11), scintigraphy (n = 8), autopsy (n = 5), criteria of the World Health Organization or European Society of Cardiology (n = 4), sudden death (n = 3), coronary angiogram (n = 2), echocardiography (n = 2), or urgent revascularisation (n = 1). In some studies, at least two tests were required. History alone was always insufficient to diagnose an acute myocardial infarction.
Reference tests for unstable angina were: history (pain: frequency, worse, new) (n = 5), ECG changes without enzymes rises (n = 3), unproven acute myocardial infarction (n = 2), Canadian Cardiovascular Society classification criteria (n = 1), and clinical judgement (n = 1).
One study46 gave only reference tests for acute coronary syndrome: troponin rise, cardiac death, acute myocardial infarction, new onset heart failure, life-threatening arrhythmia, or coronary revascularisation.
Prevalence
Two large studies36,37 provided 50% of the subjects. When the results of all the studies were combined, there were 5067 (11.6%) patients with acute myocardial infarction out of a group of 43 138, and 4594 (26.3%) patients with acute coronary syndrome out of a group of 17 416. Of these 17 416, 13 108 (75.3%) belonged to a group also examined for acute myocardial infarction. There are approximately 50% more patients with unstable angina than acute myocardial infarction.
The varying prevalence of acute myocardial infarction depended on the setting and inclusion criteria. Graff et al's37 low prevalence of about 2% was caused by the very large inclusion criteria, that is, ‘all patients with possible acute myocardial infarction for whom a rapid ECG was performed’. The chest pain observation unit, to which patients with unclear signs and symptoms and without clear ECG abnormalities and/or blood abnormalities were admitted, had a prevalence of 4%.40 In other studies executed in emergency departments, prevalences of between 6% and 18% were found. The group transported by ambulance in Svenson et al's study,44 with a prevalence of 29%, situates itself between the patients seen in an emergency department and those admitted to a coronary care unit (prevalence from 36–50%). In the study of Van der Does et al21 the prevalence of referred patients was as low as 7%.
Heterogeneity in the non-selected group
For acute myocardial infarction there was homogeneity in the LR+ of oppressive pain and in the LR− for tenderness. For acute coronary syndrome there was homogeneity in the LR+ of left-arm pain and the LR− for sweating and tenderness. For the other analyses, a moderate to high level of heterogeneity was found.
Indications of diagnostic accuracy in the non-selected group
Appendix 5 (for the diagnosis of acute myocardial infarction) and Appendix 6 (for a diagnosis acute coronary syndrome) (both subgroups separately) show the pooled sensitivity, specificity, LR+, LR−, and OR with their 95% CI and I2. The sensitivity and specificity per sign or symptom varied greatly.
The sensitivity of absence of tenderness was high, namely 92% (95% CI = 85.5 to 96.4) for acute myocardial infarction and 94% (95% CI = 91.4 to 96.1) for acute coronary syndrome. Oppressive pain followed with a sensitivity of 60% (95% CI = 53.7 to 66.0 for acute myocardial infarction). Sweating had the highest LR+, namely 2.92 (95% CI = 1.97 to 4.32 for acute myocardial infarction).
The LR+ of right arm or shoulder pain was 2.89 (95% CI = 1.40 to 5.98) for acute myocardial infarction (one study). The other LR+ fluctuated between 1.05 and 1.49 for acute coronary syndrome.
Absence of tenderness had a LR- of 0.23 (95% CI = 0.18 to 0.29) for acute myocardial infarction and 0.17 (95% CI = 0.11 to 0.26) for acute coronary syndrome. Other LR− varied between 0.69 (oppressive pian and sweating for acute myocardial infarction) and 0.98 (epigastric pain) for acute coronary syndrome.
Publication bias
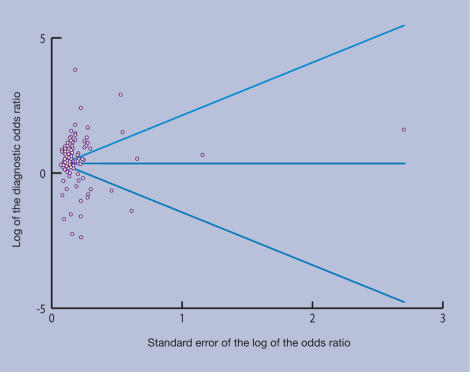
A funnel plot, signifying publication bias, is shown in Figure 2. The plot appears symmetrical, suggesting absence of publication bias. This was confirmed by a non-significant Egger test (0.79).
Figure 2.
Funnel plot for evaluation of publication bias in all studies.
DISCUSSION
Studies included
More than half of the studies dated from after Mant et al's10 selection. Sixteen new articles about several signs and symptoms that were not included in Chun and McGee's review11 were included, indicating the necessity for a new systematic review.
Although the authors aimed to find all relevant studies, some could have been missed. However, the search strategy will have detected most studies. As only two studies were performed in a general practice setting, it was decided to look for ‘primary care’ studies, which were defined as settings in which patients who had not been referred by other medical practitioners were seen. In Europe and some other parts of the world this mostly concerns general practice. In the US and a number of other countries however, this frequently includes the emergency department and patients admitted by paramedics. The information on these unselected patients is certainly relevant for GPs.
Quality
A fair amount of research dates from several decades ago. It could be argued that these older studies are disadvantaged in terms of quality as empirical research on design-related bias and the STARD (Standards for Reporting of Diagnostic Accuracy) initiative to improve quality are fairly recent.47–49 Although new research on the quality of diagnostic accuracy studies confirms that quality is still not optimal, the quality of the studies included was good according to the QUADAS criteria.
A good reference test is essential in diagnostic research. It was unclear how frequently these criteria were used for the definite diagnosis of acute myocardial infarction. The acceptance of a broad range of inclusion criteria (autopsy, sudden death, scintigraphy, echocardiography, and angiography) as reference tests increased the number of real positives at the risk of spectrum bias.10 Verification bias was not a major problem because almost all patients received a reference standard. The increasing sensitivity of the blood tests used over the years — starting with transaminase via lactate dehydrogenase, creatine kinase (CK) and the isoenzyme CK-MB, and recently troponin T — has caused a rise in real positives. However, no increase in the prevalence of acute myocardial infarction in the emergency departments or the coronary care units was noticed over the course of time. The reference test for unstable angina was not as clearly defined as that for acute myocardial infarction because it often depended on the clinical picture and its interpretation. All of this could cause either over- or underestimation of the prevalence found.
Although ‘acute myocardial infarction + unstable angina = acute coronary syndrome’, a distinction was made between acute myocardial infarction and acute coronary syndrome to ensure that no data was left out of those studies that dealt only with acute myocardial infarction.
Prevalences
In 2000, the definition of acute myocardial infarction changed to: ‘typical rise and gradual fall of cardiac troponin, or more rapid fall of CK-MB, with at least one of the following: ischemic symptoms; the development of pathological Q waves; ECG changes indicative of ischemia (ST-segment elevation or depression); coronary artery intervention’.50 This definition of acute myocardial infarction will increase the sensitivity of diagnosing acute myocardial infarction and thereby increase the findings of its incidence. The increased specificity of troponin, however, should decrease the number of false-positive diagnoses. The combined effect that these two factors may have on the case-fatality rate is currently unclear.51
The larger number of acute myocardial infarctions in patients transported by ambulance compared to those in patients transported to the emergency department by other means has been documented previously.4 The low prevalence of referred patients in the study by van der Does et al21 is possibly explained by the underestimation of the number of acute myocardial infarctions due to the absence of sensitive blood analyses in 1972.
It should also be noted that in most studies the true population prevalence of acute myocardial infarction was higher because patients who died at home could not be included.
Heterogeneity
Most of the pooled results were heterogeneous, due to different settings, inclusion criteria, and reference standards. The non-homogenous pooled results must be interpreted very carefully.
Diagnostic accuracy of signs and symptoms in the non-selected patients group
Absence of tenderness was highly sensitive for acute myocardial infarction (92%) and acute coronary syndrome (94%). The presence of palpation pain greatly reduces the chance of acute myocardial infarction and acute coronary syndrome with a LR− of 0.23 and 0.17 respectively. Similar pleuretic or positional thoracic pain was not selected in this study. In Mant's et al's study the absence of pleuretic pain had a LR− of 0.19 and the absence of positional pain a LR− of 0.27.10 Oppressive pain, with a pooled sensitivity of 60% and a specificity of 58% has almost no influence on the likelihood of acute myocardial infarction. The sensitivities of the other signs and symptoms were even lower and could not be used to exclude acute myocardial infarction or acute coronary syndrome. The differences in sensitivity and specificity between acute myocardial infarction and acute coronary syndrome remained small and were therefore not relevant.
It is true that, even in unselected settings such as general practice, patients have a reason for visiting their GP with chest pain. Fear of having a myocardial infarction may be one such reason. Anyone not visiting their doctor will not be diagnosed with acute myocardial infarction so the classical signs and symptoms of chest pain and irradiation are always part of the diagnostic work-up.
Clinical implications
To summarise the interpretation of signs and symptoms in the clinical context, consider a patient in a low-, intermediate-, and high-prevalence setting. For the sake of clarity the highest pooled LR+ and the lowest LR− found for acute myocardial infarction, namely sweating (LR+ 2.92, LR− 0.69), and absence of chest-wall tenderness (LR+ 1.47, LR− 0.23) were used.
In a low pre-test probability situation of 5%, which may be regarded as the prevalence in those patients who are unselected and contact a GP with chest pain, these LR+ translate to a 13% and 7% post-test probability of a positive test result.4 In low pre-test settings the presence of the signs and symptoms listed above is insufficient to definitively confirm acute myocardial infarction. In their absence the post-test probability is lowered to 4% and 1%. Absence of sweating should scarcely affect GPs' management; the presence of chest-wall tenderness results in a post-test probability of 1.1% and so largely rules out acute myocardial infarction for clinical purposes.
In an intermediate-prevalence setting of 15%, which is the prevalence expected in a patient seen by the GP during an urgent home visit or in an emergency department, the same reasoning produces a post-test probability of 34% and 21%. If these symptoms are absent this becomes 11% and 4%. These results should barely influence GPs' treatment strategy.
In a high-prevalence setting of 40%, such as a coronary care unit the same signs or symptoms represent a post-test probability of 66% and 49% if positive, and 32% and 13% if negative. These results will also add nothing to the diagnostic process.
Each of these signs and symptoms may also trigger consideration of acute myocardial infarction or acute coronary syndrome: non-specific complaints such as back pain or vomiting/nausea can also be caused by acute myocardial infarction or acute coronary syndrome. In Goodacre et al's45 study of patients with undifferentiated chest pain (with normal ECG and without a clear clinical diagnosis of acute coronary syndrome) the final diagnosis was acute coronary syndrome in 7.9%. This group of missed acute coronary syndrome probably still has a higher mortality than patients without acute coronary syndrome.52
Previous meta-analyses of signs and symptoms
All the pooled results were situated within the 95% CIs of the previous investigations, except in the absence of chest-wall tenderness. Here a LR+ of 1.47 (95% CI = 1.23 to 1.75) was found, which was somewhat higher than in Mant et al's10 research (1.18; 95% CI = 1.16 to 1.22).
Limitations of the review
Some studies suggested a difference in the diagnostic accuracy of signs and symptoms according to age27,38 or sex,43 but there were not enough studies to perform a subgroup analysis. Although the combination of signs and symptoms, their context, the severity, and the progression from the start influence the interpretation, it was impossible to examine this because there were almost no included studies that investigated the diagnostic accuracy of combinations of signs and symptoms. Only three of the selected studies combined different signs and symptoms: Short22 (previous or not-previous history of acute coronary syndrome and studied signs and symptoms), Lee et al23 (sharp or stabbing pain and pain pleuretic, positional or reproduced by palpation and no prior acute coronary syndrome), and Hargarten et al25 (radiating pain and sweating, difficult breathing, and nausea/vomiting).
Persons with chest pain can also be subject to serious, even life-threatening, diseases other than acute myocardial infarction or acute coronary syndrome, such as pulmonary embolism, and stomach bleeding. This requires analysis with CART-type models in the individual studies. CART is a statistical package that produces decision trees using variables (coded signs and symptoms) directing to classes (diagnostic categories). At each node of the decision tree, the programme calculates which variable is the ‘most discriminating’ and constructs, at that node, a bifurcation of two branches. For each resulting branch, CART calculates the next most discriminating variable and continues in this way until either the subgroups or the discriminating power become ‘too small’. A final statistical pruning technique results in an optimal tree, where optimality is measured by various criteria.53–55 As far as the authors know no systematic review has been published pooling the results of such studies.
In 2005 a new multilevel method for the bivariate pooling of combined sensitivity and specificity was published.56 This method may be superior to classic pooling. The authors were asked to do the calculations on the current study's results of the absence of chest-wall tenderness on palpation in relation to acute myocardial infarction. Because of the minimal differences, the previous calculations were not reworked.
Conclusions
Based on this meta-analysis, it was not possible to define an important role for signs and symptoms in the diagnosis of acute myocardial infarction or acute coronary syndrome. Only chest-wall tenderness on palpation largely ruled out acute myocardial infarction or acute coronary syndrome in low-prevalence settings.
Acknowledgments
We are grateful to the authors of the primary studies who sent additional data as well as Dr Johannes Reitsma for doing the bivariate analysis of combined sensitivity and specificity
Appendices
Appendix 1.
The QUADAS tool.12
|
Appendix 2.
Qualification of the articles with the QUADAS tool.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Säwe, 197119 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | yes | no |
| Säwe, 197220 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | yes | no |
| Van der Does, 198021 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes |
| Short, 198122 | yes | yes | yes | no | yes | yes | yes | no | yes | yes | no | yes | yes | yes |
| Lee, 198523 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | no |
| Tierney, 198624 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no |
| Hargarten, 198725 | yes | yes | yes | yes | yes | yes | yes | yes | no | ? | no | yes | no | no |
| Herlihy, 198726 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | no | yes |
| Solomon, 198927 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | yes |
| Berger, 199028 | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | yes | no |
| Jonsbu, 199129 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | no |
| Gaston-Johansson, 199130 | yes | no | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | no | no |
| Hartford, 199331 | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | yes |
| Grijseels, 199532 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | yes |
| Everts, 199633 | yes | no | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | no | no |
| Pfister, 199734 | yes | yes | yes | yes | yes | yes | yes | no | no | yes | no | yes | no | yes |
| Lopez-Jimniez, 199835 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | no |
| Pope, 199836 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | yes | no |
| Graf, 200037 | yes | yes | no | yes | ? | ? | yes | yes | no | ? | ? | yes | no | no |
| Milner, 200138 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | yes | no | yes |
| Herlitz, 200239 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | no |
| Goodacre, 200240 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | yes |
| Baxt, 200241 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | yes | no |
| Albarran, 200242 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | no | no |
| Vodopiutz, 200243 | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | ? | yes | no | yes |
| Svensson, 200344 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | no | no |
| Goodacre, 200345 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ? | yes | no | yes |
| Christenson, 200646 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
Appendix 3.
Characteristics of acute myocardial infarction (AMI) included studies.
| Study | Design | Sample size | % AMI | Mean age | % males | Setting | Inclusion | Exclusion | Reference standard AMI |
|---|---|---|---|---|---|---|---|---|---|
| Säwe, 197119,a | Prospective consecutive | 137 | 39 | 62 | 67 | CCU | Central chest pain (>15 m, <48 hr) or pulmonary oedema or shock or syncope or status anginosus | Known valvular lesion, acute hypovolaemia or intoxication, syncope without ECG evidence of AMI | Q-wave and/or ST elevation or GOT, GPT, LDH changes, necropsy |
| Säwe, 197220,a | Prospective consecutive | 921 | 49 | 65 | 60 | CCU | Central chest pain (>15 m, <48 hr) or pulmonary oedema or shock or syncope or status anginosus | Known valvular lesion, acute hypovolaemia or intoxication, syncope without ECG evidence of AMI | Q-wave and/or ST elevation or GOT, GPT, LDH changes or autopsy findings myocardial necrosis |
| Van der Does, et al, 198021 | Prospective consecutive | 1343 | 7 | 54 | 55 | GP | Recent chest pain or dyspnoea, palpitations or dizziness or syncope upper abdominal pain or mood changes | <25 yr women, <20 yr men | WHO criteria for AMI, at least 4 pts score: ECG typical-2pt, suspect-1pt, ditto symptoms and enzymes |
| Short, 198122 | Prospective ? | 456 | 40 | 62 | 57 | Car | One or more attacks of spontaneous chest pain and who were referred for cardiology opinion | Ill enough for hospitalisation or diagnosis of coronary disease regarded as definite | History and ECG (Minnesota code) or twice limit AAT at 24–48 hr after onset |
| Lee, et al, 198523 | Prospective consecutive | 596 | 17 | 56 | 48 | ED | Chief complaint of anterior, precordial or left lateral chest pain | Local trauma, abnormalities on chest X-ray, <25 yr | One of: characteristic evolution of enzyme levels (CK-MB or LDH or CK) or Q-waves of scintiscan |
| Tierney, et al, 198624 | Prospective ? | 492 | 12 | ? | ? | ED | Anterior chest pain as one of their complaints | <30 yr men, <40 yr female | When cardiac enzyme CK elevated and CK-MB >4% or LDH1> (or equal) LDH or when no enzyme: new abnormal Q-wave |
| Herlihy, et al, 198726 | Prospective consecutive | 265 | 44 | ? | ? | CCU | Chest pain and electrographic changes | Illness or medication that could produce nausea, with thrombolytic medication | CK and ECG |
| Solomon, et al, 198927 | Prospective consecutive | 7734 | 14 | ? | 50 | ED | Chief complaint of anterior, precordial or left lateral chest pain | Local trauma, abnormalities on chest X-ray, <30 yr, >4 visit | One of: characteristics evolution of enzyme levels (CK-MB or LDH or CK), Q-waves, scintiscan, sudden unexplained death within 72 hr |
| Berger, et al, 199028 | Prospective consecutive | 278 | 36 | 57 | 69 | CCU | Admitted to the hospital, complaining chiefly of chest pain | Trauma, transferred from other hospital with a diagnosis | Chest pain, ECG changes indicating myocardial infarction, significant CK elevation |
| Jonsbu, et al, 199129 | Prospective consecutive | 200 | 37 | ? | ? | CCU | Admitted to hospital with suspected acute heart disease | Unable to give reliable medical history | Clinical history, ECD signs, enzyme activity, ventriculography, scintigraphy, autopsy |
| Gaston-Johansson, et al, 199130 | Prospective consecutive | 94 | 40 | ? | 71 | CCU | Chest pain suggesting AMI | >75 yr, cardiogenic shock | Two of: typical clinical symptoms and chest pain > 15 mins, AAT or CK elevations, Q waves ST elevation or T inversion |
| Hartford, et al, 199331 | Prospective consecutive | 226 | 48 | ? | ? | CCU | Because of suspected AMI | Very poor clinical condition, does not understand Swedish | Two of three: chest pain > 15 min, aminotransferase, new Q-waves in two leads |
| Everts, et al, 199633 | Prospective consecutive | 902 | 50 | 64 | 71 | CCU | Chest pain with possible AMI | Hypotension, severe congestive heart failure, severe UA, cognitive limitation, language | Two of three: chest pain >15 min, aminotranferase, new Q-waves in two leads |
| Pfister, et al, 199734 | Prospective consecutive | 327 | 18 | 64 | 65 | ED | Chest pain (>10 min), irradiation (epigastric, jaw, L extremity) during angina, dyspnoea, non-traumatic or toxic cardiac arrest | <20 yr, trauma | At least two of: history, ECG, CK-MB |
| Lopez-Jiminez et al, 199835 | Prospective consecutive | 2694 | 6 | ? | 45 | ED | Chief complaint of chest pain | Local trauma, abnormalities on chest X-ray, <30 yr, >4 visit, prior AMI, A, PTCA, bypass | One of: characteristics evolution of enzyme levels (CK-MB or LDH or CK), Q-waves, scintiscan, sudden unexplained death within 72 hr |
| Pope, et al, 199836 | Prospective consecutive | 10 689 | 8 | 59 | 52 | ED | Chief complaint chest, left arm, jaw or epigastric pain or discomfort, dyspnoea, dizziness, palpitations or other symptoms suggestive of acute ischemia | <30yr, 18yr if suspected to have used cocaine | WHO criteria for AMI |
| Graff, et al, 200037 | Prospective consecutive | 10 678 | 2 | ? | ? | ED | All patients with possible AMI were a rapid ECG was performed | No | ICD-9-CM 410. 01/11/21/31/41/51/61/71/81/91 |
| Herlitz, et al, 200239 | Retrospective consecutive | 930 | 14 | 71 | 51 | Para | Chest pain or slightest suspicion of an acute coronary syndrome | No | Two of: chest pain >15 min, CK more than twice upper limit, Q-waves |
| Goodacre, et al, 200240 | Prospective consecutive | 893 | 4 | 53 | 62 | CPOU | Chest pain (patients at low risk) | <25 yr, trauma, new ECG changes consistent with ischemia, comorbidity necessitating hospitalisation, definite unstable angina | WHO criteria for AMI |
| Baxt, et al, 200241 | Prospective 16/ day | 2204 | 6 | 53 | 40 | ED | Anterior chest pain prompting an ECG | <24 yr | European Society of Cardiology criteria |
| Albarran, et al, 200242 | Prospective consecutive | 541 | 48 | ? | 68 | CCU | Acute chest pain | Pain >24 hr, <18 yr, no English | Troponin I >6 ng/ml and ECG changes |
| Svensson, et al, 200344 | Prospective consecutive | 538 | 29 | 69 | 58 | Para | Chest pain or discomfort >15 min, within last 6 hr, dyspnoea, or any condition suggesting acute coronary syndrome | Lung disease | Two of: typical symptoms, Q-waves, CK-MB> 10 ng/ml or troponin >0.05 ng/ml |
The patients of the first article are part of the second study. The signs and symptoms discussed in the two studies are different. Car = cardiologist; CCU = coronary care unit or admitted to hospital, CPOU = chest pain observation unit, ECD = electrocardiogram, ED = emergency department, Para = paramedics of an ambulance. A = angina. AAT = aspartate aminotransferase. AMI = acute myocardial infarction. CK = creatine kinase. CK-MB = CK isoenzyme. ECG = echocardiogram. GOT = aspartate aminotransferase. GPT = alanine transferase. ICD = International Classification of Diseases. LDH = lactate dehydrogenase. LDH1 = lactate dehydrogenase isoenzyme 1. UA = unstable angina. WHO = World Health Organization.
Appendix 4.
Characteristics of acute coronary syndrome = acute myocardial infarction + unstable angina included studies.
| Study | Design | Sample size | % ACS | Mean age | % Males | Setting | Inclusion | Exclusion | Reference Standard |
|---|---|---|---|---|---|---|---|---|---|
| Lee, et al, 198523 | Prospective consecutive | 596 | 41 | 56 | 48 | ED | Chief complaint of anterior, precordial or left lateral chest pain | Local trauma, abnormalities on chest X-ray, <25 yr | AMI: one of characteristic evolution of enzyme levels (CK-MB, LDH, CK), Q-waves, scintiscan. UA: chest pain worse or new and diagnosis was made by a senior clinician |
| Hargarten, et al, 198725 | Retrospective consecutive | 401 | 57 | 65 | ? | Para | ‘Stable’ chest pain | Heart failure, rhythm problems, hypotension | AMI: ST-elevation followed by T-inversion (at least two leads), CPK-MB, LDH ration, autopsy pyrophosphate scan UA: no |
| Grijseels, et al, 199532 | Prospective consecutive | 1005 | 42 | 67 | 54 | GP | Symptoms of possible cardiac origin seen by GP and transferred | No | AMI: standard history, ECG, enzyme criteria UA: angina with increasing frequency and severity and new recent onset with documentation of ST-T changes at rest, abnormal stress test or coronary arteriogram |
| Pope, et al, 199836 | Prospective consecutive | 10689 | 23 | 59 | 52 | ED | Chief complaint of chest, left arm, jaw or epigastric pain or discomfort, dyspnoea, dizziness, palpitations or other symptoms suggestive of acute ischemia | <30 yr, 18 yr if suspected to have used cocaine | AMI: WHO criteria for AMI UA: Canadian Cardiovascular Society classification criteria |
| Milner, et al, 200138 | Prospective consecutive | 531 | 40 | 60 | 53 | ED | >45 yr and one symptom suggestive of ACS, or 18–44 yr if diabetes and two risk factors | <45 yr without diabetes or <18 yr with diabetes | AMI: elevated cardiac enzymes (CK-MB). UA: ECG changes (ST, T) and no cardiac enzymes elevation |
| Herlitz, et al, 200239 | Retrospective consecutive | 930 | 30 | 71 | 51 | Para | Chest pain or slightest suspicion of an acute coronary syndrome | No | AMI: two of chest pain>15 min, CK more than twice upper limit, Q-wave A: according to clinical judgement |
| Goodacre, et al, 200240 | Prospective consecutive | 893 | 9 | 53 | 62 | CPOU | Chest pain (patients at low risk) | <25 yr, trauma, new ECG changes consistent with ischemia, comorbidity necessitating hospitalisation, definite unstable angina | AMI: WHO criteria for AMI. ACS: present or in following 6 months: AMI, cardiac death, arrhythmia or revascularisation |
| Vodopiutz, et al, 200243 | Prospective at random | 92 | 47 | 62 | 48 | CCU | Admitted because of chest pain as main symptom | Refused, too sick, language problems | AMI: angio, autopsy, scintigraphy, echocardio, ECG and enzyme kinetics UA: no |
| Svensson, et al, 200344 | Prospective consecutive | 538 | 57 | 69 | 58 | Para | Due to chest pain or discomfort >15 min, within last 6 hr, dyspnoea, or any condition suggesting acute CS | Lung disease | AMI: two of: typical symptoms, Q-waves, CK-MB >10 ng/ml or troponin >0.05 ng/ml Myocardial ischemia: dynamic changes ECG, no increase biochemical markers |
| Goodacre, et al, 200345 | Prospective consecutive | 972 | 8 | 50 | 64 | ED | ‘Undifferentiated chest pain’ all patients attending with chest pain or related complaint (low risk) | Evidence of ACS (ECG or clear clinical) requiring admission, clear non-cardiac cause no informed consent | ACS: any elevation of T (after 2 days) or after 30 days: cardiac death, non-fatal myocardial infarction, new-onset heart failure, life-threatening arrhythmia or coronary revascularisation procedure |
| Christenson, et al, 200646 | Prospective 7am–10pm | 769 | 21 | 58 | 62 | ED | Primary complaint of anterior or lateral chest pain | <25 yr, traumatic or XR-evident cause, enrolled in study 30 days previously, communication problems, no fixed address in British Columbia, without available telephone contact | AMI: one of 1) CK-MB increase definite for AMI (specific hospital criteria) or troponin I >1.0 μg/l 2) troponin I increase (<1.0) and ECG changes (ischemia), coronary angiogram >70% lesion, positive stress test or urgent revascularisation 3) ECG evolution consistent AMI 4) fibrinolytic therapy or angioplasty with clinical diagnosis of AMI 5) death with no other definite cause. UA: chest pain of 20 min at least and one of: 1) troponin I increase to 0.99 maximum and no other AMI criteria 2) dynamic ECG changes (ischemia) (ST or T), but not persistent ST elevation 3) coronary angiogram (70% lesions) and hospital admission 4) positive stress test and hospital admission |
Car = cardiologist; CCU = coronary care unit or admitted to hospital, CPOU = chest pain observation unit, ECD = electrocardiogram, ED = emergency department, Para = paramedics of an ambulance. ACS = acute coronary syndrome. AMI = acute myocardial infarction. CK = creatine kinase. CK-MB = CK isoenzyme. CS = coronary syndrome. ECG = echocardiogram. LDH = lactate dehydrogenase. UA = unstable angina. WHO = World Health Organization. XR = X-rays.
Appendix 5.
Pooled sensitivity, specificity, positive and negative likelihood ratios, odds ratios of signs and symptoms for acute myocardial infarction in patient groups.
| Acute myocardial infarction Non-selected patients | Acute myocardial infarction Selected patients | ||||||
|---|---|---|---|---|---|---|---|
| Symptom | 95% CI | I2a (%) | 95% CI | I2a (%) | |||
| Pain in left arm and/or shoulder | |||||||
| Not selected41 | Sensitivity | 33 | 25.4 to 41.8 | – | 54 | 50.2 to 56.9 | 0 |
| Selected19,28,30,33,42 | Specificity | 76.3 | 74.5 to 78.2 | – | 65 | 56.4 to 72.8 | 87 |
| LR+ | 1.42 | 1.10 to 1.83 | – | 1.49 | 1.20 to 1.85 | 71 | |
| LR− | 0.87 | 0.77 to 0.99 | – | 0.76 | 0.66 to 0.88 | 57 | |
| OR | 1.631 | 12 to 2.39 | – | 2.00 | 1.39 to 2.88 | 65 | |
| Pain in right arm and/or shoulder | |||||||
| Not selected24 | Sensitivity | 15 | 5.9 to 23.7 | – | 32 | 25.1 to 40.8 | 77 |
| Selected19,28,30,33,42 | Specificity | 95 | 92.8 to 97.0 | – | 86 | 78.4 to 91.2 | 85 |
| LR+ | 2.89 | 1.40 to 5.98 | – | 2.35 | 1.44 to 3.84 | 80 | |
| LR− | 0.90 | 0.81 to 1.00 | – | 0.81 | 0.66 to 1.00 | 96 | |
| OR | 3.22 | 1.41 to 7.36 | – | 3.09 | 1.63 to 5.85 | 80 | |
| Pain in both arms | |||||||
| Not selected (n/a) | Sensitivity | 32 | 25.1 to 40.8 | 77 | |||
| Selected19 | Specificity | 86 | 78.4 to 91.2 | 85 | |||
| LR+ | 2.35 | 1.44 to 3.84 | 80 | ||||
| LR− | 0.81 | 0.66 to 1.00 | 96 | ||||
| OR | 3.09 | 1.63 to 5.85 | 80 | ||||
| Pain in neck | |||||||
| Not selected41 | Sensitivity | 14 | 8.2 to 20.4 | – | 24 | 18.3 to 30.2 | 65 |
| Selected30,33,42 | Specificity | 90 | 89.0 to 91.6 | – | 75 | 71.6 to 77.7 | 0 |
| LR+ | 1.48 | 0.94 to 2.31 | – | 0.99 | 0.83 to 1.17 | 0 | |
| LR− | 0.95 | 0.88 to 1.02 | – | 1.00 | 0.95 to 1.07 | 0 | |
| OR | 1.55 | 0.92 to 2.61 | – | 0.98 | 0.78 to 1.23 | 0 | |
| Pain in back | |||||||
| Not selected (n/a) | Sensitivity | 25 | 22.0 to 28.2 | 0 | |||
| Selected30,33,42 | Specificity | 71 | 66.4 to 75.6 | 45 | |||
| LR+ | 0.84 | 0.62 to 1.14 | 59 | ||||
| LR− | 1.07 | 0.96 to 1.19 | 60 | ||||
| OR | 0.78 | 0.52 to 1.19 | 59 | ||||
| Epigastric pain | |||||||
| Not selected23 | Sensitivity | 10 | 3.9 to 15.3 | – | 5 | 2.1 to 10.8 | 89 |
| Selected34,36,37,39 | Specificity | 93 | 91.1 to 95.2 | – | 91 | 85.0 to 95.4 | 99 |
| LR+ | 1.44 | 0.73 to 2.83 | – | 0.73 | 0.61 to 0.87 | 0 | |
| LR− | 0.97 | 0.91 to 1.04 | – | 1.04 | 1.02 to 1.05 | 0 | |
| OR | 1.49 | 0.71 to 3.12 | – | 0.69 | 0.57 to 0.85 | 0 | |
| Oppressive pain | |||||||
| Not selected23,24,27,35,41 | Sensitivity | 60 | 53.7 to 66.0 | 77 | 77 | 71.3 to 81.2 | 0 |
| Selected28,29,31 | Specificity | 58 | 55.0 to 60.2 | 87 | 35 | 28.7 to 41.3 | 48 |
| LR+ | 1.42 | 1.32 to 1.53 | 36 | 1.79 | 1.07 to 1.30 | 0 | |
| LR− | 0.69 | 0.61 to 0.80 | 64 | 0.70 | 0.52 to 0.86 | 0 | |
| OR | 2.06 | 1.60 to 2.53 | 51 | 1.77 | 1.25 to 2.51 | 0 | |
| Vomiting and/or nausea | |||||||
| Not selected24,36,39,41 | Sensitivity | 34 | 25.3 to 44.1 | 84 | 29 | 12.5 to 51.5 | 97 |
| Selected20,22,26,28,29 | Specificity | 77 | 71.1 to 81.3 | 97 | 81 | 76.6 to 85.1 | 73 |
| LR+ | 1.41 | 1.17 to 1.72 | 64 | 1.42 | 0.76 to 2.64 | 92 | |
| LR− | 0.83 | 0.83 to 0.96 | 52 | 0.82 | 0.66 to 1.03 | 94 | |
| OR | 1.62 | 1.22 ro 2.14 | 59 | 1.73 | 0.71 to 4.12 | 93 | |
| Sweating | |||||||
| Not selected21,24,27,39,41,44 | Sensitivity | 45 | 36.0 to 54.0 | 91 | 41 | 22.9 to 60.5 | 95 |
| Selected20,22,29,31 | Specificity | 84 | 78.6 to 88.0 | 97 | 85 | 69.2 to 94.7 | 98 |
| LR+ | 2.92 | 1.97 to 4.32 | 95 | 2.44 | 1.42 to 4.20 | 81 | |
| LR− | 0.69 | 0.60 to 0.78 | 81 | 0.72 | 0.56 to 0.91 | 90 | |
| OR | 4.54 | 2.47 to 8.36 | 94 | 3.81 | 1.88 to 7.70 | 83 | |
| Absence of chest wall tenderness | |||||||
| Not selected23,24,27,35 | Sensitivity | 92 | 85.5 to 96.4 | 89 | |||
| Selected (n/a) | Specificity | 36 | 20.5 to 51.8 | 99 | |||
| LR+ | 1.47 | 1.23 to 1.75 | 97 | ||||
| LR− | 0.23 | 0.18 to 0.29 | 0 | ||||
| OR | 0.17 | 0.12 to 0.23 | 26 | ||||
I2 = 100% × (Q−df)/Q, where Q is Cochran's heterogeneity statistic and df the degrees of freedom. LR+ = positive likelihood ratio. LR− = negative likelihood ratio. OR = odds ratio.
Appendix 6.
Pooled sensitivity, specificity, positive and negative likelihood ratios, odds ratios of signs and symptoms for acute coronary syndrome in patients groups.
| Acute coronary syndrome Non-selected patients | Acute coronary syndrome Selected patients | ||||||
|---|---|---|---|---|---|---|---|
| Symptom | 95% CI | I2a (%) | 95%CI | I2a (%) | |||
| Pain in left arm and/or shoulder | |||||||
| Not selected38,45,46 | Sensitivity | 38 | 18.6 to 59.5 | 95 | |||
| Selected (n/a) | Specificity | 71 | 56.9 to 82.6 | 97 | |||
| LR+ | 1.30 | 1.13 to 1.47 | 0 | ||||
| LR− | 0.88 | 0.78 to 1.00 | 58 | ||||
| OR | 1.50 | 1.19 to 1.90 | 0 | ||||
| Pain in right arm and/or shoulder | |||||||
| Not selected45 | Sensitivity | 18 | 9.6 to 26.2 | – | 23 | 10.6 to 35.9 | – |
| Selected43 | Specificity | 95 | 93.8 to 96.1 | – | 94 | 87.2 to 100 | – |
| LR+ | 3.78 | 2.17 to 6.60 | – | 3.80 | 1.12 to 12.91 | – | |
| LR− | 0.86 | 0.77 to 0.96 | – | 0.82 | 0.98 to 0.98 | – | |
| OR | 4.40 | 2.29 to 8.48 | – | 46.5 | 1.19 to 18.20 | – | |
| Pain in neck | |||||||
| Not selected46 | Sensitivity | 35 | 27.9 to 42.4 | – | |||
| Selected (n/a) | Specificity | 76 | 72.2 to 79.1 | – | |||
| LR+ | 1.44 | 1.12 to 1.86 | – | ||||
| LR− | 0.86 | 0.76 to 0.97 | – | ||||
| OR | 1.69 | 1.16 to 2.44 | – | ||||
| Pain in back | |||||||
| Not selected38,45 | Sensitivity | 13 | 2.8 to 34.3 | 86 | 29 | 15.3 to 43.2 | – |
| Selected43 | Specificity | 76 | 26.7 to 98.6 | 98 | 49 | 35.0 to 63.0 | – |
| ACS38,43,45 | LR+ | 1.49 | 0.62 to 3.56 | 80 | 0.57 | 0.33 to 0.99 | – |
| LR− | 0.93 | 0.77 to 1.13 | 87 | 1.44 | 1.02 to 2.04 | – | |
| OR | 1.59 | 0.58 to 4.37 | 80 | 0.40 | 0.17 to 0.90 | – | |
| Epigastric pain | |||||||
| Not selected23,36,39,45 | Sensitivity | 12 | 5.4 to 20.8 | 97 | |||
| Selected (n/a) | Specificity | 89 | 82.9 to 94.1 | 98 | |||
| LR+ | 1.05 | 0.35 to 3.20 | 97 | ||||
| LR− | 0.98 | 0.88 to 1.08 | 97 | ||||
| OR | 1.08 | 0.31 to 3.74 | 97 | ||||
| Oppressive pain56 | |||||||
| Not selected23 | Sensitivity | 56 | 49.7 to 62.1 | – | 79 | 66.9 to 91.2 | – |
| Selected43 | Specificity | 67 | 61.8 to 71.7 | – | 39 | 25.1 to 52.4 | – |
| LR+ | 1.68 | 1.40 to 2.02 | – | 1.29 | 0.99 to 1.69 | – | |
| LR− | 0.66 | 0.56 to 0.77 | – | 0.54 | 0.27 to 1.06 | – | |
| OR | 2.54 | 1.82 to 3.56 | – | 2.39 | 0.94 to 6.08 | – | |
| Vomiting and/or nausea | |||||||
| Not selected25,36,38,39,44,45 | Sensitivity | 26 | 20.7 to 32.2 | 91 | |||
| Selected (n/a) | Specificity | 82 | 74.1 to 88.4 | 98 | |||
| LR+ | 1.32 | 1.09 to 1.65 | 68 | ||||
| LR− | 0.93 | 0.89 to 0.96 | 35 | ||||
| OR | 1.43 | 1.14 to 1.81 | 63 | ||||
| Sweating | |||||||
| Not selected32,38,39,44 | Sensitivity | 43 | 32.2 to 64.9 | 98 | |||
| Selected (n/a) | Specificity | 68 | 44.0 to 86.5 | 99 | |||
| LR+ | 1.34 | 1.09 to 1.65 | 76 | ||||
| LR− | 0.85 | 0.79 to 0.92 | 40 | ||||
| OR | 1.65 | 1.39 to 1.95 | 0 | ||||
| Absence of chest-wall tenderness | |||||||
| Not selected23,24 | Sensitivity | 94 | 91.4 to 96.1 | 0 | |||
| Selected (n/a) | Specificity | 33 | 19.7 to 47.9 | 96 | |||
| LR+ | 1.41 | 1.12 to 1.78 | 94 | ||||
| LR− | 0.17 | 0.11 to 0.26 | 0 | ||||
| OR | 0.12 | 7.0 to 21.0 | 34 | ||||
Note: Pain in both arms — not applicable.
I2=100% × (Q−df)/Q, where Q is Cochran's heterogeneity statistic and df the degrees of freedom. ACS = acute coronary syndrome. LR+ = positive likelihood ratio. LR− = negative likelihood ratio. OR = odds ratio.
Competing interests
The authors have stated that there are none
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Boersma E, Maas AC, Deckers JW, Simoons M. Early thrombolytic treatment in acute myocardial infarction: re-appraisal of the golden hour. Lancet. 1996;348(9030):771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 2.British Heart Foundation. British Heart Foundation Statistics Database. www.heartstats.org (accessed 3 Jan 2008)
- 3.Bartholomeeussen S, Truyers C, Buntinx F. Ziekten in de huisartspraktijk in Vlaanderen. [Diseases in General Practices in Flanders.] Leuven: Academisch Centrum voor Huisartsgeneeskunde; 2004. [Google Scholar]
- 4.Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18(6):586–589. doi: 10.1093/fampra/18.6.586. [DOI] [PubMed] [Google Scholar]
- 5.Erhardt L, Herlitz J, Bossaert L, et al. Task force on the management of chest pain. Eur Heart J. 2002;23(15):1153–1176. doi: 10.1053/euhj.2002.3194. [DOI] [PubMed] [Google Scholar]
- 6.DeVon H, Zerwic J. Symptoms of acute coronary syndromes: are there gender differences? A review of the literature. Heart Lung. 2002;31(4):235–245. doi: 10.1067/mhl.2002.126105. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Woods S, Puntillo K. Gender differences in symptoms associated with acute myocardial infarction: a review of the research. Heart Lung. 2005;34(4):240–247. doi: 10.1016/j.hrtlng.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294(20):2623–2629. doi: 10.1001/jama.294.20.2623. [DOI] [PubMed] [Google Scholar]
- 9.Panju A, Hemmelgarn B, Guyatt G, Simel D. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280(14):1256–1263. doi: 10.1001/jama.280.14.1256. [DOI] [PubMed] [Google Scholar]
- 10.Mant J, McManus R, Oakes R, et al. Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care. Health Technol Assess. 2004;8(2):iii, 1–158. doi: 10.3310/hta8020. [DOI] [PubMed] [Google Scholar]
- 11.Chun A, McGee S. Bedside diagnosis of coronary artery disease: a systematic review. Am J Med. 2004;117(5):334–343. doi: 10.1016/j.amjmed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devillé W, Buntinx F, Bouter L, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests: recommended methods. Cochrane Collaboration. 1996 http://www.cochrane.org/docs/sadtdoc1.htm (accessed 4 Jan 2008)
- 15.Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323(7305):157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. 2002;31(1):88–95. doi: 10.1093/ije/31.1.88. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Säwe U. Pain in acute myocardial infarction. A study of 137 patients in a coronary care unit. Acta Med Scand. 1971;190(1–2):79–81. [PubMed] [Google Scholar]
- 20.Säwe U. Early diagnosis of acute myocardial infarction with special reference to the diagnosis of the intermediate coronary syndrome. A clinical study. Acta Med Scand Suppl. 1972;545:1–76. [PubMed] [Google Scholar]
- 21.Van der Does E, Lubsen J, Pool J. Acute myocardial infarction: an easy diagnosis in general practice? J R Coll Gen Pract. 1980;30(216):405–409. [PMC free article] [PubMed] [Google Scholar]
- 22.Short D. Diagnosis of slight and subacute coronary attacks in the community. Br Heart J. 1981;45(3):299–310. doi: 10.1136/hrt.45.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee T, Cook F, Weisberg M, et al. Acute chest pain in the emergency room. Identification and examination of low-risk patients. Arch Intern Med. 1985;145(1):65–69. [PubMed] [Google Scholar]
- 24.Tierney WM, Fitzgerald J, McHenry R, et al. Physicians' estimates of the probability of myocardial infarction in emergency room patients with chest pain. Med Decis Making. 1986;6(1):12–17. doi: 10.1177/0272989X8600600103. [DOI] [PubMed] [Google Scholar]
- 25.Hargarten KM, Aprahamian C, Stueven H, et al. Limitations of prehospital predictors of acute myocardial infarction and unstable angina. Ann Emerg Med. 1987;16(12):1325–1329. doi: 10.1016/s0196-0644(87)80412-2. [DOI] [PubMed] [Google Scholar]
- 26.Herlihy T, McIvor M, Cummings CC, et al. Nausea and vomiting during acute myocardial infarction and its relation to infarct size and location. Am J Cardiol. 1987;60(1):20–22. doi: 10.1016/0002-9149(87)90976-3. [DOI] [PubMed] [Google Scholar]
- 27.Solomon CG, Lee TH, Cook EF, et al. Comparison of clinical presentation of acute myocardial infarction in patients older than 65 years of age to younger patients: the Multicenter Chest Pain Study experience. Am J Cardiol. 1989;63(12):772–776. doi: 10.1016/0002-9149(89)90040-4. [DOI] [PubMed] [Google Scholar]
- 28.Berger JP, Buclin T, Haller E, et al. Right arm involvement and pain extension can help to differentiate coronary diseases from chest pain of other origin: a prospective emergency ward study of 278 consecutive patients admitted for chest pain. J Intern Med. 1990;227(3):165–172. doi: 10.1111/j.1365-2796.1990.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 29.Jonsbu J, Rollag A, Aase O, et al. Rapid and correct diagnosis of myocardial infarction: standardized case history and clinical examination provide important information for correct referral to monitored beds. J Intern Med. 1991;229(2):143–149. doi: 10.1111/j.1365-2796.1991.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 30.Gaston-Johansson F, Hofgren C, Watson P, Herlitz J. Myocardial infarction pain: systematic description an analysis. Intensive Care Nurs. 1991;7(1):3–10. doi: 10.1016/0266-612x(91)90028-p. [DOI] [PubMed] [Google Scholar]
- 31.Hartford M, Karlson B, Sjölin M, et al. Symptoms, thoughts, and environmental factors in suspected acute myocardial infarction. Heart Lung. 1993;22(1):64–70. [PubMed] [Google Scholar]
- 32.Grijseels EW, Deckers JW, Hoes AW, et al. Pre-hospital triage of patients with suspected myocardial infarction: evaluation of previously developed algorithms and new proposals. Eur Heart J. 1995;16(3):325–332. doi: 10.1093/oxfordjournals.eurheartj.a060914. [DOI] [PubMed] [Google Scholar]
- 33.Everts B, Karlson B, Wahrborg P, et al. Localization of pain in suspected acute myocardial infarction in relation to final diagnosis, age and sex, and site and type of infarction. Heart Lung. 1996;25(6):430–437. doi: 10.1016/s0147-9563(96)80043-4. [DOI] [PubMed] [Google Scholar]
- 34.Pfister R, Gaillet R, Saner H, et al. Die Vorspitalphase von Patienten mit Verdacht auf akuten Myokardinfarkt: Ergebnisse der ‘Oltner Herznotfallstudie’. [The prehospital phase of patients with suspected acute myocardial infarct: results of the Oltner Cardiac Emergency Study] Schweiz Med Wochenschr. 1997;127(12):479–488. [PubMed] [Google Scholar]
- 35.Lopez-Jiminez F, Goldman L, Johnson PA, et al. Effect of diabetes mellitus on the presentation and triage of patients with acute chest pain without known coronary artery disease. Am J Med. 1998;105(6):500–505. doi: 10.1016/s0002-9343(98)00327-1. [DOI] [PubMed] [Google Scholar]
- 36.Pope JH, Ruthazer R, Beshansky JR, et al. Clinical features of emergency department patients presenting with symptoms suggestive of acute cardiac ischemia: a multicenter study. J Thromb Thrombolysis. 1998;6(1):63–74. doi: 10.1023/A:1008876322599. [DOI] [PubMed] [Google Scholar]
- 37.Graff L, Palmer AC, LaMonica P, Wolf S. Triage of patients for a rapid (5-minute) electrocardiogram: a rule based on presenting chief complaints. Ann Emerg Med. 2000;36(6):554–560. doi: 10.1067/mem.2000.111057. [DOI] [PubMed] [Google Scholar]
- 38.Milner KA, Funk M, Richards S, et al. Symptom predictors syndromes in younger and older patients. Nurs Res. 2001;50(4):233–241. doi: 10.1097/00006199-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Herlitz J, Starke M, Hansson E, et al. Early identification of patients with an acute coronary syndrome as assessed by dispatchers and the ambulance crew. Am J Emerg Med. 2002;20(3):196–201. doi: 10.1053/ajem.2002.33003. [DOI] [PubMed] [Google Scholar]
- 40.Goodacre S, Locker T, Morris F, Campbell S. How useful are clinical features in the diagnosis of acute, undifferentiated chest pain? Acad Emerg Med. 2002;9(3):203–208. doi: 10.1111/j.1553-2712.2002.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 41.Baxt WG, Shofer FS, Sites FD, Hollander JE. A neural computational aid to the diagnosis of acute myocardial infarction. Ann Emerg Med. 2002;39(4):366–373. doi: 10.1067/mem.2002.122705. [DOI] [PubMed] [Google Scholar]
- 42.Albarran J, Durham B, Gowers J, et al. Is the radiation of chest pain a useful indicator of myocardial infarction? A prospective study of 541 patients. Accid Emerg Nurs. 2002;10(1):2–9. doi: 10.1054/aaen.2001.0304. [DOI] [PubMed] [Google Scholar]
- 43.Vodopiutz J, Poller S, Schneider B, et al. Chest pain in hospitalized patients: cause-specific and gender specific differences. J Women Health (Larchmt) 2002;11(8):719–727. doi: 10.1089/15409990260363670. [DOI] [PubMed] [Google Scholar]
- 44.Svensson L, Isaksson L, Axelsson C, et al. Predictors of myocardial damage prior to hospital admission among patients with acute chest pain or other symptoms raising a suspicion of acute coronary syndrome. Coron Artery Dis. 2003;14(3):225–231. doi: 10.1097/01.mca.0000063503.13456.0d. [DOI] [PubMed] [Google Scholar]
- 45.Goodacre SW, Angelini K, Arnold J, et al. Clinical predictors of acute coronary syndromes in patients with undifferentiated chest pain. Q J Med. 2003;96(12):893–898. doi: 10.1093/qjmed/hcg152. [DOI] [PubMed] [Google Scholar]
- 46.Christenson J, Innes G, McKnight D, et al. A clinical rule for early discharge of patients with chest pain. Ann Emerg Med. 2006;47(1):1–10. doi: 10.1016/j.annemergmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Reid MC, Lachs MS, Feinstein AR. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995;274(8):645–651. [PubMed] [Google Scholar]
- 48.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282(11):1061–1066. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 49.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326(7379):41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined — a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 51.Newby LK, Alpert JS, Ohman EM, Thygesen K, Califf RM. Changing the diagnosis of acute myocardial infarction: implications for practice and clinical investigations. Am Heart J. 2002;144(6):957–980. doi: 10.1067/mhj.2002.129778. [DOI] [PubMed] [Google Scholar]
- 52.Sanchis J, Bodí V, Nunez J, et al. New risk score for patients with acute chest pain, non-ST-segment deviation, and normal troponin concentrations: a comparison with the TIMI risk score. J Am Coll Cardiol. 2005;46(3):443–449. doi: 10.1016/j.jacc.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 53.Buntinx F, Truyen J, Embrechts P, et al. Evaluating patients with chest pain using classification and regression trees. Fam Pract. 1992;9(2):149–153. doi: 10.1093/fampra/9.2.149. [DOI] [PubMed] [Google Scholar]
- 54.Marshall RJ. The use of classification and regression trees in clinical epidemiology. J Clin Epidemiol. 2001;54(6):603–609. doi: 10.1016/s0895-4356(00)00344-9. [DOI] [PubMed] [Google Scholar]
- 55.Van den Bruel A, Aertgeerts B, Bruyninckx R, et al. Signs and symptoms for diagnosis of serious infections in children: a prospective study in primary care. Br J Gen Pract. 2007;57(540):538–546. [PMC free article] [PubMed] [Google Scholar]
- 56.Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]