Abstract
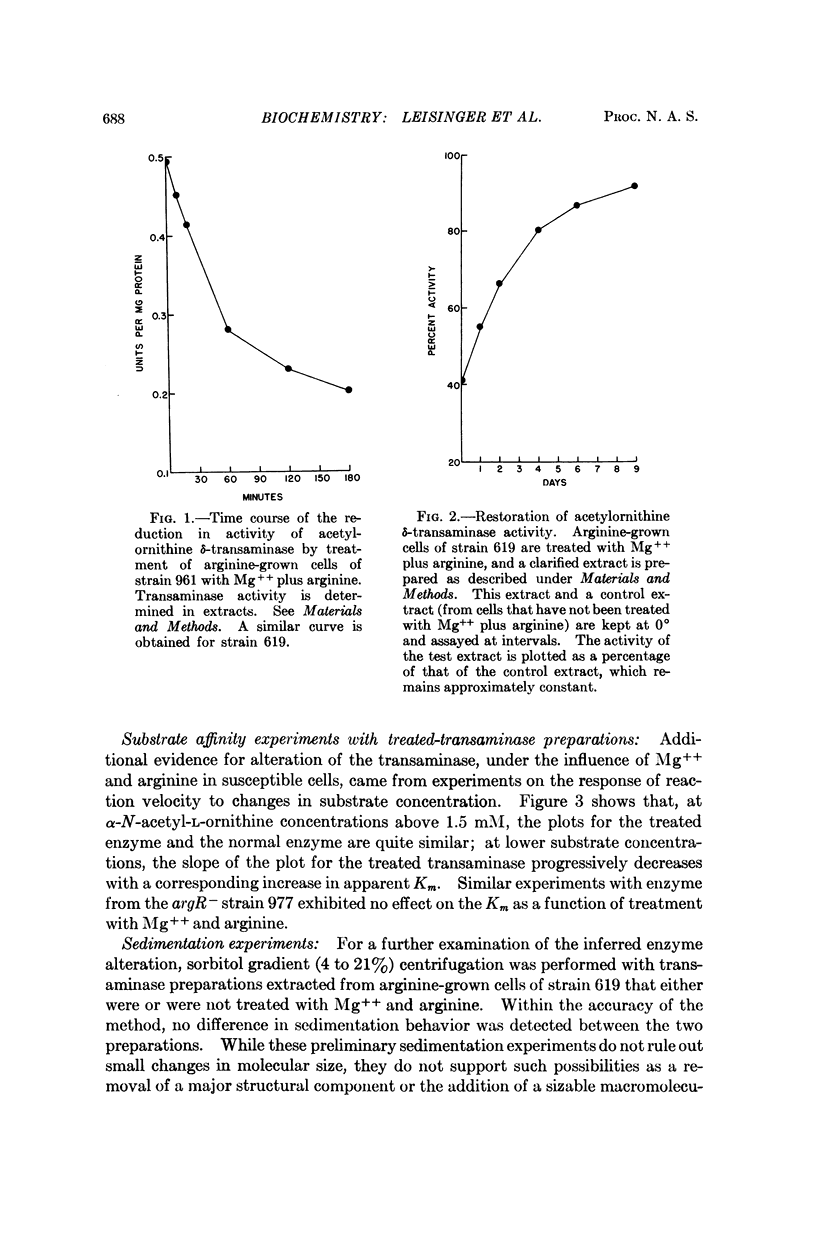
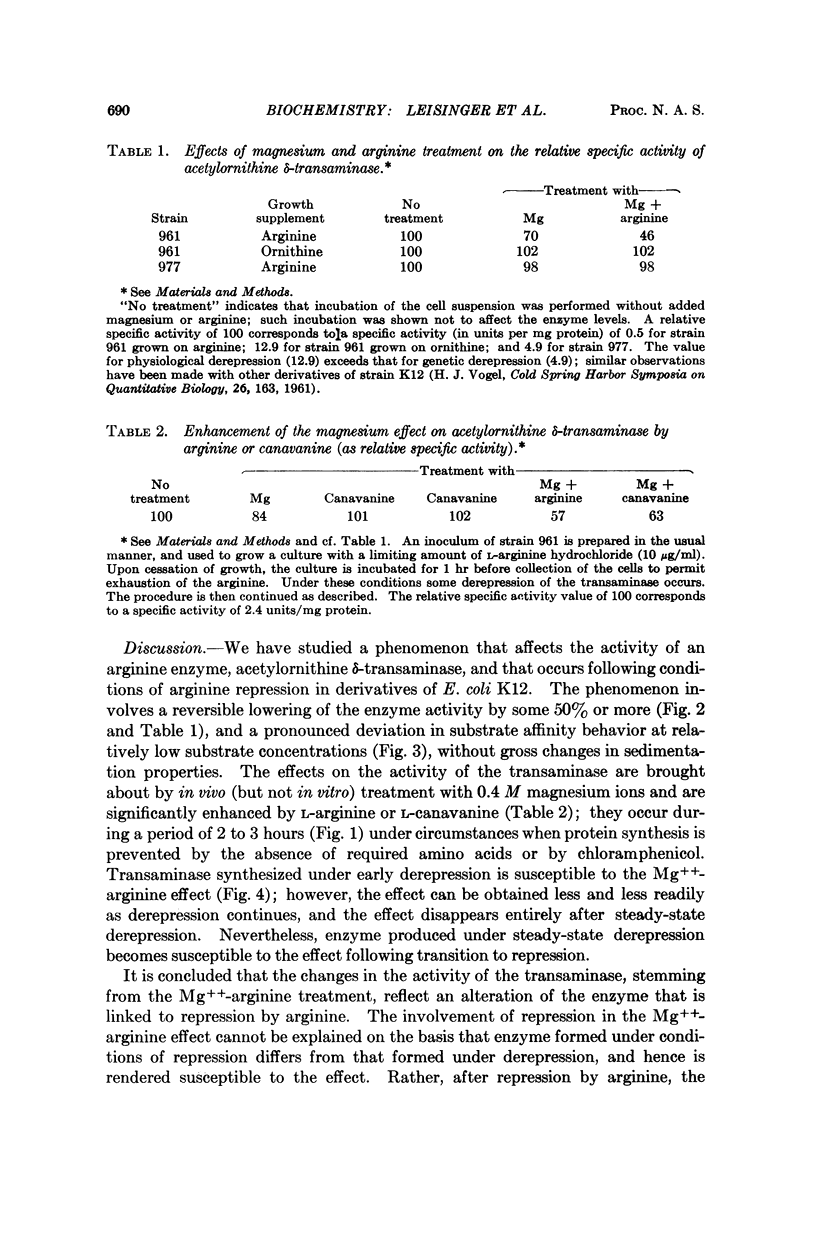
Treatment of susceptible Escherichia coli K12 derivatives with 0.4 M Mg++ at 37°, potentiated by L-arginine or L-canavanine, leads to alteration of acetylornithine δ-transaminase. The alteration, obtained in the absence of protein synthesis and reversible at 0 or 37°, is manifested in extracts by lowered activity and modified substrate affinity behavior of the enzyme without gross changes in sedimentation properties. Cells grown under arginine repression are susceptible to the treatment; cells grown under genetic or steady-state physiological derepression are not. Transaminase synthesized during early derepression can be altered, although to progressively diminishing extents. Enzyme formed under steady-state derepression becomes alterable following transition to repression. The Mg++ -dependent alteration can be thought to arise while the enzyme, arginine (or canavanine), and aporepressor are in contact, and to reflect a physiological process such as the participation of the enzyme in the repressive complex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Baumberg S., Bacon D. F., Vogel H. J. Individually repressible enzymes specified by clustered genes of arginine synthesis. Proc Natl Acad Sci U S A. 1965 May;53(5):1029–1032. doi: 10.1073/pnas.53.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Cline A. L., Bock R. M. Translational control of gene expression. Cold Spring Harb Symp Quant Biol. 1966;31:321–333. doi: 10.1101/sqb.1966.031.01.042. [DOI] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Roles of arginine and canavanine in the synthesis and repression of ornithine transcarbamylase by Escherichia coli. J Bacteriol. 1968 Aug;96(2):409–420. doi: 10.1128/jb.96.2.409-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leisinger T., Vogel H. J. Repression by arginine in Escherichia coli: a comparison of arginyl transfer RNA profiles. Biochim Biophys Acta. 1969 Jun 17;182(2):572–574. doi: 10.1016/0005-2787(69)90212-3. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]



