Abstract
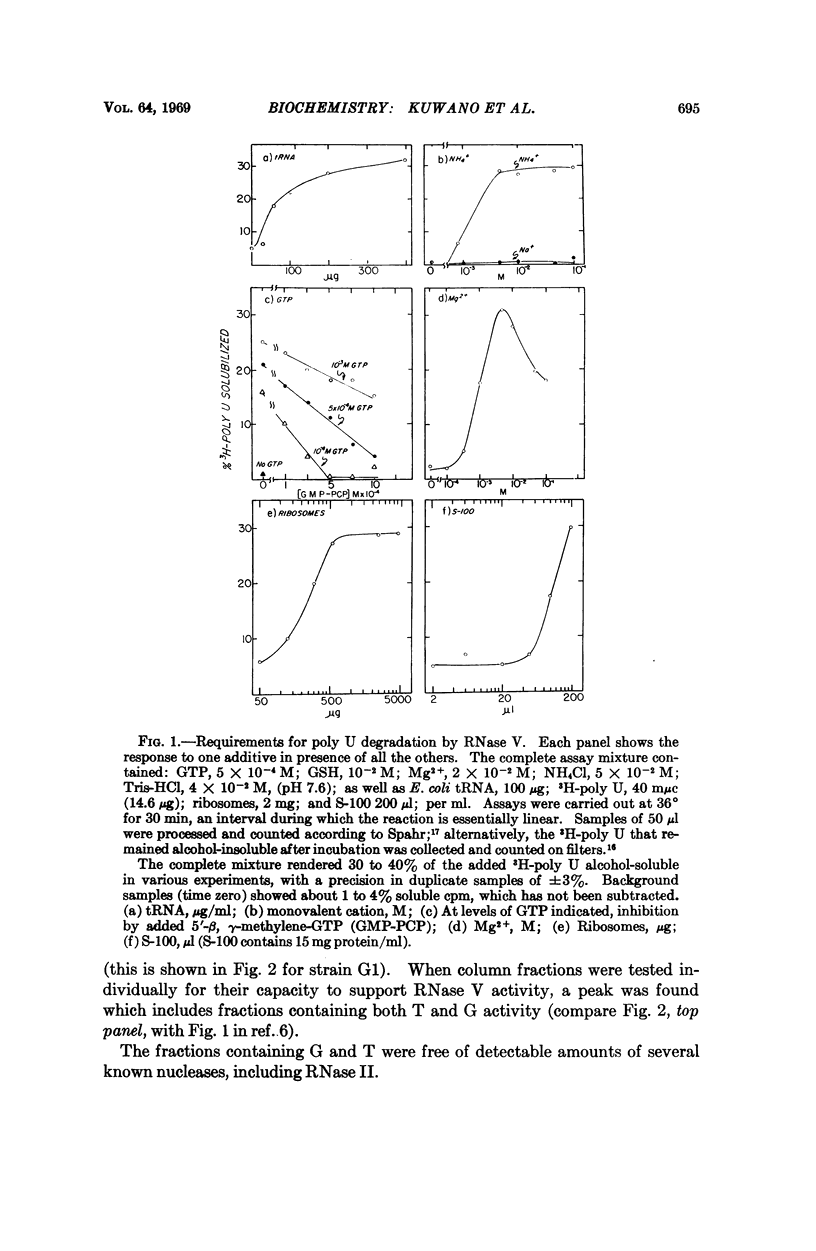
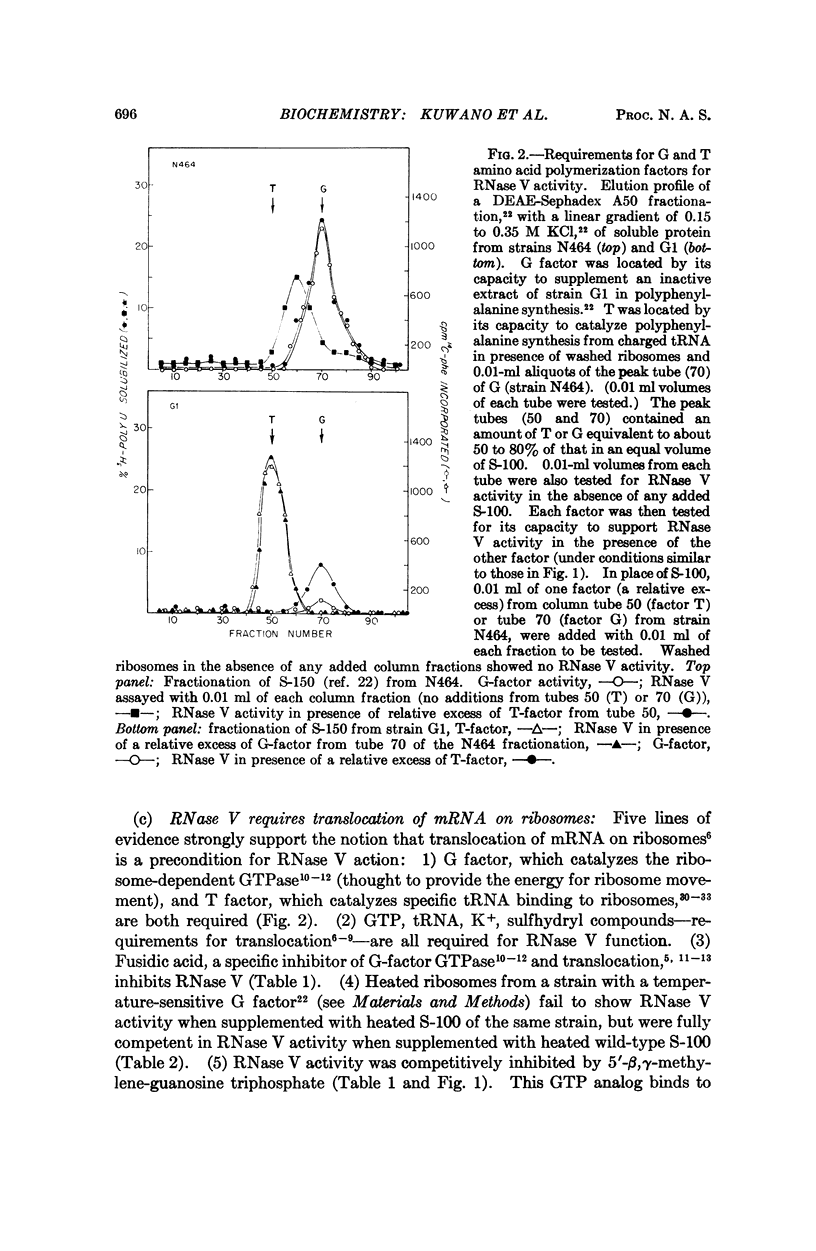
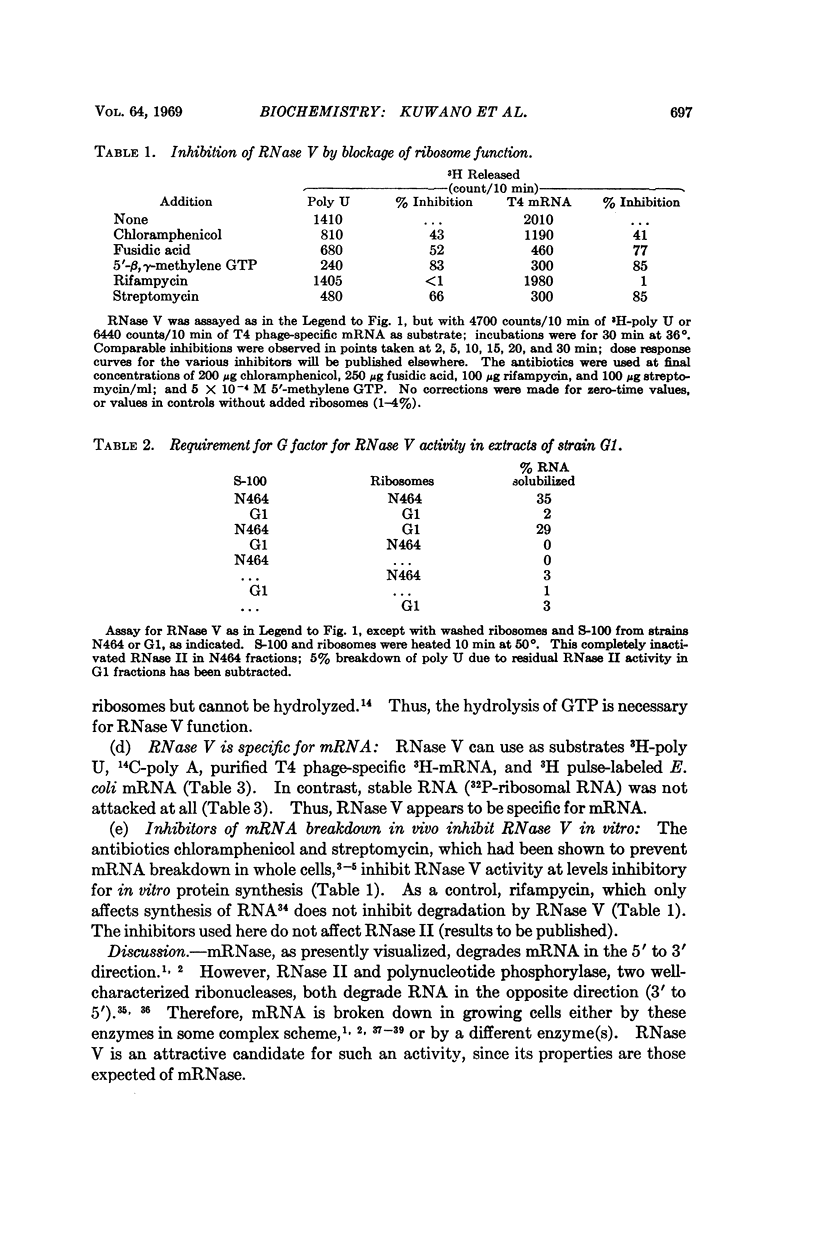
A new RNase activity, tentatively named RNase V, was found in cell-free extracts of E. coli. This activity requires ribosomes, G and T factors, tRNA, K+ or NH4+, Mg2+, GTP, and a sulfhydryl compound to degrade poly U, poly A, T4 phage mRNA, or E. coli mRNA. RNase V is specific for mRNA; it does not attack ribosomal RNA. It is inhibited by antibiotics that decrease breakdown of mRNA in vivo, such as chloramphenicol and streptomycin, and by such agents as 5′-β, γ-methylene-guanosine triphosphate, and fusidic acid, which inhibit ribosome-dependent GTPase and translocation of ribosomes along mRNA. The evidence suggests that RNase V is either an integral part of the ribosome or is tightly associated with it, and that it selectively degrades mRNA in intact cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY T. W. ON THE ROLE OF AMMONIUM OR POTASSIUM ION IN AMINO ACID POLYMERIZATION. Proc Natl Acad Sci U S A. 1964 Jun;51:1216–1220. doi: 10.1073/pnas.51.6.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles J. J., Singer M. F. Degradation of polyuridylic acid by ribonuclease II: protection by ribosomes. J Mol Biol. 1969 Feb 28;40(1):1–17. doi: 10.1016/0022-2836(69)90292-7. [DOI] [PubMed] [Google Scholar]
- Castles J. J., Singer M. F. Some properties of the polynucleotide phosphorylase and ribonuclease II of Escherichia coli 1113B. Biochem Biophys Res Commun. 1968 Aug 21;32(4):715–722. doi: 10.1016/0006-291x(68)90298-2. [DOI] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- Erbe R. W., Nau M. M., Leder P. Translation and translocation of defined RNA messengers. J Mol Biol. 1969 Feb 14;39(3):441–460. doi: 10.1016/0022-2836(69)90137-5. [DOI] [PubMed] [Google Scholar]
- Ertel R., Brot N., Redfield B., Allende J. E., Weissbach H. Binding of guanosine 5'-triphosphate by soluble factors required for polypeptide synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):861–868. doi: 10.1073/pnas.59.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNBERG-MANAGO M., ORTIZ P. J., OCHOA S. Enzymic synthesis of polynucleotides. I. Polynucleotide phosphorylase of azotobacter vinelandii. Biochim Biophys Acta. 1956 Apr;20(1):269–285. doi: 10.1016/0006-3002(56)90286-4. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Gordon J. A stepwise reaction yielding a complex between a supernatant fraction from E. coli, guanosine 5'-triphosphate, and aminoacyl-sRNA. Proc Natl Acad Sci U S A. 1968 Jan;59(1):179–183. doi: 10.1073/pnas.59.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo C., Apirion D., Schlessinger D. Effects of chloramphenicol and fusidic acid on polyribosome metabolism in escherichia coli. FEBS Lett. 1969 Apr;3(1):34–36. doi: 10.1016/0014-5793(69)80089-x. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Hershey J. W., Monro R. E. A competitive inhibitor of the GTP reaction in protein synthesis. J Mol Biol. 1966 Jun;18(1):68–76. doi: 10.1016/s0022-2836(66)80077-3. [DOI] [PubMed] [Google Scholar]
- Ibuki F., Gasior E., Moldave K. The interaction of aminoacyl soluble ribonucleic acid and aminoacyl transferase I. J Biol Chem. 1966 May 25;241(10):2188–2193. [PubMed] [Google Scholar]
- Ito J., Imamoto F. Sequential derepression and repression of the tryptophan operon in E. coli. Nature. 1968 Nov 2;220(5166):441–444. doi: 10.1038/220441a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Kawano G., Tanaka N. Association of fusidic acid sensitivity with G factor in a protein-synthesizing system. Biochem Biophys Res Commun. 1968 Dec 9;33(5):769–773. doi: 10.1016/0006-291x(68)90226-x. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Singer M. F. The processive degradation of individual polyribonucleotide chains. II. Micrococcus lysodeikticus polynucleotide phosphorylase. J Biol Chem. 1968 Mar 10;243(5):923–927. [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Apirion D., Schlessinger D. Polyribosome depletion and blockage of the ribosome cycle by streptomycin in Escherichia coli. J Mol Biol. 1969 Jun 14;42(2):315–335. doi: 10.1016/0022-2836(69)90046-1. [DOI] [PubMed] [Google Scholar]
- MATTHAEI J. H., NIRENBERG M. W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E., Traut R. R., Monro R. E. Ribosome-catalysed peptidyl transfer: the polyphenylalanine system. J Mol Biol. 1968 Jul 28;35(2):333–345. doi: 10.1016/s0022-2836(68)80028-2. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Imamoto F. Degradation of tryptophan messenger. On the degradation of messenger RNA for the tryptophan operon in Escherichia coli. Nature. 1969 Jul 5;223(5201):37–40. doi: 10.1038/223037a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Baker R. F., Yanofsky C. Translation of the tryptophan messenger RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1428–1435. doi: 10.1073/pnas.60.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R., Baker R. F., Yanofsky C. Direction of in vivo degradation of tryptophan messenger RNA--a correction. Nature. 1969 Jul 5;223(5201):40–43. doi: 10.1038/223040a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. VI. Oligopeptide synthesis and translocation on ribosomes in the presence and absence of soluble transfer factors. J Biol Chem. 1969 Mar 25;244(6):1533–1539. [PubMed] [Google Scholar]
- Ravel J. M., Shorey R. L., Shive W. Evidence for a guanine nucleotide-aminoacyl-RNA complex as an intermediate in the enzymatic transfer of aminoacyl-RNA to ribosomes. Biochem Biophys Res Commun. 1967 Oct 11;29(1):68–73. doi: 10.1016/0006-291x(67)90542-6. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Characterization of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1437–1443. doi: 10.1128/jb.97.3.1437-1443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- SCHLESSINGER D. REQUIREMENT FOR K+ AND ATP IN PROTEIN SYNTHESIS BY ESCHERICHIA COLI RIBOSOMES. Biochim Biophys Acta. 1964 Mar 23;80:473–477. doi: 10.1016/0926-6550(64)90150-1. [DOI] [PubMed] [Google Scholar]
- SPAHR P. F. PURIFICATION AND PROPERTIES OF RIBONUCLEASE II FROM ESCHERICHIA COLI. J Biol Chem. 1964 Nov;239:3716–3726. [PubMed] [Google Scholar]
- Singer M. F., Tolbert G. Purification and properties of a potassium-activated phosphodiesterase (RNAase II) from Escherichia coli. Biochemistry. 1965 Jul;4(7):1319–1330. doi: 10.1021/bi00883a016. [DOI] [PubMed] [Google Scholar]
- Spahr P. F., Gesteland R. F. Specific cleavage of bacteriophage R17 RNA by an endonuclease isolated from E. coli MRE-600. Proc Natl Acad Sci U S A. 1968 Mar;59(3):876–883. doi: 10.1073/pnas.59.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A., Niyogi S. K. Hydrolysis of oligoribonucleotides by an enzyme fraction from Escherichia coli. Biochem Biophys Res Commun. 1967 Nov 30;29(4):550–555. doi: 10.1016/0006-291x(67)90520-7. [DOI] [PubMed] [Google Scholar]
- Tissieres A., Schlessinger D., Gros F. AMINO ACID INCORPORATION INTO PROTEINS BY ESCHERICHIA COLI RIBOSOMES. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1450–1463. doi: 10.1073/pnas.46.11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Mattoccia E. A mutant of E. coli with an altered supernatant factor. Proc Natl Acad Sci U S A. 1968 Sep;61(1):146–151. doi: 10.1073/pnas.61.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. Distinct cistrons for the two ribosomal RNA components. Proc Natl Acad Sci U S A. 1963 Apr;49:538–544. doi: 10.1073/pnas.49.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]



