Abstract
We studied the regulation of nucleoside transporters in intestinal epithelial cells upon exposure to either differentiating or proliferative agents. Rat intestinal epithelial cells (line IEC-6) were incubated in the presence of differentiating (glucocorticoids) or proliferative (EGF and TGF-α) agents. Nucleoside uptake rates and nucleoside transporter protein and mRNA levels were assessed. The signal transduction pathways used by the proliferative stimuli were analyzed. We found that glucocorticoids induce an increase in sodium-dependent, concentrative nucleoside transport rates and in protein and mRNA levels of both rCNT2 and rCNT1, with negligible effects on the equilibrative transporters. EGF and TGF-α induce an increase in the equilibrative transport rate, mostly accounted for by an increase in rENT1 activity and mRNA levels, rENT2 mRNA levels remaining unaltered. This effect is mimicked by another proliferative stimulus that functions as an in vitro model of epithelial wounding. Here, rENT1 activity and mRNA levels are also increased, although the signal transduction pathways used by the two stimuli are different. We concluded that differentiation of rat intestinal epithelial cells is accompanied by increased mature enterocyte features, such as concentrative nucleoside transport (located at the brush border membrane of the enterocyte), thus preparing the cell for its ultimate absorptive function. A proliferative stimulus induces the equilibrative nucleoside activities (mostly through ENT1) known to be located at the basolateral membrane, allowing the uptake of nucleosides from the bloodstream for the increased demands of the proliferating cell.
Keywords: drug bioavailability, cell differentiation, adenosine, concentrative transport, equilibrative transport
INTRODUCTION
Plasma membrane transporters play a crucial role in the function of absorptive epithelia, since they mediate intestinal absorption and renal reabsorption and the secretion of numerous physiological and pharmacological compounds. Among these transport systems, nucleoside transporters are responsible for the uptake of natural nucleosides and most nucleoside-derived drugs (Pastor-Angalda et al., 1998; Casado et al., 2002).
There are two main families of nucleoside transporters, concentrative (CNT) and equilibrative (ENT) transporters (Griffith and Jarvis, 1996; Ritzel et al., 1998; Pastor-Angalda and Baldwin, 2001). Concentrative nucleoside transport activities have been identified in intestinal epithelial cells in several species, including mouse (Vijayalakshmi and Belt, 1988), rat (Iseki et al., 1996), human (Chandrasena et al., 1997; Patil and Unadkat, 1997), and pig (Scharrer et al., 2002). These activities, at least in humans, seem to follow a proximal-to-distal gradient, with maximal transport rates in the jejunum (Ngo et al., 2001). Alongside this activity, the mRNA and protein of the pyrimidine-preferring CNT1 tansporter (Huang et al., 1994) and the purine-preferring CNT2 transporter (Ritzel et al., 1998; Valdés et al., 2000) have also been detected in the small intestine. In the kidney, the pattern of nucleoside transporter expression appears to be more complex, since CNT2 has been detected in human kidney (Ward and Tse, 1999), but its activity is not always measurable (Gutierrez et al., 1992); its protein has not been detected in crude homogenates from rat kidney (Valdés et al., 2000). Additionally, a novel concentrative nucleoside transporter activity, termed N4, has been described in human kidney, but still remains to be identified at the molecular level (Gutierrez and Giacomini, 1993).
The presence of equilibrative transporters (both ENT1 and ENT2) in absortive epithelial cells suggests the possibility of a transepithelial, vectorial transfer of nucleosides (Williams et al., 1989; Mangravite et al., 2003a). This hypothesis is controversial, however, since the intracellular metabolism of nucleosides appears too fast to allow such a flow from the apical to the basal membrane. Concentrative transporters seem to be restricted to the apical membrane of polarized epithelial cells (Patil and Unadkat, 1997; Ward and Tse, 1999), but there remains some controversy about the localization of equilibrative transporters (Trimble and Coulson, 1984; Doherty and Jarvis, 1993; Williams et al., 1989). The use of fluorescently tagged nucleoside transporters has not produced unequivocal results, since hCNT1 seems restricted to the apical membrane (Lai et al., 2002) and hENT2 to the basolateral domain (Mangravite et al., 2003b); hENT1 was described in the former report as being localized in the basolateral membrane and in the latter as being in both the apical and the basolateral membranes.
To date, the regulation of nucleoside transporters in epithelia has not been studied in depth. Nucleoside transport is sensitive to substrate availability in the small intestine. rCNT1 protein levels and activity are increased during fasting (Valdés et al., 2000), and this regulation is exerted, at least partially, by nucleosides, since the administration of a semipurified, nucleoside-free diet induced an increase in jejunal CNT1 expression and activity in rats (Valdés et al., 2000). The high sensitivity of CNT1 to nutrient availability may have clinical relevance, since it is involved in the uptake of most fluoropyrimidines used in cancer treatment (Pastor-Angalda et al., 1998; Baldwin et al., 1999), and gemcitabine uptake is enhanced in brush border vesicles from fasted rats (Valdés et al., 2000), although this drug is not administered orally. Endocrine regulation of these transporters in intestinal cells has only been reported once, where it was shown that glucocorticoids, insulin, and epidermal growth factor increase CNT2 activity in the rat small intestine–derived cell line IEC-6 (Jakobs et al., 1990).
In the present study, we have fully characterized the expression pattern of nucleoside transporters in IEC-6 cells. We have also shown that glucocorticoids, well-known differentiation agents for intestinal cells, increase CNT2 expression and activity, while epidermal growth factor (EGF) and transforming growth factor (TGF) α, potent mitogens for enterocytes, do not affect concentrative transport but instead induce the expression and activity of ENT1, an effect mimicked by another enterocyte proliferative stimulus such as epithelial wounding. These results may be important for the improvement of nucleoside-derived drug delivery in anti-tumoral and anti-viral therapies.
MATERIALS AND METHODS
Materials
Cell culture reagents were purchased from Invitrogen. PD98059 was obtained from Calbiochem. SP600125 was from Biotrend. Antibodies against total or phosphorylated forms of extracellularly-regulated kinase (ERK) 1/2 were purchased from Promega. 3H-Uridine and 3H-guanosine were from Amersham Biosciences while 3H-cytidine was purchased from Nucliber. Dexamethasone, EGF, and TGF-α were from Sigma-Aldrich. All other reagents were of analytical grade.
Cell Culture and Treatments
IEC-6 cells (derived from crypt cells of the rat small intestine) were cultured in DMEM supplemented with 5% (by volume) FCS (or 0.2% BSA in some treatments), 1 mM sodium pyruvate, 4 mM glutamine, 0.1 U/ml bovine insulin, and a mixture of antibiotics and antimicotics. For dexamethasone treatment, cells were kept overnight in a serum-free, BSA-containing medium, and then dexamethasone was added at a final concentration of 100 nM for 24–48 h. Transport of 1 μM guanosine was then measured and protein and RNA extracts were obtained. Protein extracts were prepared in a lysis buffer containing 0.3 M sucrose, 25 mM imidazole (pH 7.2), 1 mM EDTA, aprotinin (2 μg/ml), leupeptin (10 μM), and AEBSF (1 mM). A 30-μg aliquot was used for analysis of rCNT2 protein by Western blot using a monospecific polyclonal rabbit antibody characterized elsewhere (Valdés et al., 2000). RNA extracts taken at different time points were prepared with the SV Total Isolation System (Promega) according to the manufacturer's instructions.
For EGF and TGF-α treatments, cells were also serum deprived overnight and then either EGF (final concentration 20 ng/ml) or TGF-α (10 ng/ml) was added. In some experiments, the ERK1/2 inhibitor PD98059 or the JNK1/2/3 inhibitor SP600125 was added at a final concentration of 10 μM and 20 μM, respectively, 30 min before growth factor addition. The epithelial wounding model (McCormack et al., 1992; Ciacci et al., 1993) consisted of a series of parallel cuts performed with a razor blade in the monolayer of cells (50–70 cuts per 2 cm2 dish), which had been deprived of serum for 12 h; PD98059 was also added to some dishes under the same conditions as stated before.
Measurement of Transport Activity
Nucleoside transport activity was measured at a substrate concentration of 1 μM for all nucleosides and a specific activity of 30 Ci/mmol (for uridine), 15 Ci/mmol (for guanosine), or 21.5 Ci/mmol (for cytidine), in the presence of either 137 mM sodium chloride or 137 mM choline chloride, as described elsewhere (del Santo et al., 1998). Uridine was used as a common permeant for CNTs and ENTs (ENT1 and ENT2 activities were discriminated using 1 μM nitrobenzyl-thioinosine [NBTI]), and, since CNT3 was shown to be absent, sodium-dependent guanosine transport could be used as a CNT2-specific measure and sodium-dependent cytidine transport as a CNT1-specific measure. The uptake medium also contained 5.4 mM KCl, 108 mM CaCl2, 1.2 mM MgSO4, and 10 mM HEPES, (pH 7.4). Transport measurement was stopped after 1 min incubation by washing the cells twice with 2 ml of a cold buffer containing 137 mM NaCl and 10 mM Tris-HEPES, pH 7.4. Cells were then dissolved in 100 ml of 100 mM NaOH, 0.5% Triton X-100, and aliquots were taken for protein determination (Bradford assay; BioRad Laboratories) and for radioactivity measurements.
Reverse Transcription and RT-PCR
1 μg of total RNA was used for cDNA synthesis using the TaqMan Reverse Transcription System (Applied Biosystems), according to the manufacturer's instructions. Oligonucleotide primer sequences for RT-PCR were as follows: CNT1 forward 702–725 (tttgcaggcatctgtgtgttcct) and reverse 1272–1295 (caacgcacaaggggcggccatgac); CNT2 forward 776–799 (gctcaaaggccagagcagctgatc) and reverse 1442–1445 (cagcttcactccctccttgctctt); ENT1 forward 5–25 (atgacaaccagtcacc) and reverse 1352–1375 (tccttcttgttaagggcacttgtg); ENT2 forward 356–376 (aacaactgggtgacactgctgt) and reverse 1379–1400 (ggctacttcgtgtctctcacca). RT-PCR was performed in a Thermocycler (Applied Biosystems), and the polymerase used was TaqDNA polymerase (Promega).
Real-time RT-PCR
The primers and probes used to amplify cDNA by real-time RT-PCR were designed using Primer Express software and the sequences employed were as follows: rGAPDH forward 30–50 (gatggtgaaggtcggtgtcaa), reverse 115–91 (caaatgtccacttgtcacaagagaa), probe 70–89 (ccgcctggtcaccagggctgc); rCNT2 forward 785–803 (tgcgggaatctgcatgtt), reverse 854–836 (ctccagctcaccgcactgt), probe 805–831 (atcctcatcctctttgcctgctccaaa); rENT1 forward 765–785 (gtgaaggagaggagccaagag), reverse 836–817 (tgttggcgggtagagagagagttg), probe 807–789 (accccagattcctctcttc); and rENT2 forward 923–943 (ggcccaactctggatcttgac), reverse 990–979 (cctggcttctgtggttcct), probe 968–945 (ctccagctccttctctg). Real-time monitoring of PCR amplification of cDNAs was performed with the TaqMan Universal Master Mix (Applied Biosystems) using 150 nM probe and 700 nM each primer, in the ABI Prism 7700 Sequence Detection System (Applied Biosystems). The relative quantification of gene expression was performed according to the manufacturer's instructions using rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The threshold cycle (CT) is defined as the cycle number at which the fluorescence corresponding to the amplified PCR product is first detected. The results are given as PCR arbitrary units of each transporter after normalizing the mRNA levels of these genes to the GAPDH expression levels.
Analysis of ERK1/2 Phosphorylation
IEC-6 cells were incubated under the various conditions tested and then treated with Triton lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 100 mM NaF, 10 mM EDTA, 10 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 2 μg/ml aprotinin, 10 μM leupeptin, and 1 mM AEBSF) at selected times for the first 30 min after treatment. Extracts were then centrifuged for 5 min at 12,000 rpm, their protein contents measured, and 15 μg or 40 μg of the supernatants used for analysis of total or active ERK1/2, respectively, by Western blot.
RESULTS
Characterization of Nucleoside Transport in IEC-6 Cells
The pattern of nucleoside transporters present in confluent monolayers of IEC-6 cells was studied in different ways. 3H-Uridine was used as a common permeant for the main nucleoside transport systems and its uptake was inhibited by adding nonradioactive nucleosides to the transport medium (Fig. 1 A). As deduced from these results, IEC-6 cells exhibit both sodium-dependent and sodium-independent nucleoside transport activities that are compatible with the coexistence of at least three different transport systems: a purine, sodium-dependent activity and both NBTI-sensitive and NBTI-resistant systems. This functional evidence was confirmed by studying the expression pattern of nucleoside transporters in IEC-6 cells. CNT2, ENT1, and ENT2 were easily detected by RT-PCR (Fig. 1 B), while CNT1 only appeared as a faint band that was not always detectable. CNT expression was also analyzed by Western blot (Fig. 1 C). CNT2 protein was the predominant concentrative nucleoside transporter observed, although some expression of CNT1 protein was also detected.
Figure 1.
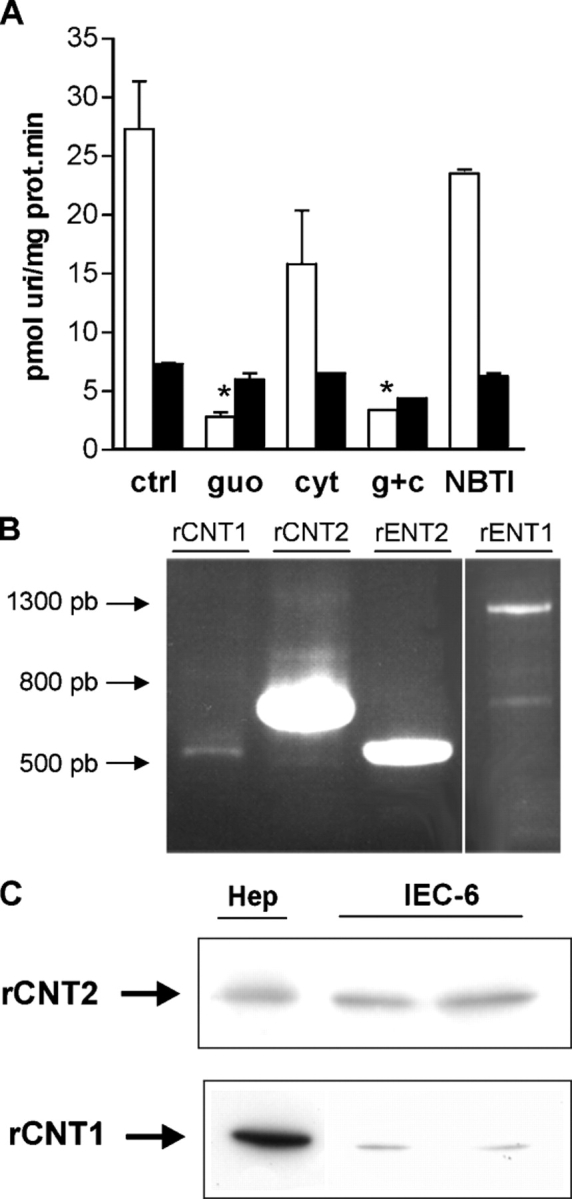
Characterization of nucleoside transporter activity and expression in IEC-6 cells. (A) Inhibition of uridine uptake by selected nucleosides and derivatives: guo, guanosine; cyt, cytidine; thy, thymidine; NBTI, nitrobenzyl thioinosine. Open bars, sodium-dependent uridine uptake; solid bars, sodium-independent uridine uptake. (B) RT-PCR of rCTN1, rCNT2, rENT1, and rENT2 performed on RNA from monolayers of IEC-6 cells. (C) Western blot of rCNT1 and rCNT2 performed on protein extracts from monolayers of IEC-6 cells. Protein extracts from rat hepatocytes were used as positive controls.
Effect of Dexamethasone Treatment on Nucleoside Transport in IEC-6 Cells
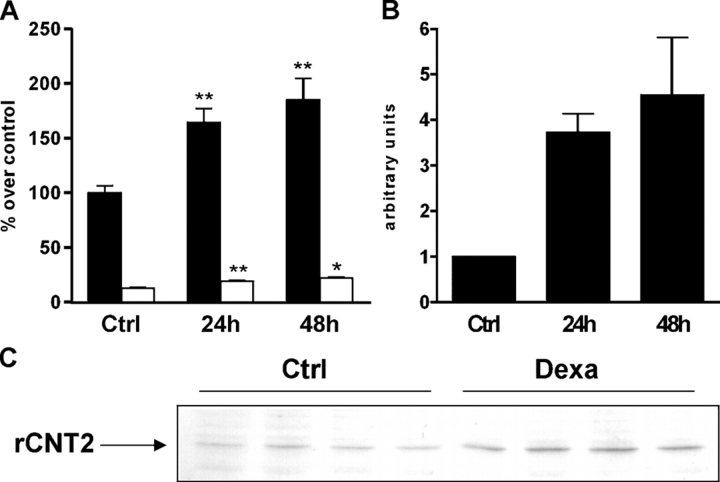
Guanosine transport was measured in IEC-6 cells at different time intervals (24 and 48 h) after addition of 100 nM dexamethasone (Fig. 2 A). Sodium-dependent guanosine transport (i.e., CNT2 activity) predominated, representing >90% of the total transport capacity of the cells, and it was further increased by dexamethasone (∼60% at 24 h and near to 100% at 48 h). Although much lower in absolute value, equilibrative transport of guanosine was also increased by a similar proportion in response to dexamethasone. In accordance with these data, CNT2 mRNA and protein levels were also increased by dexamethasone treatment (three- and fourfold at 24 and 48 h, respectively), as shown by the results obtained with real-time RT-PCR (Fig. 2 B) and Western blot (Fig. 2 C). Dexamethasone treatment also increased the levels of CNT1 mRNA and activity, although this latter effect (measured as sodium-dependent cytidine transport) remained at almost undetectable levels (2.9 ± 0.3 vs. 1.5 ± 0.1, in the presence or absence of dexamethasone, respectively), corresponding to <2% of the activity observed for CNT2.
Figure 2.
Effect of dexamethasone on nucleoside transport in IEC-6 cells. (A) Effect of different times of dexamethasone treatment in the uridine uptake of IEC-6 cells. Cells were treated with dexamethasone for 24 or 48 h and then nucleoside uptake was measured. Solid bars, CNT2 activity measured as sodium-dependent guanosine uptake (control uptake rate: 111 ± 7 pmol/min/mg protein). Open bars, equilibrative activity measured as sodium-independent guanosine uptake (control uptake rate: 14 ± 1 pmol/min/mg protein). Statistical analysis by Student's t test: *, P < 0.05; **, P < 0.01, vs. control values. (B) rCNT2 mRNA expression levels after dexamethasone treatment of IEC-6 cells determined by real-time PCR. Values are corrected by PCR internal control and normalized to control values (expressed as unity). Measurements were performed in triplicate on RNA from IEC-6 monolayers. (C) rCNT2 protein analysis by Western blot on IEC-6 cells after no exposure (ctrl) or after 24 h exposure to dexamethasone (dexa).
Effect of Proliferation Induction on Nucleoside Transport in IEC-6 Cells
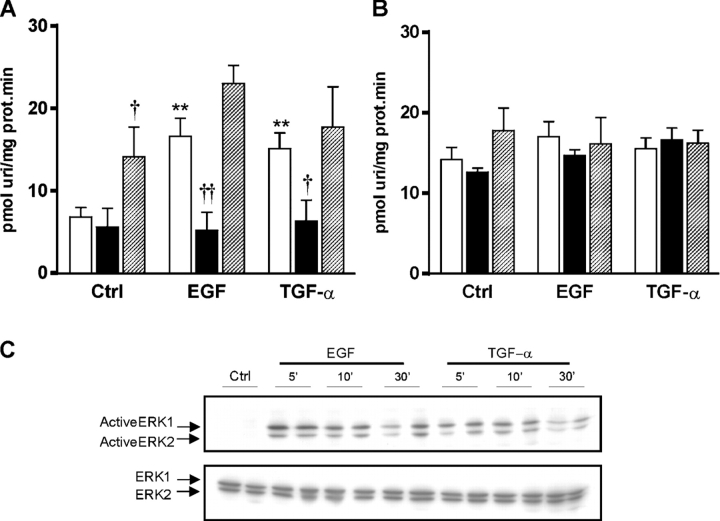
Neither CNT2 activity nor its protein and mRNA levels were altered after a 10-h treatment of IEC-6 cells with either EGF or TGF-α (unpublished data). Instead, treatment with either growth factor resulted in an increase in sodium-independent uridine transport. When analyzed in more detail, only the NBTI-sensitive component of equilibrative uridine transport (i.e., ENT1 activity) was responsible for this increase (Fig. 3 A), while the NBTI-resistant component (i.e., ENT2 activity) was unaltered by either treatment, at least at the growth factor concentrations used (Fig. 3 B). By real-time RT-PCR, it could be shown that only ENT1 mRNA levels were increased in response to growth factor addition, while the levels of ENT2 mRNA remained constant (Table I).
Figure 3.
Effect of EGF and TGFα on nucleoside transport in IEC-6 cells. (A) Effect of growth factors on ENT1 activity in monolayers of IEC-6 cells and the effect of ERK1/2 or JNK/1/2/3 inhibition. Open bars, no inhibitor; solid bars, 30 min preincubation with the ERK1/2 inhibitor PD98059; striped bars, 30 min preincubation with the JNK/1/2/3 inhibitor SP600125. Statistical analysis by Student's t test: **, P < 0.01, growth factor vs. control; †, P < 0.05, ††, P < 0.01, inhibitor vs. no inhibitor. (B) Effect of growth factors on ENT2 activity in monolayers of IEC-6 cells and the effect of ERK1/2 inhibition. Open bars, no inhibitor; solid bars, 30 min preincubation with the ERK1/2 inhibitor PD98059; striped bars, 30 min preincubation with the JNK/1/2/3 inhibitor SP600125. (C) Effect of growth factor treatment on ERK1/2 activation in IEC-6 cells. Monolayers of IEC-6 cells were exposed to the different treatments and, at given times, protein extracts were prepared and total ERK1/2 (15 μg of protein extract) and phosphorylated ERK1/2 (40 μg of protein extract) levels were analyzed by Western blot.
TABLE I.
Effect of EGF and TGFα on Nucleoside Transporter mRNA Levels in IEC-6 Cells and the Effect of ERK1/2 and JNK1/2/3 Inhibition
| No inhibitor | PD98059 | SP600125 | |
|---|---|---|---|
| Control | 0.97 ± 0.02 | 0.56 ± 0.06f | 4.21 ± 0.26f |
| EGF | 5.01 ± 0.31c | 1.72 ± 0.14b , f | 8.40 ± 0.96a |
| TGFα | 4.46 ± 0.63b | 1.59 ± 0.19b , d | 9.29 ± 0.88b , e |
Real-time PCR data are given in arbitrary units, normalized to the values obtained with untreated (control) cells in the absence of any inhibitor. Statistical analysis was by Student's t test.
P < 0.05, growth factor vs. control.
P < 0.01, growth factor vs. control.
P < 0.001, growth factor vs. control.
P < 0.05, vs. no inhibitor.
P < 0.01, vs. no inhibitor.
P < 0.001, vs. no inhibitor.
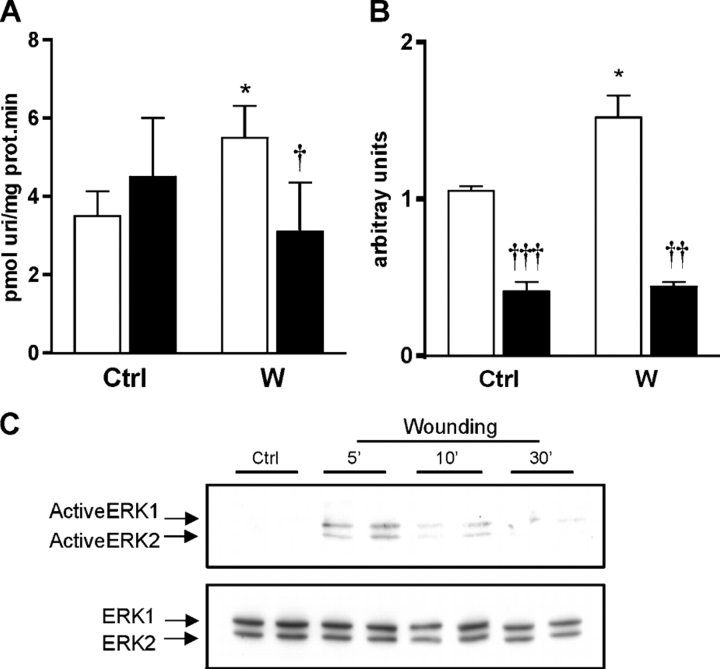
The effects of EGF and TGF-α on ENT1 expression and activity were mimicked by a model of epithelial wounding (Fig. 4). Again, an increase in ENT1, but not in ENT2 (not depicted), activity was detected 10 h after injuring the cells (Fig. 4 A), and this change was paralleled by an increase in ENT1 mRNA levels (Fig. 4 B). Since all these proliferative stimuli are known to activate the mitogen-activated protein (MAP) kinase pathway in the intestine, the possible involvement of these protein kinases in this ENT1 response was tested. Both growth factor incubation and epithelial wounding resulted in an increase in the phosphorylated form of ERK1/2 as early as 5–10 min after the beginning of the stimulus (Fig. 3 C and Fig. 4 C). Blocking the activity of ERK1/2 with the inhibitor PD98059 resulted in a loss of the effect on ENT1 activity in response to either growth factors (Fig. 3 A) or epithelial wounding (Fig. 4 A). The response of ENT1 mRNA levels, however, was different in both models. PD98059 caused a decrease in the basal levels of ENT1 mRNA in IEC-6 cells (Table I). However, while it completely suppressed the induction of ENT1 mRNA in the epithelial injury model (Fig. 4 B), the threefold induction of ENT1 mRNA in response to EGF and TGF-α treatments was unaltered (Table I). JNK1/2/3 inhibition had a different effect: the addition of the inhibitor SP600125 induced an increase in ENT1 mRNA levels and activity (Table I), but had no effect on the response to either growth factor.
Figure 4.
Effect of epithelial wounding on nucleoside transport in IEC-6 cells. (A) Effect of an epithelial wounding model on rENT1 activity in monolayers of IEC-6 cells and the effect of ERK1/2 inhibition. Open bars, no inhibitor; solid bars, 30 minute pre-incubation with the ERK1/2 inhibitor PD98059. Statistical analysis by Student's t-test: *, P < 0.05, wound vs. control; †, P < 0.05, inhibitor vs. no inhibitor. (B) Effect of epithelial wounding on rENT1 mRNA levels and the effect of ERK1/2 inhibition, determined by real-time PCR. Open bars, no inhibitor; solid bars, 30 minute preincubation with the ERK1/2 inhibitor PD98059. Statistical analysis by Student's t-test: *, P < 0.05, wound vs. control; ††, P < 0.01, †††, P < 0.001 inhibitor vs. no inhibitor. (C) Effect of epithelial wounding on ERK1/2 activation in IEC-6 cells. Monolayers of IEC-6 cells were exposed to the different treatments and, at given times, protein extracts were prepared and total ERK1/2 (15 mg of protein extract) and phosphorylated ERK1/2 (40 mg of protein extract) levels were analyzed by Western blot.
DISCUSSION
Absorptive epithelial cells rely on membrane transporters to accomplish their most characteristic function. For some metabolites like glucose, the presence of sodium-dependent, concentrative transporters at the apical membrane ensures full absorption of nutrients, while sodium-independent equilibrative transporters release these nutrients to the bloodstream at the basal membrane (Quaroni et al., 1979). The human intestine expresses both concentrative and equilibrative nucleoside transporters (Chandrasena et al., 1997; Patil and Unadkat, 1997); the sodium-dependent nucleoside transporters are distributed along a longitudinal gradient, with higher activities in the most proximal regions of the small intestine (Ngo et al., 2001). It has recently been suggested that such a dual distribution of concentrative and equilibrative transporters exists in intestinal epithelial cells (Williams et al., 1989), although in renal epithelial cells this pattern is less clear (Lai et al., 2002; Mangravite et al., 2003b). This differential location of nucleoside transporters at both membranes supports the suggestion of a transepithelial flux of nucleosides, as already shown for some substrates in kidney cells (Mangravite et al., 2003a; Lai et al., 2002), although the rapid intracellular metabolization of nucleosides argues against this possibility. Thus, the need for good absorptive cell models to study nucleoside transport is evident.
The expression pattern of nucleoside transporters in the rat intestinal epithelial cell line IEC-6 has been poorly characterized (Jakobs et al., 1990). In this study, we have performed a full characterization of this cell model, confirming the existence of a purine-preferring, sodium-dependent activity that is consistent with the expression of CNT2, shown by RT-PCR and Western blot. Furthermore, we have demonstrated the existence of two equilibrative activities, both NBTI sensitive and resistant, consistent with the expression of ENT1 and ENT2, respectively. Although some CNT1 mRNA and protein can be detected by RT-PCR and Western blot, no significant activity could be measured using cytidine as substrate.
The acquisition of intestinal digestive and absorptive properties is intimately linked to the degree of differentiation. Thus, immature crypt cells express few mature enterocytic features, such as sucrase–isomaltase complex activity or even a well-organized brush border at the luminal surface (Quaroni et al., 1979; Isselbacher, 1974). These functions are acquired during the migration of the cells along the crypt–villus axis. The IEC-6 cell line is derived from crypt cells and so it shares many of the undifferentiated characters typical of the immature enterocyte (Quaroni et al., 1979). One of the best characterized maturation agents for intestinal cells are glucocorticoids (Blake and Henning, 1983), either directly or through the stimulation of cell–cell and cell–matrix interactions (Louvard et al., 1992; Simo et al., 1992). IEC-6 cells also respond to glucocorticoids with cell cycle withdrawal and ultrastructural changes (Quaroni et al., 1999), although no induction of brush border enzymatic activities could be detected, possibly due to the failure to establish contacts with the basement membrane or with mesenchymal cells. Glucocorticoids did not elicit the appearance of sodium-dependent glucose transport in IEC-6 cells either (Inui et al., 1980), but they seem to increase the levels of previously expressed transporters (Iannoli et al., 1998). In agreement with this latter report, we have shown that dexamethasone treatment of IEC-6 cells clearly increases both CNT2 expression and activity and also boosts CNT1 expression. The fact that CNT1 activity is not measurable even after dexamethasone treatment might be explained by a failure to insert the transporter molecules into the membrane correctly. Intracellular trafficking of CNT1 molecules is much more complex than that of CNT2 and most of the protein remains in intracellular structures (Duflot et al., 2002). Our results would thus support a direct role of glucocorticoids in the regulation of some mature enterocyte features in IEC-6 cells and are in agreement with our previous results showing that CNT expression is directly dependent upon glucocorticoid regulation (del Santo et al., 2001).
A very different response is observed after EGF or TGF-α treatment of IEC-6 cells. EGF and TGF-α are among the most potent proliferative agents for intestinal epithelial cells, both in vivo (Koyama and Podolsky, 1989; Hirano et al., 1995; Dignass et al., 1996) and in vitro (Rokutan et al., 1994; Riegler et al., 1996; Dionne et al., 1998). The proliferation induced by TGF-α seems to be regulated by the autocrine production of TGF-α. Thus, it is initially self-maintained, but is ultimately down-regulated by the production of the growth-arresting TGF-β signal (Suemori et al., 1991). Treatment of IEC-6 cells with either EGF or TGF-α had little effect on concentrative nucleoside transporters, but elicited a more than twofold increase in ENT1 activity and mRNA levels. This result is in clear support of a role for ENT1 as the main nucleoside purveyor for cell proliferation, as previously reported in macrophages (Soler et al., 2001).
Epithelial intestinal cell proliferation can be elicited in vivo by mucosal damage (Feil et al., 1987; Lacy, 1988), and an in vitro model of epithelial wounding has been developed in IEC-6 cells (McCormack et al., 1992; Ciacci et al., 1993). Many growth factors and cytokines have been shown to mediate the proliferative response to mucosal wounding, such as TGF-α, TGF-β, EGF, hepatocyte growth factor, acidic and basic fibroblast growth factors, and IL-1β (Dignass and Podolsky, 1993; Dignass et al., 1994), although EGF does not seem to reduce chemotherapy-induced intestinal injury (Huang et al., 2002). In vitro wounding of IEC-6 cells also resulted, as in the treatments with EGF or TGF-α, in an increase in ENT1 activity, but without any effect on concentrative transporters. Thus, both proliferative stimuli induce the equilibrative transport that has been reported previously to be present in the basolateral membrane of the epithelial cell (Lai et al., 2002; Mangravite et al., 2003b), presumably to ensure nucleoside supply for proliferation that would have been derived from the bloodstream had this stimulus occurred in vivo. In this context, it has been shown that supplementation of parenteral nutrition formulae with nucleosides or nucleotides greatly ameliorates intestinal mucosa atrophy caused by total parenteral nutrition (Iwasa et al., 2000).
Both TGF-α and epithelial injury are known to stimulate the mitogen-activated protein (MAP) kinase cascade in IEC-6 cells (Dionne et al., 1998; Oliver et al., 1994; Göke et al., 1998). In our model, both kinds of stimuli increased the phosphorylation level of ERK1/2, and, as might have been expected, blocking of ERK1/2 activity resulted in a loss of the effect of wounding on ENT1 mRNA and activity. However, the same effect was not observed after growth factor treatment, where only a decrease in ENT1 activity, but not in mRNA expression level, was observed. So, while the wounding effects on ENT1 can be explained by an increase in its gene transcription rate mediated by ERK1/2, the effects triggered by EGF and TGFα must use a different signal transduction pathway. This alternate pathway is not mediated by JNK, since its inhibition had no effect on growth factor induction of ENT1 expression. Although we have tested a battery of different possible pathways (PI3 kinase, PKC, PKA, Tor kinase, and p38; unpublished data), the identity of this mediator still remains elusive.
In conclusion, induction of differentiation of IEC-6 cells results in an increase in the expression and activity of mature enterocyte nucleoside transporters such as CNT2 (and to a lesser extent CNT1), concentrative transporters located at the apical membrane of the enterocyte where they participate in the absorption of dietary nucleosides. In contrast, a proliferative signal such as epithelial wounding or growth factor exposure induces an increase in the expression and activity of ENT1, a basolateral nucleoside transporter, probably involved in the release of absorbed nucleosides or, as in this case, in the uptake of circulatory nucleosides to support the increased proliferation rate of these cells.
Acknowledgments
This work was supported by grant PI020934, from Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, Spain to F. Javier Casado, grant SAF2002-00717 from Ministerio de Ciencia y Tecnología, Spain to Marçal Pastor Anglada, and fellowship AP2001-1892 from Formación de Profesorado Universitario, Ministerio de Educación y Cultura to Ivette Aymerich.
David C. Gadsby served as editor.
Abbreviations used in this paper: CNT, concentrative nucleoside transporter; ENT, equilibrative nucleoside transporter; NBTI, nitrobenzyl-thioinosine; EGF, epidermal growth factor; ERK, extracellularly regulated kinase; TGF, transforming growth factor.
References
- Baldwin, S.A., J.R. Mackey, C.E. Cass, and J.D. Young. 1999. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol. Med. Today. 5:216–224. [DOI] [PubMed] [Google Scholar]
- Blake, H.H., and S.J. Henning. 1983. Weaning in the rat: a study of hormonal influences. Am. J. Physiol. 244:R537–R543. [DOI] [PubMed] [Google Scholar]
- Casado, F.J., M.P. Lostao, I. Aymerich, I.M. Larráyoz, S. Duflot, S. Rodríguez-Mulero, and M. Pastor-Anglada. 2002. Nucleoside transporters in absorptive epithelia. J. Physiol. Biochem. 58:207–216. [DOI] [PubMed] [Google Scholar]
- Chandrasena, G., R. Giltay, S.D. Patil, A. Bakken, and J.D. Unadkat. 1997. Functional expression of human intestinal Na+-dependent and Na+-independent nucleoside transporters in Xenopus laevis oocytes. Biochem. Pharmacol. 53:1909–1918. [DOI] [PubMed] [Google Scholar]
- Ciacci, C., S.E. Lind, and D.K. Podolsky. 1993. Transforming growth factor β regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology. 105:93–101. [DOI] [PubMed] [Google Scholar]
- del Santo, B., R. Valdés, J. Mata, A. Felipe, F.J. Casado, and M. Pastor-Anglada. 1998. Differential expression and regulation of nucleoside transport systems in rat liver parenchymal and hepatoma cells. Hepatology. 28:1504–1511. [DOI] [PubMed] [Google Scholar]
- del Santo, B., G. Tarafa, A. Felipe, F.J. Casado, and M. Pastor-Anglada. 2001. Developmental regulation of the concentrative nucleoside transporters CNT1 and CNT2 in rat liver. J. Hepatol. 34:873–880. [DOI] [PubMed] [Google Scholar]
- Dignass, A.U., and D.K. Podolsky. 1993. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 105:1323–1332. [DOI] [PubMed] [Google Scholar]
- Dignass, A.U., S. Tsunekawa, and D.K. Podolsky. 1994. Fibroblast growth factors modulate intestinal epithelal cell growth and migration. Gastroenterology. 106:1254–1262. [DOI] [PubMed] [Google Scholar]
- Dignass, A.U., J.L. Stow, and M.W. Babyatsky. 1996. Acute epithelial injury in rat small intestine in vivo is associated with expanded expression of transforming growth factor α and β. Gut. 38:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne, S., I.D. D'Agata, F.M. Ruemmele, E. Levy, J. St-Louis, A.K. Srivastava, D. Levesque, and E.G. Seidman. 1998. Tyrosine kinase and MAPK inhibition of TNF-α- and EGF-stimulated IEC-6 cell growth. Biochem. Biophys. Res. Commun. 242:146–150. [DOI] [PubMed] [Google Scholar]
- Doherty, A.J., and S.M. Jarvis. 1993. Na+-dependent and -independent uridine uptake in an established renal epithelial cell line, OK, from opossum kidney. Biochim. Biophys. Acta. 1147:214–222. [DOI] [PubMed] [Google Scholar]
- Duflot, S., M. Calvo, F.J. Casado, C. Enrich, and M. Pastor-Anglada. 2002. Concentrative nucleoside transporter (rCNT1) is targeted to the apical membrane through the hepatic transcytotic pathway. Exp. Cell Res. 281:77–85. [DOI] [PubMed] [Google Scholar]
- Feil, W., E. Wenzl, P. Vattay, M. Starlinger, T. Sogukoglu, and R. Schiessel. 1987. Repair of rabbit duodenal mucosa after acid injury in vivo and in vitro. Gastroenterology. 92:1973–1986. [DOI] [PubMed] [Google Scholar]
- Göke, M., M. Kanai, K. Lynch-Devaney, and D.K. Podolsky. 1998. Rapid mitogen-activated protein kinase activation by transforming growth factor a in wounded rat intestinal epithelial cells. Gastroenterology. 114:697–705. [DOI] [PubMed] [Google Scholar]
- Griffith, D.A., and S.M. Jarvis. 1996. Nucleoside and nucleobase transport systems of mammalian cells. Biochim. Biophys. Acta. 1286:153–181. [DOI] [PubMed] [Google Scholar]
- Gutierrez, M.M., and K.M. Giacomini. 1993. Substrate selectivity, potential sensitivity and stoichiometry of Na+-nucleoside transport in brush border membrane vesicles from human kidney. Biochim. Biophys. Acta. 1149:202–208. [DOI] [PubMed] [Google Scholar]
- Gutierrez, M.M., C.M. Brett, R.J. Ott, A.C. Hui, and K.M. Giacomini. 1992. Nucleoside transport in brush border membrane vesicles from human kidney. Biochim. Biophys. Acta. 1105:1–9. [DOI] [PubMed] [Google Scholar]
- Hirano, M., R. Iwakiri, K. Fujimoto, H. Sakata, T. Ohyama, T. Sakai, T. Joh, and M. Itoh. 1995. Epidermal growth factor enhances repair of rat intestinal mucosa damaged by oral administration of methotrexate. J. Gastroenterol. 30:169–176. [DOI] [PubMed] [Google Scholar]
- Huang, Q.Q., S.Y.M. Yao, M.W.L. Ritzel, A.R.P. Paterson, C.E. Cass, and J.D. Young. 1994. Cloning and functional expression of a complementary DNA encoding a mammalian nucleoside transport protein. J. Biol. Chem. 269:17757–17760. [PubMed] [Google Scholar]
- Huang, F.S., C.J. Kemp, J.L. Williams, C.R. Erwin, B.W. Warner. 2002. Role of epidermal growth factor and its receptor in chemotherapy-induced intestinal injury. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G432–G442. [DOI] [PubMed] [Google Scholar]
- Iannoli, P., J.H. Miller, C.K. Ryan, and H.C. Sax. 1998. Glucocorticoids upregulate intestinal nutrient transport in a time-dependent and substrate-specific fashion. J. Gastrointest. Surg. 2:449–457. [DOI] [PubMed] [Google Scholar]
- Inui, K.-I., A. Quaroni, L.G. Tillotson, and K.J. Isselbacher. 1980. Amino acid and hexose transport by cultured crypt cells from rat small intestine. Am. J. Physiol. 239:C190–C196. [DOI] [PubMed] [Google Scholar]
- Iseki, K., M. Sugawara, T. Fujiwara, I. Naasani, M. Kobayashi, and K. Miyazaki. 1996. Transport mechanisms of nucleoside and the derivative, 6-mercaptopurine riboside across intestinal brush-border membranes. Biochim. Biophys. Acta. 1278:105–110. [DOI] [PubMed] [Google Scholar]
- Isselbacher, K.J. 1974. The intestinal cell surface: some properties of normal, undifferentiated, and malignant cells. Ann. Intern. Med. 81:681–686. [DOI] [PubMed] [Google Scholar]
- Iwasa, Y., M. Iwasa, Y. Ohmori, T. Fukutomi, and S. Ogoshi. 2000. The effect of the administration of nucleosides and nucleotides for parenteral use. Nutrition. 16:598–602. [DOI] [PubMed] [Google Scholar]
- Jakobs, E.S., D.J. Van Os-Corby, and A.R.P. Paterson. 1990. Expression of sodium-linked nucleoside transport activity in monolayer cultures of IEC-6 intestinal epithelial cells. J. Biol. Chem. 265:22210–22216. [PubMed] [Google Scholar]
- Koyama, S.Y., and D.K. Podolsky. 1989. Differential expression of transforming growth factors α and β in rat intestinal epithelial cells. J. Clin. Invest. 83:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy, E.R. 1988. Epithelial restitution in the gastrointestinal tract. J. Clin. Gastroenterol. 10:S72–S77. [DOI] [PubMed] [Google Scholar]
- Lai, Y., A.H. Bakken, and J.D. Unadkat. 2002. Simultaneous expression of hCNT1-CFP and hENT1-YFP in Madin-Darby canine kidney cells. Localization and vectorial transport studies. J. Biol. Chem. 277:37711–37717. [DOI] [PubMed] [Google Scholar]
- Louvard, D., M. Kedinger, and H.P. Hauri. 1992. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu. Rev. Cell Biol. 8:157–195. [DOI] [PubMed] [Google Scholar]
- Mangravite, L.M., I. Badagnani, and K.M. Giacomini. 2003. a. Nucleoside transporters in the disposition and targeting of nucleoside analogs in the kidney. Eur. J. Pharmacol. 479:269–281. [DOI] [PubMed] [Google Scholar]
- Mangravite, L.M., G. Xiao, and K.M. Giacomini. 2003. b. Localization of human equilibrative transporters, hENT1 and hENT2, in renal epithelial cells. Am. J. Physiol. Renal Physiol. 284:F902–F910. [DOI] [PubMed] [Google Scholar]
- McCormack, S.A., M.J. Viar, and L.R. Johnson. 1992. Migration of IEC-6 cells: a model for mucosal healing. Am. J. Physiol. 263:G426–G435. [DOI] [PubMed] [Google Scholar]
- Ngo, L.Y., S.D. Patil, and J.D. Unadkat. 2001. Ontogenic and longitudinal activity of Na+-nucleoside transporters in the human intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G475–G481. [DOI] [PubMed] [Google Scholar]
- Oliver, B.L., R.I. Sha'afi, and J.J. Hajjar. 1994. Transforming growth factor-α increases tyrosine phosphorylation of microtubule-associated protein kinase in a small intestinal crypt cell line (IEC-6). Biochem. J. 303:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Angalda, M., and S.A. Baldwin. 2001. Recent advances in the molecular biology and physiology of nucleoside and nucleobase transporters. Drug Dev. Res. 52:431–437. [Google Scholar]
- Pastor-Angalda, M., A. Felipe, and F.J. Casado. 1998. Transport and mode of action of nucleoside derivatives used in chemical and antiviral therapies. Trends Pharmacol. Sci. 19:423–430. [DOI] [PubMed] [Google Scholar]
- Patil, S.D., and J.D. Unadkat. 1997. Sodium-dependent nucleoside transport in the human intestinal brush-border membrane. Am. J. Physiol. 272:G1314–G1320. [DOI] [PubMed] [Google Scholar]
- Quaroni, A., J. Wands, R.L. Trelstad, and K.J. Isselbacher. 1979. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J. Cell Biol. 80:248–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni, A., J.Q. Tian, M. Göke, and D.K. Podolsky. 1999. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am. J. Physiol. 277:G1027–G1040. [DOI] [PubMed] [Google Scholar]
- Riegler, M., R. Sedivy, T. Sogukoglu, E. Cosentini, G. Bischof, B. Teleky, W. Feil, R. Schiessel, G. Hamilton, and E. Wenzl. 1996. Epidermal growth factor promotes rapid response to epithelial injury in rabbit duodenum in vitro. Gastroenterology. 111:28–36. [DOI] [PubMed] [Google Scholar]
- Ritzel, M.W.L., S.Y.M. Yao, A.M.L. Ng, J.R. Macke, C.E. Cass, and J.D. Young. 1998. Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Mol. Membr. Biol. 15:203–211. [DOI] [PubMed] [Google Scholar]
- Rokutan, K., A. Sakai, F. Teramoto, Y. Kido, F. Shizuka, and K. Kishi. 1994. Epidermal growth factor-induced mitogen signals in cultured intestinal epithelial cells. J. Gastroenterol. 29:59–62. [PubMed] [Google Scholar]
- Scharrer, E., K.S. Rech, and B.J. Grenacher. 2002. Characteristics of Na+-dependent intestinal nucleoside transport in the pig. J. Comp. Physiol. 172:309–314. [DOI] [PubMed] [Google Scholar]
- Simo, P., P. Simon-Assmann, C. Arnold, and M. Kedinger. 1992. Mesenchyme-mediated effect of dexamethasone on laminin in cocultures of embryonic gut epithelial cells and mesenchyme-derived cells. J. Cell Sci. 101:161–171. [DOI] [PubMed] [Google Scholar]
- Soler, C., J. García-Manteiga, R. Valdés, J. Xaus, M. Comalada, F.J. Casado, M. Pastor-Anglada, A. Celada, and A. Felipe. 2001. Macrophages require different nucleoside transport systems for proliferation and activation. FASEB J. 15:1979–1988. [DOI] [PubMed] [Google Scholar]
- Suemori, S., C. Ciacci, and D.K. Podolsky. 1991. Regulation of transforming growth factor expression in rat intestinal epithelial cell lines. J. Clin. Invest. 87:2216–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble, M.E., and R. Coulson. 1984. Adenosine transport in perfused rat kidney and renal cortical membrane vesicles. Am. J. Physiol. 246:F794–F803. [DOI] [PubMed] [Google Scholar]
- Valdés, R., M.A. Ortega, F.J. Casado, A. Felipe, A. Gil, A. Sánchez-Pozo, and M. Pastor-Anglada. 2000. Differential regulation of nucleoside transporter expression in rat liver and small intestine by dietary nucleotides. Gastroenterology. 119:1623–1630. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi, D., and J.A. Belt. 1988. Sodium dependent nucleoside transport in mouse intestinal epithelial cells. Two transport systems with differing substrate specificities. J. Biol. Chem. 263:19419–19423. [PubMed] [Google Scholar]
- Ward, J.L., and C.M. Tse. 1999. Nucleoside transport in human colonic epithelial cell lines: evidence for two Na+-independent transport systems in T84 and Caco-2 cells. Biochim. Biophys. Acta. 1419:15–22. [DOI] [PubMed] [Google Scholar]
- Williams, T.C., A.J. Doherty, D.A. Griffith, and S.M. Jarvis. 1989. Characterization of sodium-dependent and sodium-independent nucleoside transport systems in rabbit brush-border and basolateral plasma-membrane vesicles from the renal outer cortex. Biochem. J. 264:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]