Abstract
Extracellular ATP regulates several elements of the mucus clearance process important for pulmonary host defense. However, the mechanisms mediating ATP release onto airway surfaces remain unknown. Mitochondrial voltage-dependent anion channels (mt-VDACs) translocate a variety of metabolites, including ATP and ADP, across the mitochondrial outer membrane, and a plasmalemmal splice variant (pl-VDAC-1) has been proposed to mediate ATP translocation across the plasma membrane. We tested the involvement of VDAC-1 in ATP release in a series of studies in murine cells. First, the full-length coding sequence was cloned from a mouse airway epithelial cell line (MTE7b−) and transfected into NIH 3T3 cells, and pl-VDAC-1-transfected cells exhibited higher rates of ATP release in response to medium change compared with mock-transfected cells. Second, ATP release was compared in cells isolated from VDAC-1 knockout [VDAC-1 (−/−)] and wild-type (WT) mice. Fibroblasts from VDAC-1 (−/−) mice released less ATP than WT mice in response to a medium change. Well-differentiated cultures from nasal and tracheal epithelia of VDAC-1 (−/−) mice exhibited less ATP release in response to luminal hypotonic challenge than WT mice. Confocal microscopy studies revealed that cell volume acutely increased in airway epithelia from both VDAC-1 (−/−) and WT mice after luminal hypotonic challenge, but VDAC-1 (−/−) cells exhibited a slower regulatory volume decrease (RVD) than WT cells. Addition of ATP or apyrase to the luminal surface of VDAC-1 (−/−) or WT cultures with hypotonic challenge produced similar initial cell height responses and RVD kinetics in both cell types, suggesting that involvement of VDAC-1 in RVD is through ATP release. Taken together, these studies suggest that VDAC-1, directly or indirectly, contributes to ATP release from murine cells. However, the observation that VDAC-1 knockout cells released a significant amount of ATP suggests that other molecules also play a role in this function.
Keywords: voltage-dependent anion channel, ATP release, osmotic cell swelling, regulatory volume decrease, airway epithelia
INTRODUCTION
Extracellular ATP acts as a signaling molecule in an autocrine fashion in a wide variety of cells (Dubyak and el-Moatassim, 1993; Ralevic and Burnstock, 1998), including airway epithelial cells. Physiologic, noncytolytic release of ATP from cells in response to mechanical stress (Grygorczyk and Hanrahan, 1997; Watt et al., 1998; Homolya et al., 2000) or hypotonic challenge (Wang et al., 1996b; Hazama et al., 1999) is well documented. In airway epithelia, released ATP acts through purinergic receptors (P2Y2 receptors) to initiate phospholipase C–mediated increases in intracellular Ca2+ and diacylglycerol-mediated activation of protein kinase C. These actions promote Cl− secretion via Ca2+-activated Cl− channels (Clarke et al., 1992) and CFTR (Jia et al., 1997), inhibit amiloride-sensitive epithelial Na+ absorption (Mall et al., 2000), increase ciliary beat frequency (Geary et al., 1995), and stimulate mucin release (Davis et al., 1992; Lethem et al., 1993). Extracellular ATP is broken down by a complex array of hydrolytic activities, and adenosine, the final product of ATP metabolism, promotes cAMP-regulated CFTR activity via A2b adenosine receptors (Huang et al., 2001). Whereas the roles of ATP and adenosine in modulating the integrated actions of mucociliary clearance important for pulmonary host defense have been elucidated, it is still largely unknown how ATP reaches the airway epithelial cell surface.
In general terms, the physiologic release of ATP into the extracellular environment has been postulated to be mediated by vesicular release or channels. In addition to ATP release by regulated exocytosis from excitatory or secretory tissues such as neurons, chromaffin cells, platelets, and mast cells (Burnstock, 1997), vesicular transport of ATP has been reported recently also in nonexcitatory tissues such as endothelial cells (Bodin and Burnstock, 2001), intestinal 407 cells (van der Wijk et al., 2003), urothelial cells (Knight et al., 2002), and biliary cells (Gatof et al., 2004). Channel-mediated mechanisms have included ATP binding cassette (ABC) transporters including CFTR (Reisin et al., 1994; Schwiebert et al., 1995) and the multiple drug resistance (MDR) protein (Roman et al., 2001), connexin hemichannels (Cotrina et al., 1998), maxi-anion channels (Sabirov et al., 2001; Bell et al., 2003), and stretch-activated channels (Caldwell et al., 1998; Braunstein et al., 2001).
Voltage-dependent anion channels (VDACs) are pore-forming proteins (porins) in the mitochondrial outer membrane, where they represent 5% of the total protein (rat liver mitochondria; Linden et al., 1984), and transport a variety of purine nucleotides, including ATP and ADP (Rostovtseva and Colombini, 1996). At physiological pH, most intracellular ATP molecules exist in anionic forms (MgATP2−). It is, therefore, possible that anion channels can conduct ATP across the plasma membrane, similar to the VDAC function in the mitochondrial outer membrane. Recent electrophysiological studies have suggested that “VDAC-like” maxi (large conductance)-anion channels are detected in the plasma membranes of Chinese hamster ovary cells (Mangel et al., 1993), rat bile duct epithelial cells (McGill et al., 1993), rabbit renal cortical collecting duct cells (Light et al., 1990; Schwiebert et al., 1994), HeLa cells (Schwarzer et al., 2000), and bovine brain astrocytes (Dermietzel et al., 1994; Guibert et al., 1998). Moreover, hypotonicity-induced ATP release through volume-sensitive anion channels has been reported in bovine aortic endothelial cells (Hisadome et al., 2002), mouse mammary carcinoma (C127i) cells (Sabirov et al., 2001), and in rabbit macula densa cells (Bell et al., 2003). Collectively, these results suggest that plasmalemmal VDAC-like maxi-anion channels could conduct ATP in response to physiologic stresses.
Whether VDAC itself mediates plasmalemmal maxi-anion channel activity, however, remains controversial. Linden et al. (1984) demonstrated the exclusive localization of rat liver VDAC in the mitochondrial outer membrane by subcellular fractionation and immunoblotting techniques using monospecific antisera against the porin isolated from the outer membrane of rat liver mitochondria. Several subsequent studies reported extramitochondrial locations of VDAC, mostly by immunocytochemical techniques (Cole et al., 1992; Dermietzel et al., 1994; Jakob et al., 1995). However, the potential for artifactual localization of VDAC in plasma membrane in those studies, such as nonspecific binding of antibodies or protein redistribution during the fractionation of cellular membranes, was raised by Yu et al. (1995) (Yu and Forte, 1996). Indeed, Yu et al. demonstrated that FLAG-tagged VDAC-1 protein overexpressed in COS cells was exclusively localized to mitochondria by immunoelectron microscopy. Of note, more recently, VDAC-1 protein was isolated from caveolae-like domains of biotinylated plasma membrane fractions by biochemical techniques and exhibited similar properties as those of mitochondrial porin in planar bilayers (Bathori et al., 1999). Furthermore, a splice variant of the mitochondrial VDAC-1 (mt-VDAC-1) gene, which encodes a leader peptide of 13 amino acids at NH2 terminus, was isolated from a murine brain cDNA library by 5′-RACE-PCR (Buettner et al., 2000). The protein from this splice variant exhibited extramitochondrial trafficking to the endoplasmic reticulum, the Golgi apparatus, and possibly the plasma membrane, and thus was named plasmalemmal VDAC-1 (pl-VDAC-1). Antisense oligonucleotides directed against the specific membrane leader sequence of pl-VDAC-1 markedly reduced both plasmalemmal VDAC immunostaining and maxi Cl− currents in C1300 mouse neuroblastoma cells (Bahamonde et al., 2003). In sum, it remains unclear whether VDAC participates in ATP release and whether such activity may require plasma membrane localization.
Accordingly, in the present study, our primary goal was to test the simplest hypothesis, i.e., whether VDAC is associated with physiologically significant extracellular ATP release, before initiating studies on its mechanism and site of action. Thus, we transfected pl-VDAC-1 cDNA in NIH 3T3 cells and tested whether transfection affected release of ATP from the intracellular to external environment. Subsequent studies focused on primary fibroblasts and airway cells from mice in which the VDAC-1 gene was disrupted by gene targeting (Anflous et al., 2001). We investigated the ATP release properties of these cells and the contribution of VDAC-1 to ATP release–mediated airway epithelial cell volume regulation in response to luminal hypotonic challenge.
MATERIALS AND METHODS
Cloning of Full-length pl-VDAC-1 Coding Sequence
Primers designed to amplify the full-length pl-VDAC-1 were as follows: forward primer, VD-F, 5′-aga gaa ttc cac cat gtg ttc att ctt tct cgt gct t-3′; reverse primer, VD-R, 5′-gtg tgg atc cgc tta tgc ttg aaa ttc cag tcc tag gcc aag-3′ (Fig. 1 A). Total RNA was prepared from an immortalized mouse tracheal epithelial cell line (MTE7b−; Thomas et al., 2000) using an RNAeasy kit (QIAGEN) and reverse transcribed with random primers using Superscript II (Invitrogen). The resulting cDNA was used as a template, and PCR was performed using high fidelity PFU enzyme (Promega) according to the following protocol: 94°C 2 min, 5 cycles of 94°C 45 s–58°C 45 s–72°C 1 min, 35 cycles of 94°C 45 s–65°C 45 s–72°C 1 min, and 72°C 5 min.
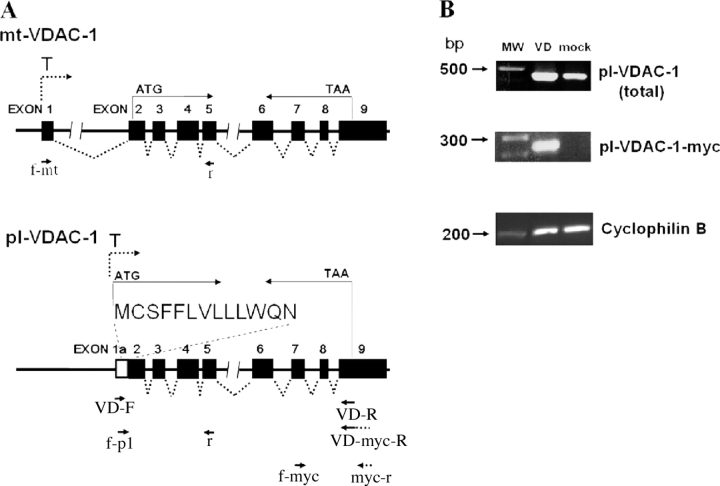
Figure 1.
Schematic of mt-VDAC-1 and pl-VDAC-1 genomic regions and expression analysis of pl-VDAC-1 mRNA. (A) In the genomic schema, boxes indicate exons and T stands for transcription start site. The leader sequence in pl-VDAC-1 is encoded by Exon 1a/2 as indicated. Primers VD-F and VD-R were designed to detect the presence of full-length pl-VDAC-1 mRNA; VD-F was designed to hybridize specifically to pl-VDAC-1 sequence and not to mt-VDAC-1. VD-R hybridized 3′ to the translation termination codon. Primers f-pl and r, f-2 and myc-r, and f-mt (and r) were designed to amplify pl-VDAC-1, pl-VDAC-1-myc, and mt-VDAC-1, respectively. (B) Top, expression of total (endogenous and exogenous) pl-VDAC-1; middle, exogenous pl-VDAC-1-myc; bottom, cyclophilin B mRNA in mock- and pl-VDAC-1-myc–transfected NIH 3T3 cells 14 d after transfection. In each lane from left to right, molecular weight markers (MW), and pl-VDAC-1-myc–transfected (VD) and mock-transfected (mock) cell cDNAs were loaded. Top, primers f-pl and r, predicted size of pl-VDAC-1 = 420 bp; middle, primers f-myc and myc-r, predicted size of pl-VDAC-1-myc = 250 bp; bottom, cyclophilin B primers, predicted size = 200 bp.
The full-length pl-VDAC-1 cDNA was cloned into pCR-Blunt II-TOPO (Invitrogen). A pl-VDAC-1 cDNA was also constructed that contained the c-myc tag (A-S-M-Q-K-L-I-S-E-D-L) on the COOH terminus (pl-VDAC-1-myc) by the addition of the c-myc tag sequence to the reverse primer (VD-myc-R, 5′-gtg tgg atc cgc tta cag gtc ctc ctc tga gat cag ctt ctg cat tga tgc tgc ttg aaa ttc cag tcc tag gcc aag-3′) and amplification with the same protocol as above. Both constructs were subsequently cloned into a retroviral expression plasmid with a puromycin-resistant cassette (pLXPIP; Olsen et al., 1992) for vector production.
Overexpression of pl-VDAC-1 in NIH 3T3 Cells
Mouse NIH 3T3 cells were grown in Dulbecco's modified eagles medium (DMEM) with 10% FBS and penicillin/streptomycin. To transfect NIH 3T3 cells with retroviral vectors, cells were seeded at 2.5 × 105 cells per 35-mm dish. Retroviruses carrying pl-VDAC-1 or pl-VDAC-1-myc, or viruses produced from pLXPIP without gene insert (“mock” viruses), were added to cells (105 infectious units/ml, 1 ml/plate) and incubated with polybrene (8 μg/ml) for 2 h at 37°C. Virus-containing medium was subsequently replaced with fresh medium. Selection with puromycin (5 μg/ml) was initiated 24 h after the removal of virus and was continued for a week.
To establish clonal cell lines, cells from virally transfected populations were trypsinized and plated in serial dilutions in 96-well plates. Wells that yielded a colony from a single cell were selected and expanded. A 25-mm2 flask with confluent cells was obtained ∼3 wk after the plating of single cells.
Primary Culture Preparation from WT, VDAC-1 (−/−), and FOXJ1/EGFP Transgenic Mice
Lung fibroblasts were isolated from wild-type (WT) and VDAC-1 (−/−) mice as previously published (Homolya et al., 1999) from explant cultures. Outgrown fibroblasts were trypsinized and passaged in 25-cm2 flasks (Falcon).
Primary cultures of tracheal and nasal epithelial tissue were generated from WT, VDAC-1 (−/−), and FOXJ1/EGFP transgenic mice (Ostrowski et al., 2003) with a protocol modified based on a previous report (You et al., 2002). VDAC-1 (−/−) mice and their WT littermate controls (CD1 background), or FOXJ1/EGFP transgenic mice (C3H x C57B1/6J F1 background), were killed at 4–8 wk of age. Resected tracheal and nasal tissues were incubated separately in Ham's F-12 containing 1.5 mg/ml pronase (Roche Molecular Biochemicals) for 4 h at 4°C with vigorous shaking every 30 min. Isolated cells were resuspended (100 μl/trachea and 500 μl/nose) in METC/Plus media (You et al., 2002). Yields were ∼2.0 × 105 cells/trachea and ∼5.0 × 105 cells/nose from a 15–20-g mouse. Harvested cells (1.5 × 105 cells/cm2) were seeded on 6.5-mm collagen-coated transwells (0.4 μm pore diameter; Corning-Costar) and incubated with MTEC/Plus media in both chambers. After the transmembrane resistance (Rt) exceeded 1,000 Ohms.cm2 (EVOM; World Precision Instruments), medium was removed from the upper chamber to establish an air–liquid interface. Cultures were studied in the fully differentiated state 3–4 wk after seeding. All cultures were maintained at 37°C in a humidified incubator supplemented with 5% CO2.
Expression Analysis of pl-VDAC-1 and mt-VDAC-1
Primers were designed to test for mRNA expression of pl-VDAC-1 (f-pl and r), pl-VDAC-1-myc (f-myc and myc-r), and mt-VDAC-1 (f-mt and r) in pl-VDAC-1–transduced 3T3 cells and primary mouse cultures: f-pl, 5′-tgt gtt cat tct ttc tcg tgc-3′; r, 5′-cca gtg ttc ggc gag aat gac-3′; f-myc, 5′-ctt cgg aat agc caa-3′; myc-r, 5′-ag ctt ctg cat tga tgc t-3′; f-mt, 5′-gct gct ccc gcc gtc acc gcc-3′ (Fig. 1 A). Total RNA prepared from mock- and pl-VDAC-1–transfected NIH 3T3 cells and cultures from WT and VDAC-1 (−/−) mice was reverse transcribed as described above. PCR was performed according to the following protocol: 94°C 7 min, 35 cycles of 94°C 30 s–55°C 30 s–72°C 30 s, and 72°C 7 min. For each cDNA pool, cyclophilin B mRNA expression was studied as a housekeeping mRNA control.
Incubations and Sample Collection
Fibroblasts
Fibroblasts were seeded on 10 mm diameter wells of 24-well plates and grown to near confluence (∼90%) in DMEM supplemented with 10% FBS and penicillin/streptomycin. Cultures were rinsed and preincubated with 300 μl serum-free DMEM for 1 h, and aliquots of 100 μl gently removed (∼100 μl/s) to determine basal ATP concentrations. For the mechanical stimulation (medium change) studies, the remaining medium was gently aspirated and prewarmed new medium (300 μl) added (∼100 μl/s). 30-μl samples were obtained at each indicated sampling interval. Each sample was immediately placed on ice, heated to 98°C for 2 min, and stored at −20°C. To measure intracellular ATP, cells were lysed with 0.5 ml ice-cold 5% trichloroacetic acid (TCA), extracted three times with 6× volume ethyl ether, and the samples were stored at −20°C until assaying for ATP levels as described below.
Airway Epithelial Cultures
Polarized airway epithelial cultures on transwells were rinsed bilaterally with PBS and incubated with 120 μl Hank's balanced salt solution (HBSS) on the basolateral and 30 μl HBSS on the apical surface for 1 h at 37°C to establish basal conditions for ATP release. Cultures were gently placed onto the microsampling stage (Lazarowski et al., 2004) and equilibrated for 10 min. For measurement of apical steady-state ATP levels, 10-μl samples were obtained with a capillary (World Precision Instruments, 1.0 mm diameter) interfaced to a microscope-mounted micromanipulator. Basolateral samples (20 μl) were collected using gel loading tips. Then, 10 μl H2O or 10 μl 300 mOsm mannitol was gently added to the mucosal compartment to generate an apical 200 mOsm or an isosmotic control solution, respectively. 2 and 10 min later, samples from apical surface (15 μl) and from basolateral bath (20 μl) were obtained and processed as described above. Airway epithelial intracellular ATP was extracted as described above.
Luciferin/Luciferase ATP Assay
The luciferin–luciferase assay was performed as described previously (Watt et al., 1998). In brief, 3–30-μl aliquots from each sample were added to test tubes containing 300 μl H2O. The tubes were placed in the light chamber of an LB953 AutoLumat luminometer (Berthold GmbH), and a luciferin–luciferase cocktail (luciferin 80 μg/ml and luciferase 4 μM, 100 μl/assay) was added to each sample with a built-in injector. Luminescence was recorded for 10 s and compared with an ATP calibration curve performed in parallel.
Derivatization of Adenyl Purines
Adenine nucleotides/nucleosides were derivatized to fluorescent 1, N 6-ethenopurines by reaction with 1.0 M 2-chloroacetaldehyde 25 mM Na2HPO4 (pH 4.0) and analyzed by HPLC (Waters 717plus Autosampler HPLC) as described previously (Huang et al., 2001; Lazarowski et al., 2004).
Cell Viability Assay
A calcein and ethidium homodimer–based assay was employed to test cell viability using LIVE/DEAD viability/cytotoxicity kit (Molecular Probes, Inc.) according to the manufacturer's instructions. The number of dead cells amongst 500 cells was counted before and after medium change in mock- and pl-VDAC-1–transfected cells.
Confocal Microscopy Assessment of Cellular Volume Regulation
Well-differentiated cultures from VDAC-1 (−/−) and WT mice were loaded bilaterally with 5 μM calcein-AM (Molecular Probes, Inc.) for 30 min at 37°C. These cultures, as well as cultures from FOXJ1/EGFP mice, were equilibrated for 10 min with 20 μl of Krebs-bicarbonate ringer (KBR) on the apical surface and 5 μl of KBR on the basolateral surface before study. They were then analyzed by a confocal fluorescence microscope (model 510, PL APO 40×/1.20 mm water lens; Carl Zeiss MicroImaging, Inc.) by placing the culture on a 25 mm coverslip that formed the bottom of an open chamber. H2O (10 μl) was added to apical surface to generate a 200 mOsm solution, and xz-scanning images were obtained every 3 s for 30 s. Analyses of EGFP expression in FOXJ1/EGFP cultures revealed that the cultures were pseudo-stratified, i.e., the lumen-facing cultured cells extended to the Transwell membrane. This distance was equivalent to the distance from the apex of the lumen-facing cells to the Transwell membrane in the calcein-AM–loaded WT and VDAC-1 (−/−) cultures. Accordingly, the distance between the apex of the lumen-facing cells and the Transwell membrane was defined as luminal cell height, and distances before and after addition of H2O were quantitated with Metamorph software (three measurements per image). Experiments were also performed on cultures by exposing them to ATP (100 μM in 10 μl H2O) or apyrase (10 U/ml in 10 μl H2O) as compared with H2O alone (10 μl).
Statistical Analysis
Data were expressed as mean values ± SEM of observations. Where appropriate, data were analyzed by paired Student's unpaired t test (to compare two unpaired groups of measurement data) with Statview software, and statistical significance was defined as P < 0.05.
RESULTS
Overexpression of pl-VDAC-1 in NIH 3T3 Cells
The full-length pl-VDAC-1 cDNA (0.9 kb) was amplified from a mouse tracheal epithelial cell line (MTE7b−) by RT-PCR and verified by sequencing. Pl-VDAC-1 and pl-VDAC-1-myc, cloned into pLXPIP retroviral vectors, were transfected into NIH 3T3 cells. 14 d after transfection, pl-VDAC-1 expression was evaluated by RT-PCR (Fig. 1 B). Endogenous pl-VDAC-1 mRNA was detected in mock-transfected NIH 3T3 cells, and increased pl-VDAC-1 levels were observed in pl-VDAC-1–transfected cells.
Pl-VDAC-1–transfected cells exhibited greater extracellular ATP accumulation after a medium change relative to mock-transfected cells 14 d after transfection (Fig. 2 A). Basal extracellular ATP levels, however, were similar in both groups. As a measure of extracellular ATP metabolism, exogenous ATP decayed at a similar rate in both groups (from 200 nM at t = 0 to 105.2 ± 4.3 nM in mock- and 101.7 ± 3.6 nM in pl-VDAC-1–transfected cells 2 h later, n = 4). Intracellular ATP levels were also not significantly different between the two groups (9.3 ± 0.2 and 9.6 ± 0.2 nmol/million cells in pl-VDAC-1- and mock-transfected cells, respectively, n = 4). Cell viability, as measured by calcein and ethidium homodimer–based assays, did not differ significantly between groups before or after the medium change: the percentage of dead cells was 0.35 ± 0.11% before medium change and 0.45 ± 0.15% after medium change in mock-transfected cells, and 0.25 ± 0.11% before medium change and 0.35 ± 0.11% after medium change in pl-VDAC-1–transfected cells (mean ± SEM of four separate experiments). These findings indicate that the greater extracellular ATP accumulation in pl-VDAC-1–transfected cells did not reflect differences in extracellular ATP metabolism, intracellular ATP levels, or cell lysis, but rather an increased rate of ATP release.
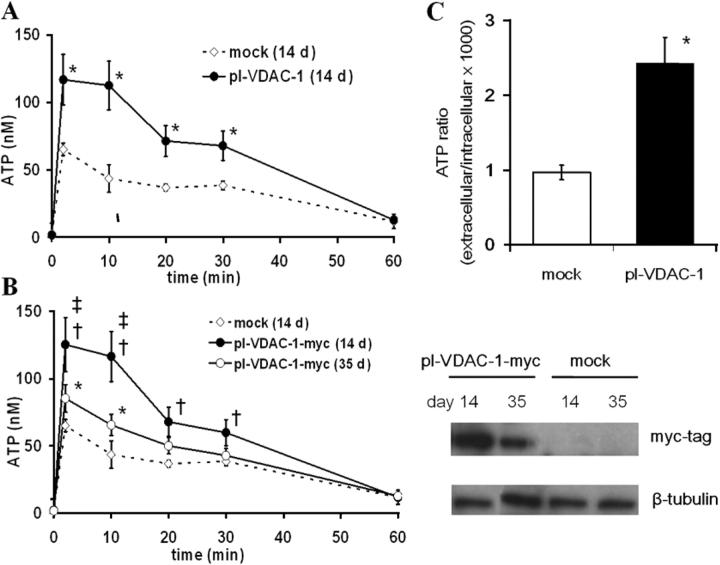
Figure 2.
ATP release from mock (empty virus)- and pl-VDAC-1–transfected NIH 3T3 cells in response to medium change. (A) 14 d after transfection by retroviral vectors and puromycin selection, heterogenous populations of mock-transfected (⋄) or pl-VDAC-1–transfected (•) NIH 3T3 cells were studied for ATP release. After medium change, extracellular medium was collected at each indicated time and ATP was measured by luminometry (n = 4). * indicates significant difference between pl-VDAC-1– and mock-transfected, P < 0.05. (B) ATP release properties after medium change and expression levels of transfected protein in pl-VDAC-1-myc–transfected cells. (Left) ATP levels were measured from mock- and pl-VDAC-1-myc–transfected cells 14 and 35 d after transfection with the same protocol used in A (n = 4). †, ‡, and * indicate significant differences (P < 0.05) between mock- and pl-VDAC-myc (14 d after) transfection, pl-VDAC-1-myc (14 d after) and pl-VDAC-1-myc (35 d after) transfection, and mock and pl-VDAC-1-myc (35 d after) transfection, respectively. (Right) Western blots of exogenous pl-VDAC-1-myc expression. In top panel, whole cell lysates (30 μg/lane) were probed with an anti-myc tag rabbit polyclonal antibody and an HRP-conjugated goat anti-rabbit IgG. Bottom panel is anti–β-tubulin as a marker of total protein loading. A band of the expected size of ∼39 kD was detected only in pl-VDAC-1-myc–transfected cells. The band had a higher intensity 14 d after transfection compared with 35 d after transfection. (C) ATP release from mock- or pl-VDAC-1–transfected clonal cell populations. Bars represent the mean values of extracellular ATP, 2 min after medium change for 11 clones/group. The data were normalized for intracellular ATP as follows: extracellular ATP (mol) in bulk medium/intracellular ATP (mol) per well × 1,000 (n = 4). * indicates significant difference (P < 0.05) between mock- and pl-VDAC-1-myc–transfected clones.
A persistent problem with pl-VDAC-1 overexpression studies has been selection against pl-VDAC-1 expression (Buettner et al., 2000). To test whether such a phenomenon existed in pl-VDAC-1–transfected NIH 3T3 cells, we measured extracellular ATP levels after medium change and pl-VDAC-1-myc protein expression in pl-VDAC-1-myc–transfected cells 14 and 35 d after transfection. As compared with measurements 14 d after transfection, decreased extracellular ATP accumulation and pl-VDAC-1-myc protein expression were observed in cells 35 d after transfection (Fig. 2 B). As above, pl-VDAC-1-myc–transfected cells exhibited similar extracellular ATP hydrolysis rates (from 200 nM at t = 0 to 103.7 ± 3.8 nM 14 d after transfection and 105.1 ± 3.5 nM 35 d after transfection 2 h later, n = 4), intracellular ATP levels (9.7 ± 0.3 and 9.5 ± 0.3 nmol/million cells 14 and 35 d after transfection, respectively, n = 4), and cell viability (0.31 ± 0.13% before medium change and 0.42 ± 0.15% after medium change, n = 4) as mock-transfected cells. These results suggest that changes in pl-VDAC-1 expression correlate with medium change–induced extracellular ATP levels.
Because of the possible bias in comparing heterogeneous pools of puromycin–selected cells, clonal cell populations were isolated and established from mock- or pl-VDAC-1-myc–transfected cells. ATP release levels varied between pl-VDAC-1-myc–transfected clones, which may be due to the variability in pl-VDAC-1 expression levels. The mean value of ATP release levels from pl-VDAC-1-myc–transfected clones exceeded those of mock-transfected clones in response to a medium change (11 clones from each group; Fig. 2 C), consistent with results from heterogeneous populations shortly after transfection (Fig. 2 A). 9 out of 11 pl-VDAC-1-myc–transfected clones exhibited significantly higher ATP levels (ranging from 150 to 420%) compared with the average of mock-transfected clones. Intracellular ATP and basal extracellular ATP levels were not different between these two groups.
VDAC-1 Expression in Tissues from VDAC-1 (−/−) and WT Mice
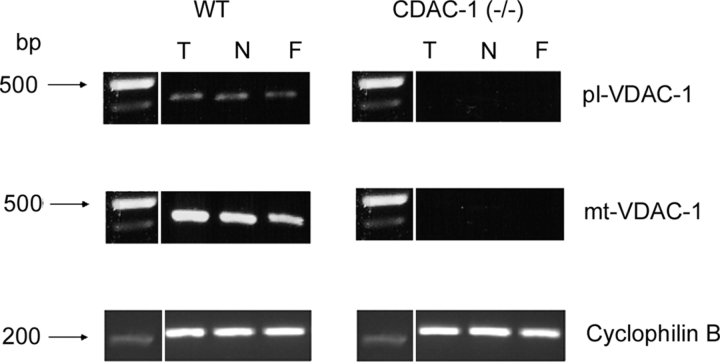
By using designed primers (Fig. 1 A), we confirmed by RT-PCR that WT mice expressed both mt- and pl-VDAC-1 mRNA in nasal and tracheal epithelial cultures and in lung fibroblasts, whereas cells from VDAC-1 (−/−) mice exhibited neither VDAC transcript (Fig. 3).
Figure 3.
Expression of pl-VDAC-1 and mt-VDAC-1 mRNA in primary cultures from WT and VDAC-1 (−/−) mice. RNA was prepared from 4-wk-old well-differentiated tracheal (T) and nasal (N) cultures and lung fibroblasts (F) for RT-PCR amplification of pl-VDAC-1 or mt-VDAC-1. Primer sets f-pl and r, and f-mt and r were used to amplify pl-VDAC-1 and mt-VDAC-1, respectively. Cyclophilin B mRNA was amplified as a housekeeping control. Both pl-VDAC-1 and mt-VDAC-1 were detected from tracheal (T) and nasal (N) cultures and from fibroblasts (F) of WT mice. Bands on the gel were extracted and the sequence was confirmed for each transcript. RT-PCR of RNA from VDAC-1 (−/−) cultures exhibited no detectable VDAC transcripts.
ATP Release from VDAC-1 (−/−) and WT Fibroblasts
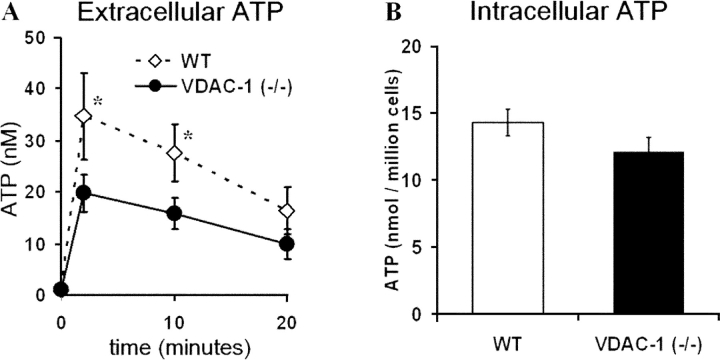
Both VDAC-1 (−/−) and WT fibroblasts responded robustly when stimulated mechanically by a medium change (Fig. 4 A). However, WT cells exhibited significantly higher extracellular ATP levels compared with VDAC-1 (−/−) cells 2 and 10 min after medium change. No differences were observed in basal extracellular ATP levels. Intracellular ATP levels were not different between VDAC-1 (−/−) and WT cells (Fig. 4 B).
Figure 4.
Extracellular and intracellular ATP levels from WT and VDAC-1 (−/−) mouse fibroblasts. (A) Time course for ATP release after medium change. Points represent mean, ±SEM, of experiments conducted on three separate litters with n = 4 transwells/genotype/litter. * indicates significant difference between WT (⋄) and VDAC-1 (−/−) (•) mice at P < 0.05. (B) Intracellular ATP levels after extraction by TCA. Bars represent mean, ±SEM, of three litters with n = 4 transwells/genotype/litter.
Hypotonicity-induced ATP Release from VDAC-1 (−/−) and WT Airway Epithelial Cells
Apical and basolateral surface liquids bathing well-differentiated tracheal and nasal cultures were sampled and ATP concentrations measured before and during hypotonic challenge by two complementary methods, luciferin-luciferase derived luminometry and etheno-derivatization coupled to HPLC.
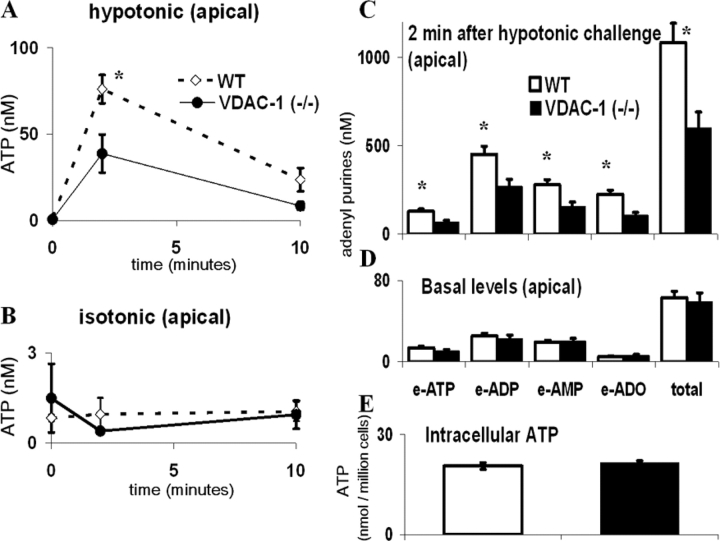
With luminometry, no differences in ATP concentrations were observed between VDAC-1 (−/−) and WT tracheal cells in the basal (resting) state in either the apical (Fig. 5 A) or basolateral (0.1 ± 0.03 nM in VDAC (−/−) and 0.11 ± 0.02 nM in WT, three experiments on three separate litters with n = 4 transwells/genotype/litter) liquids. 2 min after 10 μl H2O was gently added to the apical surface to generate a 66% hypotonic challenge, a robust increase in ATP in apical surface liquid was observed in WT cells, whereas VDAC-1 (−/−) mouse cultures exhibited a smaller increase in levels of released ATP (Fig. 5 A). Basolateral bath ATP concentrations showed no change in response to apical hypotonic challenge for either genotype (ΔATP at 2 min = 0.02 ± 0.005 nM in VDAC-1 (−/−) and 0.03 ± 0.01 nM in WT, three experiments on three separate litters with n = 4 transwells/genotype/litter).
Figure 5.
Extracellular ATP concentrations in the apical surface liquid of tracheal cultures from WT and VDAC-1 (−/−) mice after apical hypotonic challenge. (A) Apical hypotonic challenge: H2O was added gently to the apical surface at t = 0 to generate a 200 mOsm solution, and samples were collected from the apical solution at each indicated time and analyzed by luminometry. (B) Apical isosmotic challenge: a 300 mOsm mannitol solution was added to the lumen at t = 0 and samples obtained and processed as in A. Points represent mean, ±SEM, of experiments conducted on three separate litters with n = 8 transwells/genotype/litter. * indicates significant difference between WT (⋄) and VDAC-1 (−/−) (•) mice (P < 0.05). (C and D) Profile of adenyl purines in the apical surface liquid covering (D) basal (t = 0) and (C) hypotonicity-stimulated (2 min after challenge) tracheal epithelia. Samples at each point were analyzed by etheno-derivertization. Bars represent mean, ±SEM, of experiments conducted on three separate litters with n = 12 transwells/genotype/litter. * indicates significant difference between WT (□) and VDAC-1 (−/−) (▪) mice (P < 0.05). (E) Intracellular ATP levels determined after extraction by TCA. Bars represent mean, ±SEM, of three litters with n = 4 transwells/genotype /litter.
Based on the measurements with mouse tracheal cells that (a) intracellular ATP was ∼1.8 nmol/well-differentiated cells confluent on a 6.5-mm transwell (Fig. 5 E, ∼20 nmol ATP/million cells × ∼9 × 104 cells/transwell); (b) the resting state ATP concentration as microsampled was ∼1 nM, and was increased to ∼75 nM by hypotonic challenge (Fig. 5 A); and (c) apical surface liquid was 30 μl, our calculations demonstrated that the amount of ATP released under basal and stimulated conditions represented only 0.0017 and 0.12% of intracellular ATP, respectively.
To control for the effect of the mechanical stress due to liquid addition, isosmotic liquid (10 μl mannitol) was added to the apical surface, which had no effect on apical (Fig. 5 B) or basolateral (remained <0.2 nM at all time points) surface liquid ATP levels for both genotypes. In separate experiments, basolateral hypotonic challenge (66%) caused increase in basolateral ATP levels (5.2 ± 0.5 fold in WT and 2.8 ± 0.4 fold in VDAC-1 (−/−), 2 min after hypotonic challenge, three experiments on three separate litters with n = 4 transwells/genotype/litter) with no change in apical ATP levels.
Luminometry is highly sensitive. However, since ATP is rapidly hydrolyzed by ecto-nucleotidases (Picher et al., 2003), measurement of ATP alone could underestimate the total amount of released ATP and lead to inaccurate comparisons of ATP release between cell populations with different cell surface ecto-nucleotidase activities. Thus, the etheno-derivertization protocol, which allowed us to quantitate all adenyl purine species, was useful to estimate the total amount of released ATP, irrespective of its conversion to ADP, AMP, and adenosine. With etheno-derivertization, no differences in basal accumulation of individual or total adenyl purine species were detected between VDAC-1 (−/−) and WT tracheal cultures (Fig. 5 D). However, 2 min after hypotonic challenge, an increased accumulation of adenyl purines was observed in both VDAC-1 (−/−) and WT cells, but the accumulation of ATP, ADP, AMP, and adenosine was greater in WT cells (Fig. 5 C). Intracellular ATP levels were not different between VDAC-1 (−/−) and WT mice cells (Fig. 5 E).
A profile similar to that of tracheal cultures was observed in nasal cultures: apical ATP concentrations (nM) at stationary state were 1.5 ± 0.3 in VDAC-1 (−/−) and 1.2 ± 0.3 in WT; 2 min after hypotonic challenge, apical ATP concentrations were 14.0 ± 2.4 in VDAC-1 (−/−) and 33.3 ± 5.1 in WT (with luminometry, three experiments on three separate litters with n = 6 transwells/genotype/litter). As in tracheal cells, intracellular ATP levels did not differ between the two groups of nasal cells; 25.4 ± 0.1 and 27.8 ± 0.6 nmol/million cells in VDAC-1 (−/−) and WT cells, respectively (with luminometry, three litters with n = 4 transwells/genotype/litter).
Role of ATP Release in Airway Epithelial Regulatory Volume Decrease
The observation that airway epithelial cells responded to luminal hypotonic challenge with ATP release raised the question as to the physiological relevance of hypotonicity-induced ATP release and the role of pl-VDAC-1 in this physiology. Because a role for extracellular ATP in facilitating regulatory volume decrease (RVD) after hypotonic cell swelling via P2Y2 receptor–mediated Cl− and K+ channel activation has been reported in HTC rat hepatoma cells (Wang et al., 1996b; Roman et al., 1997; Feranchak et al., 1998), human intestine 407 cells (Dezaki et al., 2000) and human cholangiocarcinoma cells (Gatof et al., 2004), we investigated whether similar mechanisms were present in mouse airway epithelia and whether pl-VDAC-1 was involved.
Tracheal cells from FOXJ1/EGFP Transgenic Mice
Airway epithelial cultures exhibit a complex phenotype with differentiated surface cells, including ciliated cells and goblet cells, covering a single to several layers of basal cells. To investigate the role of ATP release in epithelial cell volume regulatory responses, we initiated studies with a model system that allowed accurate measurements of the volume responses of lumen-facing cells to luminal hypotonicity. Well-differentiated tracheal cultures from FOXJ1/EGFP transgenic mice that selectively express EGFP in ciliated cells (Ostrowski et al., 2003) were used. The height of each fluorescent ciliated cell was measured by confocal microscopy before (t = 0) and at designated times after dilution of the apical liquid to form a hypotonic (200 mOsm) solution. Tracheal epithelial cells exhibited a rapid increase in height after hypotonic challenge (Fig. 6 A). This initial increase (∼25%) was less than the expected level of volume increase (50%) if these cells acted as perfect osmometers in response to the dilution of apical liquid (300 to 200 mOsm) (Fig. 6 B). In comparison to previous reports describing the absence of regulatory volume increase (RVI) in human airway epithelia after hypertonic challenge (Willumsen et al., 1994; Matsui et al., 2000), mouse airway cells exhibited an RVD after hypotonic challenge.
Figure 6.
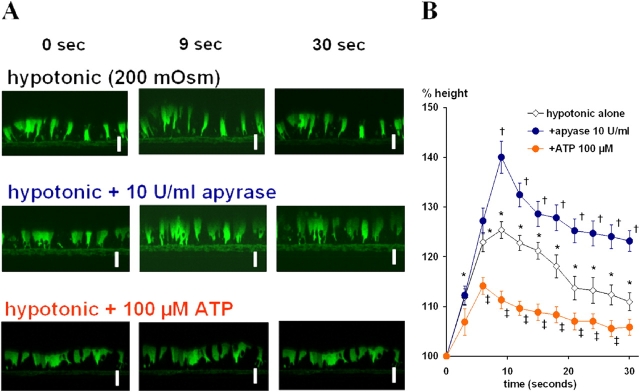
Hypotonicity-induced swelling and regulatory volume decrease (RVD) of tracheal cultures from FOXJ1/EGFP transgenic mice as measured by confocal microscopy. The cytosol of ciliated cells is defined by green fluorescence due to the ciliated cell–specific FOXJ1-driven expression of EGFP. Bar, 10 μm. (A) In the top panels, at t = 0, H2O was added gently onto the apical surface to generate 200 mOsm of hypotonicity. In the middle panels, cultures were preincubated with apyrase (10 U/ml) for 5 min, and H2O including apyrase (10 U/ml) was gently added to generate luminal hypotonicity at time 0. In the bottom panels, ATP (100 μM) was gently added together with luminal H2O challenge at t = 0. (B) Summary data for protocols illustrated in A. Points indicate mean relative tracheal cell height compared with t = 0, of cultures from two separate litters with n = 4 transwells/genotype/litter. * indicates significant difference (P < 0.05) of points for hypotonic challenge (H2O alone) group compared with t = 0. † indicates significant difference between hypotonic challenge alone (white) and apyrase pretreatment (blue) P < 0.05). ‡ indicates significant difference between hypotonic challenge alone and exogenous 100 μM ATP addition (red) (P < 0.05).
To test the role of luminally released ATP in RVD, cultures were pretreated with 10 U/ml apyrase (Fig. 6, A and B). After this treatment, the initial swelling in response to hypotonic challenge increased to a level closer to the response of a perfect osmometer (∼40%). Further, RVD was less effective in returning cell heights to baseline. Conversely, when the cultures were exposed to 100 μM ATP with hypotonic challenge, the peak cell height responses to hypotonic challenge decreased with more rapid return to baseline cell height (Fig. 6, A and B). Collectively, these data suggested a role for extracellular ATP to facilitate the cell volume recovery during and after luminal hypotonicity–induced cell swelling.
Tracheal Cells from VDAC-1 (−/−) and WT Mice
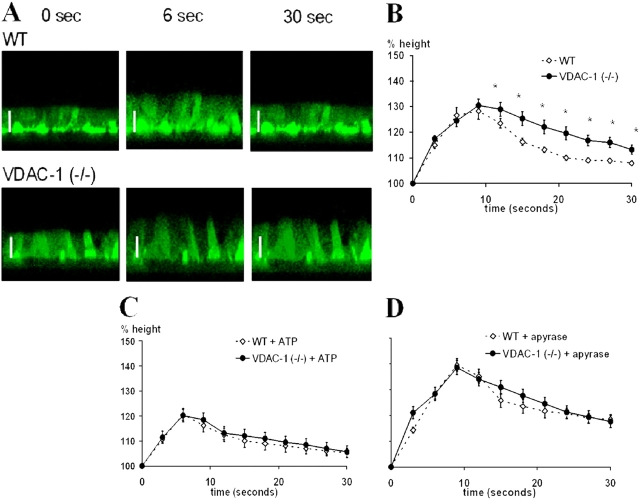
Having demonstrated that ciliated cells undergo swelling and RVD upon luminal hypotonic challenge, we next addressed whether pl-VDAC-1 was involved in the process. Well-differentiated tracheal cultures from VDAC-1 (−/−) and WT mice were studied with the same protocol as used in FOXJ1/EGFP cultures, except that cells were labeled with calcein-AM and cell volume change measured as change of luminal cell height, defined as the distance from the transwell membrane to the top of the cytoplasm (see materials and methods). Tracheal epithelia from both genotypes exhibited increases in cell height and RVD after hypotonic challenge (Fig. 7 A). The initial volume increase of tracheal cultures was similar for VDAC-1 (−/−) and WT mice, but RVD was consistently slower in VDAC-1 (−/−) cells (Fig. 7 B).
Figure 7.
Hypotonicity-induced swelling and RVD of WT and VDAC-1 (−/−) mouse tracheal epithelial cultures measured in calcein-AM–labeled cells with confocal microscopy. (A) Representative time course for swelling and RVD of (top) WT and (bottom) VDAC-1 (−/−) tracheal cultures. At t = 0, H2O was added gently onto the apical surface to generate a 200 mOsm solution. Bar, 10 μm. (B) Summary data describing time course of cell volume response to apical hypotonic stimulus. * indicates significant difference (P < 0.05) between WT (⋄) and VDAC-1 (−/−) (•). (C and D) Hypotonicity-induced swelling and RVD of WT and VDAC-1 (−/−) cultures with exogenous addition of (C) ATP or (D) apyrase. The same protocol for treatment and measurement were used as in Fig. 6. In B–D, points indicate percent initial cell height. Each point depicts the mean, ±SEM, of data from experiments on three separate litters with n = 4 transwells/genotype/litter.
We speculated that the slower rate of RVD in VDAC-1 (−/−) mouse tracheal cultures reflected the reduced rate of ATP release, and hence, lower ATP concentration at the cell surface. Tests of this hypothesis included whether an excess or an absence of ATP eliminated the difference in RVD kinetics between VDAC-1 (−/−) and WT tracheal cells. As shown in Fig. 7 C, addition of ATP to the lumen of VDAC-1 (−/−) and WT cultures resulted in reduced initial cell height responses, and importantly similar RVD kinetics, in both cell types. Conversely, addition of apyrase to the lumen led to similar increases in initial cell height, and again, similar kinetics of RVD in both cell types (Fig. 7 D). Thus, we conclude that the response of both cell types to each maneuver is similar, and hence, the differences in RVD kinetics between VDAC-1 (−/−) and WT epithelia in the absence of these maneuvers (Fig. 7 B) likely reflect differences in ATP release rates.
DISCUSSION
Our experiments explored the potential relationship of VDAC-1 to ATP release from murine cells. In a first series of studies, this relationship was investigated in NIH 3T3 fibroblasts. Functionally, greater ATP release was observed from pl-VDAC-1–transfected fibroblasts compared with mock-transfected cells in response to medium change 14 d after transfection (Fig. 2). Thus, it appears that the flow-induced shear stress induced by a medium change interacted with pl-VDAC-1 to generate ATP release. Of note, mock-transfected cells released ATP, albeit in lower amounts, in response to medium change, which may partially reflect endogenous pl-VDAC-1 expression or other mechanisms of ATP release. The absence of differences in basal ATP levels between pl-VDAC-1– and mock-transfected cells suggests that pl-VDAC-1–independent mechanisms regulate basal ATP release.
When pl-VDAC-1-myc–transfected fibroblasts were maintained in culture longer than 35 d after transfection, the enhanced ATP release after mechanical stress was reduced in parallel with a decreased level of pl-VDAC-1-myc expression (Fig. 2 B). This result may reflect the cytotoxicity resulting from pl-VDAC-1 overexpression (Buettner et al., 2000), and the correlation of pl-VDAC-1 protein expression levels and enhanced ATP release levels argues for a role of pl-VDAC-1 in ATP release.
As required for all studies of extracellular ATP release, we investigated whether the higher rate of ATP release from pl-VDAC-1–transfected fibroblasts resulted from cell lysis, e.g., cell fragility due to pl-VDAC-1 transfection. However, from the morphological observations and cell viability assays, cell lysis was minimal and not different between mock- and pl-VDAC-1–transfected populations. These data, plus data demonstrating no effect of pl-VDAC-1 transfection on extracellular ATP metabolism, led us to conclude that pl-VDAC-1 transfection conferred higher shear stress–induced rates of ATP release from NIH 3T3 fibroblasts.
As a second approach to address the role of pl-VDAC-1 in cellular ATP release, we used the VDAC-1 (−/−) mice model, which provided a null background for both mt-VDAC-1 and pl-VDAC-1. In mice, three VDAC genes (VDAC-1, VDAC-2, and VDAC-3) have been identified that are 65–70% identical to one another (Sampson et al., 1997). Targeted disruption of VDAC-1 exhibited a partial, strain-specific embryonic lethality, whereas VDAC-2 (−/−) embryos were nonviable and VDAC-3 (−/−) mice were viable (Anflous et al., 2001; Sampson et al., 2001; Weeber et al., 2002; Cheng et al., 2003). The lethality of VDAC-2 disruption is explained by its function as a component of the mitochondrial permeability transition pore that participates in apoptotic and necrotic cell death (Cheng et al., 2003). VDAC-1 (−/−) mice of viable strains exhibited altered mitochondrial ADP translocation into mitochondria in striated muscles (ventricle, soleus, and gastrocnemius) (Anflous et al., 2001) as well as disrupted fear conditioning and spatial learning (Weeber et al., 2002). The reason why lethality of VDAC-1 is only partial, despite the facts that it is the most abundant isoform and plays a critical role in maintaining cellular homeostasis by transporting purine metabolites across mitochondrial outer membrane, may be explained by redundancy. In support of this notion, we found that the intracellular ATP concentrations in fibroblasts and tracheal/nasal epithelia grown in vitro were not affected by VDAC-1 deficiency (Fig. 4 B and Fig. 5 E).
Hypotonic challenge, which induces membrane shear stress secondary to membrane expansion, is an effective stimulus for ATP release from various epithelia (Wang et al., 1996a; Hazama et al., 1999). Hypotonic challenge of the lumen of tracheal and nasal epithelia greatly increased the ATP concentrations in the luminal liquid bathing WT cultures without affecting the ATP concentration of the basolateral baths (Fig. 5 A). The levels of ATP in the luminal liquid of VDAC-1 (−/−) cultures after a hypotonic challenge were reduced by ∼50% compared with WT cultures, in both luminometry and etheno-derivertization, suggesting a true reduction in ATP release from VDAC-1 (−/−) cultures rather than a difference in ectonucleotidase activities (Fig. 5, A and C). The observation that the intracellular ATP levels in VDAC-1 (−/−) cells were similar to WT cells (Fig. 5 E) argued against the possibility that decreased ATP release from VDAC-1 (−/−) cells reflected a decreased chemical driving force resulting from decreased intracellular ATP concentrations. Thus, we conclude that VDAC-1 contributes to hypotonicity-induced ATP release into apical surface liquid in WT mouse airway epithelia.
Several possible mechanisms may mediate the contribution of VDAC-1 to ATP release. It is possible that VDAC-1 itself serves as a plasmalemmal ATP release channel. Our calculations demonstrated that the amount of ATP released under basal and stimulated conditions represented only 0.0017 and 0.12% of intracellular ATP, respectively. This rate of release may require no more than a few active channels/cell and, likely, would not be cytotoxic to cells. It is also possible that VDAC could regulate the activity of ATP release molecules within the plasma membrane. Alternatively, VDAC-1 may not need to localize on plasma membrane to regulate ATP release. However, our data argue that VDAC-1 does not affect ATP release by regulating intracellular ATP concentration and hence altering the chemical driving force for translocation (Fig. 4 B and Fig. 5 E).
We should note that VDAC-1 (−/−) airway epithelia and fibroblasts exhibited basal ATP levels similar to those of WT cells and released ∼50% less, but significant, amounts of ATP in response to stimuli. These findings suggest that VDAC-1 (−/−) cells have mechanisms for basal and stimulated ATP release independent from VDAC-1. Future studies are required to assess whether these mechanisms involve other VDAC-like channels, transporters, or as suggested by recent studies, vesicular exocytosis of ATP (Knight et al., 2002; Schwiebert et al., 2002; van der Wijk et al., 2003; Gatof et al., 2004).
The interaction of extracellular ATP with purinoreceptors facilitates RVD after hypotonic cell swelling in various cell types (Hwang et al., 1996; Gatof et al., 2004). Based on the finding that VDAC-1 (−/−) airway cells released ∼50% less ATP compared with WT cells in response to hypotonic challenge, we investigated the role of ATP release in cellular swelling and RVD in mouse airway epithelia. Data derived from EGFP-expressing ciliated cells, which allowed for the most accurate measurement of changes in cell height, revealed that mouse airway cells responded to hypotonic challenge with cell swelling (Fig. 6 A). Importantly, confocal microscopy confirmed that the cellular responses to luminal hypotonicity were initial swelling and subsequent RVD, not irreversible cell lysis, supporting the concept that ATP release after hypotonic challenge did not reflect cytolytic release. The data from these cells were consistent with a role for released ATP both in regulating the height of the apparent initial increase in cell height and the magnitude of RVD. For example, the initial height change in response to hypotonic challenge was less (∼25%; Fig. 6 B) than predicted if the apical membrane water permeability were limiting (50%; Matsui et al., 2000). The addition of apyrase or ATP increased or reduced the initial cell height change, respectively (Fig. 6 B). These data suggest that the mouse airway epithelial volume response to luminal hypotonicity is mediated by two mechanisms. First, the initial cell swelling reflects the movement of water into the cell across the highly water-permeable apical membrane in response to the imposed osmotic gradient (Matsui et al., 2000). Second, there is a component of cell volume regulation that is mediated by loss of cellular osmotic content. This latter response is mediated by ATP release and activation of P2Y2 receptors. P2Y2 receptors, via increase in intracellular Ca2+ and possibly protein kinase C α activation (Hermoso et al., 2004), activate both apical Cl− and K+ channels to release KCl into the apical medium, which produces RVD (Clarke and Boucher, 1992; Clarke et al., 1997; Homolya et al., 1999; Okada et al., 2001; Darby et al., 2003). This second component (ATP-mediated KCl movement) is likely to occur simultaneously with water movement into the cell, resulting in smaller than theoretical initial cell height increase (i.e., ∼25 compared with 50%).
The role of VDAC-1 in the ATP signaling component of hypotonicity-induced RVD was investigated in VDAC-1 (−/−) cultures. The initial swelling of tracheal or nasal epithelia in response to hypotonicity was similar for VDAC-1 (−/−) and WT mice, but the recovery from the peak of swelling (“RVD phase”) was markedly slower in VDAC-1 (−/−) cells than in WT cells (Fig. 7 B). The observations that VDAC-1 (−/−) cells (a) did not exhibit detectably larger initial swelling responses than WT cells and (b) responded to apyrase treatment with an increased peak cell height and a slower RVD (Fig. 7 D) may reflect the partial ATP release in VDAC-1 (−/−) cultures in response to hypotonicity (Fig. 5 A). When extracellular ATP levels were pharmacologically reduced or increased by addition of apyrase or ATP, respectively, VDAC-1 (−/−) and WT cells exhibited similar peak cell height response and RVD in response to hypotonic challenge (Fig. 7, C and D). From these data, we conclude that VDAC-1 plays a physiological role in the RVD response to luminal hypotonicity by mediating luminal extracellular ATP release.
It is important to note that not all species may have a putative plasmalemmal splice variant of VDAC. Mouse and human VDAC-1s are 98% identical at the amino acid level. However, bioinformatics sequence analyses have not identified a pl-VDAC-1 splice site in the human gene. We attempted to identify such a splice variant isoform of human mitochondrial VDAC-1 by 5′-RACE-PCR, but 100 out of 100 clones isolated were identical to mitochondrial VDAC-1, without a coding sequence for the NH2-terminal signal peptide expected for plasmalemmal VDAC-1. Thus, the role of pl-VDAC-1 in ATP release in murine cells may be served by other proteins in humans. It is, however, possible that mt-VDAC-1 is expressed also in the plasma membrane in humans. Recent biochemical observations suggest the expression of VDAC-1 (identical to the mitochondrial form) in the plasma membranes of human endothelial cells (Gonzalez-Gronow et al., 2003) and human B lymphocytes (Baker et al., 2004). Thus, we cannot distinguish amongst the hypotheses that human airway epithelia (a) do not express VDAC-1 or a related protein at the plasma membrane; (b) some mt-VDAC-1 enters the secretory pathway to reach the plasma membrane and mediate ATP release; or (c) a related VDAC-like protein is expressed at the plasma membrane and mediates ATP release.
In summary, we conclude that VDAC-1 contributes to ATP transport across the plasma membrane of murine cells. Studies of pl-VDAC-1 overexpression in NIH 3T3 cells produced evidence for both increased ATP release in response to mechanical stimuli. Studies of airway epithelial cells from VDAC-1–deficient mice revealed reduced ATP release and reduced ATP-mediated cell volume responses to luminal hypotonicity as compared with littermate controls. Our studies do not resolve the issue of whether VDAC-1 exerts those activities by being expressed at the plasma membrane as maxi-anion channels, via regulation of vesicular exocytosis, or as a regulator of other ATP transport proteins. Further, our data suggest that molecules other than VDAC are involved in basal ATP release and a component (∼50%) of stress-induced ATP release. However, our studies ruled out other explanations to account for reduced extracellular ATP levels in VDAC-1 (−/−) cells, e.g., reduced intracellular ATP concentrations, facilitated extracellular ATP hydrolysis, or decreased cell lysis. Thus, our data argue that VDAC-1 protein is a candidate molecule to control the release of ATP into the extracellular environment of murine cells.
Acknowledgments
The authors are grateful to Dr. John Olsen (The University of North Carolina at Chapel Hill) for providing retroviral vectors; to Dr. Barbara Grubb for access to microsampling apparatus; to Dr. Hirotoshi Matsui for technical and intellectual suggestions regarding confocal microscopy; to Dr. Jaclyn Stonebreaker, Chuanwen Sun, Dan Gillie, and Catharina van Heusden for excellent technical support and advice; to Drs. Rolf Dermietzel, David Spray, Eliana Scemes, Vito De Pinto, Gyorgy Bathori, Miguel Valverde, and Shawn Ahmed for helpful discussions and critical reading of the manuscript.
This work has been supported by the National Institutes of Health (NIH) and the Cystic Fibrosis Foundation (CFF) through grants to R.C. Boucher (NIH/NHLBI 5 P01 HL 34322-18, CFF R026-CR02).
Olaf S. Andersen served as editor.
P. Huang's present address is Department of Biology, Hong Kong University of Science and Technology, Kowloon, Hong Kong.
Abbreviations used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator; DMEM, Dulbecco's modified eagles medium; mt, mitochondrial; pl, plasmalemmal; RVD, regulatory volume decrease; TCA, trichloroacetic acid; VDAC, voltage-dependent anion channel; WT, wild type.
References
- Anflous, K., D.D. Armstrong, and W.J. Craigen. 2001. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 276:1954–1960. [DOI] [PubMed] [Google Scholar]
- Bahamonde, M.I., J.M. Fernandez-Fernandez, F.X. Guix, E. Vazquez, and M.A. Valverde. 2003. Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated maxi Cl− currents in C1300 neuroblastoma cells. J. Biol. Chem. 278:33284–33289. [DOI] [PubMed] [Google Scholar]
- Baker, M.A., D.J. Lane, J.D. Ly, V. De Pinto, and A. Lawen. 2004. VDAC1 is a transplasma membrane NADH-ferricyanide reductase. J. Biol. Chem. 279:4811–4819. [DOI] [PubMed] [Google Scholar]
- Bathori, G., I. Parolini, F. Tombola, I. Szabo, A. Messina, M. Oliva, V. De Pinto, M. Lisanti, M. Sargiacomo, and M. Zoratti. 1999. Porin is present in the plasma membrane where it is concentrated in caveolae and caveolae-related domains. J. Biol. Chem. 274:29607–29612. [DOI] [PubMed] [Google Scholar]
- Bell, P.D., J.Y. Lapointe, R. Sabirov, S. Hayashi, J. Peti-Peterdi, K. Manabe, G. Kovacs, and Y. Okada. 2003. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc. Natl. Acad. Sci. USA. 100:4322–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin, P., and G. Burnstock. 2001. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J. Cardiovasc. Pharmacol. 38:900–908. [DOI] [PubMed] [Google Scholar]
- Braunstein, G.M., R.M. Roman, J.P. Clancy, B.A. Kudlow, A.L. Taylor, V.G. Shylonsky, B. Jovov, K. Peter, T. Jilling, I.I. Ismailov, et al. 2001. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J. Biol. Chem. 276:6621–6630. [DOI] [PubMed] [Google Scholar]
- Buettner, R., G. Papoutsoglou, E. Scemes, D.C. Spray, and R. Dermietzel. 2000. Evidence for secretory pathway localization of a voltage-dependent anion channel isoform. Proc. Natl. Acad. Sci. USA. 97:3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. 1997. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 36:1127–1139. [DOI] [PubMed] [Google Scholar]
- Caldwell, R.A., H.F. Clemo, and C.M. Baumgarten. 1998. Using gadolinium to identify stretch-activated channels: technical considerations. Am. J. Physiol. 275:C619–C621. [DOI] [PubMed] [Google Scholar]
- Cheng, E.H., T.V. Sheiko, J.K. Fisher, W.J. Craigen, and S.J. Korsmeyer. 2003. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 301:513–517. [DOI] [PubMed] [Google Scholar]
- Clarke, L.L., and R.C. Boucher. 1992. Chloride secretory response to extracellular ATP in human normal and cystic fibrosis nasal epithelia. Am. J. Physiol. 263:C348–C356. [DOI] [PubMed] [Google Scholar]
- Clarke, L.L., T. Chinet, and R.C. Boucher. 1997. Extracellular ATP stimulates K+ secretion across cultured human airway epithelium. Am. J. Physiol. 272:L1084–L1091. [DOI] [PubMed] [Google Scholar]
- Clarke, L.L., B.R. Grubb, S.E. Gabriel, O. Smithies, B.H. Koller, and R.C. Boucher. 1992. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 257:1125–1128. [DOI] [PubMed] [Google Scholar]
- Cole, T., L.A. Awni, E. Nyakatura, H. Gotz, G. Walter, F.P. Thinnes, and N. Hilschmann. 1992. Studies on human porin. VIII. Expression of “Porin 31HL” channels in the plasmalemma of the acute-lymphoblastic-leukemia cell line KM3 as revealed by light- and electron-microscopy. Biol. Chem. Hoppe Seyler. 373:891–896. [DOI] [PubMed] [Google Scholar]
- Cotrina, M.L., J.H. Lin, and M. Nedergaard. 1998. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J. Neurosci. 18:8794–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby, M., J.B. Kuzmiski, W. Panenka, D. Feighan, and B.A. MacVicar. 2003. ATP released from astrocytes during swelling activates chloride channels. J. Neurophysiol. 89:1870–1877. [DOI] [PubMed] [Google Scholar]
- Davis, C.W., M.L. Dowell, M. Lethem, and M. Van Scott. 1992. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am. J. Physiol. 262:C1313–C1323. [DOI] [PubMed] [Google Scholar]
- Dermietzel, R., T.K. Hwang, R. Buettner, A. Hofer, E. Dotzler, M. Kremer, R. Deutzmann, F.P. Thinnes, G.I. Fishman, D.C. Spray, et al. 1994. Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc. Natl. Acad. Sci. USA. 91:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezaki, K., T. Tsumura, E. Maeno, and Y. Okada. 2000. Receptor-mediated facilitation of cell volume regulation by swelling-induced ATP release in human epithelial cells. Jpn. J. Physiol. 50:235–241. [DOI] [PubMed] [Google Scholar]
- Dubyak, G.R., and C. el-Moatassim. 1993. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 265:C577–C606. [DOI] [PubMed] [Google Scholar]
- Feranchak, A.P., R.M. Roman, E.M. Schwiebert, and J.G. Fitz. 1998. Phosphatidylinositol 3-kinase contributes to cell volume regulation through effects on ATP release. J. Biol. Chem. 273:14906–14911. [DOI] [PubMed] [Google Scholar]
- Gatof, D., G. Kilic, and J.G. Fitz. 2004. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G538–G546. [DOI] [PubMed] [Google Scholar]
- Geary, C.A., C.W. Davis, A.M. Paradiso, and R.C. Boucher. 1995. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am. J. Physiol. 268:L1021–L1028. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow, M., T. Kalfa, C.E. Johnson, G. Gawdi, and S.V. Pizzo. 2003. The voltage-dependent anion channel is a receptor for plasminogen kringle 5 on human endothelial cells. J. Biol. Chem. 278:27312–27318. [DOI] [PubMed] [Google Scholar]
- Grygorczyk, R., and J.W. Hanrahan. 1997. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am. J. Physiol. 272:C1058–C1066. [DOI] [PubMed] [Google Scholar]
- Guibert, B., R. Dermietzel, and D. Siemen. 1998. Large conductance channel in plasma membranes of astrocytic cells is functionally related to mitochondrial VDAC-channels. Int. J. Biochem. Cell Biol. 30:379–391. [DOI] [PubMed] [Google Scholar]
- Hazama, A., T. Shimizu, Y. Ando-Akatsuka, S. Hayashi, S. Tanaka, E. Maeno, and Y. Okada. 1999. Swelling-induced, CFTR-independent ATP release from a human epithelial cell line: lack of correlation with volume-sensitive Cl− channels. J. Gen. Physiol. 114:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso, M., P. Olivero, R. Torres, A. Riveros, A.F. Quest, and A. Stutzin. 2004. Cell volume regulation in response to hypotonicity is impaired in HeLa cells expressing a protein kinase C α mutant lacking kinase activity. J. Biol. Chem. 279:17681–17689. [DOI] [PubMed] [Google Scholar]
- Hisadome, K., T. Koyama, C. Kimura, G. Droogmans, Y. Ito, and M. Oike. 2002. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J. Gen. Physiol. 119:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolya, L., T.H. Steinberg, and R.C. Boucher. 2000. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J. Cell Biol. 150:1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolya, L., W.C. Watt, E.R. Lazarowski, B.H. Koller, and R.C. Boucher. 1999. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. J. Biol. Chem. 274:26454–26460. [DOI] [PubMed] [Google Scholar]
- Huang, P., E.R. Lazarowski, R. Tarran, S.L. Milgram, R.C. Boucher, and M.J. Stutts. 2001. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc. Natl. Acad. Sci. USA. 98:14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, T.H., E.M. Schwiebert, and W.B. Guggino. 1996. Apical and basolateral ATP stimulates tracheal epithelial chloride secretion via multiple purinergic receptors. Am. J. Physiol. 270:C1611–C1623. [DOI] [PubMed] [Google Scholar]
- Jakob, C., H. Gotz, T. Hellmann, K.P. Hellmann, S. Reymann, H. Florke, F.P. Thinnes, and N. Hilschmann. 1995. Studies on human porin: XIII. The type-1 VDAC ‘porin 31HL’ biotinylated at the plasmalemma of trypan blue excluding human B lymphocytes. FEBS Lett. 368:5–9. [DOI] [PubMed] [Google Scholar]
- Jia, Y., C.J. Mathews, and J.W. Hanrahan. 1997. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J. Biol. Chem. 272:4978–4984. [DOI] [PubMed] [Google Scholar]
- Knight, G.E., P. Bodin, W.C. De Groat, and G. Burnstock. 2002. ATP is released from guinea pig ureter epithelium on distension. Am. J. Physiol. Renal Physiol. 282:F281–F288. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., R. Tarram, B.R. Grubb, C.A. van Heusden, S. Okada, and R.C. Boucher. 2004. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 279:36855–36864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethem, M.I., M.L. Dowell, M. Van Scott, J.R. Yankaskas, T. Egan, R.C. Boucher, and C.W. Davis. 1993. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 9:315–322. [DOI] [PubMed] [Google Scholar]
- Light, D.B., E.M. Schwiebert, G. Fejes-Toth, A. Naray-Fejes-Toth, K.H. Karlson, F.V. McCann, and B.A. Stanton. 1990. Chloride channels in the apical membrane of cortical collecting duct cells. Am. J. Physiol. 258:F273–F280. [DOI] [PubMed] [Google Scholar]
- Linden, M., G. Andersson, P. Gellerfors, and B.D. Nelson. 1984. Subcellular distribution of rat liver porin. Biochim. Biophys. Acta. 770:93–96. [DOI] [PubMed] [Google Scholar]
- Mall, M., A. Wissner, T. Gonska, D. Calenborn, J. Kuehr, M. Brandis, and K. Kunzelmann. 2000. Inhibition of amiloride-sensitive epithelial Na+ absorption by extracellular nucleotides in human normal and cystic fibrosis airways. Am. J. Respir. Cell Mol. Biol. 23:755–761. [DOI] [PubMed] [Google Scholar]
- Mangel, A.W., J.R. Raymond, and J.G. Fitz. 1993. Regulation of high-conductance anion channels by G proteins and 5-HT1A receptors in CHO cells. Am. J. Physiol. 264:F490–F495. [DOI] [PubMed] [Google Scholar]
- Matsui, H., C.W. Davis, R. Tarran, and R.C. Boucher. 2000. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J. Clin. Invest. 105:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill, J.M., T.W. Gettys, S. Basavappa, and J.G. Fitz. 1993. GTP-binding proteins regulate high conductance anion channels in rat bile duct epithelial cells. J. Membr. Biol. 133:253–261. [DOI] [PubMed] [Google Scholar]
- Okada, Y., E. Maeno, T. Shimizu, K. Dezaki, J. Wang, and S. Morishima. 2001. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 532:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, J.C., L.G. Johnson, M.J. Stutts, B. Sarkadi, J.R. Yankaskas, R. Swanstrom, and R.C. Boucher. 1992. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum. Gene Ther. 3:253–266. [DOI] [PubMed] [Google Scholar]
- Ostrowski, L.E., J.R. Hutchins, K. Zakel, and W.K. O'Neal. 2003. Targeting expression of a transgene to the airway surface epithelium using a ciliated cell-specific promoter. Mol. Ther. 8:637–645. [DOI] [PubMed] [Google Scholar]
- Picher, M., L.H. Burch, A.J. Hirsh, J. Spychala, and R.C. Boucher. 2003. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J. Biol. Chem. 278:13468–13479. [DOI] [PubMed] [Google Scholar]
- Ralevic, V., and G. Burnstock. 1998. Receptors for purines and pyrimidines. Pharmacol. Rev. 50:413–492. [PubMed] [Google Scholar]
- Reisin, I.L., A.G. Prat, E.H. Abraham, J.F. Amara, R.J. Gregory, D.A. Ausiello, and H.F. Cantiello. 1994. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J. Biol. Chem. 269:20584–20591. [PubMed] [Google Scholar]
- Roman, R.M., N. Lomri, G. Braunstein, A.P. Feranchak, L.A. Simeoni, A.K. Davison, E. Mechetner, E.M. Schwiebert, and J.G. Fitz. 2001. Evidence for multidrug resistance-1 P-glycoprotein-dependent regulation of cellular ATP permeability. J. Membr. Biol. 183:165–173. [DOI] [PubMed] [Google Scholar]
- Roman, R.M., Y. Wang, S.D. Lidofsky, A.P. Feranchak, N. Lomri, B.F. Scharschmidt, and J.G. Fitz. 1997. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J. Biol. Chem. 272:21970–21976. [DOI] [PubMed] [Google Scholar]
- Rostovtseva, T., and M. Colombini. 1996. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J. Biol. Chem. 271:28006–28008. [DOI] [PubMed] [Google Scholar]
- Sabirov, R.Z., A.K. Dutta, and Y. Okada. 2001. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J. Gen. Physiol. 118:251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson, M.J., W.K. Decker, A.L. Beaudet, W. Ruitenbeek, D. Armstrong, M.J. Hicks, and W.J. Craigen. 2001. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 276:39206–39212. [DOI] [PubMed] [Google Scholar]
- Sampson, M.J., R.S. Lovell, and W.J. Craigen. 1997. The murine voltage-dependent anion channel gene family. Conserved structure and function. J. Biol. Chem. 272:18966–18973. [DOI] [PubMed] [Google Scholar]
- Schwarzer, C., S. Becker, L.A. Awni, T. Cole, R. Merker, S. Barnikol-Watanabe, F.P. Thinnes, and N. Hilschmann. 2000. Human voltage-dependent anion-selective channel expressed in the plasmalemma of Xenopus laevis oocytes. Int. J. Biochem. Cell Biol. 32:1075–1084. [DOI] [PubMed] [Google Scholar]
- Schwiebert, E.M., M.E. Egan, T.H. Hwang, S.B. Fulmer, S.S. Allen, G.R. Cutting, and W.B. Guggino. 1995. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 81:1063–1073. [DOI] [PubMed] [Google Scholar]
- Schwiebert, E.M., J.W. Mills, and B.A. Stanton. 1994. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J. Biol. Chem. 269:7081–7089. [PubMed] [Google Scholar]
- Schwiebert, L.M., W.C. Rice, B.A. Kudlow, A.L. Taylor, and E.M. Schwiebert. 2002. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am. J. Physiol. Cell Physiol. 282:C289–C301. [DOI] [PubMed] [Google Scholar]
- Thomas, E.J., S.E. Gabriel, M. Makhlina, S.P. Hardy, and M.I. Lethem. 2000. Expression of nucleotide-regulated Cl− currents in CF and normal mouse tracheal epithelial cell lines. Am. J. Physiol. Cell Physiol. 279:C1578–C1586. [DOI] [PubMed] [Google Scholar]
- van der Wijk, T., S.F. Tomassen, A.B. Houtsmuller, H.R. de Jonge, and B.C. Tilly. 2003. Increased vesicle recycling in response to osmotic cell swelling. Cause and consequence of hypotonicity-provoked ATP release. J. Biol. Chem. 278:40020–40025. [DOI] [PubMed] [Google Scholar]
- Wang, Y., R. Roman, S.D. Lidofsky, and J.G. Fitz. 1996. a. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc. Natl. Acad. Sci. USA. 93:12020–12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., A. Sostman, R. Roman, S. Stribling, S. Vigna, Y. Hannun, J. Raymond, and J.G. Fitz. 1996. b. Metabolic stress opens K+ channels in hepatoma cells through a Ca2+- and protein kinase Cα-dependent mechanism. J. Biol. Chem. 271:18107–18113. [DOI] [PubMed] [Google Scholar]
- Watt, W.C., E.R. Lazarowski, and R.C. Boucher. 1998. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J. Biol. Chem. 273:14053–14058. [DOI] [PubMed] [Google Scholar]
- Weeber, E.J., M. Levy, M.J. Sampson, K. Anflous, D.L. Armstrong, S.E. Brown, J.D. Sweatt, and W.J. Craigen. 2002. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J. Biol. Chem. 277:18891–18897. [DOI] [PubMed] [Google Scholar]
- Willumsen, N.J., C.W. Davis, and R.C. Boucher. 1994. Selective response of human airway epithelia to luminal but not serosal solution hypertonicity. Possible role for proximal airway epithelia as an osmolality transducer. J. Clin. Invest. 94:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, Y., E.J. Richer, T. Huang, and S.L. Brody. 2002. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- Yu, W.H., and M. Forte. 1996. Is there VDAC in cell compartments other than the mitochondria? J. Bioenerg. Biomembr. 28:93–100. [DOI] [PubMed] [Google Scholar]
- Yu, W.H., W. Wolfgang, and M. Forte. 1995. Subcellular localization of human voltage-dependent anion channel isoforms. J. Biol. Chem. 270:13998–14006. [DOI] [PubMed] [Google Scholar]