Abstract
We investigated the effect of protein kinase A (PKA) on passive force in skinned cardiac tissues that express different isoforms of titin, i.e., stiff (N2B) and more compliant (N2BA) titins, at different levels. We used rat ventricular (RV), bovine left ventricular (BLV), and bovine left atrial (BLA) muscles (passive force: RV > BLV > BLA, with the ratio of N2B to N2BA titin, ∼90:10, ∼40:60, and ∼10:90%, respectively) and found that N2B and N2BA isoforms can both be phosphorylated by PKA. Under the relaxed condition, sarcomere length was increased and then held constant for 30 min and the peak passive force, stress-relaxation, and steady-state passive force were determined. Following PKA treatment, passive force was significantly decreased in all muscle types with the effect greatest in RV, lowest in BLA, and intermediate in BLV. Fitting the stress-relaxation data to the sum of three exponential decay functions revealed that PKA blunts the magnitude of stress-relaxation and accelerates its time constants. To investigate whether or not PKA-induced decreases in passive force result from possible alteration of titin–thin filament interaction (e.g., via troponin I phosphorylation), we conducted the same experiments using RV preparations that had been treated with gelsolin to extract thin filaments. PKA decreased passive force in gelsolin-treated RV preparations with a magnitude similar to that observed in control preparations. PKA was also found to decrease restoring force in skinned ventricular myocytes of the rat that had been shortened to below the slack length. Finally, we investigated the effect of the β-adrenergic receptor agonist isoprenaline on diastolic force in intact rat ventricular trabeculae. We found that isoprenaline phosphorylated titin and that it reduced diastolic force to a degree similar to that found in skinned RV preparations. Taken together, these results suggest that during β-adrenergic stimulation, PKA increases ventricular compliance in a titin isoform-dependent manner.
Keywords: myocardium, connectin, elasticity, passive force, muscle mechanics
INTRODUCTION
β-Adrenergic stimulation activates PKA in cardiac muscle, resulting in phosphorylation of a number of proteins, including L-type Ca2+ channels, ryanodine receptors, phospholamban, troponin I (TnI), and myosin-binding protein C (My-BPC) (for a recent review with original citations see Bers, 2001, 2002). Accordingly, myocardial function is associated with increased developed force and increased rates of rise and fall of developed force. Changes in intracellular Ca2+ handling are generally thought to be important for these effects (Kurihara and Konishi, 1987; Endoh and Blinks, 1988; Li et al., 2000; Bers, 2001, 2002). However, accumulating evidence suggests that myocardial properties are modulated, at least in part, at the myofilament level following β-adrenergic stimulation, by accelerating crossbridge cycling (Hoh et al., 1988; Hongo et al., 1993; Kentish et al., 2001), enhancing power output (Herron et al., 2001; Layland and Kentish, 2002) and facilitating dissociation of Ca2+ from troponin C (TnC) via phosphorylation of TnI (Robertson et al., 1982; Zhang et al., 1995), resulting in decreased Ca2+ sensitivity of force (Garvey et al., 1988; Wattanapermpool et al., 1995; Fentzke et al., 1999).
In striated muscle, titin functions as a molecular spring that is responsible for most passive stiffness within the physiological sarcomere length (SL) range (Granzier and Labeit, 2002). Titin also plays a role as a molecular scaffold in the sarcomere (Gregorio et al., 1999) and was recently proposed to be a factor in the Frank-Starling mechanism of the heart because it promotes actomyosin interaction in response to stretch (Fukuda and Granzier, 2004). In the mammalian heart, titin exists in two isoforms with different molecular lengths, i.e., the shorter N2B isoform (high passive force) and longer N2BA isoform (low passive force) (Granzier and Labeit, 2002).
Recently, we reported that titin's unique cardiac-specific N2B segment is phosphorylated by PKA, resulting in a reduction of passive force in rat cardiomyocytes in response to stretch (Yamasaki et al., 2002). To understand the physiological role of titin phosphorylation in the heart's diastolic function, we investigated the effects of PKA on passive properties of myocardial tissues with different passive stiffness resulting from differential titin expression profiles. It is important to note that the expression ratio of N2B titin to N2BA titin markedly varies in a species-specific and chamber-specific manner, with an ∼50:50 ratio occurring in the ventricles of large mammals, including humans (Cazorla et al., 2000). Furthermore, adjustments in the coexpression ratio take place in patients with coronary artery disease or dilated cardiomyopathy (Wu et al., 2002; Neagoe et al., 2002; Makarenko et al., 2004; Nagueh et al., 2004). It is important to know therefore whether or not N2BA titin is phosphorylated as shown for N2B titin (Yamasaki et al., 2002), and if so, to what extent passive properties are modulated by PKA in myocardial tissues with different titin expression profiles.
Results show that PKA phosphorylates both N2B and N2BA titins, resulting in decreases in passive force, with greater effects in muscles that express N2B titin at higher levels. Furthermore, the PKA-induced decrease in passive force is seen in muscles from which thin filaments are extracted by gelsolin, and our results reveal that titin is phosphorylated and diastolic force is reduced in intact cardiac muscle in response to β-adrenergic stimulation.
MATERIALS AND METHODS
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington D.C., 1996).
Skinned Muscle Preparation
Skinned muscles were prepared based on previously published procedures (bovine muscle, Wu et al., 2000 and Fukuda et al., 2003; rat muscle, Fukuda et al., 2000, 2005).
Myocardium from the left ventricle and left atrium of the adult cow (∼1.5 yr) was cut into small pieces and placed into a modified Krebs solution (see Wu et al., 2000 for exact composition; containing 30 mM 2,3-butanedione monoxime [BDM] to inhibit actomyosin interaction) with propranolol (10 μM) to block nonspecific β-adrenergic stimulation and with carbamyl choline (10 μM) to enhance phosphatase activity (Gupta et al., 1994). BDM is also known to enhance dephosphorylatoin of myofibrillar proteins (Venema et al., 1993; Turnbull et al., 2002) and may thus increase the likelihood that titin is in a dephosphorylated state. The presence of propranolol, carbamyl choline, and BDM, however, does not exclude basal levels of protein phosphorylation.
Muscle strips (diameter, 1–2 mm; length, ∼10 mm) were next dissected and skinned in relaxing solution (5 mM MgATP, 40 mM BES, 1 mM Mg2+, 10 mM EGTA, 1 mM dithiothreitol, 15 mM phosphocreatine, 15 U/ml creatine phosphokinase, 180 mM ionic strength [adjusted by K-propionate], pH 7.0) containing 1% (wt/vol) Triton X-100 overnight at ∼3°C. Muscles were then washed with relaxing solution and stored (for 1 mo or less) at −20°C in relaxing solution containing 50% (vol/vol) glycerol. Small preparations (100–200 μm in diameter; ∼2 mm in length) were dissected from these strips for force measurement.
The heart was quickly removed from male LBNF-1 rats (200–250 g) anesthetized with sodium pentobarbital (50 mg/kg, IP) and perfused through the aorta with the Krebs solution (see above). Thin trabeculae (100–200 μm in diameter; 1–2 mm in length) were then dissected from the right ventricle and skinned for 2 h in relaxing solution containing 1% Triton X-100 at ∼3°C. Muscles were then washed with relaxing solution, stored overnight at ∼3°C in relaxing solution, and used in the following day. To prevent protein degradation, all solutions used in the present study contained protease inhibitors (PMSF, 0.5 mM; leupeptin, 0.04 mM; E64, 0.01 mM).
Skinned Muscle Mechanics
First, we activated the preparation at the slack SL (SL 1.90 μm) with a saturating Ca2+ concentration (pCa 4.5) to secure the ends of the preparation and to determine the quality of the contractile machinery. The values of maximal Ca2+-activated force (see below) were similar to those obtained in our previous studies conducted under the same experimental condition (Fukuda et al., 2003, 2005). The preparation was stretched from the slack SL (i.e., 1.90 μm) to various SLs at a constant velocity of 0.1 muscle length/s and held for 30 min (15 min for gelsolin experiments; see Fig. 5), followed by a release to the slack SL (SL measured by later diffraction, Granzier and Irving, 1995; Wu et al., 2000; Fukuda et al., 2003, 2005). After 1 h, the preparation was stretched again at the same velocity to determine the reproducibility of passive force. Only when the passive force development was reproducible (<3% reduction; used as a criterion), the preparation was incubated for 50 min at 22°C with purified PKA (catalytic subunit from bovine heart; Sigma-Aldrich) at a concentration of 1 U/μl, based on our previous study (Yamasaki et al., 2002). In some experiments, we used PKA-specific inhibitor (PKI; Sigma-Aldrich) at a concentration of 50 μM. Then, the same stretch-hold protocol was repeated and stress-relaxation data were obtained.
Figure 5.

Effect of PKA on passive force in gelsolin-treated RV preparations. (A) Typical chart recording showing the effect of PKA on total and titin-based passive force. SL was increased from 1.90 to 2.25 μm as indicated. Black, before PKA; red, after PKA. Titin-based passive force was obtained by subtracting collagen-based passive force from total passive force. Arrows indicate the onset and end of stress-relaxation. (B) Summarized data from six preparations showing the effect of PKA on titin-based passive force. SL was increased from 1.90 to 2.25 μm. The value of maximal Ca2+-activated force before PKA treatment was 3.12 ± 1.38 mN/mm2 (at SL 1.90 μm). Data fitted to three exponential decays (compare Table I). (C) Bar graph comparing the PKA-induced decreases in passive force in control (C; data taken from Fig. 2 B) and gelsolin-treated preparations (G). No significant differences were observed (NS). (D) Summary of stress-relaxation measurements. *, P < 0.05 compared with pre-PKA values.
Finally, according to previous reports (Granzier and Irving, 1995; Wu et al., 2000; Fukuda et al., 2003, 2005), the preparation was treated with KCl/KI, and titin-based passive force was obtained as total passive force minus collagen-based (KCl/KI insensitive) passive force. We found in collagen strips (prepared from rat ventricular trabeculae with KCl/KI treatment) that passive force is unaffected by PKA treatment (not depicted). Throughout the study, to minimize contraction-induced structural damage on the preparation and to ensure high reproducibility of passive force (Fukuda et al., 2003, 2005), we measured passive and active forces at 12°C.
To extract thin filaments, some rat ventricular (RV) preparations were incubated overnight at ∼4°C in relaxing solution containing gelsolin fragment FX-45 (∼1 mg/ml) during continuous agitation. Maximal Ca2+-activated force was decreased to ∼5% of the control value with no significant difference in the value of steady-state passive force (see below). To ensure high reproducibility of passive force, we tested the effect of PKA on passive force (as described above), using a shorter (i.e., 15 min) hold period.
Restoring Force Measurement
Myocytes were enzymatically isolated from the hearts of male Wistar rats (200–250 g) and anesthetized with sodium pentobarbital (50 mg/kg, IP) as described in our previous study (Granzier and Irving, 1995). The myocytes were skinned with relaxing solution (see above) containing 0.3% (wt/vol) Triton X-100 for 50 min at room temperature (22°C) (Granzier and Irving, 1995). We measured relengthening velocity of skinned cardiomyocytes following rigor contraction, as a surrogate of restoring force, using methods based on the work by Sawyer and colleagues (Helmes et al., 2003). In brief, a single myocyte was perfused using a multibarrel pipette perfusion setup with a fast-step switcher (SF-77B; Warner Instruments Corporation). The multibarrel pipette was attached to a stepper motor that could rapidly switch the adjacent barrel (barrel diameter 800 μm) over the myocyte (in 20 ms). One of the pipettes was continuously perfused with relaxing solution and the other with rigor solution (relaxing solution without ATP and creatine phosphokinase and additional K-propionate to adjust ionic strength to 180 mM). Thus, the cell bathed in relaxing solution could be perfused with rigor solution within ∼20 ms, and vice versa.
SL was measured by a commercially available SL detection system (IonOptix Corp.) (Helmes et al., 2003). The cell image was digitized at a sampling speed of 240 Hz and displayed on a computer screen. A user defined region of interest was selected within the myocyte (∼30% of the cell surface area) and the video lines within this region were averaged vertically, followed by a fast-Fourier transformation. The first-order peak in the spectrum was converted to SL with a spatial resolution of ∼5 nm per sarcomere.
At the onset of the experiment, the cell was in relaxing solution and had an SL of ∼1.90 μm. Ca2+-independent activation was induced in the myocyte by switching relaxing solution to ATP-free rigor solution (Helmes et al., 2003). Then, rigor solution was switched back to relaxing solution, allowing for a rapid relengthening of the myocyte back to the slack SL (∼1.90 μm). Because of the close relationship between the maximum acceleration (dSL·dt−2 max) and maximum velocity of sarcomere relengthening (dSL·dt−1 max) when plotted against minimal SL (SLmin) attained during rigor contraction (Helmes et al., 2003), we used in the present study dSL·dt−1 max as an index of restoring force (dSL·dt−1 max is less sensitive to noise and is therefore preferred). Each myocyte was subjected to multiple rigor contractions to various SLs (down to SL ∼ 1.65 μm) and dSL·dt−1 max was plotted against SLmin. The obtained relation was fitted with a linear line with R2 usually >0.95. Experiments were conducted at room temperature (22°C).
Intact Muscle
Thin trabeculae (diameter < 250 μm) were dissected from the right ventricle of the rat and experiments were performed according to previous reports (ter Keurs et al., 1980; Kentish et al., 1986; Stuyvers et al., 2002). We used a modified Tyrode solution (130 mM NaCl, 1.2 mM MgCl2, 5 mM [15 mM for dissection] KCl, 2.8 mM sodium acetate, 10 mM HEPES, 10 mM glucose, 1.0 mM CaCl2, 10 U/ml insulin, pH 7.4, 25°C) bubbled with 95% O2–5% CO2. We also added 10 μM EGTA to the Tyrode solution to inhibit oxidation of catecholamines catalyzed by heavy metal ions (Layland and Kentish, 2002). The muscle was stimulated with a square pulse at 1.3-fold threshold with 5 ms duration at 0.5 Hz. We measured SL during diastole by laser diffraction and initially set it at ∼1.90 μm, the length at which no detectable diastolic force was developed (Kentish et al., 1986). We increased SL in a stepwise manner (seven steps up to SL ∼2.20 μm; each step in 200 ms), followed by a release of SL to the original ∼1.90 μm. We added 1 μM isoprenaline 5 min after the protocol was completed (the time at which developed force recovers to the original value; see Fig. 7 A). The same stretch protocol was repeated 10 min after the isoprenaline application. At each SL, developed and diastolic force of the fourth twitch was used for analysis (diastolic force was averaged before and after this twitch). Preparations that developed spontaneous oscillations were excluded from analysis.
Figure 7.

Effect of β-adrenergic stimulation on systolic and diastolic properties of intact RV preparations. (A) Typical chart recording showing the effect of 1 μM isoprenaline (ISO). Top, effect of isoprenaline on twitch contractions at the slack SL (∼1.90 μm). Middle, twitch contractions at different lengths before (left) and after (right) isoprenaline. For this preparation, SL was varied from 1.904 to 2.205 μm and from 1.902 to 2.201 μm before and after isoprenaline, respectively. Bottom, changes in diastolic force are shown on a larger scale. Diastolic force is decreased by isoprenaline (arrows indicate diastolic force for the last three stretches). (B) Top, the effect of isoprenaline on developed force. Data from each preparation were fitted by a linear regression line (for each set of data, R > 0.97; P < 0.0001). Developed force was increased with isoprenaline, especially at the long SL range, and, consequently, the slope of the linear regression line was significantly increased (P < 0.05). Before isoprenaline: y = 167.85x − 312.05. After isoprenaline: y = 270.70x − 505.93. Bottom, the effect of isoprenaline on diastolic force. Data fitted to an exponential function as described in text. The values at the longest SL (seventh step in the protocol) are as follows. Developed force, 56.84 ± 4.98 and 82.97 ± 8.97 mN/mm2 (P < 0.05), before and after isoprenaline, respectively. Diastolic force, 7.93 ± 1.26 and 6.22 ± 1.11 mN/mm2 (P < 0.05), before and after isoprenaline, respectively. SL, 2.17 ± 0.01 before and after isoprenaline. n = 6. (C) Data showing reproducibility of developed and diastolic force without isoprenaline. Top, developed force. First protocol: y = 124.89x − 219.14. Second protocol: y = 125.89x − 223.75. Slopes are not significantly different. Bottom, diastolic force. k: 8.18 ± 0.65 and 8.21 ± 0.89, respectively, in the first and second protocols (P > 0.05). SL0: 1.88 ± 0.02 and 1.88 ± 0.03 μm, respectively, in the first and second protocols (P > 0.05). Neither parameter is significantly different between first and second protocols. The values at the longest SL (seventh step in the protocol) are as follows. Developed force, 49.28 ± 4.89 and 47.63 ± 5.05 mN/mm2 (P > 0.05), in the first and second protocols, respectively. Diastolic force, 7.84 ± 0.60 and 7.87 ± 0.48 mN/mm2 (P > 0.05), in the first and second protocols, respectively. SL, 2.16 ± 0.01 for first and second protocols. n = 6.
Gel Electrophoresis and Autoradiography
Skinned myocardial preparations prepared as described above were incubated for 50 min at 22°C in relaxing solution containing 1000 cpm/pmol [γ-32P]ATP (ICN Biomedicals, Inc.) with 1 U/μl of purified PKA (Yamasaki et al., 2002). The preparations were then solubilized and electrophoresed on 1.8–6% acrylamide gradient gels, stained with Coomasie brilliant blue (CBB), destained, dried, and exposed for 24 h to the autoradiographic film at 22°C. Phosphorylation was determined by phosphoscanner (Cyclone; Packard Instrument Company). The CBB-stained gel and autoradiograph were scanned, and the integrated optical density (OD) of N2B and N2BA titins was determined (Yamasaki et al., 2002).
In another set of experiments, intact rat ventricular trabeculae, prepared as described above, were continuously perfused for 10 min with the modified Tyrode solution (for composition see above) bubbled with 95% O2–5% CO2, containing either 1 μM isoprenaline (to enhance phosphorylation of myofibrillar proteins) or 10 μM propranolol and 10 μM carbamyl choline (to enhance dephosphorylation of myofibrillar proteins). Then, the preparations were skinned for 2 h and incubated for 50 min at 22°C in relaxing solution containing 1000 cpm/pmol [γ-32P]ATP with 1 U/μl of purified PKA. The preparations were then solubilized and electrophoresed on 2–7% acrylamide gradient gels and autographs were obtained accordingly. Samples were electrophoresed on the gel with four different loadings, and integrated OD-loading relations were obtained (slope obtained from regression analysis; see Yamasaki et al., 2002). The slope ratio obtained from CBB-stained gels reflects differences in the relative protein concentrations of the two groups and was used to correct the slope ratios of corresponding autoradiographs (compare Yamasaki et al., 2002).
Statistical Analysis
Significant differences were assigned using the paired or unpaired Student's t test as appropriate. Regression analysis was performed using the least-square method. All data are expressed as mean ± SEM, with n representing the number of muscles. Statistical significance was verified at P < 0.05.
RESULTS
PKA Phosphorylates N2B and N2BA Titins
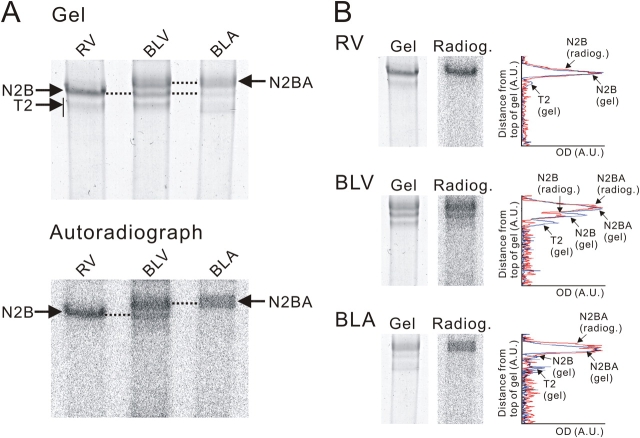
Fig. 1 shows CBB-stained gels of titin and autoradiographs for RV, bovine left ventricular (BLV), and bovine left atrial (BLA) muscles. As is well known, titin consists of T1 and T2, the full-length protein and a large degradation product, respectively, and T1 of N2BA titin migrates slower than that of N2B titin. Consistent with previous studies (e.g., Cazorla et al., 2000), the ratio of N2B to N2BA titin is highest in RV, lowest in BLA and intermediate in BLV. Radiographs (exposure 24 h) revealed phosphorylation of both N2B and N2BA isoforms (Fig. 1 A). Densitometric analyses (Fig. 1 B) revealed that T1 peaks of both N2B and N2BA titins overlap with the corresponding phosphorylated bands in the autoradiograph, but not for T2, leading us to conclude that PKA phosphorylates both cardiac titin isoforms but not their T2 degradation products.
Figure 1.
Titin phosphorylation in various muscle types. (A) Top, CBB-stained gel of solubilized RV, BLV, and BLA preparations incubated with [γ-32P]ATP in the presence of PKA; bottom, 24-h autoradiographic exposure. Note phosphorylation of T1 of N2B and N2BA titins and no phosphorylation of T2. (B) Side-by-side comparison of gel and autoradiograph and densitometric scan of gel (blue) and autoradiograph (red) for RV, BLV, and BLA.
PKA Decreases Passive Force in Cardiac Muscle with Different Titin Expression Profiles
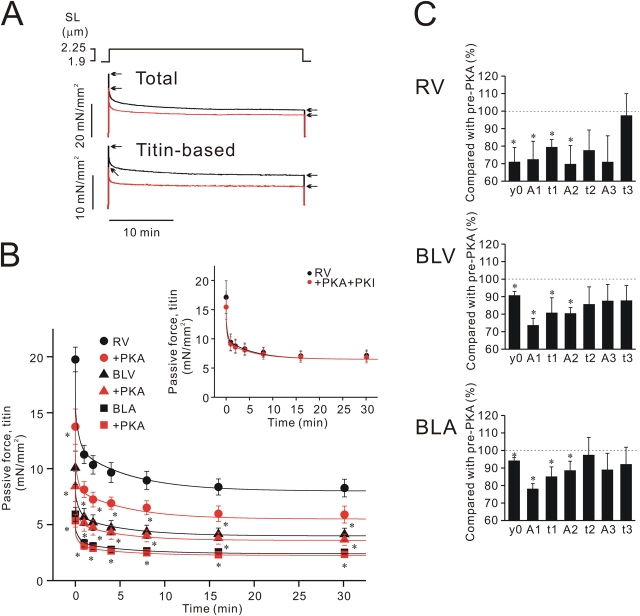
Fig. 2 A is a typical chart trace showing stress-relaxation of a skinned RV preparation before and after PKA treatment (SL increased from 1.9 to 2.25 μm). It is clearly seen that passive force, both total and titin-based, is reduced following incubation with PKA. Fig. 2 B summarizes the effect of PKA on titin-based passive force from six to eight preparations for RV, BLV, and BLA (SL increased from 1.9 to 2.25 μm). As reported previously (Cazorla et al., 2000; Wu et al., 2000; Fukuda et al., 2003), because of differential expressions of titin (compare Fig. 1), passive force was highest in RV, intermediate in BLV, and lowest in BLA. PKA significantly decreased passive force in all types of muscle, and the magnitude of force decrease (both peak and steady-state force) was greatest in RV, smallest in BLA, and intermediate in BLV. The PKA-induced decreases in passive force are relatively small in BLV and especially in BLA, but changes are statistically significant. Therefore, PKA decreases passive force in cardiac muscle regardless of the expression profile of titin, and its effect is more pronounced when N2B expression levels are high. PKI inhibited PKA-induced decreases in passive force in RV (Fig. 2 B, inset) and also in BLV and BLA (not depicted).
Figure 2.
Effect of PKA on passive force in various types of skinned cardiac muscle. (A) Typical chart recording showing the effect of PKA on total and titin-based passive force in RV. SL was increased from 1.90 to 2.25 μm as indicated. Black, before PKA; red, after PKA. Titin-based passive force was obtained by subtracting collagen-based passive force from total passive force. Arrows indicate the onset and end of stress-relaxation. (B) Effect of PKA on titin-based passive force in RV, BLV, and BLA. SL was increased from 1.90 to 2.25 μm. The values of maximal Ca2+-activated force before PKA treatment were 51.52 ± 8.53, 26.08 ± 2.56, and 18.42 ± 1.63 mN/mm2 in RV, BLV, and BLA, respectively (at SL 1.90 μm). Data obtained from each preparation (see A for example) were fitted to three exponential decays as described in text, and curves with mean values of exponential parameters are shown (compare Table I). RV, n = 6; BLV, n = 6; BLA, n = 8. Inset, effect of PKA+PKI on titin-based passive force in RV preparations (SL increased from 1.90 to 2.25 μm; n = 6). PKA+PKI does not significantly affect passive force. (C) Summary of stress-relaxation measurements. Percent changes compared with pre-PKA values (offsets, amplitudes, and time constants) are shown. *, P < 0.05 compared with pre-PKA values.
To quantify PKA-induced changes in stress-relaxation, we fitted the stress-relaxation data to three exponential decays of the type y = y0 + A1exp(−x/t1) + A2exp(−x/t2) + A3exp(−x/t3), where y0 is the offset, A1, A2, and A3 are decay amplitudes, and t1, t2, and t3 are decay time constants (compare Kulke et al., 2001). The fact that stress-relaxation can be fitted well with a three-order exponential decay function suggests the existence of multiple sources of titin-based viscoelasticity (Trombitas et al., 2003). The fitting revealed that offset, decay amplitudes, and decay time constants were significantly decreased and that changes in the amplitudes, as well as in time constants, were greatest for the fastest component (Fig. 2 C; Table I). These findings suggest that PKA affects titin's elasticity (y0) as well as viscoelasticiy (decay amplitudes and time constants) (see discussion). Changes in these parameters were greatest in RV, followed respectively by BLV and BLA.
TABLE I.
Stress-relaxation Values Obtained before PKA Treatment
| y0 | A1 | t1 | A2 | t2 | A3 | t3 | ||
|---|---|---|---|---|---|---|---|---|
| mN/mm2 | mN/mm2 | s | mN/mm2 | min | mN/mm2 | min | ||
| Fig. 2 | ||||||||
| RV | 7.72 ± 0.99 | 4.15 ± 0.59 | 0.87 ± 0.06 | 3.49 ± 0.33 | 0.42 ± 0.08 | 3.87 ± 0.43 | 5.95 ± 0.88 | |
| BLV | 4.00 ± 0.54 | 2.03 ± 0.38 | 1.15 ± 0.08 | 1.95 ± 0.31 | 0.50 ± 0.03 | 1.75 ± 0.26 | 5.59 ± 0.28 | |
| BLA | 2.40 ± 0.24 | 1.20 ± 0.16 | 1.16 ± 0.11 | 1.21 ± 0.16 | 0.51 ± 0.09 | 0.99 ± 0.15 | 5.97 ± 0.81 | |
| Fig. 3 | ||||||||
| BLV | 6.40 ± 0.29 | 4.61 ± 0.36 | 1.12 ± 0.15 | 4.22 ± 0.32 | 0.52 ± 0.09 | 2.94 ± 0.23 | 6.79 ± 0.98 | |
| BLA | 3.97 ± 0.30 | 2.46 ± 0.28 | 1.24 ± 0.07 | 2.56 ± 0.33 | 0.63 ± 0.11 | 1.59 ± 0.14 | 7.48 ± 0.78 | |
| Fig. 5 | ||||||||
| RV | 7.19 ± 0.71 | 2.66 ± 0.22a | 0.64 ± 0.10 | 2.53 ± 0.18a | 0.27 ± 0.09 | 3.01 ± 0.30 | 5.29 ± 1.14 |
P < 0.05 compared with the corresponding values obtained with no gelsolin treatment (from Fig. 2).
It has been reported that large mammals have relatively long end-diastolic SL (∼2.40 μm, Rodriguez et al., 1992) compared with small rodents (∼2.20 μm, Grimm et al., 1980; MacKenna et al., 1994). To ascertain whether PKA affects passive force and stress-relaxation at the upper limit of the physiological SL range of large mammals where maximal levels of passive force are generated (Wu et al., 2000), we conducted stress-relaxation experiments at SL 2.40 μm on BLV and BLA. As shown in Fig. 3 A, titin-based passive force was approximately twofold higher than that at SL 2.25 μm in these preparations. Passive force was decreased by PKA throughout the course of stress-relaxation, and the effect was significant for both BLV and BLA. As found at SL 2.25 μm, fitting the data to three exponential decays revealed that (a) offset, decay amplitudes, and the fastest decay time constant (t1) were all decreased, and (b) changes in the amplitudes were greatest for the fastest component (Fig. 3 B; Table I). Again, PKA accelerates stress-relaxation of passive cardiac muscle.
Figure 3.

Effect of PKA on passive force at the upper limit of the physiological SL range in BLV and BLA. (A) Titin-based passive force before and after PKA treatment in BLV and BLA. SL was increased from 1.90 to 2.40 μm. The values of maximal Ca2+-activated force before PKA treatment were 27.49 ± 2.93 and 19.48 ± 1.62 mN/mm2 in BLV and BLA, respectively (at SL 1.90 μm). Data fitted to three exponential decays (compare Table I). BLV, n = 8; BLA, n = 8. (B) Summary of stress-relaxation measurements. *, P < 0.05 compared with pre-PKA values.
To more fully investigate the SL dependencies of the PKA effect on passive force in preparations that express different isoforms of titin, we performed stress-relaxation experiments at the shorter range of SLs (i.e., 2.10 and 2.20 μm) and obtained relationships of SL vs. percent reduction of passive force for peak and steady-state passive force (Fig. 4). We found that in all muscle types, PKA decreased passive force more markedly at the short SL range than at the long SL range and that the effect was inversely correlated with SL for peak as well as for steady-state total and titin-based passive force. Thus, the PKA effect on passive force is SL dependent as the effect is more pronounced at the shorter SL range. We clearly see that the PKA-induced reduction in passive force (both total and titin-based) is in the following order for peak as well as for steady-state passive force (SL ≤ 2.25 μm): RV > BLV > BLA.
Figure 4.
SL dependence of the effect of PKA on passive force. Percent reduction is shown. (A) SL dependence for peak force. Data fitted to linear regression line. Left, total passive force. The slopes are −55.10 (R2 = 0.98; P < 0.05), −14.56 (R2 = 0.97; P < 0.01), and −6.65 (R2 = 0.98; P < 0.005), for RV, BLV, and BLA, respectively. Right, titin-based passive force. The slopes are −92.20 (R2 = 0.99; P < 0.05), −12.49 (R2 = 0.91; P < 0.05), and −4.25 (R2 = 0.94; P < 0.05), for RV, BLV, and BLA, respectively. (B) SL dependence for steady-state passive force (data obtained 30 min after stretch). Left, total passive force. The slopes are −38.61 (R2 = 0.98; P < 0.01), −6.69 (R2 = 0.97; P < 0.05), and −5.58 (R2 = 0.89; P < 0.05), for RV, BLV, and BLA, respectively. Right, titin-based passive force. The slopes are −30.64 (R2 = 0.99; P < 0.05), −5.10 (R2 = 0.89; P < 0.05), and −3.29 (R2 = 0.92; P < 0.05), for RV, BLV, and BLA, respectively. *, P < 0.05 compared with BLA; #, P < 0.05 compared with BLV. n = 6–8.
We also found that the magnitude of the SL dependence, as judged by the slope of the regression line, varies depending on the expression profile of titin, and it is highest in RV (N2B titin dominant) and lowest in BLA (N2BA titin dominant). This suggests that the SL dependence of the PKA effect is greater in N2B titin than in N2BA titin.
Effect of PKA on Passive Force in Gelsolin-treated Cardiac Muscle
To clarify whether the PKA-induced reduction in passive force results from an altered thin filament–titin interaction or is solely titin based, we treated RV preparations with gelsolin to extract thin filaments (maximal Ca2+-activated force, ∼5% of the control value) and examined the effect of PKA on passive force in these preparations. Fig. 5 A is a typical chart record showing stress-relaxation of gelsolin-treated RV before and after PKA treatment. It has been reported that actin removal with gelsolin almost completely abolishes stress-relaxation in experiments on rat cardiac myofibrils (Kulke et al., 2001). In the present study, however, gelsolin treatment depressed peak passive force but did not completely abolish stress-relaxation. Data from six muscles (Fig. 5 B) showed that under the control condition before PKA treatment, the value of peak titin-based passive force was significantly smaller when compared with that obtained with control preparations in Fig. 2 B (P < 0.05). However, the value of steady-state passive force was not significantly affected by gelsolin treatment (P > 0.05). Also, fitting stress-relaxation with a three-order exponential decay revealed that gelsolin treatment decreases amplitudes with no significant effect on time constants (Table I).
PKA significantly decreased passive force throughout the course of stress-relaxation (Fig. 5 B). Importantly, the magnitude of force reduction was similar to what was found for control preparations in Fig. 2 B (Fig. 5 C). Exponential analysis revealed a significant reduction in various parameters similar to those found for control preparations (Fig. 5 D; compare Fig. 2 C). As in control preparations with no gelsolin treatment, significant changes in passive force were not found throughout stress-relaxation with PKA+PKI (unpublished data). It is therefore likely that the PKA-induced decrease in passive force, as well as the PKA-induced acceleration of stress-relaxation, is not due to altered titin–thin filament interaction but instead is purely titin based.
Effect of PKA on Restoring Force
Titin produces not only passive force but also restoring force at SLs where myocytes are shortened to below the slack SL (Helmes et al., 1996, 2003). We investigated the effect of PKA on skinned rat ventricular myocytes that had been shortened to below their slack length (using rigor contractions), followed by relaxation and measurement of the relengthening velocity (dSL.dt−1 max) as an index of restoring force.
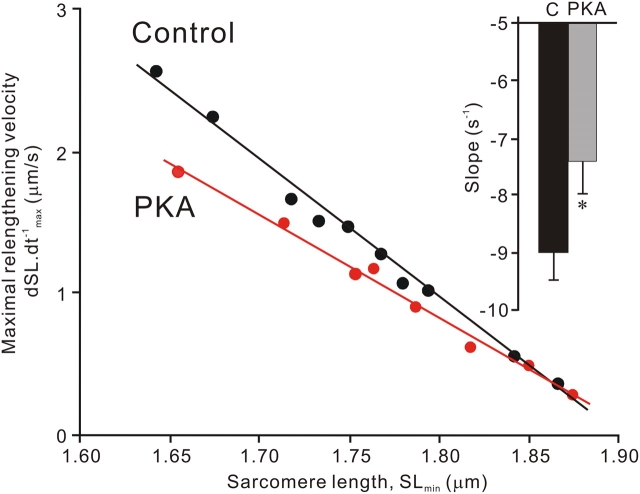
Fig. 6 compares the typical data of the relationship of dSL.dt−1 max vs. SLmin for control and PKA-treated myocytes (obtained from the same heart). The relationship was linear for both control and PKA-treated myocytes (R2 > 0.95) at the SL range of ∼1.90 to ∼1.65 μm, and the slope of the relationship was significantly less for the PKA-treated myocyte (i.e., lower relengthening velocity). The inset summarizes the slope of the dSL.dt−1 max vs. SLmin relationship for control and PKA-treated myocytes with the value ∼20% less in the PKA-treated group compared with the control group (P < 0.05).
Figure 6.
Effect of PKA treatment on relengthening velocity of skinned rat ventricular myocytes following rigor contraction to below the slack length. Examples of the relationship between maximum relengthening velocity (dSL.dt−1 max) and the minimum SL (SLmin) in control (black) and PKA-treated myocytes (red). The relationship of dSL.dt−1 max vs. SLmin is fitted well with a linear regression line (R2 > 0.95). Inset, bar graph showing slopes of dSL.dt−1 max vs. SLmin relationship of 93 control myocytes (from 12 animals) and 71 PKA-treated myocytes (from 10 animals). C, control (no PKA). *, P < 0.05 compared with control.
Effect of β-Adrenergic Stimulation on Diastolic Properties of Intact Muscle
We investigated whether diastolic force is decreased upon β-adrenergic stimulation in stimulated intact rat ventricular trabeculae. In this series of experiments, we increased SL in seven steps (completed in 200 ms) and measured diastolic force for the fourth twitch (diastolic force averaged before and after the twitch), the time after which the decline in diastolic force was small (i.e., decline in diastolic force <∼10%). Thus, the diastolic force measured in the present study mainly reflects elasticity of living trabeculae (see discussion). Fig. 7 A (top) illustrates the effect of 1 μM isoprenaline on twitch contractions at the slack SL. As is well known, developed force is increased and twitch duration is shortened with isoprenaline. Developed force increased in response to the addition of isoprenaline, reached its plateau in ∼2 min, and then remained stable (compare de Tombe and ter Keurs, 1991). Fig. 7 A (middle and bottom) shows a typical chart trace for the SL dependence of the effect of isoprenaline on twitch contractions (same preparation used throughout experiment shown in Fig. 7 A). It is clearly seen that developed force is increased at each step after isoprenaline application, while diastolic force is decreased. Note that with (Fig. 7 A, middle right) and without isoprenaline (Fig. 7 A, middle left as control), developed force recovers to the original level in ∼2 min upon release of muscle length.
Fig. 7 B summarizes the results from six preparations before and after isoprenaline application. Because developed force increased linearly in response to an increase in SL above ∼1.90 μm, the data were fitted to a linear regression line. The slope of the linear regression line increased by ∼1.6-fold in isoprenaline (P < 0.05), indicating that the Frank-Starling mechanism is enhanced upon β-adrenergic stimulation, as reported for ferret ventricular muscle by Komukai and Kurihara (1997). Consistent with previous findings by others on intact rat ventricular trabeculae (van Heuningen et al., 1982; Kentish et al., 1986), diastolic force was nearly zero at SL ∼1.90 μm and increased rapidly as SL was increased to ∼2.20 μm. Here, to minimize the likelihood of spontaneous oscillations that frequently occurred upon isoprenaline application when SL was elongated to ∼2.25 μm and that could potentially affect diastolic properties, we stretched preparations up to SL ∼2.20 μm (shorter than in skinned muscle experiments) in all preparations. According to a previous report on intact muscle (Stuyvers et al., 2002), diastolic force data from each preparation were analyzed with an exponential function, i.e., diastolic force = exp (k*(SL − SL0)) − 1, where k is the exponent of diastolic stiffness in mN/mm2 and SL0 is the intercept on the SL axis, i.e., the zero load SL. We found that isoprenaline reduced diastolic force to a degree similar to what PKA did in skinned RV preparations (i.e., ∼20% at SL ∼2.20 μm; compare Fig. 4) with no significant changes in SL0 (1.92 ± 0.02 and 1.93 ± 0.02 μm, respectively, before and after isoprenanline; P > 0.05) and that it decreased the k value (8.99 ± 1.29 and 8.14 ± 1.24, respectively, before and after isoprenaline; P < 0.05). There was a trend for SL dependence in the effect of isoprenaline on diastolic force (i.e., greater effect at shorter SLs), but this was not statistically significant.
Control experiments without isoprenaline showed that developed force and diastolic force were consistent in the first and second stretch protocol (Fig. 7 C), making it unlikely that decreases in diastolic force via β-adrenergic stimulation result from rundown of myocardial diastolic properties. We therefore conclude that β-adrenergic stimulation decreases diastolic force of stimulated cardiac muscle.
β-Adrenergic Stimulation-dependent Titin Phosphorylation in Intact Cardiac Muscle
Fig. 8 shows 32P incorporation by titin in skinned rat ventricular trabeculae, after 10 min perfusion of intact trabeculae with either 10 μM propranolol and 10 μM carbamyl choline or 1 μM isoprenaline, skinning, and performing back phosphorylation assays (see materials and methods). We also included a study of myosin-binding protein C (MyBP-C) for comparative reasons. Results show that at similar quantities of loaded protein, isoprenaline-treated preparations incorporate less 32P in both titin and MyBP-C (Fig. 8 A). Note no phosphorylation for T2, as in Fig. 1.
Figure 8.
Effect of propranolol and carbamyl choline (Pro) and isoprenaline (ISO) on titin and MyBP-C phosphorylation by using the back phosphorylation assay. Intact rat right ventricular trabeculae were treated with Pro or ISO, followed by skinning and incubation with [γ-32P]ATP in the presence of PKA. (A) Gel and corresponding autoradiograph for titin (top) and MyBP-C bands (bottom). Each sample was electrophoresed with four different loadings (two loadings are shown here). Integrated OD was determined for each band and plotted against loading volume. (B) Pro vs. ISO slope ratio determined from autoradiographs after correction for protein loading differences between Pro and ISO-treated samples. Results indicate significantly less 32P incorporation after ISO treatment in titin as well as in MyBP-C.
The mean results of five experiments are shown in Fig. 8 B. The differences in 32P incorporation in propranolol (plus carbamyl choline) and isoprenaline-treated preparations were statistically significant for both titin (74.7 ± 8.5%) and My-BPC (69.8 ± 6.4%) at P < 0.01. These differences in 32P incorporation are similar to those obtained in our previous study with ventricular myocytes of the rat (Yamasaki et al., 2002). These results support the notion that in intact muscle, titin is phosphorylated by PKA in response to β-adrenergic stimulation.
DISCUSSION
β-Adrenergic stimulation results in marked changes in the contractile behavior of the heart. This includes increased rates of rise and fall of developed pressure and an increased heart rate. The heart's systolic and diastolic functions are interrelated, and the positive chronotropic effects are likely to necessitate adjustments of diastolic properties. In the present study, we found that myocardial compliance is increased upon β-adrenergic stimulation and that the effect is titin isoform dependent (i.e., greater in muscles that express N2B titin at higher levels). These findings reveal a novel function of titin that allows for more rapid and complete cardiac chamber filling during β-adrenergic stimulation.
Phosphorylation of Titin
Consistent with previous findings on rat cardiomyocytes (Yamasaki et al., 2002), only T1, but not T2, was phosphorylated by PKA in skinned myocardial preparations with different titin expression profiles (Fig. 1). Since T2 is a proteolytic cleavage product of titin that constitutes largely the A-band region of the molecule, these findings are consistent with the notion that the phosphorylation site is located in the extensible I-band region of titin. In previous work on human N2B titin using recombinant protein fragments that represent the various spring elements (i.e., tandem Ig segments, PEVK segment, and N2B unique sequence), the N2B unique sequence was the only element that was phosphorylated by PKA, and titin phosphorylation resulted in a reduction of passive force in response to ramp stretch (Yamasaki et al., 2002). Since the N2B element is present in both N2B and N2BA isoforms, it is reasonable to propose that phosphorylation of the N2B unique sequence occurs in both N2B and N2BA titins. However, considering that the extensible region of N2BA titin includes additional sequences not present in the N2B isoform (such as additional PEVK sequences and unique sequences in the N2A element; Granzier and Labeit, 2002) and that there is variation in the sequence of the N2B element of different animal species (Greaser et al., 2002), this proposal requires future testing.
Effect of PKA-based Phosphorylation on Passive and Restoring Forces
At physiological SLs above the slack SL, both titin and collagen contribute to passive force of cardiac muscle, with collagen's role more dominant at long SLs (Granzier and Irving, 1995). The present study revealed that titin-based passive force is decreased by PKA and that the effect is large enough to result in a reduction of total passive force at all SLs (Fig. 4).
It has been reported that titin interacts with thin filaments and that this interaction provides a source of viscosity in cardiac muscle (Kulke et al., 2001; Yamasaki et al., 2001). It would therefore be possible that PKA-induced phosphorylation of TnI impacts the thin filament–titin interaction and possibly decreases passive force upon stretch. To investigate this possibility, we used gelsolin to extract thin filaments. Peak passive force and amplitudes of the three exponential decays (A1 and A2) were significantly smaller after thin filament extraction with no significant changes in steady-state passive force (see results and Table I). The finding that in thin filament–extracted preparations, PKA significantly decreased passive force throughout the course of stress-relaxation, to a magnitude similar to that observed in control preparations (Fig. 5 C), indicates that the PKA effect does not involve titin–thin filament interaction but is solely titin based.
Passive force reduction due to PKA-dependent phosphorylation might be explained by assuming that phosphorylation destabilizes native structures within titin, possibly formed by the N2B unique sequence, resulting in contour length gain and an ensuing reduction in fractional extension of titin's extensible region. Furthermore, since some of these structures might have a low stability and exist mainly at short SLs (Yamasaki et al., 2002), phosphorylation is expected to have the greatest effect at short SLs (i.e., greater passive reduction at shorter SLs), consistent with the findings of the present work (Fig. 4). In previous work on cardiac myocytes, we showed that for native conformations within titin to recover from effects of stretch, a prolonged period of rest (>∼200 s) at the slack SL is required (Helmes et al., 1999). Because the work on intact preparations (Fig. 7) did not impose such rest periods (indeed, due to internal shortening during the twitch contractions, sarcomeres are undergoing continuous cycles of shortening/lengthening), it is likely that native structures of low stability are absent in these preparations, possibly explaining the absence of significant SL dependence in the effect of β-adrenergic stimulation on diastolic force.
The present finding that passive force reduction is less in muscles that have a lower N2B:N2BA ratio (BLA < BLV < RV) indicates that the effect of phosphorylation on force generated by N2BA titin is less than that of N2B titin (SL ≤ 2.25 μm). This is consistent with the notion that the passive force reduction is due to a reduction in the fractional extension (end-to-end length divided by contour length) of titin's extensible region. Because the N2BA isoform has a much longer PEVK segment and contains an additional tandem Ig segment (Granzier and Labeit, 2002), its contour length is longer than that of N2B titin, diminishing the effect of the phosphorylation-induced contour length gain of the N2B unique sequence on the fractional extension of titin's extensible region.
An interesting phenomenon observed in the present study is that stress-relaxation is accelerated by PKA, with the effect being most sensitive for the fastest component (t1) and that, importantly, the accelerating effect was observed in thin filament–extracted preparations (Fig. 5 D). Therefore, the phosphorylation-sensitive acceleration of stress-relaxation is a phenomenon that resides in titin per se. It is worth noting that the PEVK-thin filament interaction is not the only source for viscosity of passive cardiac muscle and that the unique N2B sequence is an additional source (Helmes et al., 1999). Thus, PKA-induced changes in the native structure of the N2B sequence might be responsible for the acceleration of stress-relaxation.
We also studied the restoring force by inducing rigor contraction followed by relaxation and using the maximal relengthening velocity as an index of restoring force (compare Helmes et al., 2003). Restoring force was reduced upon PKA treatment (Fig. 6), with the magnitude of reduction similar to that observed for passive force (compare Fig. 4). This finding suggests that PKA-based phosphorylation of titin reduces not only passive force but also restoring force, with as possible mechanism a phosphorylation-induced contour length gain of the N2B unique sequence and resultant reduction of fractional extension of titin's I-band region.
Recent studies suggest that titin plays a role in length-dependent activation, possibly via interfilament lattice spacing modulation (for review see Fukuda and Granzier, 2004). Currently, the effect of PKA on length-dependent changes in Ca2+ sensitivity is unsolved. Kajiwara et al. (2000) reported that PKA diminishes length-dependent changes in Ca2+ sensitivity of force, while Konhilas et al. (2003) reported that length-dependent changes in Ca2+ sensitivity are either enhanced (when [Ca2+] was expressed on an absolute scale) or unchanged (when pCa was used), in skinned rat ventricular muscle. Changes in length-dependent activation and, possibly, deactivation (Helmes et al., 2003) of cardiac muscle with PKA-based titin phosphorylation are areas for future research.
Physiological Significance
To address whether the results on skinned muscle are physiologically relevant, we used a twitch protocol on intact muscle. In these experiments, we measured diastolic force at a time when the rate of decline in diastolic force was small (see results) and, therefore, the obtained values are likely to reflect mainly the passive elasticity of living cardiac muscle (compare Chiu et al., 1982). This explains why diastolic force at SL ∼2.20 μm (9.02 ± 0.45 mN/mm2; Fig. 7 B, middle) is similar to total passive force obtained in skinned RV preparations at SL 2.20 μm at the steady state (10.26 ± 1.95 mN/mm2, 30 min after stretch, n = 6). This similarity also indicates that the contribution of actomyosin interaction to diastolic force is likely to be low or absent. This conclusion is consistent with the work of Layland and Kentish (2002) that showed that in rat ventricular trabeculae at low stimulation frequencies (<0.75 Hz) and a temperature of 25°C (as in the present study), actomyosin interaction does not contribute to diastolic force. It is, thus, reasonable to assume that the diastolic force under baseline conditions is solely due to passive muscle force and that the decrease in diastolic force with β-adrenergic stimulation (Fig. 7, A and B) is due to a decrease in passive muscle force. In the present study, we found that titin can be phosphorylated upon β-adrenergic stimulation in intact rat ventricular trabeculae under the experimental condition used for the twitch protocol (Fig. 8). Therefore, the present findings suggest that β-adrenergic stimulation phosphorylates titin and that this results in a decrease in diastolic force in intact cardiac muscle. Reduced Ca2+ sensitivity of actomyosin-based force (e.g., Okazaki et al., 1990) upon β-adrenergic stimulation, coupled possibly with enhanced Ca2+ dissociation from TnC due to TnI phosphorylation (Robertson et al., 1982; Zhang et al., 1995), may also result in a decrease in diastolic force, especially at higher stimulation frequencies. The comparison of diastolic force with passive force (see above) suggests, however, that the effect of titin phosphorylation might be dominant in the present study.
The positive chronotropic effect of β-adrenergic stimulation could potentially cause incomplete ventricular filling (due to the shortened diastolic interval), and this might be compensated by multiple mechanisms including increased venous return (e.g., Imai et al., 1978; Leenen and Reeves, 1987). We propose that one such mechanism is based on titin phosphorylation that promotes ventricular filling via a reduction of ventricular wall stiffness. Our finding that the effect of phosphorylation on passive force is largest in tissues that express high levels of N2B titin might reflect the high beat frequency encountered in species (e.g., rat and mouse) that express high levels of N2B titin (Cazorla et al., 2000) and their short baseline diastolic interval. Here, one may point out that titin produces restoring force in early diastole (the systolic–diastolic transition) (Helmes et al., 1996), possibly to facilitate rapid ventricular filling via deactivating myofilaments (Helmes et al., 2003), and, therefore, PKA-induced reduction in titin-based restoring force (Fig. 6) may tend to delay myofibrillar deactivation. This possible unbeneficial effect might be offset, however, at the cardiac muscle level via acceleration of myocardial relaxation due to PKA-based phosphorylation of phospholamban and TnI (e.g., Bers, 2001, 2002) and reduction of ventricular wall stiffness due to titin phosphorylation (Yamasaki et al., 2002 and present study). Clearly, more work is required to fully understand the effect of titin phosphorylation on the filling of the heart.
To our knowledge, a consensus has not been achieved regarding the effect of β-adrenergic stimulation on diastolic pressure at the isolated heart level. For example, Layland et al. (2004) and Grieve et al. (2004) reported no effect in mouse, whereas van Kerckhoven et al. (2000) observed dose-dependent decreases in diastolic pressure in rat. The view that phosphorylation of titin might improve ventricular filling is consistent, however, with multiple studies that revealed that the diastolic pressure–volume relationship falls upon β-adrenergic stimulation in vivo (humans, Udelson et al., 1989; Parker et al., 1991; Nagata et al., 1995; rats, Salyers et al., 1988). The importance of the present study is further highlighted by the recent reports of changes in titin isoform expression during heart failure (Neagoe et al., 2002; Wu et al., 2002; Makarenko et al., 2004; Nagueh et al., 2004), since our findings predict that the sensitivity of diastolic pressure to β-adrenergic stimulation will vary accordingly.
Acknowledgments
This work was supported by National Institutes of Health grant HL62881/61497/67274 to H. Granzier. N. Fukuda is a recipient of the grant from the Uehara Memorial Foundation, Tokyo, Japan.
Olaf S. Andersen served as editor.
N. Fukuda's present address is Department of Physiology II, The Jikei University School of Medicine, 3-25-8 Nishi-shinbashi, Minato-ku, Tokyo 105-8461, Japan.
Abbreviations used in this article: BDM, 2,3-butanedione monoxime; BLA, bovine left atrium; BLV, bovine left ventricle; CBB, Coomassie brilliant blue; MyBP-C, myosin-binding protein C; OD, optical density; PKI, PKA inhibitor; RV, rat ventricular; SL, sarcomere length; TnC, troponin C; TnI, troponin I.
References
- Bers, D.M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Second edition. Kluwer-Academic, Dordrecht, Netherlands. 427 pp.
- Bers, D.M. 2002. Cardiac excitation–contraction coupling. Nature. 415:198–205. [DOI] [PubMed] [Google Scholar]
- Cazorla, O., A. Freiburg, M. Helmes, T. Centner, M. McNabb, Y. Wu, K. Trombitas, S. Labeit, and H. Granzier. 2000. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ. Res. 86:59–67. [DOI] [PubMed] [Google Scholar]
- Chiu, Y.L., E.W. Ballou, and L.E. Ford. 1982. Internal viscoelastic loading in cat papillary muscle. Biophys. J. 40:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe, P.P., and H.E.D.J. ter Keurs. 1991. Lack of effect of isoproterenol on unloaded velocity of sarcomere shortening in rat cardiac trabeculae. Circ. Res. 68:382–391. [DOI] [PubMed] [Google Scholar]
- Endoh, M., and J.R. Blinks. 1988. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through α- and β-receptors. Circ. Res. 62:247–265. [DOI] [PubMed] [Google Scholar]
- Fentzke, R.C., S.H. Buck, J.R. Patel, H. Lin, B.M. Wolska, M.O. Stojanovic, A.F. Martin, R.J. Solaro, R.L. Moss, and J.M. Leiden. 1999. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J. Physiol. 517:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, N., H. Kajiwara, S. Ishiwata, and S. Kurihara. 2000. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ. Res. 86:e1–e6. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., Y. Wu, T.C. Irving, and H. Granzier. 2003. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J. Physiol. 553:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, N., and H. Granzier. 2004. Role of the giant elastic protein titin in the Frank-Starling mechanism of the heart. Curr. Vasc. Pharmacol. 2:135–139. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., Y. Wu, G. Farman, T.C. Irving, and H. Granzier. 2005. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch. 449:449–457. [DOI] [PubMed] [Google Scholar]
- Garvey, J.L., E.G. Kranias, and R.J. Solaro. 1988. Phosphorylation of C-protein, troponin I, and phospholamban in isolated rabbit hearts. Biochem. J. 249:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier, H., and T.C. Irving. 1995. Passive tension in cardiac muscle: contribution of collagen, titin, microtubles, and intermediate filaments. Biophys. J. 68:1027–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier, H., and S. Labeit. 2002. Cardiac titin: an adjustable multifunctional spring. J. Physiol. 541:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser, M.L., M. Berri, C.M. Warren, and P.E. Mozdziak. 2002. Species variations in cDNA sequence and exon splicing patterns in the extensible I-band region of cardiac titin: relation to passive tension. J. Muscle Res. Cell Motil. 23:473–482. [DOI] [PubMed] [Google Scholar]
- Gregorio, C.C., H. Granzier, H. Sorimachi, and S. Labeit. 1999. Muscle assembly: a titanic achievement? Curr. Opin. Cell Biol. 11:18–25. [DOI] [PubMed] [Google Scholar]
- Grieve, D.J., A.C. Cave, J.A. Byrne, J. Layland, and A.M. Shah. 2004. Analysis of ex vivo left ventricular pressure-volume relations in the isolated murine ejecting heart. Exp. Physiol. 89:573–582. [DOI] [PubMed] [Google Scholar]
- Grimm, A.F., H.L. Lin, and B.R. Grimm. 1980. Left ventricular free wall and intraventricular pressure-sarcomere length distributions. Am. J. Physiol. 239:H101–H107. [DOI] [PubMed] [Google Scholar]
- Gupta, R.C., J. Neumann, P. Boknik, and A.M. Watanabe. 1994. M2-specific muscarinic cholinergic receptor-mediated inhibition of cardiac regulatory protein phosphorylation. Am. J. Physiol. 266:H1138–H1144. [DOI] [PubMed] [Google Scholar]
- Helmes, M., K. Trombitas, and H. Granzier. 1996. Titin develops restoring force in rat cardiac myocytes. Circ. Res. 79:619–626. [DOI] [PubMed] [Google Scholar]
- Helmes, M., K. Trombitas, T. Centner, M. Kellermayer, S. Labeit, W.A. Linke, and H. Granzier. 1999. Mechanically driven contour-length adjustment in rat cardiac titin's unique N2B sequence: titin is an adjustable spring. Circ. Res. 84:1339–1352. [DOI] [PubMed] [Google Scholar]
- Helmes, M., C.C. Lim, R. Liao, A. Bharti, L. Cui, and D.B. Sawyer. 2003. Titin determines the Frank-Starling relation in early diastole. J. Gen. Physiol. 121:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron, T.J., F.S. Korte, and K.S. McDonald. 2001. Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes. Circ. Res. 89:1184–1190. [DOI] [PubMed] [Google Scholar]
- Hoh, J.F.Y., G.H. Rossmanith, L.J. Kwan, and A.M. Hamilton. 1988. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ. Res. 62:452–461. [DOI] [PubMed] [Google Scholar]
- Hongo, K., E. Tanaka, and S. Kurihara. 1993. Alterations in contractile properties and Ca2+ transients by β- and muscarinic receptor stimulation in ferret myocardium. J. Physiol. 461:167–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y., K. Satoh, and N. Taira. 1978. Role of the peripheral vasculature in changes in venous return caused by isoproterenol, norepinephrine, and methoxamine in anesthetized dogs. Circ. Res. 43:553–561. [DOI] [PubMed] [Google Scholar]
- Kajiwara, H., S. Morimoto, N. Fukuda, I. Ohtsuki, and S. Kurihara. 2000. Effect of troponin I phosphorylation by protein kinase A on length-dependence of tension activation in skinned cardiac muscle fibers. Biochem. Biophys. Res. Commun. 272:104–110. [DOI] [PubMed] [Google Scholar]
- Kentish, J.C., H.E.D.J. ter Keurs, L. Ricciardi, J.J.J. Bucx, and M.I.M. Noble. 1986. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influcence of calcium concentrations on these relations. Circ. Res. 58:755–768. [DOI] [PubMed] [Google Scholar]
- Kentish, J.C., D.T. McCloskey, J. Layland, S. Palmer, J.M. Leiden, A.F. Martin, and R.J. Solaro. 2001. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ. Res. 88:1059–1065. [DOI] [PubMed] [Google Scholar]
- Komukai, K., and S. Kurihara. 1997. Length dependence of Ca2+-tension relationship in aequorin-injected ferret papillary muscles. Am. J. Physiol. 273:H1068–H1074. [DOI] [PubMed] [Google Scholar]
- Konhilas, J.P., T.C. Irving, B.M. Wolska, E.E. Jweied, A.F. Martin, R.J. Solaro, and P.P. de Tombe. 2003. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J. Physiol. 547:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke, M., S. Fujita-Becker, E. Rostkova, C. Neagoe, D. Labeit, D.J. Manstein, M. Gautel, and W.A. Linke. 2001. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ. Res. 89:874–881. [DOI] [PubMed] [Google Scholar]
- Kurihara, S., and M. Konishi. 1987. Effects of β-adrenoceptor stimulation on intracellular Ca transients and tension in rat ventricular muscle. Pflugers Arch. 409:427–437. [DOI] [PubMed] [Google Scholar]
- Layland, J., and J.C. Kentish. 2002. Myofilament-based relaxant effect of isoprenaline revealed during work-loop contractions in rat cardiac trabeculae. J. Physiol. 544:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland, J., D.J. Grieve, A.C. Cave, E. Sparks, R.J. Solaro, and A.M. Shah. 2004. Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J. Physiol. 556:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen, F.H., and R.A. Reeves. 1987. β-Receptor-mediated increase in venous return in humans. Can. J. Physiol. Pharmacol. 65:1658–1665. [DOI] [PubMed] [Google Scholar]
- Li, L., J. Desantiago, G. Chu, E.G. Kranias, and D.M. Bers. 2000. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation. Am. J. Physiol. Heart Circ. Physiol. 278:H769–H779. [DOI] [PubMed] [Google Scholar]
- MacKenna, D.A., J.H. Omens, A.D. McCulloch, and J.W. Covell. 1994. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. Am. J. Physiol. 266:H1007–H1018. [DOI] [PubMed] [Google Scholar]
- Makarenko, I., C.A. Opitz, M.C. Leake, C. Neagoe, M. Kulke, J.K. Gwathmey, F. del Monte, R.J. Hajjar, and W.A. Linke. 2004. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ. Res. 95:708–716. [DOI] [PubMed] [Google Scholar]
- Nagata, K., M. Iwase, T. Sobue, and M. Yokota. 1995. Differential effects of dobutamine and a phosphodiesterase inhibitor on early diastolic filling in patients with congestive heart failure. J. Am. Coll. Cardiol. 25:295–304. [DOI] [PubMed] [Google Scholar]
- Nagueh, S.F., G. Shah, Y. Wu, G. Torre-Amione, N.M. King, S. Lahmers, C.C. Witt, K. Becker, S. Labeit, and H.L. Granzier. 2004. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 110:155–162. [DOI] [PubMed] [Google Scholar]
- Neagoe, C., M. Kulke, F. del Monte, J.K. Gwathmey, P.P. de Tombe, R.J. Hajjar, and W.A. Linke. 2002. Titin isoform switch in ischemic human heart disease. Circulation. 106:1333–1341. [DOI] [PubMed] [Google Scholar]
- Okazaki, O., N. Suda, K. Hongo, M. Konishi, and S. Kurihara. 1990. Modulation of Ca2+ transients and contractile properties by β-adrenoceptor stimulation in ferret ventricular muscles. J. Physiol. 423:221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J.D., J.S. Landzberg, J.A. Bittl, I. Mirsky, and W.S. Colucci. 1991. Effects of β-adrenergic stimulation with dobutamine on isovolumic relaxation in the normal and failing human left ventricle. Circulation. 84:1040–1048. [DOI] [PubMed] [Google Scholar]
- Robertson, S.P., J.D. Johnson, M.J. Holroyde, E.G. Kranias, J.D. Potter, and R.J. Solaro. 1982. The effects of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J. Biol. Chem. 257:260–263. [PubMed] [Google Scholar]
- Rodriguez, E.K., W.C. Hunter, M.J. Royce, M.K. Leppo, A.S. Douglas, and H.F. Weisman. 1992. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am. J. Physiol. 263:H293–H306. [DOI] [PubMed] [Google Scholar]
- Salyers, A.K., L.F. Rozek, S.E. Bittner, and G.M. Walsh. 1988. Simultaneous determination of ventricular function and systemic hemodynamics in the conscious rat. J. Pharmacol. Methods. 19:267–274. [DOI] [PubMed] [Google Scholar]
- Stuyvers, B.D., A.D. McCulloch, J. Guo, H.J. Duff, and H.E.D.J. ter Keurs. 2002. Effect of stimulation rate, sarcomere length and Ca2+ on force generation by mouse cardiac muscle. J. Physiol. 544:817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs, H.E., W.H. Rijnsburger, R. van Heuningen, and M.J. Nagelsmit. 1980. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ. Res. 46:703–714. [DOI] [PubMed] [Google Scholar]
- Trombitas, K., Y. Wu, M. McNabb, M. Greaser, M.S. Kellermayer, S. Labeit, and H. Granzier. 2003. Molecular basis of passive stress relaxation in human soleus fibers: assessment of the role of immunoglobulin-like domain unfolding. Biophys. J. 85:3142–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull, L., J.F.Y. Hoh, R.I. Ludowyke, and G.H. Rossmanith. 2002. Troponin I phosphorylation enhances crossbridge kinetics during β-adrenergic stimulation in rat cardiac tissue. J. Physiol. 542:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udelson, J.E., R.O. Cannon III, S.L. Bacharach, T.F. Rumble, and R.O. Bonow. 1989. β-Adrenergic stimulation with isoproterenol enhances left ventricular diastolic performance in hypertrophic cardiomyopathy despite potentiation of myocardial ischemia. Comparison to rapid atria pacing. Circulation. 79:371–382. [DOI] [PubMed] [Google Scholar]
- van Heuningen, R., W.H. Rijinsburger, and H.E.D.J. ter Keurs. 1982. Sarcomere length control in striated muscle. Am. J. Physiol. 242:H411–H420. [DOI] [PubMed] [Google Scholar]
- van Kerckhoven, R., E.A.J. Kalkman, P.R. Saxena, and R.G. Schoemaker. 2000. Altered cardiac collagen and associated changes in diastolic function of infracted rat hearts. Cardiovasc. Res. 46:316–323. [DOI] [PubMed] [Google Scholar]
- Venema, R.C., R.L. Raynor, T.A.J. Noland, and J.F. Kuo. 1993. Role of protein kinase C in the phosphorylation of cardiac myosin light chain 2. Biochem. J. 294:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanapermpool, J., X. Guo, and R.J. Solaro. 1995. The unique amino-terminal peptide of cardiac troponin I regulates myofibrillar activity only when it is phosphorylated. J. Mol. Cell. Cardiol. 27:1383–1391. [DOI] [PubMed] [Google Scholar]
- Wu, Y., O. Cazolra, D. Labeit, S. Labeit, and H. Granzier. 2000. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J. Mol. Cell. Cardiol. 32:2151–2162. [DOI] [PubMed] [Google Scholar]
- Wu, Y., S.P. Bell, K. Trombitas, C.C. Witt, S. Labeit, M.M. LeWinter, and H. Granzier. 2002. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 106:1384–1389. [DOI] [PubMed] [Google Scholar]
- Yamasaki, R., M. Berri, Y. Wu, K. Trombitas, M. McNabb, M.S. Kellermayer, C. Witt, D. Labeit, S. Labeit, M. Greaser, and H. Granzier. 2001. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys. J. 81:2297–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, R., Y. Wu, M. McNabb, M. Greaser, S. Labeit, and H. Granzier. 2002. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ. Res. 90:1181–1188. [DOI] [PubMed] [Google Scholar]
- Zhang, R., J. Zhao, A. Mandveno, and J.D. Potter. 1995. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ. Res. 76:1028–1035. [DOI] [PubMed] [Google Scholar]