Abstract
A selective and sensitive LC/MS/MS assay was developed for the quantification of d2-nicotine and d2-cotinine in plasma of current and past smokers administered d2-nicotine. After solid phase extraction and liquid liquid extraction, HPLC separation was achieved on a capillary hydrophilic interaction chromatography phase column. The analytes were monitored by tandem mass spectrometry with electrospray positive ionization. Linear calibration curves were generated for d2-nicotine (0.03 to 6.0 ng/ml plasma) and d2-cotinine (0.15 to 25 ng/ml plasma). The lower limits of quantitation were 0.15 ng/ml and 0.25 ng/ml for d2-nicotine and d2-cotinine, respectively. The coefficient of variation was 3.7% for d2-nicotine and 2.5% for d2-cotinine. The method was applied to two ongoing studies of d2-nicotine metabolism in prior and current smokers. Preliminary analysis of a subset of subjects from these studies detected a significantly lower rate of nicotine conversion to cotinine by past smokers compared to current smokers.
Introduction
Nicotine is the main addictive agent in tobacco [1;2]. Smokers typically adjust their nicotine intake to maintain plasma nicotine levels. However, plasma nicotine concentrations vary among smokers due to both the extent of smoking and to individual differences in nicotine metabolism [3]. In smokers, nicotine is extensively metabolized, primarily by the hepatic enzyme P450 2A6, although P450 2A13, expressed in the respiratory tract, may also contribute [3;4]. P450 2A6 and P450 2A13 catalyze nicotine 5′-oxidation to the corresponding iminium ion that is then converted, by either aldehyde oxidase or P450 enzymes, to cotinine [5]. In both tobacco users and individuals using nicotine replacement therapies greater than 70% of the nicotine dose is converted to cotinine, which is subsequently oxidized to trans-3′-hydroxycotinine [3]. Plasma cotinine concentrations are routinely used to assess both tobacco use and environmental tobacco smoke exposure (ETS) [6–8]. However, plasma cotinine levels vary as much as two fold due to individual differences in nicotine metabolism [3]. There are many studies being carried out to assess the role of nicotine metabolism in smoking behavior and nicotine addiction [9–11].
To study nicotine metabolism in both smokers and non-smokers, Benowitz and co-workers have carried out a number of elegant studies with [3′,3′-D2]-nicotine (d2-nicotine). The majority of these studies have analyzed d2-nicotine and d2-cotinine by GC/MS [12]. However, more recently the liquid chromatography-atmospheric pressure ionization tandem mass spectrometry (LC-APCI MS/MS) method developed by Bernert and co-workers for the determination of serum cotinine concentrations [13] has been applied to the analysis of d2-cotinine [14–16]. Two of these studies also quantified urine concentrations of d2-nicotine by LC/MS/MS [15;16], but GC/MS analysis was used to quantify plasma d2-nicotine, suggesting that the LC/MS/MS method used was not sufficiently sensitive for the analysis of the lower d2-nicotine concentrations in plasma.
A number of LC/MS/MS methods have been developed for the measurement of nicotine and cotinine in biological fluids. These methods were recently reviewed by Heavner et al [17]. The limit of quantification ranged from 1 to 10 ng/ml for nicotine and from 0.1 to 10 ng/ml for cotinine. A lower limit of quantification for cotinine, 0.05 ng/ml plasma, was reported by Bernert et al [13]. Their method used both liquid liquid extraction and solid phase extraction for sample clean-up and analyzed cotinine by reverse phase HPLC linked to atmospheric pressure (APCI) MS/MS. This method was sensitive, selective and robust. Only a handful of studies have used electrospray ionization (ESI) MS/MS for the analysis of either nicotine or cotinine [18–21]. For compounds with a high proton affinity, APCI is reported to be the more efficient ionization process [17]. In addition, APCI is much less sensitive than is ESI to interference from matrix on ionization, specifically salts and ion pairing agents present in the mobile phase used for reverse phase HPLC analyses. However, the use of silica based columns operated under hydrophilic interaction chromatography mode (HILIC) has resulted in an increase in the sensitivity of LC-ESI/MS/MS detection of basic compounds [20–22].
In the study reported here we extend the use of HILIC-ESI/MS/MS analysis to capillary chromatography to further lower the limit of detection for nicotine and cotinine in plasma. We apply this method to the determination of plasma d2-cotinine and d2-nicotine levels in smokers and non-smokers who were administered [3′,3′-d2]-nicotine. Unlike earlier reports [13;14], this assay quantifies both deuterated and non deuterated nicotine in plasma by LC/MS/MS analysis.
2. Experimental
2.1 Chemicals and Reagents
(-)-Nicotine (98%) was obtained from Sigma (St. Louis, MO). (-)-Cotinine, (d3-methyl)-nicotine (99%), and (d3-methyl)-cotinine (99%) was purchased from Toronto Research Chemicals (New York, ON, Canada). [3′,3′-D2]-nicotine and [5′,5′-D2]-cotinine was synthesized as previously described [23]. Both di-deuterated compounds were > 99.4% pure as determined by GC/MS, LC/MS and LC/MS/MS analysis; less than 0.1% of the non-deuterated compounds were detected. The location of the label was confirmed by NMR analysis. B All other solvents and reagents were of highest analytical grade and purchased from Sigma.
2.2 Stock solutions and calibration standards
Primary stock solutions of d0, d2, and d3-nicotine and cotinine (5 µg/ml) were prepared in both water and methanol and stored at −20°C. The concentrations of the primary stock solutions were determined by UV spectrophotometry. A working solution of the internal standard (IS) was prepared in methanol and contained 0.082 ng/µl of both (d3-methyl)-nicotine and (d3-methyl)-cotinine. The primary stock solutions of d0, d2, and d3-nicotine and cotinine were diluted with methanol to working stock solutions of 0.5 ng/µl, 0.05 ng/µl, and 0.005 ng/µl and used to optimize the MS/MS response. Working stock solutions that were analyzed directly by LC/MS/MS without going through the sample preparation protocol described below were prepared from the primary stock solutions prepared in methanol. All other working stock solutions were prepared from the primary stock solutions prepared in water. Aliquots of these solutions were kept frozen at −20°C prior to use.
Quantification was based on the addition of a known amount of IS. Calibration standards were prepared by adding decreasing amounts of the d2-nicotine and d2-cotinine working stock solutions to water, plasma from a non-smoker and plasma from a smoker. IS (0.82 ng) was added to each calibration standard and the samples prepared as described in the “sample preparation” section. d2-nicotine and d2-cotinine (0.005 – 5 ng) was also added directly to 100 µl methanol containing IS (8.2 pg/µl) to determine the instrument response.
2.3 Sample preparation
The plasma samples were stored at −20°C until workup. The samples have currently been stored for up to two years without any noticeable degradation of the deuterated nicotine or cotinine. For analysis, plasma was thawed, mixed thoroughly and 100 µl or 200 µl added to 4 ml silanized vials containing 900 µl water and 2 ml Dulbecco's 10 mM phosphate buffered saline (pH 7.4, PBS). For method validation the amount of plasma used ranged from 20 µl – 1 ml. IS (10 µl) was added to each sample and the samples were vortexed gently. Samples were loaded on Oasis MCX columns (60 mg sorbent material with an average particle diameter of 53 µm and 6 ml cartridge volume (Waters Corporation, Milford, MA)), that had been prepared according to the manufacturers recommendations (the columns were activated with 3 ml methanol and equilibrated with 3 ml of water followed by 3 ml PBS). The columns were then washed with 3 ml each of water, 0.1 N HCl, and methanol, and the samples eluted with 3 ml of methylene chloride/isopropanol/ammonium hydroxide (78:20:2). The eluted cotinine and nicotine were further purified with a series of acid-base liquid-liquid extractions. Water (1–2 ml) was added to the eluted samples, which were then extracted and the aqueous layer discarded. The organic layer was then extracted with an equal volume of 1 N hydrochloric acid. The aqueous layer was removed, an equal volume of 50% potassium carbonate added to it and cotinine and nicotine extracted into methylene chloride. The methylene chloride layer was transferred to a 4-mL silanized glass vial and 200 µl of methanol was added. The samples were then dried under nitrogen gas to a volume of 50 – 100 µl methanol.
2.4 Liquid Chromatography-Tandem Mass Spectrometry Analysis
LC/MS/MS analysis was performed on an Agilent 1100 series capillary HPLC system (Agilent Technologies, Palo Alto, California) interfaced to a Finnigan TSQ Quantum Ultra AM triple quadrupole mass spectrometer (Thermo Electron, San Jose, California). The samples (0.2 – 3 µl) were injected onto a 300 µm × 100 mm Atlantis HILIC (5 µm particle size), silica-based, capillary column (Waters Corporation). Nicotine and cotinine were analyzed independently by injecting each sample twice. d0-, d2-, and d3-Nicotine were eluted isocratically with 7.5 mM ammonium acetate- acetonitrile (23:77 v/v) containing 0.4% acetic acid, at a flow rate of 15 µL/min. d0-, d2-, and d3-Cotinine were eluted isocratically with water- acetonitrile- formic acid (15:85:0.2 v/v/v) at a flow rate of 15 µL/min. The column was operated at 20°C. For the first min the eluant was diverted to waste before directing the flow into the ESI source of the mass spectrometer. The void volume of the system at the 15 µL/min flow rate was 0.84 min. Alternatively, nicotine and cotinine were analyzed simultaneously with a mobile phase gradient from 99.8% acetonitrile (0.2% formic acid) to 59.8% water: 40% acetonitrile:0.2% formic acid.
The ESI source was operated in the positive ion mode with a collision energy of 25 V, the tube lens offset set to 60V, the collision gas pressure set to 1.4 mTorr and the source CID collision energy set to 10 V. Peak area obtained from selected reaction monitoring (SRM) of the mass transitions for the various analytes were used for quantification. The scan width was 0.35 (m/z) and the scan time 0.2 seconds and the peak widths for both Q1 and Q3 were 0.30. The mass transitions were as follows: d0-Nicotine (m/z 163→130 and m/z 163→117), d2-nicotine (m/z 165→131 and m/z 165→118), d3-nicotine (m/z 166→130 and m/z 166→117), d0-cotinine (m/z 177→98 and m/z 177→80), d2-cotinine (m/z 179→100 and m/z 179→80), d3-cotinine (m/z 180→101 and m/z 180→80).
The presence of common product ions for some of the SRM transitions theoretically could result in "cross-talk" between these transitions due to incomplete clearance of the collision cell resulting in quantitation errors. The acquisition time for the SRM transitions of 0.2 seconds is long enough such as that the effect should be negligible. The injection of each analyte and internal standard independently of the others while monitoring all SRM transitions confirmed this to be the case.
2.5 Application
A smoker who had abstained from smoking overnight was administered d2-nicotine intravenously (2µg/min/kg) and blood samples were collected in plasma separator tubes (Vacutainer PST gel tube with green marble top, Fisher Scientific Corp.). Plasma was frozen at −20°C until analysis. No loss of cotinine or nicotine in the PST gel was detected when blood from non-smoking individuals was supplemented with either cotinine or nicotine. In a second on-going study, subjects, current smokers and prior smokers (abstinent for more than 1 year), were administered 2 mg d2-nicotine orally, blood samples were collected at 30, 90 and 240 min. Samples from the 30 min collection were analyzed by the method described above. Samples from both studies were analyzed in batches of 12–16 with a positive control (a pooled plasma sample from a non-smoking subject administered d2-nicotine) and a water blank. For each batch all samples and controls were analyzed in duplicate. Both protocols were approved by the University of Minnesota Human Subjects review board.
3. Results and Discussion
3.1 Chromatography
Recently, Shou and Naidong described a HILIC-ESI tandem mass spectrometry (MS/MS) method for the analysis of eight basic compounds. Nicotine was one of the eight analyzed, however, the response for nicotine was significantly less than for many of the other compounds. We report here the development of a sensitive new method for the analysis of nicotine and cotinine in plasma that utilizes capillary HILIC-ESI-MS/MS. This method has been applied to the characterization of d2-nicotine metabolism in smokers. Both undeuterated and deuterated nicotine and cotinine were quantified in plasma.
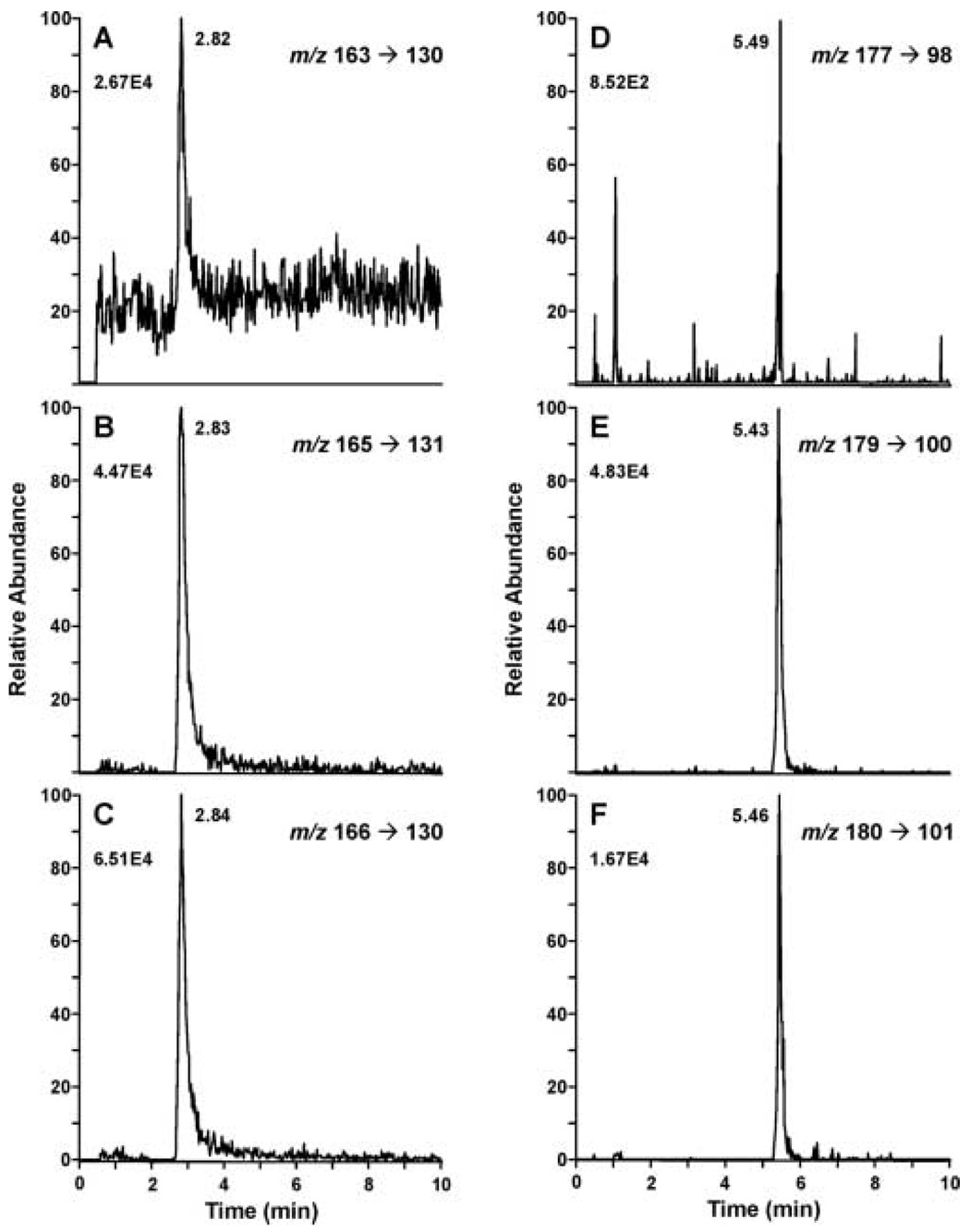
In our initial experiments we analyzed cotinine and nicotine in a single LC analysis using a gradient of water and acetonitrile containing 0.2% formic acid. However, the use of an elution gradient required significant time to reequilibrate the capillary column between injections. In addition, the conditions for optimal peak shape were different for cotinine and nicotine. Therefore, the independent analysis of nicotine and cotinine by two isocratic LC systems was both more time efficient and more reproducible than a gradient system. We began our analysis using conditions similar to those of Shou and Naidong [21] in which the mobile phase contained a small amount of trifluoroacetic acid (TFA) with either formic or acetic acid, and acetonitrile. However, we obtained good sensitivity and reproducible peak shape for nicotine with a mobile phase that was 23% 7.5 mM ammonium acetate, 76.7% acetonitrile, 0.3% acetic acid. The inclusion of ammonium acetate in the mobile phase was essential for optimum nicotine peak shape. Cotinine peak shape was best when the mobile phase did not contain ammonium acetate. In both cases a low pH (< 3) was critical for good peak shape. (Fig. 1 A–F). Cotinine peak shape was very sensitive to the presence of water in the sample. Water (< 0.5%) caused significant peak broadening and an increase in retention time (data not shown). Therefore, during sample preparation it was important not to carry any water into the final methylene chloride extraction. The liquid liquid extractions following the solid phase extraction of the samples were necessary to obtain reproducible chromatography on the capillary column and to maintain the sensitivity of the method.
Figure 1.
Capillary HILIC-ESI SRM analysis of a plasma sample from a non-smoker administered d2-nicotine orally. Six ion pair transitions were monitored d0-nicotine, m/z 163→130 (A), d2-nicotine, m/z 165→131 (B), d3-nicotine, m/z 166→130 (C), d0-cotinine, m/z 177→98 (D), d2-cotinine, m/z 179→100 (E), d3-cotinine, m/z 180→101 (F). Different chromatographic conditions were used for nicotine (A–C) and cotinine (D–F). Plasma (100 µl) was worked up for analysis and 1 µl of the 100 µl methanol preparation was injected.
3.2 LC/MS/MS Analysis
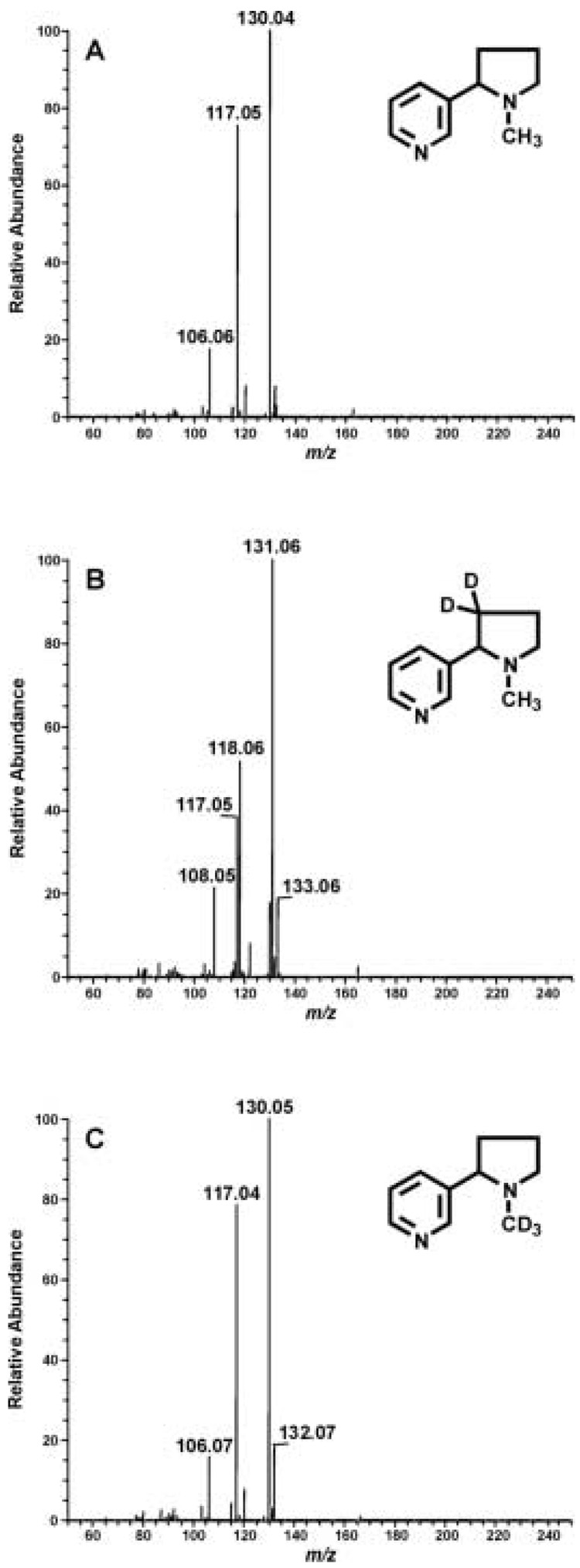
The product ion spectra obtained by ESI MS/MS analysis of the (M+H)+ ion for d0-, d2-, and d3-nicotine are illustrated in Fig. 2. The daughter spectra for d0- and d3-nicotine, m/z 163 and m/z 166, are identical; the most abundant ion is m/z 130 and the second most abundant ion is m/z 117 (Fig. 2A, 2C). The spectrum for d2-nicotine is slightly different. The major fragment is m/z 131, due to the presence of one deuterium atom (Figure 2B). The second most abundant ion is m/z 118; an ion, m/z 117 was present at a slightly lower abundance. The m/z 118 ion must contain one deuterium atom whereas the m/z 117 ion is likely the same fragment observed with d0- and d3-nicotine.
Figure 2.
ESI-MS/MS analysis of d0-nicotine (A), d2-nicotine (B) and d3-nicotine (C). One ng of each of the three nicotine isotopes was injected onto the HILIC column and daughter ion spectra were collected at m/z 163, 165 and 166 for d0-, d2- and d3-nicotine, respectively.
The daughter ion spectra for d0-, d2-, and d3-cotinine are similar to what has previously been reported for ESI-MS/MS analysis of d0-cotinine [24]. The major fragment for all three cotinine isotopes is m/z 80. The second most abundant ions were m/z 98, 100, and 101 for d0-, d2-, and d3-cotinine, respectively. These ions correspond to a d0-, d2-, and d3-N-methylpyrrolidine fragment.
To quantify d0- and d2-nicotine and d0- and d2-cotinine plasma concentrations, SRM was used. Two ion transition pairs were monitored for each analyte. The ratio between the two transitions was used as confirmation of the identity of d0- and d2-nicotine or d0- and d2-cotinine. For d2-cotinine the ratio of 100/80 was 0.54 ± 0.04 (n = 32) and for d2-nicotine the ratio of 131/118 was 1.82 ± 0.13 (n = 28). As noted previously in the literature, the ratio of two progeny ion from multiple reaction ion monitoring (MRM) are not fundamental and may vary with analytical conditions, such as operation of Q2, the collision cell [13]. Therefore, the evaluation of these ratios in samples was based on standards analyzed at the same time under the same conditions.
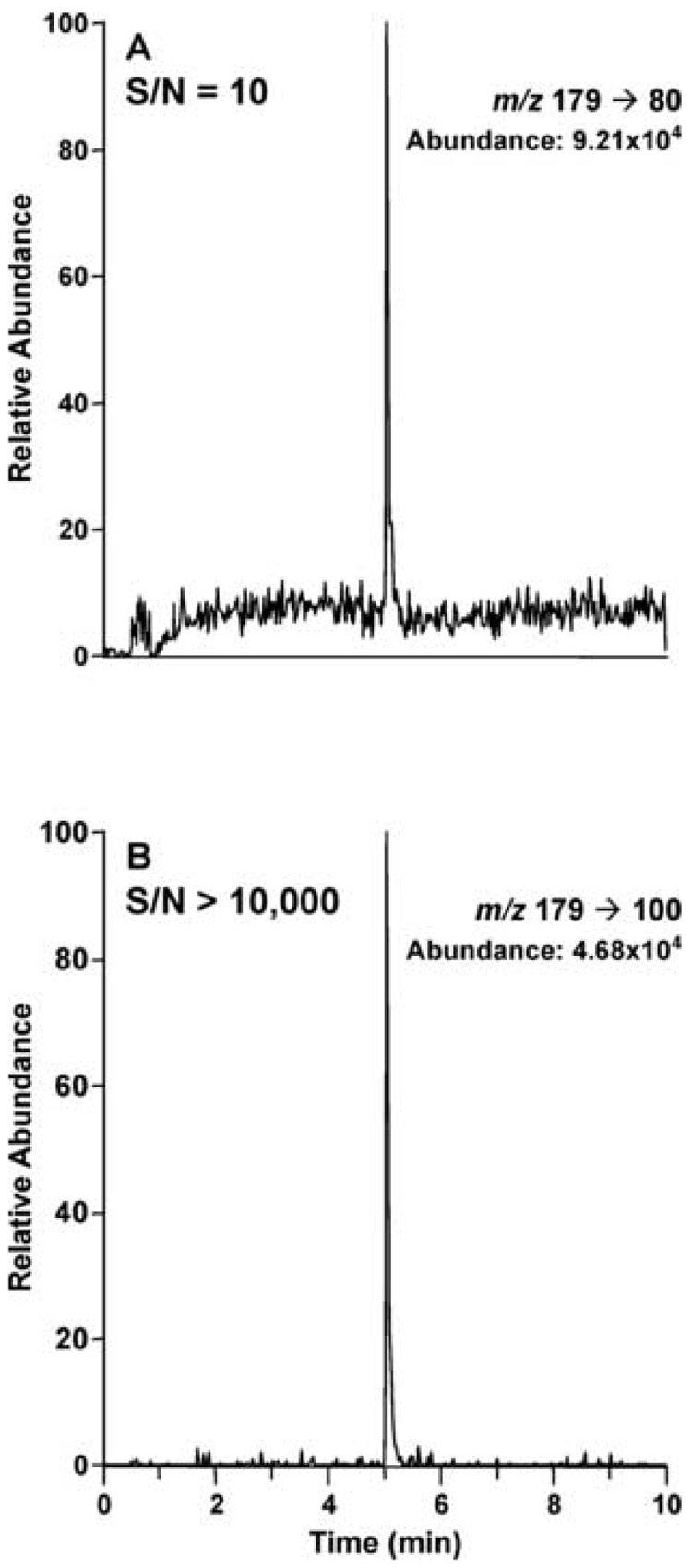
To quantify d0-, and d2-nicotine the most abundant transitions, m/z 163→130, m/z 165→131, and m/z 166→130 for d0-, d2-, and d3-nicotine, respectively, were used. However, to quantify d0-, and d2-cotinine the second most abundant ion transitions were used: m/z 177→98, m/z 179→100, m/z 180→101 for d0-, d2-, and d3-cotinine, respectively. The signal-to-noise ratio for these transitions was significantly greater than for the most abundant transitions. The difference in signal to noise for the two transition ion pairs monitored for d2–cotinine is illustrated in Fig. 3.
Figure 3.
Signal-to-noise comparison between the m/z 179→80 and 179→100 mass transitions for d2-cotinine, obtained from HILIC-ESI MS/MS analysis of plasma from a non-smoker administered d2-nicotine orally.
The presence of isotopomers of d0-cotinine with the same nominal mass as d2-cotinine (primarily due to the natural abundances of 13C (1.11%), 15N (0.37%), and 18O (0.20%)) will result in a contribution to the d2-cotinine SRM signal from d0-cotinine present in the sample. The percent contribution from the d0-cotinine is dependent upon the naturally occurring isotopic abundances, the molecular formula of the fragment ion, and any significant isotopic dependence of the fragmentation pathway. This was determined empirically using an authentic standard and is listed in Table 1. A similar contribution to the measured d3-cotinine signal from d2-cotinine is also in Table 1 as are similar analogous values for the nicotine analysis. These values were used to correct for the presence of d0-cotinine and d0-nicotine in the d2-cotinine and d2-nicotine analyses. The analysis of d2-cotinine in the plasma of smokers administered d2-nicotine is an example of when this correction was significant.
Table 1.
SRM analysis of d0-, d2-, d3-nicotine and d0-, d2-, d3-cotinine standardsa
| Analyzed compound | Mass transitions (Percent of area)b |
||
|---|---|---|---|
| 163 → 130 | 165 → 131 | 166 → 130 | |
| d0-nicotine | 99.8 | 0.16 | – |
| d2-nicotine | – | 99.6 | 0.35 |
| d3-nicotine | – | – | 100 |
| 177 → 98 | 179 → 100 | 180 → 101 | |
| d0-cotinine | 99.5 | 0.5 | 0.01 |
| d2-cotinine | – | 94.3 | 5.7 |
| d3-cotinine | – | – | 100 |
1 µl of a 5 ng/µl standard solution of each cotinine and nicotine isotope was injected onto the HILIC column and analyzed by SRM, for the three transitions listed.
The area % was calculated by dividing the area corresponding to each mass transition by the total area of the two or three mass transitions for a given standard solution. The value is the average of two independent determinations, which differed by < 2%.
3.3 Characteristics of the method
The limit of detection of the SRM capillary LC/MS/MS method was determined by analyzing the response for injections of methanol containing 0.05 pg to 500 pg of d0, d2, d3-nicotine and d0, d2, d3-cotinine. The response was linear from 0.125 pg to 500 pg injected. The signal to noise ratio for 0.05 pg cotinine or nicotine injected was < 50. Based on a SD of less than 10%, the limits of quantification of nicotine and cotinine standard injected on capillary LC were determined to be 0.125 pg and 0.24 pg, respectively. [The mean peak area ± SD (n = 5) for 0.24 pg d2-cotinine was 3020 ± 261 (CV 9.0%). The mean ± SD (n = 5) for 0.125 pg d2-nicotine was 49860 ± 3138 (CV, 6.0%)]. Therefore, if a 100 µl plasma was prepared for analysis and 1 µl of the resulting 100µl methanol sample is injected for LC/MS/MS analysis, then theoretically the lowest limit of quantitation that may be obtained is 0.24 ng/ml d2-cotinine and 0.12 ng/ml d2-nicotine.
The limit of quantification of the assay was experimentally determined by analyzing non-smoker plasma samples (100 µl) supplemented with varying amounts of d2-nicotine or d2-cotinine. Both nicotine and cotinine were linear over the range of concentrations tested, 0.15 – 25 ng/ml for cotinine and 0.03 – 6 ng/ml for nicotine. The equations of the calibration curves obtained were y = 1.09x−0.05 and y = 1.02x − 0.01 for d2-nicotine and d2-cotinine, respectively. The regression constants for both compounds were > 0.99. The accuracy of the assay was good over the range tested and the limit of quantitation (based on a SD < 20%) was 0.25 ng/ml for d2-cotinine and 0.15 ng/ml for d2-nicotine (Table 2). Ion suppression of the analyte and internal standard signal due to coeluting material from the plasma matrix should be minimal due to the extensive sample clean up procedure employed. The isotopically labeled d3-cotinine and d3-nicotine internal standards coelute with the non-deuterated and di-deuterated cotinine and nicotine analytes, respectively, eliminating any effect ion suppression would have on the ability of the assay to properly quantify the analyte levels. This is confirmed by the linearity of the standard addition experiment. The variation in the assay with different amounts of plasma was assessed using plasma volumes as low as 20 µl and as high as 1 ml. Typically 100 or 200 µl of plasma was used for this assay, but when 20 µl plasma was used the results were identical (Table 3).
Table 2.
Calculated concentrations for LS/MS/MS determination of spiked plasmaa
| Compound | Expected (ng/ml) | Observed (mean ± SD) (ng/ml) | Observed/Expected (%) |
|---|---|---|---|
| d2-Nicotineb | 0.03 | 0.03c | |
| 0.15 | 0.14 ± 0.02 | 93 | |
| 0.3 | 0.29 ± 0.02 | 97 | |
| 0.6 | 0.62 ± 0.06 | 103 | |
| 3.0 | 3.0 ± 0.32 | 100 | |
| 6.0 | 6.6c | ||
| d2-Cotinined | 0.15 | 0.18 ± 0.041 | 128 |
| 0.25 | 0.26 ± 0.042 | 104 | |
| 0.5 | 0.54 ± 0.090 | 108 | |
| 2.5 | 2.7 ± 0.08 | 112 | |
| 5.0 | 5.0 ± 0.46 | 100 | |
| 12.5 | 12.5 ± 1.07 | 100 | |
| 25.0 | 25.5 ± 1.11 | 102 | |
Either d2-nicotine or d2-cotinine was added to 100µL plasma from a non-smoker at the indicated concentrations, and the samples prepared as described in the Experimental section.
n=4
n=2
n=5
Table 3.
Determination of nicotine and cotinine concentration in varying plasma volumesa
| Plasma Volume | d2-Nicotineb (ng/ml) | d2-Cotinineb (ng/ml) |
|---|---|---|
| 20 µl | 1.4 ± 0.16 | 24.8 ± 1.37 |
| 50 µl | 1.3 ± 0.09 | 23.5 ± 0.55 |
| 200 µl | 1.2 ± 0.10 | 23.3 ± 0.49 |
Different volumes of plasma (a positive control sample prepared by pooling plasma from several individuals who were administered d2-nicotine) were analyzed for d2-nicotine and d2-cotinine according to the protocol described in the Experimental section.
The values are the means ± SD of three independent experiments performed in duplicate.
Intra- and inter-day variability was determined for a plasma sample spiked with d2-nicotine and d2-cotinine and a sample from an individual who had been administered d2-nicotine. Six injections from the same extracted sample were made on three days over a 1 month period (Table 4). The intra-day coefficients of variation (CV) ranged from 2.7 to 5.9% for d2-nicotine and from 1.8 to 12% for d2-cotinine. The higher CV at the lower cotinine concentration is primarily due to variable peak shape on the capillary column, and the difficulty in accurately integrating the peak. The inter-day CV was 0.4% for nicotine and 1.9% and 7%, respectively for low and high d2-cotinine concentrations.
Table 4.
Intra- and interday variability of the LC/MS/MS analysis
| Day 1 (n = 6) | Day 2 (n = 6) | Day 3 (n = 6) | Days 1–3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Mean (ng/ml) (Range) | %CV | Mean (ng/ml) (Range) | %CV | Mean (ng/ml) (Range) | %CV | Mean (ng/ml) | %CV | |
| Positive Controla | d2-Nicotine | 0.84 (0.78 – 0.91) | 5.4 | 0.85 (0.80 – 0.95) | 5.9 | 0.86 (0.82 – 0.92) | 5.0 | 0.85 | 0.4 |
| d2-Cotinine | 12.44 (12.14 – 12.84) | 1.8 | 12.54 (12.09 – 13.11) | 2.8 | 13.23 (12.2 – 13.99) | 5.2 | 12.74 | 1.9 | |
| Standard (1 ng/ml)b | d2-Nicotine | 0.94 (0.90 – 0.98) | 2.7 | 1.02 (0.92 – 1.06) | 5.5 | 0.94 (0.92 – 1.00) | 3.2 | 0.97 | 4.6 |
| d2-Cotinine | 0.94 (0.81 – 1.11) | 12 | 1.06 (0.97 – 1.19) | 7 | 0.94 (0.79 – 1.07) | 12 | 0.98 | 7 | |
Plasma from subject administered d2-nicotine
Plasma supplemented with 1 ng/ml d2-nicotine and d2-cotinine
The analytical method reproducibility was determined from repeated analysis of a positive control sample consisting of plasma pooled from individuals who had been administered d2-nicotine. Plasma samples were routinely analyzed in batches of 12 to 16. Each batch contained the positive control sample described above, and a water blank. The values obtained in the analysis of positive control from 5 consecutive analyses are presented in Table 5. The CV for d2-nicotine was 3.7% and for d2-cotinine was 2.5%.
Table 5.
Assay reproducibilitya
| Positive Control Sampleb | Day 1 (n = 2) | Day 2 (n = 2) | Day 3 (n = 2) | Day 4 (n = 2) | Day 5 (n = 2) | Overall, Days 1–5 (n = 10) | ||
|---|---|---|---|---|---|---|---|---|
| Average (ng/ml) | Average (ng/ml) | Std. Dev. | %CV | |||||
| Nicotine | 2.44 | 2.26 | 2.46 | 2.45 | 2.46 | 2.41 | 0.09 | 3.7 |
| Cotinine | 25.9 | 25.5 | 26.8 | 26.2 | 27.2 | 26.3 | 0.67 | 2.5 |
A plasma sample was worked up independently on 5 days and analyzed in duplicate
Plasma pooled from 3 individuals who had received d2-nicotine
3.4 Application to plasma samples of subjects administered D2-nicotine
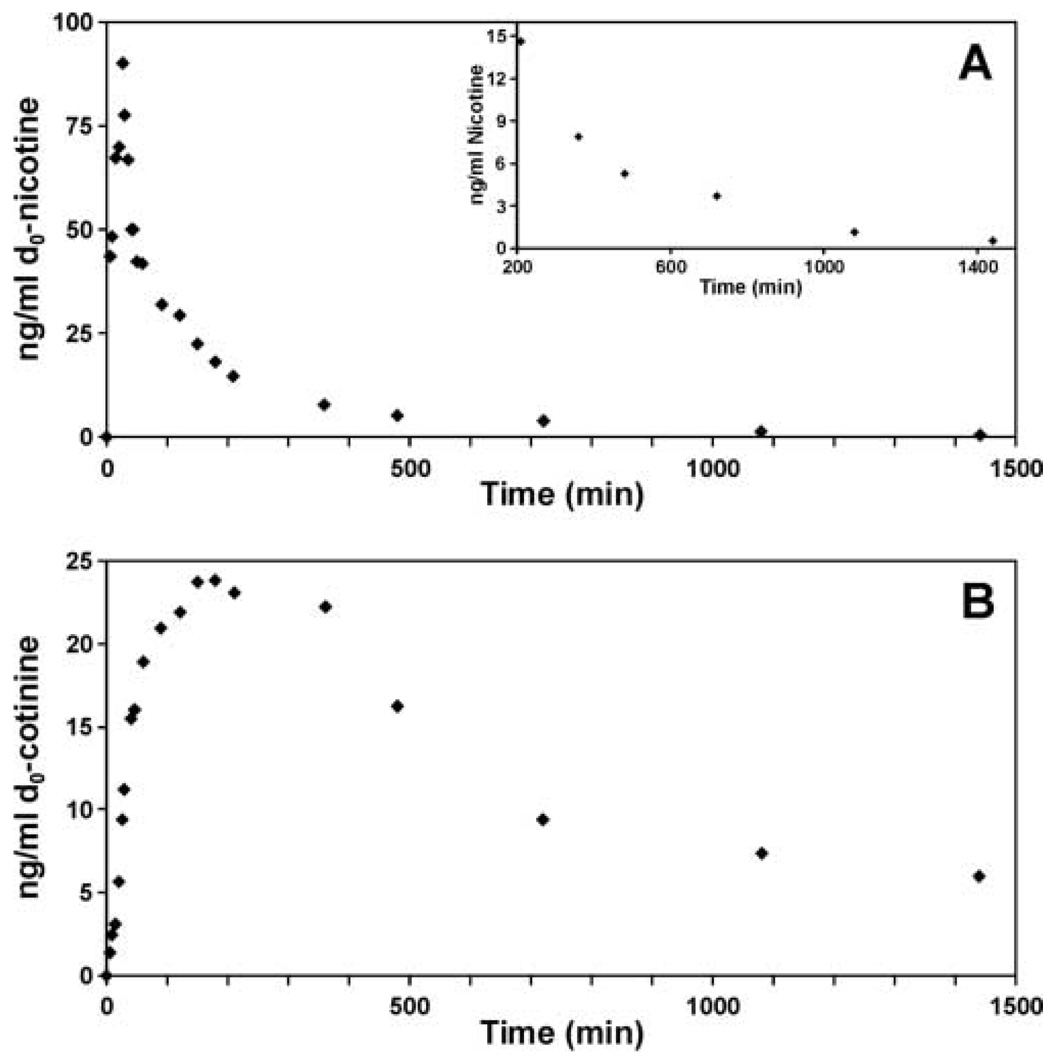
The method described above was applied to two on-going studies. In one, d2-nicotine was administered orally and plasma nicotine and cotinine concentrations were determined 30 min later. There were two groups of subjects in this study, current smokers and past smokers. The d2-nicotine concentration was similar in both groups whereas d2-cotinine levels were lower in past smokers. The data is presented for only a subset of the 200 subjects in this study. However, it illustrates the usefulness of the method for simultaneously determining the concentrations of both deuterated and non-deuterated nicotine and cotinine in current smokers, who were not required to modify there normal smoking habits. In a second on-going study d2-nicotine was infused intravenously and its concentration and that of d2-cotinine were monitored over time (figure 4). The peak plasma nicotine concentration occurred at 25 min and peak plasma cotinine concentrations at 120 to 150 min. At 23.5 h d2-nicotine was still detected in the plasma at a concentration of 0.5 ng/ml.
Figure 4.
Plasma d2-nicotine (A) and d2-cotinine concentrations (B) during and following the intravenous infusion of d2-nicotine. d2-Nicotine (2 µg/kg/min) was infused from 0 to 30 min. Insert shows plasma d2-nicotine concentrations from 210 min to 23.5 h. Values are mean of duplicate determinations that differed by < 10%.
4. Conclusions
We report here the development of an ESI-LC/MS/MS method which uses capillary HILIC to quantify d2-nicotine and d2-cotinine in plasma of current smokers and non-smokers. Analysis may be carried out on as little as 20 µl of plasma and the limits of quantitation for d2-cotinine and d2-nicotine were 0.25 ng/ml and 0.15 ng/ml, respectively. These limits are similar to what has been reported previously for the analysis of d2-cotinine in plasma by APCI LC/MS/MS [14]. The method is being applied to on-going studies of nicotine metabolism in smokers and non-smokers administered d2-nicotine. In smokers, non-deuterated nicotine and cotinine may be analyzed simultaneously with the deuterated isotopes.
Table 6.
Application of HILIC ESI MS/MS method to the analysis of plasma d2-nicotine and d2-cotinine
| Plasma concentrations (ng/ml) |
||||
|---|---|---|---|---|
| d2-nicotine | d2-cotinine | d0-nicotine | d0-cotinine | |
| Smokersa (n=12) | 2.47 ± 1.02 | 17.8 ± 6.6 | 28.8 ± 8.1 | 440 ± 81 |
| Past smokersc (n=10) | 2.7 ± 2.12 | 10.5 ± 6.4 | NDb | 0.6 ± 0.44 |
Smokers and non-smokers were orally administered 2mg d2-nicotine and plasma collected for analysis at 30 min.
ND < 1.5 ng/ml, the limit of detection is due to contamination of the blanks with a mean of 1.5 ng/ml d0-nicotine.
Subjects has smoked a cigarette 20–90 min prior to d2-nicotine administration.
Acknowledgements
This work was supported by the National Institute of Health grant P01-CA89392. LC/MS/MS analysis was carried out in the Analytical Biochemistry Core of the University of Minnesota Cancer Center, supported in part by grant CA-77598.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benowitz NL. Primary Care; Clinics In Office Practice. 1999;26:611–631. doi: 10.1016/s0095-4543(05)70120-2. [DOI] [PubMed] [Google Scholar]
- 2.Balfour DJ. Respiration. 2002;69:7–11. doi: 10.1159/000049362. [DOI] [PubMed] [Google Scholar]
- 3.Hukkanen J, Jacob P, III, Benowitz NL. Pharmacological Reviews. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Murphy SE, Raulinaitis V, Brown KM. Drug Metab Dispos. 2005;13:1166–1173. doi: 10.1124/dmd.105.004549. [DOI] [PubMed] [Google Scholar]
- 5.von Weymarn LB, Brown KM, Murphy SE. The Journal of Pharmacology and Experimental Therapeutics. 2006;316:295–303. doi: 10.1124/jpet.105.091306. [DOI] [PubMed] [Google Scholar]
- 6.Lee PN. In: Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Gorrod JW, Jacob P III, editors. Amsterdam: Elsevier Science; 1999. pp. 669–719. [Google Scholar]
- 7.Davis RA, Curvall M. In: Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Gorrod JW, Jacob P III, editors. Amsterdam: Elsevier Science; 1999. pp. 583–643. [Google Scholar]
- 8.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Environmental Health Perspectives. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swan GE, Benowitz NL, Lessov CN, Jacob P, III, Tyndale RF, Wilhelmsen K. Pharmacogenet.Genomics. 2005;15:115–125. doi: 10.1097/01213011-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N. Clinical Pharmacology and Therapeutics. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Malaiyandi V, Sellers EM, Tyndale RF. Clinical Pharmacology and Therapeutics. 2005;77:145–158. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Jacob P, III, Yu L, Wilson M, Benowitz NL. Biological Mass Spectrometry. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 13.Bernert JT, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW, Miller BB, Patterson J, Needham LL, Hannon WH, Sampson EJ. Clinical Chemistry. 1997;43:2281–2291. [PubMed] [Google Scholar]
- 14.Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Clinical Pharmacology and Therapeutics. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL, Swan GE, Jacob P, III, Lessov-Schlaggar CN, Tyndale RF. Clinical Pharmacology and Therapeutics. 2006;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Hukkanen J, Jacob P, III, Benowitz NL. Clinical Pharmacology and Therapeutics. 2006;80:522–530. doi: 10.1016/j.clpt.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Heavner DL, Richardson JD, Morgan WT, Ogden MW. Biomedical Chromatography. 2005;19:312–328. doi: 10.1002/bmc.463. [DOI] [PubMed] [Google Scholar]
- 18.Tuomi T, Johnsson T, Reijula K. Clinical Chemistry. 1999;45:2164–2172. [PubMed] [Google Scholar]
- 19.Taylor PJ, Forrest KK, Landsberg PG, Mitchell C, Pillans PI. Ther.Drug Monit. 2004;26:563–568. doi: 10.1097/00007691-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM. American Journal of Clinical Pathology. 2006;126:880–887. doi: 10.1309/LQ8U3UL956ET324X. [DOI] [PubMed] [Google Scholar]
- 21.Shou WZ, Naidong W. J.Chromatogr.B Analyt.Technol.Biomed.Life Sci. 2005;825:186–192. doi: 10.1016/j.jchromb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Naidong W, Shou W, Chen YL, Jiang X. J.Chromatogr.B Biomed.Sci.Appl. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- 23.Jacob P, III, Benowitz NL, Shulgin AT. Journal of Labelled Compounds and Radiopharmaceuticals. 1988;XXV:1117–1128. [Google Scholar]
- 24.Xu X, Iba MM, Weisel CP. Clinical Chemistry. 2004;50:2323–2330. doi: 10.1373/clinchem.2004.038489. [DOI] [PubMed] [Google Scholar]