Abstract
Lymphocyte homeostasis requires coordination of metabolic processes with cellular energetic and biosynthetic demands but mechanisms that regulate T-cell metabolism are uncertain. We show that interleukin-7 (IL-7) is a key regulator of glucose uptake in T lymphocytes. To determine how IL-7 affects glucose uptake, we analyzed IL-7 signaling mechanisms and regulation of the glucose transporter, Glut1. The IL-7 receptor (IL-7R) stimulated glucose uptake and cell-surface localization of Glut1 in a manner that required IL-7R Y449, which promoted rapid signal transducer and activator of transcription 5 (STAT5) activation and a delayed yet sustained activation of Akt. Each pathway was necessary for IL-7 to promote glucose uptake, as Akt1−/− T cells or PI3-kinase inhibition and RNAi of STAT5 led to defective glucose uptake in response to IL-7. STAT5 and Akt acted in a linear pathway, with STAT5-mediated transcription leading to Akt activation, which was necessary for STAT5 and IL-7 to promote glucose uptake and prevent cell death. Importantly, IL-7 required glucose uptake to promote cell survival. These data demonstrate that IL-7 promotes glucose uptake via a novel signaling mechanism in which STAT5 transcriptional activity promotes Akt activation to regulate Glut1 trafficking and glucose uptake that is critical for IL-7 to prevent T-cell death and maintain homeostasis.
Introduction
T-cell homeostasis is necessary to maintain immune responsiveness and depends on balanced cell proliferation and elimination.1 The availability of cell extrinsic growth factors is central for this balance2–5 and regulation of metabolism may be a critical component of the influence of extrinsic signals on T-cell fate. Glucose metabolism in particular has been shown to be regulated by cytokines,5–7 antigen receptor activation and costimulation,8–10 and developmental cues such as Notch signaling.11 In the absence of such signals, glycolytic flux decreases to a level that no longer sustains cell viability and proapoptotic Bcl-2 family proteins become activated, eliciting cell death.4,12,13 A key step in regulation of glucose metabolism is uptake of glucose through facilitative glucose transporters. In immune cells, Glut1 is the primary glucose transporter, yet little is understood about its role or means of regulation
The cytokine interleukin-7 (IL-7) can promote glycolysis6 and plays a unique role in T-cell development, survival, and establishment of immunologic memory. In development, thymocytes fail to differentiate in humans and mice deficient for IL-7 or IL-7 signals.1 Likewise, mature T cells require IL-7 for survival in the periphery14 and generation of T-cell memory is impaired in IL-7–deficient hosts.15 Regulation of cell survival is an important mechanism of IL-7 to promote thymocyte development and peripheral T-cell homeostasis. However, transgenic expression of the antiapoptotic protein Bcl-216 or deficiency of the proapoptotic proteins Bim17 or Bax18 fails to fully rescue IL-7 deficiency, indicating that other functions of IL-7 also support cell survival. One possibility is regulation of glucose uptake to support cell metabolism, yet it remains unclear how IL-7 may promote lymphocyte glucose metabolism.
The IL-7R is composed of the IL-7Rα and the γc and initiates several signaling cascades that may mediate IL-7 regulation of glucose metabolism. Tyr449 in the cytoplasmic domain of IL-7Rα is required for full and proper T-cell development in vivo19,20 and to activate the Jak/signal transducer and activator of transcription 5 (STAT5) and PI3k/Akt pathways.21 STAT5a and STAT5b (referred to together as STAT5) are closely related transcription factors that are required for lymphocyte development22,23 and can induce expression of Pim kinases, which can promote glycolysis.24 In addition to STAT5, Akt kinases are known regulators of glucose uptake and metabolism25–27 and constitutively active forms of Akt1 promote glucose consumption and Glut1 trafficking to the cell surface in a variety of systems, including lymphoid cells.10,12,28,29
The regulation of glucose metabolism may play a critical role in IL-7 functions in T-lymphocyte survival and homeostasis. Because the first step in controlling glucose metabolism is regulation of glucose uptake, we sought to determine if and how IL-7R may regulate glucose uptake and Glut1, and the role of glucose in IL-7–mediated survival. We found that IL-7 stimulated glucose uptake in both naive and activated T cells. This glucose uptake was important for IL-7 functions because IL-7 failed to efficiently prevent cell death of neglected T cells under glucose-limiting conditions despite robust Bcl-2 induction. In addition, we establish a novel signaling mechanism in which IL-7 relies on STAT5 transcriptional activity for sustained phosphorylation and stimulation of Akt, which in turn was critical for glucose uptake and survival. Thus, glucose uptake is a highly regulated aspect of IL-7 signaling that is required for IL-7 function and may play a pivotal role in maintenance of T-cell development and homeostasis.
Methods
Mice
C57Bl/6J (Jackson Laboratories, Bar Harbor, ME), Akt1−/−, and Akt2−/− mice (generously provided by Morris Birnbaum, University of Pennsylvania, PA),25,30 and pLck-Bcl-xL transgenic mice,31 were bred and housed in a specific pathogen-free environment according to approved guidelines. T cells were isolated from unmanipulated mice or from chimeric mice made by tail vein injection of fetal liver cells into lethally irradiated Rag2−/− mice (Jackson Laboratories). Mice were analyzed between 10 and 16 weeks of age or 8 and 10 weeks after injection.
T-cell purification and in vitro culture
T cells were purified from pooled spleen and mesenteric lymph nodes as described4 and cultured in RPMI 1640 with 10% fetal calf serum (Gemini Bio-Products, West Sacramento, CA). T cells were stimulated with 5 ng/mL IL-2 in plates coated with 5 μg/mL each anti-CD3 and anti-CD28 (BD Pharmingen, San Jose, CA). Recombinant murine IL-2, IL-4, and IL-7 (Peprotech, Rocky Hill, NJ) were used at 10 ng/mL except where indicated.
Plasmids
IL-7Rα was cloned from mouse T-cell cDNA into pEF6/V5-His (Invitrogen, Carlsbad, CA). IL-7Rα-Y449F mutation was made using a Quickchange kit (Stratagene, La Jolla, CA). pEF6-FLAG-Glut1, pEF6-hBcl-xL, and pEF6-MyrAkt1 have been described.28 hBcl-xL was subcloned from pEF6 into MIG-tNGFR. STAT5a-1*6 (generously provided by T. Kitamura, University of Tokyo, Japan) was cloned into pEF6. STAT5a-1*6-VVV32 was made using polymerase chain reaction (PCR) mutageneis to change codons 466-468 from GTGGTCGTT to GCGGCGGCT. Green fluorescence protein (GFP)33 or a sequence common to murine STAT5a and STAT5b (GACGCCATGTCCCAGAAGCACCTT) was targeted with shRNAi as previously described.24
Cell lines
FL5.12 cells were cultured and transfected as described previously.6 Cell lines stably expressing pEF6–FLAG-Glut1, – IL-7Rα, or – IL-7Rα-Y449F were generated by transfection and blasticidin selection. IL-7Rα/Bcl-xL stable cell line was made by retroviral transduction of IL-7Rα cells with MIG-tNGFR-hBcl-xL, which yielded a population more than 95% positive for tNGFR. LY294002 (10 μM; Calbiochem, San Diego, CA) and actinomycin D (1 μg/mL; Sigma, St Louis, MO) were used to inhibit PI3k activity and transcription, respectively.
2-deoxy-D-glucose uptake assay
Glucose uptakes of 8 minutes for FL5.12 cells or 12 minutes for T cells were measured as described previously.28 In some cases, cytokine-stimulated glucose uptake was determined by measuring glucose uptake in the presence and absence of cytokine and calculating the difference of the means of triplicate samples. The standard errors of each group were added to determine the total standard error.
Immunoblotting
Cells were lysed as previously described,28 standardized for protein content, run on 4% to 15% or 4% to 20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (BioRad, Hercules, CA), and transferred to nitrocellulose. Primary antibodies were rabbit anti-Glut1 (Abcam, Cambridge, MA), mouse anti-Actin (Sigma), rabbit anti-Actin (Santa Cruz Technologies, Santa Cruz, CA), mouse anti-Akt1, rabbit anti-Akt2, rabbit anti-Akt3, rabbit anti–phospho-Akt (S473), rabbit anti–phospho-Akt (T308), rabbit anti–phospho-mTOR (S2448), rabbit anti-p85α, rabbit anti–phospho-p85 (Y458), rabbit anti-PTEN, rabbit anti–phospho-FoxO1/FoxO3a (T24/T32), rabbit anti-STAT5, rabbit anti–phospho-STAT5 (Y694) (Cell Signaling Technology, Beverly, MA), mouse anti-STAT5, or rabbit anti–Bcl-2 (BD Pharmingen). Secondary antibodies Alexa Fluor 680 anti–rabbit IgG (Invitrogen) and IRDye 800 anti–mouse IgG (Li-Cor Biosciences, Lincoln, NE) were detected using a Li-Cor Odyssey infrared imaging system (Li-Cor Biosciences). Contrast and brightness were adjusted uniformly for each image and some were digitally rearranged for ease of viewing, as indicated by white spaces.
Flow cytometry
Viability was determined by propidium iodide exclusion (1 μg/mL; Molecular Probes) using a FACScan (Becton Dickinson, San Jose, CA) and WinMDI software. Cell-surface FLAG-Glut1 was measured as previously described.28 In some cases, cytokine-stimulated surface FLAG-Glut1 was determined by measuring the mean fluorescence of cells stained for surface FLAG-Glut1 in the presence and absence of cytokine and calculating the difference of the means of triplicate samples and the standard errors of each group were added to determine the total standard error. Surface IL-7Rα levels were measured using biotin anti-CD127/IL-7Rα (BD Pharmingen) followed by PE-Cy5-streptavidin (BD Biosciences).
Results
IL-7 regulates glucose uptake in naive and activated T cells
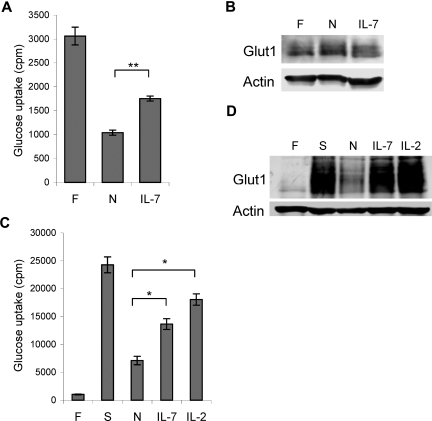
T cells require extrinsic signals to maintain sufficient glucose metabolism to prevent atrophy and spontaneous cell death.1,6 To determine if IL-7 regulated glucose uptake in lymphocytes, we measured glucose uptake in purified naive T cells immediately following isolation and after one day in culture without stimulation (neglect) or in the presence of IL-7. After one day of neglect, glucose uptake diminished 3-fold and IL-7 was sufficient to partially prevent this decrease (Figure 1A). The loss of glucose uptake in the neglected cells was not due to decreased expression of the glucose transporter Glut1 (Figure 1B), but instead neglect may have reduced Glut1 activity or surface levels.
Figure 1.
IL-7 regulates glucose uptake in naive and activated T cells. (A,B) T cells were analyzed immediately after purification (F) or after culture overnight without stimulation (N) or in IL-7 for (A) glucose uptake and (B) Glut1 protein levels. (C,D) T cells were analyzed freshly isolated (F) and after stimulation with anti-CD3 and anti-CD28 (S), then neglect (N), IL-7, or IL-2 for (C) glucose uptake and (D) Glut1 protein levels. Values represent means plus or minus the standard error of the mean (SEM) of triplicate samples. By Student t test, *P < .03, **P < .001.
In addition to naive T cells, cytokines play critical roles to support survival and differentiation in T-cell activation and may also promote glucose uptake in activated T cells. TCR and CD28 costimulation rapidly lead to increased glucose uptake and glycolytic rates within the first day of T-cell activation independent of cytokines.9 To determine if IL-2 and IL-7 regulated glucose uptake after initial phases of T-cell activation, purified naive T cells were stimulated with TCR and CD28 costimulation. Compared with freshly isolated unstimulated cells, glucose uptake and total Glut1 protein levels were increased greater than 10-fold by this time (Figure 1C,D). Cells were then washed and cultured without stimulation (neglect) for 18 hours, at which point glucose uptake decreased. Unlike neglected naive T cells, Glut1 protein was down-regulated during neglect of activated T cells (Figure 1D). Both IL-7 and IL-2 partially prevented the decrease in glucose uptake (Figure 1C) and maintained Glut1 protein levels similar to levels in stimulated cells (Figure 1D), demonstrating that cytokines such as IL-7 can regulate glucose uptake in both naive and activated T cells.
IL-7Rα–tyrosine 449 is required for IL-7 regulation of glucose uptake
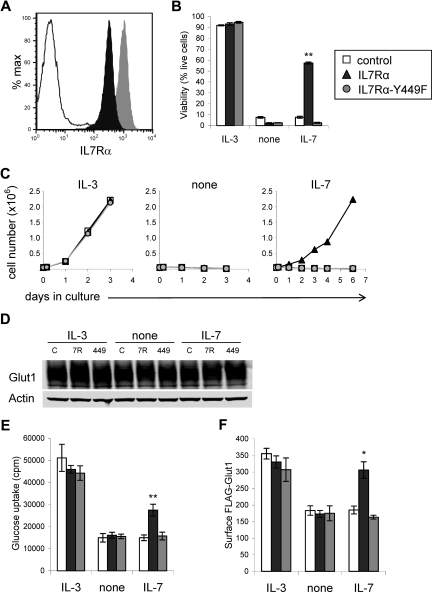
We used an early hematopoietic lymphoid cell line, FL5.12, that is dependent on IL-3 for glucose uptake and metabolism as well as survival28,33 to address the mechanism of IL-7Rα–regulated glucose uptake. These cells express γc but lack expression of endogenous IL-7Rα, allowing examination of IL-7Rα signaling pathways. Cells were transduced with wild-type IL-7Rα or mutant IL-7Rα in which tyrosine 449 was replaced with phenylalanine (IL-7Rα-Y449F).20,21 Surface expression of wild-type and mutant IL-7Rα was confirmed by flow cytometry (Figure 2A). Cells were cultured in IL-3, in the absence of cytokine, or in IL-7. In the presence of IL-3 all cells survived, and upon withdrawal from cytokine all cells underwent apoptosis within one day. IL-7 was capable of preventing death (Figure 2B) and promoting proliferation (Figure 2C) only in cells expressing wild-type IL-7Rα.
Figure 2.
Expression of IL-7Rα, but not IL-7Rα–Y449F, allows IL-7 to support cell survival, growth, glucose uptake, and surface Glut1 in cytokine-dependent cells. FL5.12 cells were transduced with control, wild-type IL-7Rα, or IL-7Rα–Y449F expression plasmids and stable clones were isolated for analysis. (A) Surface IL-7Rα levels were determined by flow cytometry. (B-E) Control, IL-7Rα–, and IL-7Rα-Y449F–expressing cells were washed and cultured in IL-3, no cytokine (none), or IL-7, and observed for (B) cell viability after one day, (C) cell growth over time, (D) Glut1 protein levels, and (E) glucose uptake after 8 hours. (F) Regulation of surface Glut1 trafficking was determined by transfecting control, IL-7Rα–, and IL-7Rα-Y449F–expressing cells with exofacially FLAG-tagged Glut1 (FLAG-Glut1). Cells were washed and cultured in IL-3, no cytokine (none), or IL-7 for 8 hours and surface FLAG-Glut1 was determined by flow cytometry. Values represent means plus or minus SEM of triplicate samples. By Student t test, *P < .02, **P < .001.
To determine how IL-7Rα may affect glucose uptake, control, IL-7Rα, and IL-7Rα-Y449F cells were cultured in IL-3, no cytokine, or IL-7 for 8 hours and evaluated for Glut1 protein content, glucose uptake, and Glut1 surface trafficking. Glut1 protein levels were similar in all cells and conditions (Figure 2D). Only cells expressing wild-type IL-7Rα responded to IL-7 with increased glucose uptake (Figure 2E). To measure surface Glut1 levels and regulation of Glut1 trafficking by IL-7, control-, IL-7Rα–, and IL-7Rα-Y449F–expressing cells were transfected with an exofacially tagged FLAG-Glut1 expression construct28 to measure regulation of Glut1 trafficking. This construct allows flow cytometric measurements of surface FLAG-Glut1 levels that represent trafficking and localization of endogenous Glut1 proteins.28 Consistent with glucose uptake results, IL-7Rα cells maintained surface Glut1 protein levels in response to IL-7 (Figure 2F). However, IL-7Rα-Y449F cells failed to maintain surface Glut1 when cultured in IL-7, indicating that the signaling pathways downstream of Tyr449 are responsible for IL-7 regulation of glucose uptake and surface trafficking of Glut1.
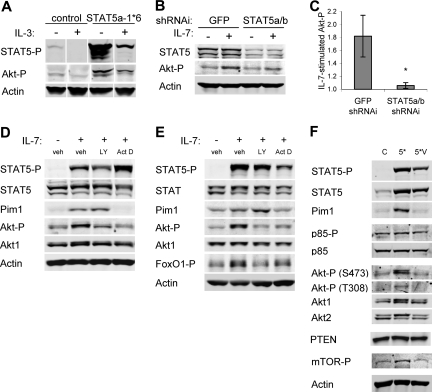
IL-7 stimulated immediate STAT5 and delayed yet sustained Akt activation
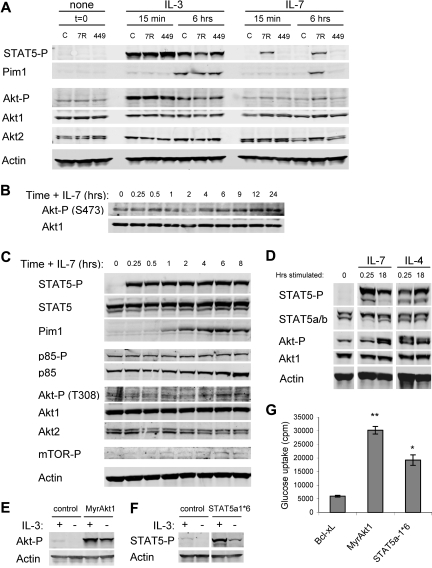
Phosphorylation of Tyr449 by Jak tyrosine kinases promotes STAT5 recruitment and phosphorylation as well as activation of PI3k and Akt.19–21 To confirm a role for Tyr449 phosphorylation in STAT5 and Akt activation in IL-7Rα–reconstituted FL5.12 cells, phospho-STAT5 and phospho-Akt levels were measured in response to cytokine stimulation. Cells were washed and cultured in the absence of cytokine for 4 hours (t = 0), and then stimulated with IL-3 or IL-7 for 15 minutes or 6 hours. IL-3 stimulated robust initial and lower sustained phosphorylation of STAT5 in all cells (Figure 3A). IL-7 stimulated phospho-STAT5 at 15 minutes and 6 hours, but only in IL-7Rα cells. Phospho-STAT5 was active, as a transcriptional target of STAT5, Pim1, was induced in all cell lines after 6 hours of IL-3 addition and in IL-7Rα cells after 6 hours of IL-7 stimulation. Similar to phospho-STAT5, IL-3 induced rapid phosphorylation of Akt that was sustained at lower levels at later time points. In contrast, IL-7 did not lead to detectable Akt phosphorylation at the early time point in any cell line. However, increased phospho-Akt was observed in IL-7Rα cells after 6 hours of IL-7 treatment. In no case did IL-7Rα-Y449 cells respond to IL-7 with phosphorylation of STAT5 or Akt. When IL-7Rα cells were stimulated with IL-7 for 15 minutes to 8 hours, phospho-STAT5 was again activated by 15 minutes and maintained over the entire time course (Figure 3C). Pim1 was detectable after 30 to 60 minutes, indicating that STAT5 activation was rapidly followed by induction of transcription. Phosphorylation of PI3k p85 and total p85 were unaffected by the presence of cytokine. Phosphorylation of Akt at both the PI3k/PDK1-dependent Thr308 and the TORC2-dependent Ser473 sites was only detectable after 4 to 6 hours of stimulation with IL-7 (Figure 3B,C). Phosphorylation of mTOR was also not apparent until this later time. These data suggest that PI3k activation and phosphorylation of Akt are significantly delayed relative to STAT5 activation in IL-7–stimulated cells.
Figure 3.
IL-7 leads to rapid STAT5 activation yet delayed and sustained Akt activation, both of which promote glucose uptake. (A) Control (C), IL-7Rα (7R)–, and IL-7Rα-Y449F (449)–expressing FL5.12 cells were washed and cultured without cytokine (t = 0) and then stimulated with IL-3 or IL-7 for 15 minutes or 6 hours. Cell lysates were analyzed by immunoblot for phospho-STAT5 (Y694), Pim1, phospho-Akt (S473), Akt1, Akt2, and Actin. (B, C) IL-7Rα cells were washed and cultured without cytokine for 6 hours, then stimulated with IL-7 for 15 minutes to 24 hours. Cell lysates were analyzed by immunoblot for (B) phospho-Akt (S473) and Akt1 and (C) phospho-STAT5 (Y694), STAT5, Pim1, phospho-p85 (Y458), p85α, phospho-Akt (T308), Akt1, Akt2, phosho-mTOR (S2448), and Actin. (D) Purified primary T cells were analyzed without stimulation or after culture for 15 minutes or 18 hours with IL-7 or IL-4. Cell lysates were analyzed by immunoblot for phospho-STAT5 (Y694), total STAT5, phospho-Akt (S473), Akt1, and Actin. (E,F) FL5.12 cells were transfected with Bcl-xL as a control, MyrAkt1, or STAT5a-1*6 expression plasmids, cultured with or without IL-3 for 10 hours, and analyzed for (E) phospho-Akt and (F) phospho-STAT5 and Actin. Note that short exposures are shown to illustrate levels of phosphorylation in constitutively active proteins. (G) One day after transfection, Bcl-xL, MyrAkt1, or STAT5a-1*6 transfected cells were cultured in the absence of cytokine for 14 hours and glucose uptake was measured. Values represent means plus or minus SEM of triplicate samples within the given experiment. By Student t test, *P < .005, **P < .001. White vertical lines have been inserted to indicate repositioned gel lanes in panels D and F.
Delayed but sustained activation of Akt by IL-7 would be consistent with reported functions of IL-7 as a survival and growth rather than stimulatory cytokine.6,14,20 IL-7 signal transduction was analyzed in naive T cells to confirm this pattern of STAT5 and Akt activation in primary lymphocytes. T cells were isolated and untreated or treated with IL-7 or IL-4 for 15 minutes or 18 hours. STAT5 phosphorylation was apparent within 15 minutes and was maintained over 18 hours (Figure 3D). In contrast, T cells stimulated with IL-7 for 15 minutes showed little phospho-Akt above the untreated control, whereas IL-4 was capable of promoting rapid Akt phosphorylation. However, phospho-Akt was readily detectable after 18 hours with IL-7. The delayed activation of Akt by IL-7 suggested that IL-7Rα may not activate PI3k/Akt directly in resting T cells, and an intermediate step may be required. Further, these data illustrate that IL-7Rα signal transduction to STAT5 and Akt in FL5.12 cells appears to faithfully represent IL-7Rα signaling in resting mature T cells.
Constitutively active Akt1 and STAT5a can promote glucose uptake
While Akt has previously been implicated in Glut1 regulation,28,29 STAT5 has not been described to affect Glut1 or glucose uptake. To test for possible roles of Akt and STAT5 in cytokine-mediated regulation of these processes, control vector or constitutively active forms of Akt and STAT5, myristoylated Akt1 (MyrAkt1) and STAT5a-1*6, respectively, were transfected into FL5.12 cells and glucose uptake was analyzed.34,35 Transfected cells were cultured for one day in IL-3, and then washed and cultured in the absence of cytokine. Expression and cytokine-independent phosphorylation of MyrAkt1 (Figure 3E) and STAT5a-1*6 (Figure 3F) were determined by immunoblot. Phosphorylation of constitutively active forms of Akt and STAT5 was markedly higher than the steady-state phosphorylation of endogenous Akt and STAT5 cultured in IL-3 for long periods of time. Instead, expression of these constitutively active proteins model the stronger yet transient acute activation of each pathway that can be observed shortly after IL-3–mediated stimulation (Figure 3A and Wieman et al28). Both MyrAkt1 and STAT5a-1*6 maintained glucose uptake in the absence of IL-3 relative to control cells, with Akt1 being more potent than STAT5a (Figure 3G).
Akt is required for IL-7–induced glucose uptake and regulation of Glut1 localization
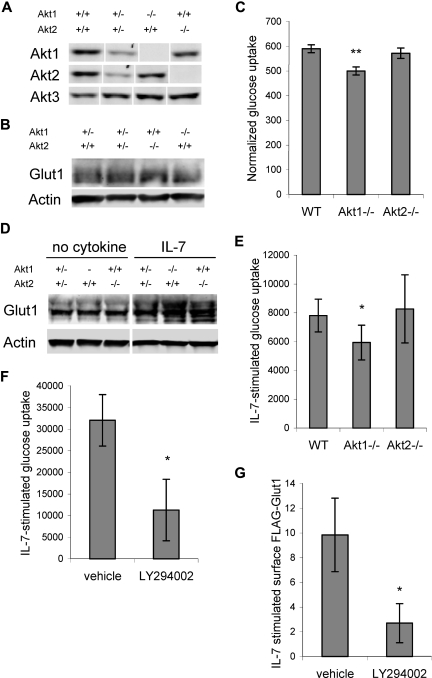
Akt kinases have been shown to play an important role in insulin-regulated glucose uptake, with Akt2 in particular being required in insulin signaling to trigger Glut4 translocation.27 To determine if Akt1 or Akt2 was required for IL-7–stimulated glucose uptake and Glut1 trafficking, Akt1- or Akt2-deficient lymphocytes were analyzed.25,30 T cells were purified from control, Akt1−/−, and Akt2−/− mice and Akt isoform expression was observed by immunoblot (Figure 4A). Glut1 expression was not altered by Akt1 or Akt2 deficiency in resting T cells (Figure 4B), yet measurement of glucose uptake revealed a mild deficit in resting Akt1−/− T cells that was statistically significant across 5 independent experiments. In contrast, Akt2−/− T cells had normal glucose uptake (Figure 4C). When stimulated, Akt1−/− and Akt2−/− T cells had similar or higher levels of total Glut1 protein than stimulated Akt1+/−Akt2+/− T cells when removed from stimulation and cultured in the absence of cytokine or when cultured in IL-7 (Figure 4D). Selective loss of a single Akt isoform, therefore, did not reduce Glut1 protein levels. Nevertheless, activated Akt1−/− T cells had a modest defect in IL-7–stimulated glucose uptake, whereas Akt2−/− T cells responded normally (Figure 4E).
Figure 4.
Akt is required for maximal IL-7–stimulated glucose uptake. (A-C) T cells were purified from control, Akt1-, and Akt2-deficient mice and analyzed for (A) Akt isoform expression, (B) Glut1 protein levels by immunoblot, and (C) glucose uptake. (D,E) Purified T cells from wild-type, Akt1-, and Akt2-deficient mice were stimulated with anti-CD3 and anti-CD28 with IL-2 for 2 days, cultured in IL-2 alone for 1 day, then washed and neglected or cultured in IL-7 for an additional day. (D) Glut1 protein levels were determined by immunoblot and (E) IL-7–stimulated glucose uptake (treated − untreated, the difference in uptake + IL-7 above uptake in untreated cells) was measured. (F) IL-7Rα–expressing FL5.12 cells that expressed Bcl-xL to maintain cell viability were washed and cultured without cytokine or with IL-7 in DMSO (vehicle) or LY294002. Glucose uptakes were measured after 14 hours and IL-7–stimulated glucose uptakes are shown. (G) FLAG-Glut1 was transfected into IL-7Rα–expressing FL5.12 cells that expressed Bcl-xL to maintain cell viability. One day after transfection, cells were washed and cultured without cytokine or with IL-7 in DMSO (vehicle) or LY294002 for an additional 14 hours and surface FLAG-Glut1 was determined. Values represent averages from 5 (C) and 4 (E) independent experiments. IL-7–stimulated glucose uptake and FLAG-Glut1 levels were determined using triplicate unstimulated and stimulated samples. Values represent means plus or minus SEM of multiple experiments (C,E) or triplicate samples within the given experiment (F,G). By Student t test, *P < .05, **P < .005. White vertical lines have been inserted to indicate repositioned gel lanes in panels A and D.
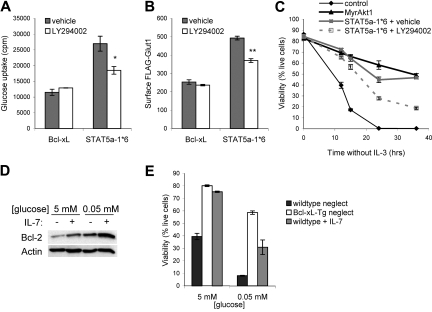
Although these data suggested a possible preferential role for Akt1, the glucose uptake defect was small and indicated that unlike Akt2-mediated regulation of Glut4 trafficking in response to insulin,27 there may be significant redundancy of Akt isoforms to regulate Glut1 in T cells. To acutely prevent activation of all 3 Akt isoforms, the effects of PI3k inhibition on IL-7–stimulated glucose uptake and Glut1 trafficking were analyzed. IL-7Rα–expressing FL5.12 cells were cultured in the absence or presence of IL-7 either with or without a PI3k inhibitor for 15 hours. Similar to findings in recent thymic emigrants,36 inhibition of PI3k diminished IL-7–stimulated glucose uptake (Figure 4F) and IL-7 regulation of Glut1 surface levels (Figure 4G). These data indicate a critical role for the PI3k/Akt pathway in IL-7 regulation of glucose uptake and Glut1 cell-surface localization.
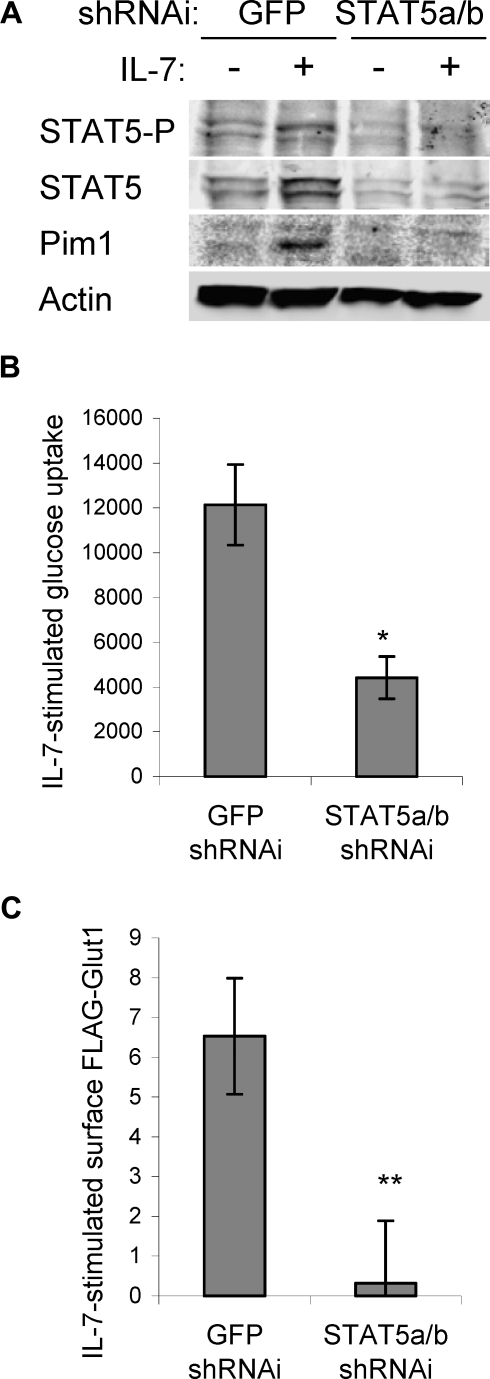
STAT5 is also required for IL-7–induced glucose uptake and regulation of Glut1 localization
Glucose uptake is regulated at multiple steps and STAT5 may also have played an essential role in this process. To test the role of STAT5 in IL-7–induced glucose uptake, we used RNAi against a sequence shared by STAT5a and STAT5b that reduced expression of both STAT5a and STAT5b, phospho-STAT5, and induction of Pim1 (Figure 5A) and led to rapid cell death (data not shown) that was prevented by expression of Bcl-xL or MyrAkt1 (data not shown). As expression of Bcl-xL affects neither glucose uptake nor Glut1 trafficking (data not shown and Wieman et al28), Bcl-xL was expressed in subsequent experiments to maintain cell survival. Similar to the effect of PI3k inhibition on glucose uptake, IL-7–stimulated glucose uptake was significantly reduced in cells transfected with STAT5a/b shRNAi relative to cells transfected with control shRNAi (Figure 5B). In addition, STAT5 deficiency prevented IL-7 regulation of surface Glut1 levels (Figure 5C). Thus, both PI3k/Akt and STAT5 signaling pathways are required for maximal IL-7–stimulated glucose uptake and surface Glut1.
Figure 5.
STAT5 is required for maximal IL-7–stimulated glucose uptake and surface Glut1 levels. IL-7Rα–expressing FL5.12 cells that expressed Bcl-xL to maintain cell viability were transfected with control (shGFP) or STAT5a/b shRNAi and cultured for 30 hours, washed, and cultured in the absence or presence of IL-7 for an additional 14 hours. (A) STAT5, phospho-STAT5 (Y694), and Pim1 were analyzed by immunoblot. (B) IL-7–stimulated glucose uptake (treated − untreated) was measured. (C) FLAG-Glut1 was cotransfected with control or STAT5a/b shRNAi and IL-7–stimulated FLAG-Glut1 level was determined by flow cytometry. Values represent means plus or minus SEM of triplicate samples within the given experiment. By Student t test, *P < .009, **P < .001.
STAT5 regulates PI3k/Akt via transcription
The dual requirement for PI3k/Akt and STAT5 in IL-7–induced glucose uptake and Glut1 surface levels may have been due to independent or interdependent mechanisms of Glut1 regulation. One mechanism by which STAT5 may promote glucose uptake is through activation of Akt. Indeed, expression of constitutively active STAT5a led to elevated levels of endogenous phospho-Akt in the presence of IL-3 and this was maintained upon cytokine withdrawal (Figure 6A). STAT5-mediated activation of Akt was also observed with endogenous STAT5, as IL-7 no longer stimulated phospho-Akt when STAT5 levels were decreased by shRNAi (representative immunoblot shown in Figure 6B and quantification from 4 independent experiments shown in Figure 6C). As a transcription factor, STAT5 may have altered expression of Akt-regulatory genes to stimulate Akt activation. To test this, the ability of IL-7 to promote Akt phosphorylation was determined in the presence of the transcriptional inhibitor, actinomycin D. The PI3k inhibitor LY294002 was used as a positive control for inhibition of PI3k/Akt activation. IL-7Rα–expressing FL5.12 cells were washed, cultured without cytokine for 6 hours, and treated with actinomycin D or the PI3k inhibitor for 30 minutes prior to treatment with IL-7 for 8 hours (Figure 6D). Freshly isolated T cells were treated with inhibitors for 30 minutes prior to treatment with IL-7 for 8 hours (Figure 6E). Inhibition of PI3k had no effect on activation of STAT5 or Pim1 induction, but did block phospho-Akt and phosphorylation of the Akt substrate FoxO1.37 Inhibition of transcription blocked induction of Pim1 and phosphorylation of Akt and FoxO1.
Figure 6.
STAT5 regulation of Akt activation requires STAT5-activated transcription. (A) Cells were transfected with control or STAT5a-1*6 expression plasmids and cultured overnight. Cells were then washed and cultured with or without IL-3 for an additional 8 hours and cell lysates were immunoblotted for phospho-STAT5, phospho-Akt, and Actin. (B,C) IL-7Rα–expressing FL5.12 cells that expressed Bcl-xL to maintain cell viability were transfected with control (GFP) or STAT5a/b shRNAi and cultured for 30 hours, washed, and cultured in the absence or presence of IL-7 for an additional 14 hours. (B) STAT5, phospho-Akt, and Actin were observed by immunoblot. (C) Phospho-Akt/Actin ratios were quantitated from untreated and IL-7–treated samples from 4 independent experiments to determine IL-7–stimulated phospho-Akt in control and STAT5a/b shRNAi treated cells. (D) IL-7Rα–expressing FL5.12 cells were washed and cultured without cytokine for 6 hours, treated with DMSO (vehicle), LY294002, or actinomycin D for 30 minutes, and stimulated with IL-7 for 8 hours. (E) T cells were treated with DMSO (vehicle), LY294002, or actinomycin D for 30 minutes, and stimulated with IL-7 for 8 hours. (D,E) Cell lysates were analyzed by immunoblot for phospho-STAT5 (Y659), STAT5, Pim1, phospho-Akt (S473), Akt1, phospho-FoxO1/FoxO3a (T24/T32), and Actin. (F) Cells were transfected with control (C), STAT5a-1*6 (5*), or STAT5a-1*6-VVV (5*V) expression plasmids and cultured overnight. Cells were then washed and cultured in the absence of cytokine for 9 hours. Cell lysates were analyzed by immunoblot for phospho-STAT5 (Y694), STAT5, Pim1, phospho-p85 (Y458), p85α, phospho-Akt (T308), Akt1, Akt2, PTEN phospho-mTOR (S2448), and Actin. Values represent means plus or minus SEM from 4 independent experiments. By Student t test, *P < .05. White vertical lines have been inserted to indicate repositioned gel lanes in panel A.
While these data revealed a general transcriptional dependence for IL-7 activation of Akt, they did not show a direct role for STAT5-mediated transcription nor exclude proposed nontranscriptional roles of STAT5 in regulating cell signaling.38,39 A DNA-binding mutant form of STAT5a (STAT5a-VVV) can be phosphorylated and participate in hetero- and homodimerization with STAT5b, but is defective for transcriptional induction.32 STAT5a-1*6-VVV would thus be unable to regulate transcription but still be phosphorylated and retain any nontranscriptional roles of STAT5.38,39 FL5.12 cells were transfected with control plasmid, STAT5a-1*6, or STAT5a-1*6-VVV and cytokine withdrawn for 9 hours. Both STAT5a-1*6 and STAT5a-1*6-VVV remained phosphorylated, but STAT5a-1*6-VVV failed to maintain Pim1 expression like STAT5a-1*6 (Figure 6F). The DNA-binding mutant was also unable to maintain phospho-Akt or phospho-mTOR. PTEN levels and Phosphorylation of PI3k p85 were similar in all samples. Thus, IL-7 activates Akt through a mechanism dependent on STAT5 transcription.
STAT5 regulates glucose uptake and survival via PI3k/Akt
It was unclear if STAT5 depended on Akt activation to promote glucose uptake, Glut1 cell-surface trafficking, and cell survival. To determine if STAT5-mediated activation of endogenous PI3k/Akt was required for STAT5-induced glucose uptake, FL5.12 cells and stable FLAG-Glut1–expressing cells were transfected with control plasmid or STAT5a-1*6 and cytokine withdrawn with or without the PI3k inhibitor. Importantly, inhibition of PI3k prevented maximal STAT5-stimulated glucose uptake and trafficking of FLAG-Glut1 to the cell surface (Figure 7A,B). To test if the antiapoptotic effects of STAT5 were also dependent on PI3k and activation of Akt, FL5.12 cells were transiently transfected with a control plasmid, MyrAkt1, or STAT5a-1*6 and cytokine withdrawn with or without a PI3k inhibitor. Cells transfected with the control plasmid died within 24 hours of withdrawal from cytokine regardless of PI3k inhibition, whereas MyrAkt1 and STAT5a-1*6 were capable of maintaining viability (Figure 7C). MyrAkt1-induced survival has been shown to be sensitive to inhibition of PI3k (data not shown and Rathmell et al12), and we show here that STAT5a-1*6 also exhibited diminished viability when PI3k was inhibited. The inability of PI3k inhibition to completely block STAT5-mediated regulation of these processes indicates that other STAT5-induced pathways also contribute to the metabolic and survival functions of STAT5. Nevertheless, these data demonstrate that IL-7 signals via STAT5 to activate Akt and this stimulation of the PI3k/Akt pathway is required for maximal glucose uptake, Glut1 trafficking, and cell survival.
Figure 7.
IL-7–mediated survival requires glucose and depends on STAT5 regulation of Akt. (A,B) Bcl-xL (control) and STAT5a-1*6 expression vectors were transfected into (A) FL5.12 cells and (B) FLAG-Glut1–expressing cells. One day after transfection, cells were washed and cultured in the absence of cytokine with DMSO (vehicle) or LY294002 for an additional 14 hours, and (A) glucose uptake and (B) surface FLAG-Glut1 were determined. (C) FL5.12 cells were transfected with control, MyrAkt1, or STAT5a-1*6 expression plasmids, withdrawn from IL-3 in the presence of absence of LY294002, and cell viability was observed over time. (D,E) Nontransgenic or Bcl-xL–transgenic T cells were purified and neglected or cultured in IL-7 in media with 5 mM or 0.05 mM glucose. (D) Bcl-2 protein levels in nontransgenic T cells and (E) cell viability in nontransgenic (wild type) and Bcl-xL–transgenic T cells were observed after one day. Values represent means plus or minus SEM of triplicate samples. By Student t test, *P < .02, **P < .005.
IL-7–mediated survival requires regulation of glucose uptake
Akt is dependent on glucose for viability,34 and we have now shown that STAT5 requires Akt for maximal glucose uptake and survival. It was unclear if regulation of glucose played a significant role in IL-7–stimulated survival. To test the role of glucose availability in IL-7 induction of Bcl-2 and survival, purified T cells were cultured in glucose-replete or -deficient media. Culture of purified T cells in low glucose media itself led to increased Bcl-2 expression that IL-7 augmented further (Figure 7D), indicating that normal glucose concentrations were not required for IL-7 induction of Bcl-2. To determine if IL-7 could promote survival independent of glucose, T cells were cultured in glucose-replete or -deficient media and viability was measured after one day (Figure 7E). T cells expressing a Bcl-xL transgene were analyzed to establish the extent to which antiapoptotic Bcl-2 family proteins can prevent T-cell apoptosis in low glucose conditions.34 Neglected T cells died rapidly at low glucose concentrations whereas Bcl-xL–transgenic T cells survived well. Despite robust induction of Bcl-2, IL-7 treatment failed to fully protect cells from apoptosis in low glucose conditions. This illustrates that adequate glucose is required for IL-7–mediated survival and suggests that IL-7 regulation of glucose uptake via STAT5 and Akt may be an important component of IL-7–induced cell survival.
Discussion
Glucose uptake is the first step in glucose metabolism. It has been unclear if and how this process may be regulated in lymphocytes and the role that glucose uptake may play in lymphocyte survival. Here we demonstrate that T-cell glucose uptake is dynamic, is essential for IL-7 to maintain cell survival, and is regulated by IL-7 in both resting and activated T cells by STAT5 and Akt. IL-7 has been shown to be a central regulator of T-cell homeostasis with roles in T-cell development, survival, and glucose metabolism.1,3 These studies show that the ability of IL-7 to promote Glut1 cell-surface levels and glucose uptake is a critical feature of IL-7–stimulated cell survival. Regulation of glucose uptake is thus likely an essential component of lymphocyte homeostasis. Furthermore, these studies identify a signaling pathway in which STAT5-dependent activation of Akt was required for maximal IL-7 control over Glut1 trafficking and glucose uptake.
Glucose uptake and trafficking can be regulated at multiple levels. Glut1 protein expression is regulated by both gene induction4,9,34 and lysosomal degradation.40 Upon withdrawal of activated T cells from cytokines, Glut1 protein levels decrease through reduced synthesis, enhanced protein degradation, or a combination of these. Both IL-7 and IL-2 were sufficient to prevent this loss of Glut1 protein (Figure 1D), indicating that these γc cytokines can affect Glut1 synthesis or degradation. However, in resting T cells and in the cell line model for IL-7 signaling used here, Glut1 protein levels did not change within the experimental time frames (Figures 1B and 2D), yet IL-7 still affected glucose uptake. Glut1 trafficking to the cell surface and activity of the transporter at the cell surface can also be regulated28,41 and it is likely that IL-7 modulated these aspects of glucose uptake.
We found that Akt1 may be particularly important for glucose uptake in T cells. Despite normal or modestly elevated Glut1 protein levels, resting Akt1−/−, but not Akt2−/−, T cells had decreased glucose uptake at baseline and defective IL-7–stimulated glucose uptake in activated T cells (Figure 4C,E). This is in contrast to the deficiency in insulin-stimulated glucose uptake via Glut4 that is observed in Akt2−/−, but not Akt1−/−, mice.27 Studies in mammary epithelial cells have also shown that Akt1−/− mice have decreased Glut1 at the cell surface during the metabolically demanding period of lactation.29 Taken together, these data suggest that Akt1 may be important among Akt isoforms in regulation of glucose uptake through control of Glut1 trafficking. However, the modest nature of the glucose uptake defect in Akt1−/− cells suggests that other Akt isoforms also contribute to regulation of glucose uptake through independent or redundant means. Supporting a general role for Akt kinases in glucose uptake, early Akt1−/−Akt2−/− thymocytes exhibit defective glucose uptake, which may have a role in their failure to mature.42 Although relative roles of the Akt isoforms are not fully elucidated, studies here indicate that Akt signaling is essential for normal Glut1 trafficking and glucose uptake in T cells.
Consistent with findings that Bcl-2 expression can only partially rescue T-cell development in IL-7Rα−/− mice16 and that T-cell numbers are reduced in IL-7Rα–Y449F mutant mice despite Bcl-2 induction,19 activation of Akt and STAT5 appear to be required for full IL-7 homeostatic function. The mechanism by which IL-7Rα leads to STAT5 and Akt activation has been thought to be through the independent recruitment of Jak43 and the p85 subunit of PI3k,44 respectively, to Tyr449. This direct recruitment of p85 would lead to rapid activation of Akt following ligation with IL-7. We did not, however, observe induction of Akt phosphorylation within the early minutes or hours of stimulation in resting primary T cells or IL-7Rα–expressing cell lines. Instead, phospho-Akt appeared only after several hours of sustained IL-7R signaling. Unlike signaling via TCR and CD28 or IL-4, which lead to immediate and robust activation of Akt and a sustained, lower level of Akt activation, IL-7 treatment of resting T cells leads only to a delayed and sustained level of Akt activation. This pattern of IL-7–induced Akt activation may be well suited to the role of IL-7 as a survival and homeostatic factor that maintains basal metabolism rather than as a stimulatory factor.6
Delayed activation of Akt by IL-7 depends on STAT5. It remains unclear, however, precisely how STAT5 may affect Akt. Although oncogenic active STAT5 has been proposed to accumulate in the cytoplasm and bind in a complex with p85 via the scaffolding protein Gab238 to directly activate PI3k and Akt, we observed a requirement for STAT5-dependent transcription for Akt phosphorylation. STAT5 may alter expression of genes influencing activation or feedback inhibition of the PI3k/Akt pathway. This sort of mechanism has been suggested for IL-2–induced lymphocyte proliferation via STAT5.45 It is unlikely, however, that primary T cells require STAT5-mediated modulation of the core PI3k/Akt signaling pathway to respond to other growth factors because p85 and PTEN levels were unaffected and, unlike IL-7, IL-4 could rapidly induce Akt phosphorylation in T cells. These data suggest that the effects of STAT5 may be specific for the IL-7 receptor or may be required to sustain PI3k/Akt signaling and allow low-level activation of PI3k/Akt to accumulate over time. The IL-7 receptor requirement for STAT5 to mediate PI3k activation may also vary depending on cell type. In contrast to what we observe in our cell line model and in resting primary peripheral T cells, leukemic models for IL-7 signaling, such as D1, TAIL7, and HPB-ALL cells, derived from murine p53−/− thymoma (D1) and human leukemia cells (TAIL7 and HPB-ALL) have shown rapid Akt phosphorylation following treatment with IL-7.44,46,47 Thus, the signaling pathways by which IL-7 activates Akt may be modulated by cellular differentiation or activation states and may potently influence the outcome of IL-7 as a homeostatic or growth-promoting cytokine. It will be important in future work to further establish the nature of this signaling mechanism and the relevant transcriptional targets, as both STAT5 and Akt have important roles in normal cell development and survival as well as oncogenesis.
Together this work demonstrates that Glut1 trafficking and glucose uptake are under dynamic regulation via STAT5 and Akt signaling pathways and are critical to support the metabolism and survival of T lymphocytes. The requirement for adequate glucose to allow IL-7–mediated cell survival suggests that IL-7 regulation of Glut1 and glucose uptake may be a critical part of IL-7 functions. Thus, a pathway that includes STAT5 and Akt in control of Glut1 trafficking and glucose uptake may be a general mechanism supporting biosynthesis and cell growth and may play a critical role in T-cell survival and homeostasis.
Acknowledgments
We thank Drs Motonari Kondo (Duke University), Morris J. Birnbaum (University of Pennsylvania, Philadelphia), and Craig Thompson (University of Pennsylvania) for helpful discussions. We also thank Dr Morris J. Birnbaum for generously providing the Akt1−/− and Akt2−/− mice and Dr T. Kitamura (University of Tokyo, Japan) for a STAT5a-1*6 expression plasmid. We also thank the Duke University Flow Cytometry shared facility.
J.C.R. was funded by a Howard Temin KO1 Career Development Award, The V Foundation for Cancer Research Scholar Award, and a Sidney Kimmel Foundation for Cancer Research Scholar Award. This work was also supported by the National Institute of Allergy and Infectious Diseases (grant no. R01-AI063345).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A.W. designed and performed research, analyzed data, and wrote the paper; H.L.W., S.R.J., and Y.Z. performed research and analyzed data; J.C.R. supervised research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey C. Rathmell, Duke University Medical Center Box 3813, Durham, NC 27710; e-mail: jeff.rathmell@duke.edu.
References
- 1.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 2.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 3.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 7.Dufort FJ, Bleiman BF, Gumina MR, et al. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179:4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- 8.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 9.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 10.Doughty CA, Bleiman BF, Wagner DJ, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 12.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275:40252–40257. doi: 10.1074/jbc.M005508200. [DOI] [PubMed] [Google Scholar]
- 14.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech S, Tan JT, Wherry E, Konieczny B, Surh C, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 16.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrini M, Bouillet P, Robati M, Belz G, Davey G, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaled A, Li W, Huang J, et al. Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity. 2002;17:561–673. doi: 10.1016/s1074-7613(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 19.Osborne LC, Dhanji S, Snow JW, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R{alpha} mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Q, Li WQ, Hofmeister RR, et al. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–535. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 22.Yao Z, Cui Y, Watford WT, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz CA, Harmon IR, O'Neil JJ, Burchill MA, Johanns TM, Farrar MA. Restricted STAT5 activation dictates appropriate thymic B versus T cell lineage commitment. J Immunol. 2005;174:7753–7763. doi: 10.4049/jimmunol.174.12.7753. [DOI] [PubMed] [Google Scholar]
- 24.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Devel. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho H, Mu J, Kim J, et al. Insulin resistance and a diabetes-mellitus-like syndrome in mice lacking protein kinase AKT2 (PKBbeta). Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 26.Garofalo R, Orena S, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBbeta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 28.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/akt regulation of glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boxer RB, Stairs DB, Dugan KD, et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metabol. 2006;4:475–490. doi: 10.1016/j.cmet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Cho H, Thorvaldsen J, Chu Q, Feng F, Birnbaum M. Akt1/PKBalpha is required for normal growth but dispensible for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 31.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilaria RL, Jr, Hawley RG, Van Etten RA. Dominant negative mutants implicate STAT5 in myeloid cell proliferation and neutrophil differentiation. Blood. 1999;93:4154–4166. [PubMed] [Google Scholar]
- 33.Zhao Y, Altman BJ, Coloff JL, et al. GSK-3{alpha}/{beta} mediate a glucose-sensitive anti-apoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plas D, Talapatra S, Edinger A, Rathmell J, Thompson C. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 35.Onishi M, Nosaka T, Misawa K, et al. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109:1034–1042. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- 37.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nyga R, Pecquet C, Harir N, et al. Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J. 2005;390:359–366. doi: 10.1042/BJ20041523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harir N, Pecquet C, Kerenyi M, et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood. 2007;109:1678–1686. doi: 10.1182/blood-2006-01-029918. [DOI] [PubMed] [Google Scholar]
- 40.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bentley J, Itchayanan D, Barnes K, et al. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem. 2003;278:39337–39348. doi: 10.1074/jbc.M305689200. [DOI] [PubMed] [Google Scholar]
- 42.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alpha-beta thymocyte survival and differentiation. Proc Natl Acad Sci U S A. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng YX, Takahashi H, Shibata M, Hirokawa K. JAK3 Janus kinase is involved in interleukin 7 signal pathway. FEBS Lett. 1994;353:289–293. doi: 10.1016/0014-5793(94)01065-x. [DOI] [PubMed] [Google Scholar]
- 44.Venkitaraman AR, Cowling RJ. Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur J Immunol. 1994;24:2168–2174. doi: 10.1002/eji.1830240935. [DOI] [PubMed] [Google Scholar]
- 45.Lockyer HM, Tran E, Nelson BH. STAT5 is essential for akt/p70s6 kinase activity during IL-2-induced lymphocyte proliferation. J Immunol. 2007;179:5301–5308. doi: 10.4049/jimmunol.179.8.5301. [DOI] [PubMed] [Google Scholar]
- 46.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–29166. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 47.Barata JT, Boussiotis VA, Yunes JA, et al. IL-7-dependent human leukemia T-cell line as a valuable tool for drug discovery in T-ALL. Blood. 2004;103:1891–1900. doi: 10.1182/blood-2002-12-3861. [DOI] [PubMed] [Google Scholar]