Abstract
We examined functional status, activation mechanisms, and biologic role of the mTORC1 signaling pathway in malignant CD4+ T cells derived from the cutaneous T-cell lymphoma (CTCL). Whereas the spontaneously growing CTCL-derived cell lines displayed persistent activation of the TORC1 as well as the PI3K/Akt and MEK/ERK pathways, the IL-2–dependent cell lines activated the pathways in response to IL-2 and IL-15 but not IL-21. Activation of mTORC1 and MEK/ERK was nutrient dependent. The mTORC1, PI3K/Akt, and MEK/ERK pathways could also be activated by IL-2 in the primary leukemic, mitogen-preactivated CTCL cells. mTORC1 activation was also detected in the CTCL tissues in the lymphoma stage–dependent manner with the highest percentage of positive cells present in the cases with a large cell transformation. Rapamycin inhibited mTORC1 signaling and suppressed CTCL cell proliferation but showed little effect on their apoptotic rate when used as a single agent. Activation of the mTORC1, PI3K/Akt, and MEK/ERK pathways was strictly dependent on the Jak3 and Jak1 kinases. Finally, mTORC1 activation was transduced preferentially through the PI3K/Akt pathway. These findings document the selective γc-signaling cytokine-mediated activation of the mTORC1 pathway in the CTCL cells and suggest that the pathway represents a therapeutic target in CTCL and, possibly, other T-cell lymphomas.
Introduction
mTOR (mammalian target of rapamycin) is a ubiquitously expressed serine/threonine kinase that affects a number of key cell functions, including protein synthesis and proliferation.1,2 mTOR associates with either protein called raptor or another named rictor to form the mTORC1 and mTORC2 complexes, respectively. The signaling pathways activated by mTORC1 have thus far been much better characterized.1–3 Accordingly, mTORC1 acts by activating p70S6 kinase 1 (p70S6K1) and inhibiting the 4E binding protein 1 (4E-BP1). In turn, p70S6K1 phosphorylates an S6 protein of the 40S ribosomal subunit (S6rp) at several sites, including serines 235 and 236. The exact mechanisms of mTOR activation are still under investigation, but at least 2 separate, distinct signals are required. Whereas the first is provided by the cell membrane receptors for growth factors, such as insulin and insulin-like growth factor (IGF), the second is generated by nutrients.4–7 The IGF and other receptors activate cell-signaling pathways PI3K/Akt8–10 and ERK/MEK.11–13 Both these signaling pathways have been implicated in mTORC1 activation through an indirect mechanism by suppressing the activity of the tuberous sclerosis complex proteins TSC1 and TSC2 which, in turn, inhibit activity of mTORC1 through inactivating the G protein Rheb. mTORC1 can be functionally inactivated by inhibitors from the rapamycin family. Rather than occupying the enzymatic kinase/ATP-binding domain, rapamycin-type compounds block the binding of mTORC1 to the FKBP12 protein and, consequently, inhibit activity of the complex. Rapamycin and its derivatives are highly potent and specific for mTORC1. They are currently used clinically as immunosuppressive drugs and are evaluated as therapeutic agents in various types of cancer.1,2
From the several cytokines such as IL-2, IL-7, IL-15, and IL-21 that signal through the receptors that share the common γ chain (γc), IL-2 is by far the best characterized. It signals through the receptor that, in addition to the γc, contains the second signaling chain β and, in the case of high-affinity receptor, also the IL-2–specific, signal nontransducing α chain. In normal immune cells, IL-2 has been shown to activate Jak/STAT signaling, PI3K/Akt and MEK/ERK signaling pathways.14 In addition to the γc, the receptor for IL-7 contains a second IL-7–specific signal-transducing α chain. IL-7 has been implicated in promoting maturation and survival of T lymphocytes.15–18 In turn, IL-15 shares with IL-2 both receptor signaling chains, γ and β, and, similar to IL-2, also has the IL-15–specific, nontransducing α chain. Consequently, IL-2 and IL-15 share a number of properties, including stimulation of the T-, natural killer (NK)–, and B-cell proliferation and functional maturation, but certain features unique to each of them have also been described.14,19
IL-21 also displays a spectrum of effects on the immune cells,20 with its ability to increase cytotoxicity of both NK21 and CD8+ T22 cells being the best defined. In addition to the γc, IL-21 receptor comprises its own distinct signal transducing α chain. All 3 cytokines activate Jak1 and Jak3 kinases that phosphorylate and, hence, activate the respective cytokine receptors as well as the signaling proteins that dock to the activated receptors.
Primary cutaneous T-cell lymphoma (CTCL) represents the most common type of T-cell lymphoma.23,24 CTCL is derived from the CD4+ helper/inducer T-cell subset and typically presents in the form of skin patches and/or patches. A subset of cases presents with or, more frequently, displays progression over time to the more advanced forms, including development of skin tumors, peripheral blood involvement (a leukemic phase called Sézary syndrome), involvement of lymph nodes, and transformation to large cell lymphoma.
In the current study we examined activation status of the mTORC1 signaling pathway in CTCL cells, tested the ability of the key γc-signaling cytokines to activate the pathway, determined the effect of mTORC1 on the CTCL cell function, and, finally, elucidated the contribution of the Jak1/Jak3 kinase complex, PI3K/Akt and MEK/ERK pathways to the mTORC1 activation in this type of T-cell lymphoma.
Methods
CTCL cell lines, freshly isolated cells, and biopsy tissues
IL-2–dependent T-cell lines Sez-4 and SeAx were derived from patients with CTCL.25,26 The cell lines were cultured at 37°C and 5% CO2 in the presence of 100 U of IL-2 in standard RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Sez-4) or 10% heat-inactivated human serum (SeAx), 1% penicillin/streptomycin/Fungizone mixture, and 2 mM l-glutamine. Peripheral blood mononuclear cells from patients were obtained after Ficoll density-gradient centrifugation from heparinized blood samples. The patients with CTCL were diagnosed as having peripheral blood involvement (leukemic phase/Sézary syndrome) on the basis of clinical, histopathologic, and immunophenotypic criteria. The leukemic CTCL cell populations of the 3 patients analyzed were more than 90% pure as determined by flow cytometry analysis using the CD4/CD8 ratio and CD7 and/or CD26 loss by the CD4+ T-cell population as the criteria. To obtain primed cells, leukemic CTCL cells were cultured for 7 days in the presence of a mitogen PHA-L (Sigma-Aldrich, St Louis, MO) used at 10 μg/mL.
A total of 25 skin biopsies and 15 lymph nodes were harvested from patients with CTCL for diagnostic purpose. Skin biopsies included 9 patch, 10 plaque, and 6 tumor stage lesions. Ten of the lymph nodes showed involvement by a standard CTCL, and 5 contained CTCL with large-cell transformation. Samples included into the study conformed to the institutional review board–approved protocols. Approval was obtained from the University of Pennsylvania Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Cytokines and kinase inhibitors
Recombinant human (rh) IL-2 was purchased from Bender MedSystems (Burlingame, CA), rhIL-7 and rhIL-15 from R&D Systems (Minneapolis, MN), and rhIL-21 from BioSource International (Camarillo, CA). An mTORC1 inhibitor rapamycin was obtained from Cell Signaling Technology (Danvers, MA), pan-Jak (Jak I)27 from Calbiochem (San Diego, CA), and Jak3 inhibitor was synthesized according to the published structure.28 PI3K inhibitor wortmannin was purchased from Calbiochem, MEK1/2 inhibitor U0126 from Promega (Madison, WI), and Syk inhibitor (inhibitor I) from Calbiochem.
Western blot
The cells were washed briefly in PBS, centrifuged, and lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) supplemented with 0.5 mM PMSF, phosphatase inhibitor cocktails I and II from Sigma-Aldrich and protease inhibitor cocktail from Roche (Indianapolis, IN). For normalization of the gel loading, the protein extracts were assayed with the Lowry method (Dc protein assay; Bio-Rad, Hercules, CA). Typically, 5 to 50 mg of the protein per lane was loaded. To examine protein phosphorylation, the membranes were incubated with antibodies specific for Akt T308, S6rp S235/236, 4E-BP1 T37/46, ERK1/2 T202/Y204, STAT3 Y705, or STAT5 Y694 (all from Cell Signaling Technology). To detect total protein, anti–actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used. The membranes were incubated with the appropriate secondary, peroxidase-conjugated antibodies. The blots were developed using the ECL Plus Western Blotting Detection System from GE Healthcare (Chalfont St Giles, United Kingdom).
Immunohistochemistry
The analysis was performed using the anti–phospho-S6rp(S235/236) and anti–(p)-4E-BP1 (S65) antibodies from Cell Signaling Technology and the ENVISION horseradish peroxidase polymer method (Dako North America, Carpinteria, CA). Formalin-fixed paraffin-embedded tissue specimens of the skin and lymph node biopsies were deparaffinized and heat treated for antigen retrieval by boiling slides in 10 mM citrate buffer pH 6 for 20 minutes. The sections were blocked for 10 minutes with the peroxidase blocking system and incubated at room temperature with the primary antibodies at the 1:50 dilution for 90 minutes, secondary biotinylated anti–rabbit IgG for 30 minutes, and the streptavidin-biotinylated horseradish peroxidase complex for 30 minutes. After washing, the slides were exposed to the chromagen DABplus from Dako North America for 5 minutes and counterstained with hematoxylin. Photographs of the stained slides were taken using an Olympus BX41 microscope (Olympus, Melville, NY) equipped with 10× super widefield eyepieces and Olympus U-PlanApo 20×/0.70 NA and 40×/0.85 NA objectives and an Olympus C-5050 digital camera and SPOT advanced image acquisition software version 4.7 (Sterling Heights, MI). The figure was prepared using Adobe Illustrator software CS2 (Adobe Systems, San Jose, CA).
siRNA assay
A mixture of 4 Jak1- or Jak3-specific siRNAs or nonsense siRNAs (all from Dharmacon RNA Technologies, Lafayette, CO) was introduced into cells at 100nM by lipofection with the new generation Lipofectamine (DMRIE-C; Invitrogen, Carlsbad, CA). The procedure was repeated after 24 hours of culture in the presence of IL-2, and the cells were cultured for an additional 24 hours. The extent of the protein knockdown was examined by Western blotting.
Cell proliferation (BrdU incorporation) assay
After cell culture for 48 hours in the presence of kinase inhibitors, and, for the IL-2–dependent lines IL-2, cell proliferation was evaluated in bromodeoxyuridine (BrdUrd) incorporation assay using the commercially available kit Cell Proliferation enzyme-linked immunoabsorbent assay (ELISA; Roche) according to the manufacturer's protocol. In brief, cells were seeded in 96-well plates (Corning, Corning, NY) at a concentration of 104 cells/well in RPMI medium supplemented with 10% FBS and labeled with BrdU (Roche) for 4 hours. After the plate centrifugation (10 minutes at 300g), supernatant removal, and plate drying, the cells were fixed, and the DNA was denaturated by the addition of 200 mL FixDenat reagent. The amount of incorporated BrdU was determined by incubation with a specific antibody conjugated with peroxidase followed by colorimetric conversion of the substrate and OD evaluation in the ELISA plate reader.
Cell apoptosis (terminal dUTP nick-end labeling) assay
We used the ApoAlert DNA Fragmentation Assay Kit from BD Biosciences (San Jose, CA) according to the manufacturer's protocol. In brief, cells were cultured at 0.5 × 106 cells/mL for 24 hours with the kinase inhibitors and, when required, IL-2. The cells were collected, washed twice in PBS, and fixed with 1% formaldehyde/PBS. After the wash, cells were permeabilized with 70% ice-cold ethanol for at least 2 hours, washed, and incubated in TdT incubation buffer for 1 hour at 37°C. The reaction was stopped by adding 20mM EDTA, and the cells were washed twice in 0.1% Triton X-100/BSA/PBS. Finally, samples were resuspended in 0.5 mL of PI/RNAse/PBS, collected, and analyzed by flow cytometry (FACSort; Becton Dickinson, Franklin Lakes, NJ) using the CellQuest PRO software.
Results
Activation of mTORC1, PI3K/Akt, MEK/ERK, and STAT signaling pathways in CTCL cells
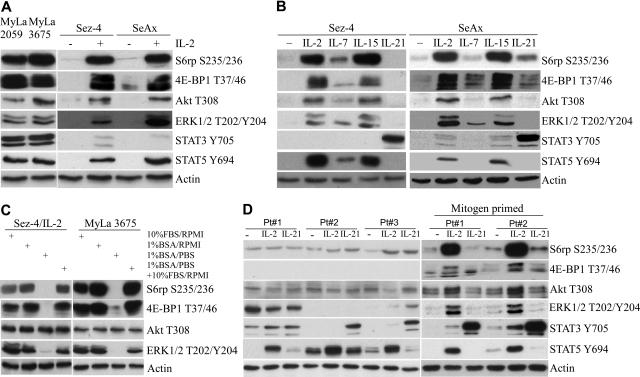
To evaluate activation status of the mTOR pathway in malignant CD4+ T cells, we examined 4 CTCL-derived cell lines, 2 of which grow independently of the exogenous cytokines and the other 2 that are IL-2 dependent.21,22 As shown in Figure 1A, the cytokine-independent cell lines displayed constitutive activation of the pathway as determined by phosphorylation of the mTOR targets S6rp and 4E-BP1. These cell lines also displayed constitutive activation of the PI3K/Akt and MEK/ERK and, as expected, the STAT3 and STAT5 pathways.22 In contrast, activation of mTORC1, as well as of the other pathways in the IL-2–dependent cell lines, was strictly dependent on the presence of the cytokine with essentially no evidence of active signaling seen in the IL-2–depleted cells. To determine whether mTORC1 and the other pathways can become also activated by other members of the γc-signaling cytokine family, we exposed the IL-2–dependent cell lines to IL-7, IL-15, or IL-21. As shown in Figure 1B, IL-2 and IL-15, which share the entire receptor signaling unit, induced comparably strong activation of mTORC1, PI3K/Akt, MEK/ERK, and STAT5 and weak to nondetectable activation of STAT3. IL-7 displayed the same pattern of the pathway activation, the degree of the activation was, however, uniformly weaker. In striking contrast, IL-21 strongly activated STAT3 but not mTORC1 or any of the other pathways.
Figure 1.
Activation of mTORC1 and other signaling pathways in CTCL cell lines and preactivated primary cells. (A) Western blots of protein lysates from 2 spontaneously growing and 2 IL-2–dependent CTCL cell lines were performed using a set of antibodies against the listed phospho-proteins from the mTORC1, PI3K/Akt, MEK/ERK, STAT3, and STAT5 signaling pathways. Antibody against actin served as a positive control. Before cell lysis, the IL-2–dependent cell lines were cultured without IL-2 for 24 hours and stimulated afterward for 30 minutes with 100 U of the cytokine or medium alone. (B) Western blots of protein lysates from the 2 IL-2–dependent/IL-2–depleted CTCL cell lines stimulated for 30 minutes with medium alone, 100 U of IL-2, 5 ng/mL of IL-7, 20 ng/mL of IL-15, or 100 ng/mL of IL-21 were performed using the same listed antibodies as in panel A. (C) The effect of serum and nutrient depletion on mTORC1 activation. The 2 CTCL cell lines were incubated for 20 hours in RPMI medium supplemented with 10% FBS or RPMI with 1% BSA (with a subset of samples cultured for the last 2 hours in PBS with 1% BSA), lysed, and analyzed for phosphorylation status of the mTORC1, PI3K, and MEK1/2 target proteins using the depicted antibodies. The 1% BSA/PBS cultures restimulated for 1 hour with the complete 10% FBS/RPMI medium served as positive controls. (D) Western blots of protein lysates from blood-circulating lymphoma cells isolated from 3 patients CTCL with an advanced leukemic (Sézary) phase and stimulated for 30 minutes with medium alone, 100 U of IL-, or 100 ng/mL of IL-21. Whereas some of the cell populations were examined directly after isolation (left), other were preactivated by a 7-day culture with a mitogen PHA (right).
We next examined the effect of serum- and nutrient-deprivation on mTORC1 activation in the CTCL cells. As shown in Figure 1C, activation of mTORC1 as well as of PI3K/Akt and MEK/ERK pathways was not affected by the overnight serum withdrawal. However, activation of mTORC1 and MEK/ERK but not of PI3K/Akt was abrogated by the brief, 2-hour depletion of nutrients. The mTORC1 and MEK/ERK activation was totally restored by the cell reexposure to the complete, nutrient-containing medium, indicating the reversible nature of the inhibition.
To show that activation of the mTORC1 pathway occurs also in the uncultured, primary malignant CD4+ T cells, we examined freshly isolated, blood-circulating malignant cells from 3 patients with the leukemic form of the lymphoma (Figure 1D left). As with the CTCL-derived cell lines, the primary CTCL cells displayed strong phosphorylation of STAT5 in response to IL-2 and strong phosphorylation of STAT3 on stimulation with IL-21. However, neither the TORC1 nor PI3K/Akt or MEK/ERK pathway could be activated by these cytokines. To understand the discrepancy between the cell lines and primary cells, we reasoned that the difference might stem from differential cell activation status between these 2 types of CTCL cell populations with the leukemic primary cells possibly being in the more quiescent state. To test this hypothesis, we preactivated the leukemic CTCL cells with a mitogen (PHA) for 7 days. As shown in Figure 1D (right), the mitogen preactivation indeed changed the cytokine response pattern of the primary cells. Similar to the CTCL-derived cell lines and in contrast to the freshly isolated cells, the primed CTCL cells activated mTORC1, PI3K/Akt, MEK/ERK, and, as expected, STAT5 but not STAT3 in response to IL-2 and IL-15. IL-21 did strongly activate STAT3 but not the remaining pathways.
Activation of mTORC1 pathway in CTCL tissues
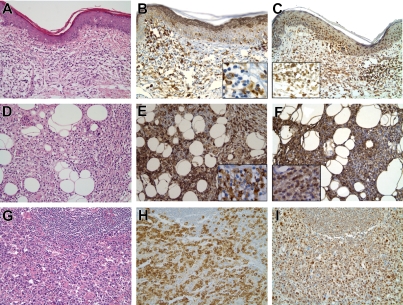
To determine whether mTORC1 activation occurs in the CTCL tissues and if so at which stage of the disease, we examined by immunohistochemistry phosphorylation of the mTORC1 targets S6rp and 4E-BP1 in biopsy tissues representing the whole spectrum of CTCL spanning from the early patch skin lesions to the advanced cases of large cell transformation occurring in the lymph nodes. Representative images from this examination are presented in Figure 2 and the entire dataset is summarized in Table 1. Only a relatively small number of atypical, seemingly enlarged lymphocytes displayed staining with the anti–phospho-S6rp (Figure 2B) and – 4E-BP1 (Figure 2C) antibodies at the patch stage with admixed reactive plasma cells and macrophages also displaying some degree of staining. Frequency of the positively staining atypical lymphocytes steadily increased at the plaque and tumor stage (Figure 2E,F) and in the areas of lymph node involvement by CTCL. Of note, the positive cells were particularly numerous in the CTCL cases with an overt large cell transformation of the lymphoma cells and clearly predominated in some cases (Figure 2G,H).
Figure 2.
Stage-dependent activation of mTORC1 signaling pathway in tissues involved by CTCL. Representative H&E stains of CTCL patch (A) and tumor (D) stage and lymph node involved by CTCL with large cell transformation (G). Immunohistochemical staining for the phosphorylated S235/236-S6rp (B) and S65-4E-BP1 (C) in the patch stage is negative in the majority of atypical lymphocytes with many of the positive cells representing plasma cells and macrophages (B). The stains are positive for the phospho-S235/236-S6rp and phospho-S65-4E-BP1 in a large subset of the atypical lymphocytes cells at the tumor stage (E and F, respectively). The percentage of positive cells was the highest among the malignant-appearing cells in lymph nodes involved by CTCL that has undergone large cell transformation with the positive cells representing a majority in some cases (H,I). The main images represent 200X magnification ×200 and the insets are magnification ×400.
Table 1.
Expression of phospho-S6rp and – 4E-BP1 at various stages of CTCL
| S6rp S235/236 | 4E-BP1 S65 | |
|---|---|---|
| Patch stage | ||
| 0% to 25% | 9 (100)* | 9 (100) |
| 26% to 50% | 0 (0) | 0 (0) |
| More than 50% | 0 (0) | 0 (0) |
| Plaque stage | ||
| 0% to 25% | 8 (80) | 9 (90) |
| 26% to 50% | 2 (20) | 1 (10) |
| More than 50% | 0 (0) | 0 (0) |
| Tumor stage | ||
| 0% to 25% | 2 (33) | 1 (17) |
| 26% to 50% | 4 (67) | 5 (83) |
| More than 50% | 0 (0) | 0 (0) |
| Lymph node | ||
| 0% to 25% | 1 (10) | 2 (20) |
| 26% to 50% | 6 (60) | 7 (70) |
| More than 50% | 3 (30) | 1 (10) |
| Large cell transformation | ||
| 0% to 25% | 0 (0) | 0 (0) |
| 26% to 50% | 3 (60) | 4 (80) |
| More than 50% | 2 (40) | 1 (20) |
Skin biopsies from the patch, plaque, and tumor stages and the involved lymph nodes were stained by immunohistochemistry for the above-mentioned proteins. The cases are grouped based on the depicted percentage of staining of the atypical lymphocytes. Values are number of cases within the group; percentage of cases within the given CTCL stage in parentheses.
Effect of rapamycin on mTORC1 signaling, apoptotic cell death, and proliferation of CTCL cells
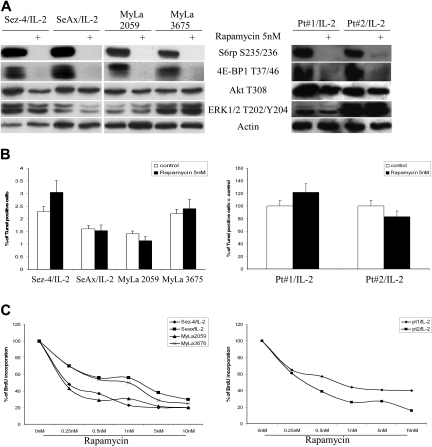
To determine whether mTORC1 function can be efficiently inhibited in the CTCL cells, we analyzed the effect of rapamycin, the very potent and seemingly totally specific inhibitor of mTORC1. As shown in Figure 3A, rapamycin used at the low dose of 5nM abrogated phosphorylation of S6rp and 4E-BP1 in the cell lines (left) and mitogen-primed native cells (right), showing high sensitivity of the CTCL cells to the drug. Phosphorylation of Akt and ERK1/2 remained essentially intact in agreement with the high specificity of the drug. To determine the biologic importance of the mTORC1 pathway in the CTCL cells, we examined rapamycin's effect on apoptotic death and proliferative rate in cell lines and native cells. Whereas the apoptotic rate has not significantly changed (Figure 3B), rapamycin profoundly suppressed proliferation of the CTCL cells (Figure 3C).
Figure 3.
Rapamycin inhibits mTORC1-transduced cell signaling and proliferation of CTCL cells. (A) The CTCL cell lines (left) and mitogen preactivated primary leukemic CTCL cells (right) were cultured for 30 minutes with medium or 100 U of IL-2, as indicated, in the presence of rapamycin at 5nM or its solvent and analyzed for phosphorylation of mTORC1 targets S6rp and 4E-BP1 using phospho-Akt, phospho-ERK1/2, and actin as negative controls. (B) The CTCL cell lines and native, mitogen-primed cells were exposed to medium containing rapamycin at the indicated doses or solvent alone and examined for the apoptotic (B) and proliferative (C) cell rate after 24-hour and 48-hour cultures, respectively. Error bars are SEM.
Role of Jak1 and Jak3 kinases in mTORC1 activation in CTCL cells
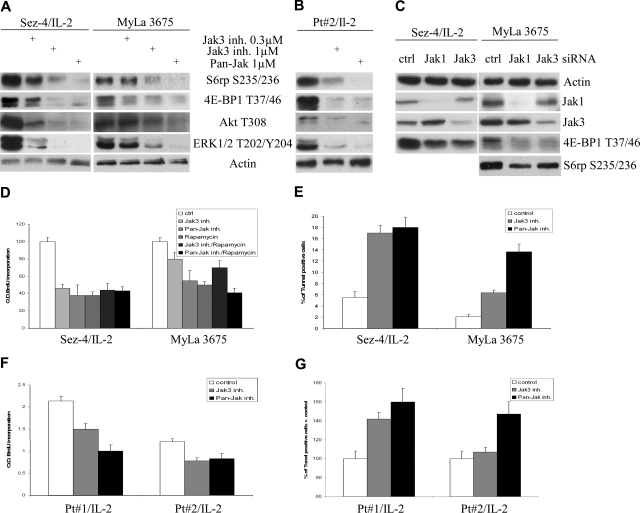
Because Jak1 and Jak3 are critical for signaling by the γc-signaling cytokines such as IL-2, we next examined the role of Jak1 and Jak3 in mTORC1 activation in the CTCL cells by taking advantage of 2 potent and highly specific Jak inhibitors. Whereas one of the inhibitors, pan-Jak, suppresses in vitro kinase activity of all 4 members of the Jak family,23 the latter is much more specific for Jak3.24 Both pan-Jak and Jak3 inhibitors suppressed activation of mTORC1 as well as PI3K/Akt and MEK/ERK pathways in the CTCL-derived cell lines (Figure 4A) and mitogen-primed native cells (Figure 4B). The overall similar effect on mTORC1 signaling was obtained on siRNA-mediated depletion of Jak1 and Jak3 (Figure 4C). The less profound effect most likely reflects incomplete inhibition of the Jaks, in particular of Jak3, because partial depletion of the target is frequently seen with this method. On the cell functional level, the pan-Jak and Jak3 inhibitors profoundly suppressed proliferation of CTCL cells, both cell lines (Figure 4D), and native cells (Figure 4F). Of note, the degree of suppression induced by the Jak inhibitors matched the one triggered by rapamycin, and the combination of rapamycin with either of the Jak inhibitors was not more potent than any of these 3 inhibitors alone. This finding indicates that mTORC1 is the key effector of the Jaks in regard to stimulation of the cell proliferation. Finally, Jak inhibitors, in particular the pan-Jak inhibitor, markedly enhanced apoptotic cell death of the cell lines (Figure 4E) and native cells (Figure 4G) supporting the critical role of Jak1 and Jak3 in the IL-2 signaling in the malignant CD4+ T cells.
Figure 4.
Jak1/Jak3 kinase-dependent activation of mTORC1 in CTCL cells. The CTCL cell lines (A) and mitogen preactivated primary leukemic CTCL cells (B) were cultured for 2 hours with medium or 100 U of IL-2, as indicated, in the presence of Jak3 or pan-Jak inhibitor used at the listed concentrations, lysed, and examined with the listed antibodies. (C) The CTCL cell lines were pretreated at 0 and 24 hours with 100 nM nonsense (control) or Jak1- or Jak3-specific siRNA, cultured for an additional 24 hours, lysed, and analyzed with the indicated antibodies. The CTCL cell lines (D) and mitogen preactivated primary leukemic CTCL cells (F) were cultured for 48 hours with medium or 100 U of IL-2, as indicated, in the presence of 1 μM Jak3 inhibitor, 1 μM pan-Jak inhibitor, or 5 nM rapamycin either alone or in combination with the Jak inhibitors as indicated, and analyzed for the proliferative cell rate. The Jak3 and pan-Jak inhibitor-treated CTCL cell lines and preactivated primary cells were also analyzed for the apoptotic cell death rate (E and G, respectively).
Role of PI3K/Akt and MEK/ERK signaling pathways in mTORC1 activation in CTCL cells
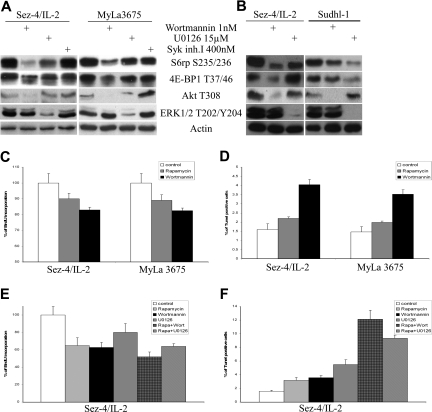
Because both PI3K/Akt8–10 and MEK/ERK11–13 have been shown to activate the mTORC1 pathway in other cell types, we decided to determine their role in activation of the pathway in the CTCL cells. We used potent PI3K inhibitor wortmannin29 and MEK inhibitor U0126.30 As shown in Figure 5A, the seemingly complete inhibition in the CTCL cells of Akt phosphorylation by wortmannin was associated with marked inhibition of phosphorylation of the mTORC1 targets S6rp and 4E-BP1. In turn, profound inhibition of ERK1/2 phosphorylation by U0126 had a much smaller effect on phosphorylation of the mTORC1 targets. Because Syk kinase was recently reported to activate mTORC1 in malignant B lymphocytes,31 we also used Syk inhibitor at the pretested saturating dose that was effective in the B-cell lymphoma cells (M.E.-S. et al, manuscript in preparation). The inhibitor had, however, no effect on mTORC1 activation in the CTCL cells (Figure 5A).
Figure 5.
mTORC1 activation is transduced in CTCL cells preferentially by PI3K/Akt compared with MEK/ERK signaling pathway. CTCL cell lines (A) and CTCL and ALK + TCL (Sudhl-1) cell lines (B) were exposed for 1 hour to solvent, PI3K inhibitor wortmannin, MEK1/2 inhibitor U0126, or Syk inhibitor I at the indicated doses and analyzed in Western blots with the listed antibodies. The CTCL cell lines were also exposed to 2 nM wortmannin for 8 hours (readministered every 2 hours), 5 nM rapamycin, and evaluated for the proliferative (C) and apoptotic (D) cell rate. The effect on cell proliferation (E) and apoptosis (F) of combining rapamycin (0.5 nM) with wortmannin (10 nM) or U0126 (0.5 μM in panel E and 15 μM in panel F) was also determined in the 48-hour and 24-hour assays, respectively.
We have recently shown mTORC1 activation in T-cell lymphoma that expresses anaplastic lymphoma kinase (ALK + TCL).32 To compare the relative contribution of the PI3K/Akt and MEK/ERK pathways in the CTCL and ALK + TCL cells to the mTORC1 activation, we treated cell lines derived from both types of lymphoma with wortmannin or U0126 in the same experiments (Figure 5B). Whereas PI3K inhibition indeed had a stronger suppressive effect on mTORC1 activation in the CTCL cells, inhibition of MEK was more effective in the ALK + TCL cells, which agreed with our previous findings.32
In the next set of experiments we compared the effect of PI3K/Akt inhibition to mTORC1 inhibition on the growth and survival of CTCL cells. Wortmannin seemed equally, if not more, effective than rapamycin in inhibiting cell proliferation under the same experimental conditions (Figure 5C). It was more effective, however, in enhancing cell apoptosis (Figure 5D). Finally, to determine whether simultaneous inhibition of PI3K/Akt or MEK/ERK pathway enhances the effect of the mTORC1 inhibition, we examined the result of combining rapamycin with either wortmannin or U0126. As shown in Figure 5E, neither combination augmented substantially the inhibitory effect of rapamycin on the cell proliferation. However, both combinations led to marked increase in the apoptotic cell rate, suggesting potential efficacy of such approach in treatment of CTCL.
Discussion
Although persistent activation of mTORC1 signaling pathway has been found in many types of cancer, the exact mechanisms that lead to the mTORC1 induction in both normal and transformed cells still remain poorly defined. In this study we identified activation of mTORC1 in CTCL cells and tissues with the lymphoma cells displaying the activation being most frequent at the advanced stages of the lymphoma. Activation of mTORC1, as well as the PI3K/Akt and MEK/ERK pathways, could be induced in the CTCL cells by stimulation with members of the γc-signaling family IL-2, IL-15, and, to the lesser degree, IL-7 with IL-21 representing a notable exception. Whereas the IL-2–triggered induction of the pathways in the cytokine-dependent CTCL cell lines did not require any additional stimuli, the freshly isolated leukemic CTCL cells needed priming with a mitogen. mTORC1 activation was strictly dependent on the second, IL-2–unrelated signal provided by nutrients. mTORC1 induction also required activity of the Jak1/Jak3 complexes that are associated with the γc-type cytokine receptors and was transduced mainly by the PI3K/Akt signaling pathway with only a minor contribution of the MEK/ERK pathway. Inhibition of mTORC1 as well as of Jak1/Jak3 complexes or PI3K/Akt signaling profoundly impaired proliferative capacity of the CTCL. In addition, inhibition of the Jaks or PI3K/Akt but not of mTORC1 alone markedly increased apoptosis of the cells. However, simultaneous inhibition of mTORC1 and either PI3K/Akt or MEK/ERK enhanced the apoptotic cell rate.
On the basis of the studies with certain types of epithelial cells, IGF-I and other members of the insulin family are believed to play the key role in triggering mTORC1 activation.1–3 However, our previous studies as well as the current one indicate that in malignant lymphocytes of both B- and T-cell lineages, these growth factors that are present at high concentration in serum are not critical for mTORC1 induction.29,32 Instead, the whole spectrum of quite diverse stimuli, including CD40 ligand,33 an oncogenic ALK tyrosine kinase,32 and notch,34 has been implicated in mTORC1 activation in lymphoid cells. Other ligands capable of inducing mTORC1 activation were described in various nonlymphoid cells such as TSH in the thyroid epithelial cells,35 fibroblast growth factor 9 (FGF-9) in endometrial stromal cells,36 polycystein-1 in renal tubular cells,37 and prostaglandin F2a in luteal cells.38 In all likelihood, even more mTORC1-activating ligands will be identified, considering the importance of mTORC1 for cell biology, on one hand, and the capability of many ligands and receptors to activate PI3K/Akt and/or MEK/ERK signaling, on the other hand. It is not particularly surprising in this context that IL-2 induces mTORC1 activation because this cytokine had been shown to activate the PI3K/Akt and MEK/ERK pathways some time ago.14 It is also not surprising that IL-15 displays the same property, given that IL-2 and IL-15 receptors share the entire signal transduction module of the β and γ chains and display overlapping signaling mechanisms.19,22,39,40 Although some differences in biologic functions between these 2 cytokines have been noted,14,19 they most likely stem from the different types of cells producing the cytokines, diverse patterns of IL-2 and IL-15 receptor expression on various immune cells, and different modes of the cytokine presentation in vivo with IL-2 acting as a soluble and IL-15 as a membrane-bound ligand.14 Furthermore, at least some of these differences seem more quantitative than qualitative in nature and tend to occur on prolonged cell stimulation apparently because of the differential ability of these cytokines to regulate sustained expression of their specific α chain in their respective receptors.19 The relatively modest effect of IL-7 on activation of mTORC1 and the other signaling pathways was somewhat surprising, given that this cytokine is able to support growth of normal T lymphocytes15–18 and CTCL cells.39,41 Furthermore, in contrast to IL-2, IL-15, and other cytokines, concentration of IL-7 is markedly increased in peripheral blood of the patients with CTCL.41 In addition, its mRNA can be easily detected in the CTCL tissue lesions.41 Whether IL-7 is a stronger activator of mTORC1 and the other pathways in vivo where it acts in the context of other cell-activating stimuli remains to be determined.
The inability of IL-21 to activate mTORC1, as well as PI3K/Akt and MEK/ERK, was rather unexpected considering not only that the IL-21 receptor shares the γc with IL-2 and IL-15 receptors but also that the IL-21 receptor's α chain displays a high degree of homology to the IL-2 and IL-15 receptors' common β chain.20 Consequently, both types of receptors have similar signaling units and activate the Jak1/Jak3 complexes. The marked difference in response to IL-21 compared with IL-2 and IL-15 suggests that changes in conformation of their receptors induced by the cytokines are most likely responsible for the differential effect of the cytokines.
It is interesting that, in contrast to the CTCL cell lines, freshly isolated leukemic CTCL cells required mitogen-mediated preactivation to efficiently activate mTORC1, PI3K/Akt, and MEK/ERK pathways in response to IL-2. This finding is reminiscent of the finding that normal murine CD4+CD25+ T cells also required priming to activate the PI3K/Akt pathway on IL-2 stimulation.42 These 2 observations combined indicate that the final effect of IL-2, and likely other ligands, is highly dependent on the discrete functional status of the targeted cells. In this context, the CTCL-derived cell lines may be more reflective of the activation status of the tissue anchored rather than the seemingly more quiescent circulating malignant T cells. Our finding that mTORC1 activation can be detected in the CTCL tissues (Figure 2; Table 1), in particular in the larger and overtly transformed malignant T cells that likely correspond to the activated normal counterparts, strongly supports this notion.
Of note, we have documented that mTOR activation in the CTCL cells is strictly nutrient dependent on signals provided by nutrients (Figure 1C). This observation indicates that CTCL cells retain the requirement for the second mTORC1-activating signal that is growth factor and cytokine independent and acts by targeting directly Rheb, the immediate upstream activator of mTORC1.43 Combined with the need for IL-2 stimulation and the highly effective inhibition of mTORC1 by rapamycin, the preserved dependence on nutrients indicates that the mTORC1 signaling pathway remains intact in the malignant CD4+ T cells and does not contain any mutations that could lead to an autonomous, constitutive activation of the pathway.44 Recently, a protein called hVps34 that belongs to the class III PI3K kinase family as well as MAP4 kinase that is related to Ste20 have been identified as the nutrient-activated intermediaries involved in the mTORC1 activation.45–47 These findings raise the interesting question of whether hVps34 and MAP4 kinases could represent novel therapeutic targets in the mTORC1-dependent malignancies.
Our findings about the signaling pathways upstream of mTORC1 in CTCL (this report) and ALK plus TCL28 (Figure 5B) not only confirm the previous observations made in different cell types that both PI3K/Akt8–10 and MEK/ERK11–13 pathways play a role in the mTORC1 activation, but they also show that both these pathways can contribute together to mTORC1 activation in the given cell type, malignant CD4+ T lymphocytes in our case. Furthermore, the relative contribution of PI3K/Akt and MEK/ERK pathways to mTORC1 activation appears to depend on the primary stimulus with the IL-2–induced γc/Jak signaling, preferentially using the former and the ALK-trigged signaling relying more on the latter pathway32 (Figure 5B). Of note, other signaling pathways also seem to be involved in mTORC1 activation with their relationship to either PI3K/Akt or MEK/ERK signaling not always being entirely clear. Accordingly, we found that mTORC1 activation in malignant B lymphocytes is largely PI3K/Akt and MEK/ERK independent29 and others have recently managed to implicate Syk kinase in the process in such cells.31 Activation of mTORC1 by FGF-936 and prostaglandin F237 have been reported as Akt independent.
Our findings suggest that mTORC1 represents a potential novel and attractive therapeutic target in CTCL and, in all likelihood, other CD4+ T-cell lymphoma malignancies. Beside rapamycin, 3 rapamycin analogs, RAD001, CCI-779, and AP23573, have been introduced and are at various stages of clinical trials in the whole spectrum of malignancies. Whereas they differ in some characteristics (eg, pharmacokinetics or availability in an oral form), their basic function of inhibiting mTORC1 with high specificity and low toxicity appears to be essentially the same. Our previous studies indicate that mTOR inhibitors hold promise as potential therapeutic agents in another type of CD4+ T-cell lymphoma ALK + TCL,32 as well as various B-cell lymphomas29 with post-transplantation lymphoproliferative disorders being so far the best validated.29,48–50 Considering that mTORC1 inhibitors are used as immunosuppressive drugs directed primarily against T lymphocytes, CTCL and other T-cell lymphomas may be particularly sensitive to therapies targeting mTORC1. Given that atypical, malignant-appearing lymphocytes that display mTORC1 activation are particularly frequent at the late stages of CTCL (Figure 2; Table 1), it could be argued that mTORC1 inhibitors may have the biggest effect in the more advanced cases of the lymphoma.
The fact that mTORC1 inhibition triggers in CTCL cells and many other malignancies,51 cytostatic (Figure 3C) rather than cytotoxic (Figure 3B) effect may limit its efficacy if administered as a single agent. Accordingly, mTORC1 inhibitors used so far alone have shown in clinical trials highly variable efficacy51 with often low response rate even for sensitive cancer types.52 Therefore, combination with other compounds that either alone or in combination with an mTOR inhibitor would induce apoptotic death of the malignant cells may be of particular value. Indeed, modeling studies indicate that combinations of effective cytostatic and cytotoxic drugs should markedly increase the cure rate by delaying development of drug resistance and preventing tumor growth in the time intervals between doses of the cytotoxic agents.53 Furthermore, the observation that mTORC1 inhibitor dramatically enhanced apoptotic cell rate of the DNA-damaging agent cisplatin indicates that the mTORC1 inhibition may also augment the effect of the cytotoxic agents.54 Our observation that combination of rapamycin with an inhibitor of either PI3K/Akt or MEK/ERK pathway (Figure 5F) increased apoptotic cell rate further supports this notion.
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01-CA89194 and R01-DE-017337 (M.A.W.) and R01-CA89442 (A.R.), and The Danish Cancer Society (N.O.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.M. designed and performed the cell signaling experiments; X.L. performed cell cultures and assisted in cell signaling experiments; M.K. designed and performed cell function experiments; A.W. interpreted and described the immunohistochemical stains; P.N.R. performed the immunohistochemical analysis; M.E-S. collected the cases and performed preliminary interpretation of the immunohistochemical stains; E.R. advised in design and performance of the molecular experiments; N.O. advised in project development and experimental design; and M.A.W. oversaw the project and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariusz A. Wasik, University of Pennsylvania Medical Center, Department of Pathology and Laboratory Medicine, 7.106 Founders, 3400 Spruce Street, Philadelphia, PA 19104; e-mail: wasik@mail.med.upenn.edu.
References
- 1.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 2.Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corradettia MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 4.Hara K, Yonezawa K, Weng Q-P, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70, S6 kinase and eIF4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334:261–267. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takano A, Usui I, Haruta T, et al. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050–5062. doi: 10.1128/MCB.21.15.5050-5062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 9.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 10.Sekulic A, Hudson CC, Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 11.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 12.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 15.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathmel JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6868–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 17.Kondrack RM, Harbertson J, Tan JT, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 20.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 21.Kasaian M, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 22.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 24.Dummer R. Future perspectives in the treatment of cutaneous T-cell lymphoma (CTCL). Semin Oncol. 2006;33:S33–36. doi: 10.1053/j.seminoncol.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Raghunath PN, Vonderheid E, Odum N, Wasik MA. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T cells results from methylation of the SHP-1 promoter. Am J Pathol. 2000;157:1137–1146. doi: 10.1016/S0002-9440(10)64629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen M, Kaltoft K, Nordahl M, et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci U S A. 2007;94:6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JE, Cubbon RM, Cummings RT, et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 28.Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 29.Wlodarski P, Kasprzycka M, Liu X, Marzec M, Slupianek A, Wasik MA. Activation of mTOR in transformed B lymphocytes is nutrient-dependent but independent of Akt, MEK, IGF-I, and serum. Cancer Res. 2005;65:7800–7808. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- 30.Marzec M, Kasprzycka M, Liu X, Raghunath PN, Wlodarski P, Wasik MA. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-Raf. Oncogene. 2007;26:813–821. doi: 10.1038/sj.onc.1209843. [DOI] [PubMed] [Google Scholar]
- 31.Leseux L, Hamdi SM, al Saati T, et al. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- 32.Marzec M, Kasprzycka M, Liu X, et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene. 2007;26:5606–5614. doi: 10.1038/sj.onc.1210346. [DOI] [PubMed] [Google Scholar]
- 33.Sakata A, Kuwahara K, Ohmura T, Inui S, Sakaguchi N. Involvement of a rapamycin-sensitive pathway in CD40-mediated activation of murine B cells in vitro. Immunol Lett. 1999;68:301–309. doi: 10.1016/s0165-2478(99)00053-x. [DOI] [PubMed] [Google Scholar]
- 34.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh JM, Song JH, Kim DW, et al. Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem. 2003;278:21960–21971. doi: 10.1074/jbc.M300805200. [DOI] [PubMed] [Google Scholar]
- 36.Wing L-Y, Chen H-M, Chuang P-C, Wu M-H, Tsai S-J. The mTOR-S6K1 but not PI3K-Akt signaling is responsible for fibroblast growth factor-9-induced cell proliferation. J Biol Chem. 2005;280:19937–19947. doi: 10.1074/jbc.M411865200. [DOI] [PubMed] [Google Scholar]
- 37.Shillingford JM, Murchid NS, Larson CH. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvisais EM, Romanelli A, Hou X, Davis JS. AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2alpha. J Biol Chem. 2006;281:26904–26913. doi: 10.1074/jbc.M605371200. [DOI] [PubMed] [Google Scholar]
- 39.Dobbeling U, Dummer R, Laine E, Potoczna N, Qin JZ, Burg G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood. 1998;92:252–258. [PubMed] [Google Scholar]
- 40.Gadina M, Sudarshan C, Visconti R, et al. The docking molecule gab2 is induced by lymphocyte activation and is involved in signaling by interleukin-2 and interleukin-15 but not other common gamma chain-using cytokines. J Biol Chem. 2000;275:26959–26966. doi: 10.1074/jbc.M004021200. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka K, Clark R, Rich B, et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;2440:2445. doi: 10.1182/blood-2005-03-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besinger SJ, Walsh PT, Zhang J, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 44.Urano J, Sato T, Matsuo T, Otsubo Y, Yamamoto M, Tamanoi F. Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc Natl Acad Sci U S A. 2007;104:3514–3519. doi: 10.1073/pnas.0608510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 46.Nobukuni T, Joaquin M, Roccio M, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signaling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majewski M, Korecka M, Kossev P, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: a potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci U S A. 2000;97:5285–4290. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majewski M, Korecka M, Joergensen J, et al. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses. Transplantation. 2003;75:1710–1717. doi: 10.1097/01.TP.0000063934.89714.19. [DOI] [PubMed] [Google Scholar]
- 50.El-Salem M, Raghunath PN, Marzec M, et al. Constitutive activation of mTOR signaling pathway in post-transplant lymphoproliferative disorders (PTLDs). Lab Invest. 2007;87:29–39. doi: 10.1038/labinvest.3700494. [DOI] [PubMed] [Google Scholar]
- 51.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 52.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 53.Gardner SN, Fernandez M. Cytostatic anticancer drug development. J Exp Ther Oncol. 2004;4:9–18. [PubMed] [Google Scholar]
- 54.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;129:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]