Abstract
Combination immunotherapy with anti-CD20 and anti-CD22 mAbs shows promising activity in non-Hodgkin lymphoma. Therefore, bispecific mAbs (bsAbs) were recombinantly constructed from veltuzumab (humanized anti-CD20) and epratuzumab (humanized anti-CD22) and evaluated in vitro and in vivo. While none of the parental mAbs alone or mixed had notable antiproliferative activity against Burkitt lymphoma cells when not cross-linked, the bsAbs [eg, anti-CD20 IgG-anti–CD22 (scFv)2] were inhibitory without cross-linking and synergistic with B-cell antigen (BCR)-mediated inhibition. The bsAbs demonstrated higher antibody-dependent cellulary cytoxicity (ADCC) activity than the parental mAbs, but not complement-dependent cytoxicity (CDC) of the parental CD20 mAb. Cross-linking both CD20 and CD22 with the bsAbs resulted in the prominent redistribution of not only CD20 but also CD22 and BCR into lipid rafts. Surprisingly, appreciable translocation of CD22 into lipid rafts was also observed after treatment with epratuzumab. Finally, the bsAbs inhibited Daudi lymphoma transplant growth, but showed a significant advantage over the parental anti-CD20 mAb only at the highest dose tested. These results suggest that recombinantly fused, complementary, bispecific, anti-CD20/22 antibodies exhibit functional features distinct from their parental antibodies, perhaps representing new candidate therapeutic molecules.
Introduction
CD20 is the principal target in the immunotherapy of B-cell lymphomas,1,2 while a mAb against CD22, epratuzumab, also has promising activity as a single agent.3–5 Despite encouraging results with these mAbs, there are ongoing efforts to improve immunotherapy, because durable responses are only achieved in a portion of patients.5 One emerging approach is combination therapy with different biologic agents,6,7 such as 2 mAbs directed against distinct cell-surface antigens. Ideally, the 2 mAbs would have distinct mechanisms of action so that the therapeutic outcome would be additive or synergistic, and the combined antitumor effects would mitigate resistance to either of the mAbs
In vitro cell-based studies have shown that the combination of epratuzumab and rituximab has a greater antiproliferative effect than either mAb alone.8 The results of 3 phase 2 clinical trials showed that epratuzumab combined with rituximab was well tolerated and suggested an improved activity in non-Hodgkin lymphoma (NHL), compared with historical reports of rituximab in similar patients.9–11 However, combination mAb therapy involves sequential administration, thus requiring lengthy infusion times and potentially higher costs. The objectives of this study were to generate a single agent, namely a bsAb that targets both CD20 and CD22 antigens, and to evaluate its properties.
A bsAb can be prepared by chemical conjugation, and this was used to prepare a bsAb Fab'x Fab' from hA20 (veltuzumab), a humanized anti-CD20 mAb,12 and hLL2 (epratuzumab), a humanized anti-CD22 mAb,13 in earlier studies. While chemical linkage to prepare bsAbs is convenient, it is a tedious, low-yield process involving multiple steps of modification and purification. Thus, recombinant engineering is preferred, with notable examples including 2 different scFv's joined with a flexible linker14; minibodies that contain 2 different scFv's joined at the C-termini via a hinge that is further fused to a helix-turn-helix15 or leucine zipper motif16 to facilitate heterodimerization; and diabodies that form heterodimers with a short linker between the VL and VH domains.17,18 However, these bsAbs have a relatively short half-life in vivo compared with IgG, because of their smaller size and lack of an Fc fragment. To increase the residence time for maximizing therapeutic effects, a variety of fusion bsAbs combining scFvs or diabodies and IgG or Fc fragments have been devised and produced in mammalian cell cultures,19–22 among which the IgG-(scFv)2 format19 is appealing for its divalency for each antigen, easy purification by protein A, a serum half-life comparable to IgG, and the presence of Fc to mediate CDC and ADCC.
Veltuzumab contains the same human IgG1/κ constant and variable framework regions as in epratuzumab, which has shown rapid and relatively safe infusion properties in clinical studies.3,5,23 CDC, ADCC, and inhibition of cell growth and induction of apoptosis have been shown for veltuzumab in vitro.12 Further, initial clinical studies have confirmed that low doses of veltuzumab can be given in shorter infusion times than rituximab, and it has shown good safety and efficacy results in NHL patients.24 In addition, enhancement of the in vitro and in vivo antitumor effects of veltuzumab was demonstrated experimentally when combined with epratuzumab.8,12
Epratuzumab is a humanized version of LL2, the murine mAb raised against the Raji Burkitt lymphoma cell line, and originally named EPB-2.13,25 LL2 is specifically reactive with B cells and NHL, and recognizes the same extracellular epitope of CD22 as the RFB4 mAb.26,27 In this study, anti-CD20/22 bsAbs composed of veltuzumab IgG and 2 entities of LL2scFv or RFB4scFv were constructed and evaluated for their growth-inhibitory effects in human B-cell lymphomas. Surprisingly, we found a unique mode of action of the bsAbs in comparison to the parental mAbs, either alone or combined. Similar results were observed with 3 alternative forms of anti-CD20/22 bsAbs.
Methods
Cell lines
The Burkitt lymphoma lines, Daudi and Ramos, were purchased from the American Type Culture Collection (Manassas, VA) and maintained in vitro in RPMI 1640 medium supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 2 mM l-glutamine, penicillin (100 units/mL), streptomycin (100 μg/mL), all from Life Technologies (Gaithersburg, MD), and 10% fetal bovine serum (Hyclone, Logan, UT). Because fluorescence microscopy revealed a heterogeneous Daudi cell population for expression of surface Ig (sIg), 2 Daudi sublines (D1-1 and D1-7) were obtained by limiting dilution, and stained uniformly compared with the original cell population. D1-1 appeared to have a higher sIg expression than D1-7, based on a protein expression assay using Guava PCA system (Guava Technologies, Hayward, CA).
Reagents
Epratuzumab, veltuzumab, chimeric RFB4 (cRFB4), and hCD22-Fc were provided by Immunomedics (Morris Plains, NJ). HRP-conjugated LL2 (HRP-LL2) was prepared using the periodate coupling method.28 Murine anti–human IgM mAb (clone SA-DA4) was purchased from SouthernBiotech (Birmingham, AL) and further dialyzed in Dulbecco phosphate-buffered saline (PBS; Life Technologies). Other commercially available agents included: biotinylated RFB4 (biotin-RFB4, Ancell, Bayport, MN), HRP-conjugated antiphosphotyrosine specific mAb 4G10 (HRP-4G10, Upstate Biotechnology, Charlottesville, VA), HRP-GAH IgM, Fc5μ specific Ab (Jackson ImmunoResearch, West Grove, PA), FITC-GAH IgG, Fc-specific Ab (Jackson ImmunoResearch), mAbs against CD79a (clone HM41) and CD79b (clone CB3.1; BD Biosciences, San Diego, CA), rabbit anti-CD22 Ab, EP498Y (Abcam, Cambridge, MA), and rabbit anti–CD20 Ab, EP459Y (Abcam), and rabbit anti–Lyn antibody (Cell Signaling Technology, Danvers, MA).
Preparation of anti-CD20/22 bsAbs
The bsAb of the IgG-(scFv)2 format was constructed by fusing VH-(GGGGS)3-VL of LL2 or RFB4 to the C-terminal end of hA20 heavy chain through a hydrophilic helical linker, SNS(EEAKK)3SNS.29 Both hA20IgG-(LL2scFv)2 and hA20IgG-(RFB4scFv)2 after purification on protein A consisted of a mixture of monomeric, dimeric, and multimeric forms, which were further separated by size-exclusion high-performance liquid chromatography (SE-HPLC) using a TSK-GEL G3000SW column (Tosoh Bioscience, Stuttgart, Germany). The fraction containing the monomeric form was used for this study unless noted otherwise.
Other anti-CD20/22 bsAb constructs used included hA20xhLL2 prepared by chemically cross-linking the Fab' fragments of veltuzumab and epratuzumab; TF3, composed of covalently linked 2 Fabs of veltuzumab and one Fab of epratuzumab made by the Dock-and-Lock (DNL) method30,31; and hA20Fab-LL2scFv and hA20Fab-RFB4scFv, both made by fusing LL2scFv and RFB4scFv to hA20Fab, respectively.
Binding assays
A recombinant fusion protein, hCD22-Fc, containing the N-terminal 3 Ig-like domains of human CD22, where the epitopes recognized by LL2 and RFB4 are located, was used to assess the binding of the hLL2-containing bsAbs. Briefly, serial dilutions of unlabeled bsAbs as the competitors ranging from 0 to 100 nM were mixed with a predetermined concentration of HRP-LL2 or biotin-RFB4, added to 96-well ELISA plates precoated with hCD22-Fc, and blocked with BSA. After incubation for 1 hour at room temperature, the plates were washed with PBS, and probed with ortho-phenylenediamine and H2O2 after the addition of HRP-labeled streptavidin for biotin-RFB4 or none for HRP-LL2.
Internalization and fluorescence microscopy
The fate of the anti-CD20/22 bsAbs on cell-surface binding was evaluated by fluorescence microscopy. Briefly, Ramos cells were incubated on ice for 1 hour in complete RPMI medium containing 10 μg/mL of hA20IgG-(RFB4scFv)2 or cRFB4, washed twice, resuspended in fresh medium, split into 2 portions, and incubated at 4° and 37°C, respectively, for 30 minutes. Thereafter, the cells were washed, fixed and permeablized in 3.7% Formald-Fresh solution (Fisher Scientific, Waltham, MA), and stained with a FITC-GAH IgG, Fc fragment-specific Ab.
In vitro cell-proliferation assays
Proliferation of Ramos, D1-1, or D1-7 cultured in the medium containing anti-CD20/22 bsAbs or control mAbs was determined by 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays according to the manufacturer's instructions. Some assays were also performed in the presence of an anti-IgM mAb (α-IgM). All assays were performed in duplicate or triplicate and the data analyzed by Student t test.
Determination of synergism by the combination index method
To determine whether there is synergism on growth inhibition of D1-7 cells from the combined action of α-IgM and anti-CD20/22 bsAb, the pertinent data obtained from the MTT assays were analyzed by the combination index (CI) method of Reynolds and Maurer,32 using the mutually exclusive model.
Immunoprecipitation and Western blotting
Cells (107) were stimulated with 100 nM each of the test articles for 15 minutes and then lysed in a buffer containing 10 mM Tris-HCl (pH 7.5), 20 mM NaCl, 0.5% 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS), 10 mM Na-pyrophosphate, 2 mM Na-orthovanadate, 10 mM NaF, 0.4 mM EDTA, and protease inhibitors. The lysates were centrifuged and the supernatants were subjected to immunoprecipitation with epratuzumab and the Catch and Release immunoprecipitation kit (Upstate). The remaining lysate pellets were washed twice and solubilized in reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The immunoprecipitated and solubilized pellet proteins were separated by SDS-PAGE, and electrotransferred to Immobilon-P membranes (Millipore, Billerica, MA). The membranes were then analyzed by immunoblotting with HRP-4G10 for phosphorylated CD22 (phosphor-CD22) or rabbit anti-CD22 Ab followed by an HRP-goat anti–rabbit Ab for total CD22.
ADCC and CDC assays
For ADCC activity, a 4-hour fluorometric lactate dehydrogenase (LDH)-release assay was used (CytoTox-One; Promega, Madison, WI). Briefly, target cells (Daudi) were incubated with bsAbs at 5 μg/mL in triplicate for 30 minutes at 37°C, 5% CO2. After informed consent was obtained in accordance with the Declaration of Helsinki, freshly isolated peripheral blood mononuclear cells (PBMCs) collected from healthy volunteers (Institutional Review Board–approved) were then added at a predetermined optimal effector to target ratio of 50:1, because prior studies with veltuzumab showed ADCC activities of 12.4% (± 2.4%), 23.0% (± 2.4%), and 34.9% (± 2.7%) for target ratios of 10:1, 20:1, and 40:1, respectively. After a further 4-hour incubation, cell lysis was assessed by release of fluorometric LDH.
CDC activity was determined via a cell viability assay using the fluorescent dye resazurin (Molecular Probes; Eugene, OR). Target cells were plated (5 × 104 cells/well) in black 96-well plates. The bsAbs in a series of 2-fold dilutions were added to the plates in the presence of human complement (Quidel; San Diego, CA). After incubation at 37°C, 5% CO2, for 2 hours, C12 resazurin substrate was added to the wells (5 μM) and the plates were read 5 hours later. Cells treated with 0.25% Triton X-100 served as the 100% lysis control and cells treated with complement alone served as background control. CDC activity was measured as a percentage of viable cells in the treated wells relative to the complement-alone control.
Isolation of lipid rafts
D1-7 cells (0.4-1 × 108) were treated and lysed as described above for immunoprecipitation, except that 40% sucrose was included in the lysis buffer, and the lysates were separated by sucrose density gradient centrifugation. Briefly, the cell lysates (∼2 mL) in centrifuge tubes (Sorvall, Asheville, NC) were first overlaid with 6 mL of the lysis buffer containing 30% sucrose, then with the same lysis buffer containing 5% sucrose (∼3.5 mL) to fill the tubes, which were centrifuged at 40 000 rpm for 3 hours. After the top 2.5-mL fraction was removed, the next 3-mL fraction crossing the interface of 5% and 30% sucrose was collected and regarded as the lipid raft fraction.33 The remainder was collected as the soluble fraction in 1-mL portions. The lipid raft fractions were diluted with the lysis buffer without sucrose and centrifuged again at 40 000 rpm for 1 hour to pellet the detergent-insoluble lipid raft particles. The pellets were resuspended in reducing SDS-PAGE sample buffer and boiled for complete solubilization. Distributions of total CD22, phosphor-CD22, CD20, and BCR complexes in the soluble fraction and lipid rafts were analyzed by immunoblotting. In separate experiments with Daudi cells, the lipid raft fraction collected between 2.5 and 5.5 mL as described above was correlated with the presence of Lyn, a known lipid raft marker, as follows. One-mL fractions were removed sequentially from the top of the sucrose density gradient and the resulting 11 fractions, after 10-fold concentration, were resolved by reducing SDS-PAGE, transferred onto polyvinylidenefluoride membranes and incubated with polyclonal rabbit anti-Lyn antibody, followed by HRP-goat anti-rabbit IgG Fc fragment-specific. The immunoblotting results showed the predominant presence of a 55 to 60 kDa band attributed to Lyn in the first 5 1-mL fractions taken from the top, with fraction 4 showing the highest intensity.
Therapeutic activity of anti-CD20/22 bsAb in SCID mice
Eight-week-old female C.B.-17 SCID mice (Taconic Farms, Germantown, NY) were inoculated intravenously with the Daudi Burkitt lymphoma cell line (1.5 × 107 cells) and randomized into 9 groups of 6 mice each. Treatment started on the following day and consisted of intraperitoneal injections, twice weekly, for 3 weeks. Four different doses of hA20IgG-(LL2scFv)2 were evaluated (7, 13, 33, and 67 μg), as well as 4 molar equivalent doses of veltuzumab (5, 10, 25, and 50 μg). The control group received saline. Animals were monitored daily, weighed weekly, and killed on the development of hind-limb paralysis or loss of greater than 20% of pretreatment weight. Animal studies were approved by the Institutional Animal Care and Use Committee. Survival curves were analyzed using Kaplan-Meier plots (log-rank) with GraphPad Prism software (GraphPad Software, San Diego, CA).
Results
Characterization of recombinant tetravalent anti-CD20/22 bsAbs
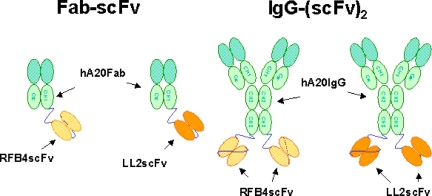
Figure 1 shows the schematic structures of the recombinant bsAbs generated for this study. Because of the instability of hLL2scFv, we elected to fuse LL2scFv or RFB4scFv to hA20 IgG. Cell clones producing hA20IgG-(LL2scFv)2 and hA20IgG-(RFB4scFv)2 were generated as described for hA20,12 and the monomeric forms were isolated by preparative SE-HPLC. The fractions of hA20IgG-(LL2scFv)2 and hA20IgG-(RFB4scFv)2 were shown by SE-HPLC to consist of 78% to 80% and more than 98% monomer, respectively. The purity of both bsAbs before and after fractionation was demonstrated by reducing SDS-PAGE, because only bands corresponding to the scFv-fused H-chain and the L-chain of hA20 were observed. Since the Ag-binding activities of the scFv moieties were of major interest, the monomeric fraction of hA20IgG-(LL2scFv)2 or hA20IgG-(RFB4scFv)2 was evaluated for binding specificity and affinity to hCD22-Fc in both direct and competitive binding assays. Both bsAbs bound to hCD22-Fc with less avidity than their corresponding parental mAbs and only slightly stronger than their respective Fab-scFv constructs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Both the dimeric and multimeric fractions of hA20IgG-(LL2scFv)2 exhibited similar binding affinity for hCD22-Fc as the monomeric fraction.
Figure 1.
Schematic structures of the recombinant bsAbs generated for this study. The structures of chemically linked hA20 Fab' x hLL2 Fab' and TF3 are not shown.
The anti-CD22 mAbs were shown to internalize within minutes after cell-surface binding,8,34 whereas anti-CD20 mAbs were generally slowly or not internalizing.35 Internalization of the anti-CD20/22 bsAbs was investigated on Daudi cells by fluorescence microscopy. Representative results with hA20IgG-(RFB4scFv)2 and cRFB4 shown in Figure S2 confirmed that cRFB4 internalized rapidly (panel A), whereas hA20IgG-(RFB4scFv)2 only aggregated extensively, as indicated by the formation of capping structures on the cell surface (panel B). These results show that physically linking an internalizing anti-CD22 mAb to a noninternalizing mAb prevents internalization of the resulting CD22 complexes.
In vitro antiproliferation activities of the anti-CD20/22 bsAbs
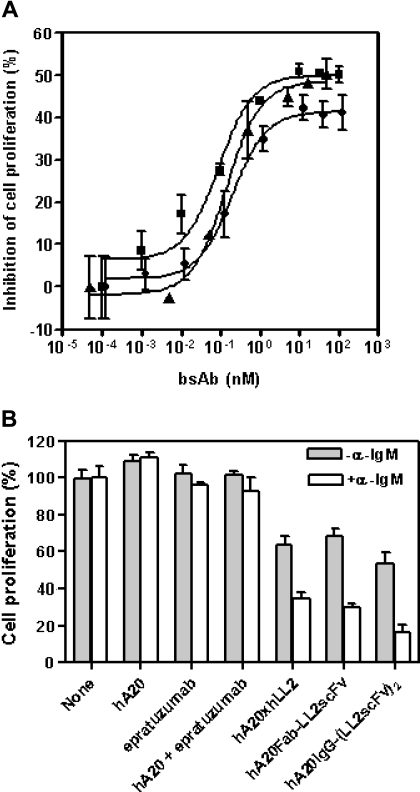
The anti-CD20/22 bsAbs were evaluated for their in vitro antiproliferative activities under various conditions described previously for the parental mAbs.8,12 Expectedly, both hA20-IgG-(LL2scFv)2 and hA20IgG-(RFB4svFv)2 were effective when immobilized or cross-linked (data not shown), because they are, in fact, composed of anti-CD20 and anti-CD22 moieties. However, unlike the parental mAbs, all 3 forms of anti-CD20/22 bsAbs (Fab' × Fab', Fab-scFv, and IgG-(scFv)2) exhibited comparable antiproliferative activity on Daudi cells without cross-linking (Figure 2A). From these dose-titration studies, we determined that the maximum growth-inhibitory effect by an anti-CD20/22 bsAb was achievable at approximately 10 nM, which was used subsequently. We also found that the antiproliferative effect of anti-CD20/22 bsAbs was enhanced when the cells were costimulated by α-IgM (Figure 2B). The anti-CD20/22 bsAbs had greater inhibitory effects when α-IgM was present (P = .006), while there was no difference between the parental mAb-treated cultures with or without α-IgM (P = .33).
Figure 2.
Effects of anti-CD20/22 bsAbs on proliferation of B-lymphoma cell lines. The cultures were started at approximately 105 cells/mL in 48-well tissue culture plates so that the untreated control cells can continuously proliferate at log-phase for 3 to 4 days. The viable cell populations were then determined by the MTT assay. (A) The inhibitory potencies of 3 different anti-CD20/22 bsAbs were determined in D1-1 cells. The cells were cultured in complete RPMI medium containing varying concentrations (up to 10 μg/mL) of hA20xhLL2 (●), hA20Fab-LL2scFv (■), or hA20IgG-(RFB4scFv)2 (▴) for 3 days and viable populations were determined. Error bars represent standard deviations of duplicate tests. (B) The growth inhibitory effects of the anti-CD20/22 bsAbs with or without α-IgM were compared. D1-1 cells were cultured in medium containing 10 nM of the parental mAb (hA20 or epratuzumab), both parental mAbs (hA20 + epratuzumab), or each of the 3 different anti-CD20/22 bsAbs as indicated under the x-axis, in the absence (shaded bars) or presence (clear bars) of SA-AD4 (1 nM), for 3 days and viable populations were determined. The results shown were normalized to those of the corresponding controls in which none of the parental mAbs or bsAbs was added. Error bars represent standard deviations of duplicate tests.
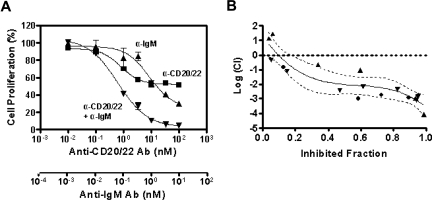
To determine whether the combined inhibitory effect of anti-CD20/22 bsAb and α-IgM is additive or synergistic, we used a formal quantitative analysis of the data to calculate the combination index (CI) based on a multiple drug-effect equation,32 and a typical plot for a 3-day study using the MTT assay is shown in Figure 3A. Based on the dose-titration curves, the median-effect dose signifying the potency of hA20IgG-(LL2scFv)2 or α-IgM as a single agent was calculated, and the median-effect equation derived to calculate the doses of hA20IgG-(LL2scFv)2 and α-IgM that inhibit cell growth at given percentages (Figure 3B). The overall CI values were determined to be less than 0.1, indicating a strong synergistic inhibitory effect by hA20IgG-(LL2scFv)2 combined with α-IgM.
Figure 3.
The synergistic antiproliferation effect of anti-CD20/22 bsAb and α-IgM. The combined antiproliferation effect of hA20IgG-(LL2scFv)2 and SA-AD4 was characterized by deriving CI values from the experimental data, that is, the dose-dependent inhibitory effects of each Ab alone and in combination, as described by Reynold and Maurer.13 (A) The dose-effect plot. Dose-dependent growth inhibition by hA20IgG-(LL2scFv)2 (▴), SA-AD4 (■), or hA20IgG-(LL2scFv)2 plus SA-AD4 with a constant molar ratio of 10:1 (▾) on D1-7 cells was determined similarly as in Figure 2. The error bars represent standard deviations of triplicate tests. The nonlinear fitting curves shown in the chart were generated with the Prism 4 software (GraphPad Software) (B). The plot of log CI values derived from the dose-dependent inhibitory curves versus fraction of inhibited cell population. The plot represents the data from 3 sets of independent experiments (each set was in triplicate), as indicated by distinct symbols (●,▴,▾). The solid curve is the best nonlinear fit of all data points. The dotted curves indicate 95% confidence interval (CI) of the nonlinear fitting. In this method, log(CI) > 0 indicates antagonism, log(CI) = 0 indicates an additive effect, and log(CI) < 0 indicates synergy.
ADCC and CDC assays
Cytotoxicity results indicated that neither of the 2 IgG-(scFv)2 constructs demonstrated CDC activity against Daudi cells, in contrast to the parental veltuzumab that had an EC50 of 1.03 nM. However, using an effector to target ratio of 50:1, both hA20IgG-(LL2scFv)2 and hA20IgG-(RFB4svFv)2 demonstrated significant ADCC activity in comparison to a nontargeting control mAb, hMN-14 IgG (33.2 ± 1.7% and 30.4 ± 9.6% vs 0.9 ± 5.0%; P < .009). Additionally, hA20IgG-(LL2scFv)2 appeared to mediate significantly higher lysis than either veltuzumab (24.4 ± 0.8%) or epratuzumab (15.1 ± 2.5%); P < .001.
Changes of CD22 phosphorylation and solubility by anti-CD20/22 bsAbs
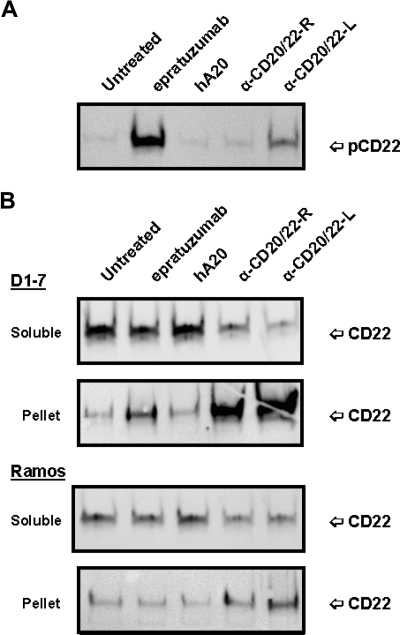
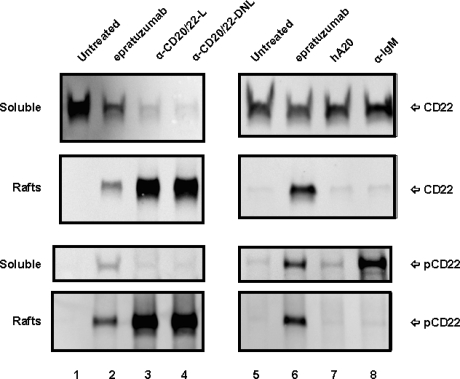
Since binding of epratuzumab to CD22 was reported to result in a rapid increase of CD22 tyrosine phosphorylation in Daudi or Ramos cells,36 the phosphorylation of CD22 in D1-7 and Ramos cells upon ligation with anti-CD20/22 bsAbs was assessed after cell lysis, solubilization with CHAPS, and centrifugation to separate the detergent-soluble proteins (supernatant fraction) from the insoluble cell debris (pellet fraction). Immunoprecipitation and Western blotting confirmed that epratuzumab induced a rapid increase in CD22 phosphorylation, as compared with untreated or veltuzumab-treated controls (Figure 4A). However, in the cells treated with hA20IgG-(RFB4scFv)2 or hA20IgG-(LL2scFv)2, there was markedly less or no phospho-CD22 detected in the supernatant fraction. Further experiments revealed that although CD22 was present predominantly in the supernatant (or soluble) fraction of the untreated or veltuzumab-treated controls (Figure 4B), epratuzumab treatment resulted not only in phosphorylation of CD22 (Figure 4A lane 2), but also migration of a fraction of CD22 into the pellet fraction in D1-7 cells, although it was not apparent in Ramos cells (Figure 4B lane 2). A more significant finding was that anti-CD20/22 bsAb treatment resulted in the majority of CD22 migrating into the pellet fraction in both D1-7 and Ramos cells (Figure 4B lanes 4,5). Similar results were obtained with the lysis buffer containing 1% Triton X-100 instead of CHAPS. By Western blotting, it was confirmed that CD22 in the pellet fraction was phosphorylated (data not shown). These results demonstrate that either epratuzumab or the anti-CD20/22 bsAbs is capable of phosphorylating CD22 and altering the solubility of CD22 in CHAPS or Triton X-100, with the anti-CD20/22 bsAbs being more effective than epratuzumab.
Figure 4.
Phosphorylation and distribution of CD22 induced by anti-CD20/22 bsAbs. D1-7 or Ramos cells were treated for 15 minutes with 100 nM of hA20IgG-(RFB4scFv)2, hA20IgG-(LL2scFv)2, epratuzumab or hA20 (veltuzumab). Cells were lysed in buffer containing 0.5% CHAPS and centrifuged to clear the supernatant. CD22 in the supernatants was immunoprecipitated by epratuzumab. The pellet (ie, insoluble cell debris) was solubilized in SDS-PAGE sample buffer. (A) Immunoprecipitated CD22 from D1-7 cells was analyzed by Western blotting with HRP-4G10 to reveal CD22 phosphorylation. The position of phosphorylated CD22 is indicated on the right. (B) Immunoprecipitated CD22 (marked as soluble on left) and insoluble pellet proteins (marked as pellet on left) from D1-7 (top 2 panels) and Ramos (bottom 2 panels) cells were analyzed by Western blotting with a rabbit anti-CD22 Ab to reveal total CD22. The positions of CD22 are indicated on the right. Lanes labeled as α-CD20/22-R and α-CD20/22-L are hA20IgG-(RFB4scFv)2 and hA20IgG-(LL2scFv)2 treated samples, respectively.
Translocation of CD22 into lipid rafts by anti-CD20/22 bsAbs
The observation that CD22 becomes detergent-insoluble in the cells treated with epratuzumab or the anti-CD20/22 bsAbs prompted us to investigate the membrane distribution of CD22 in lipid rafts using the established method involving sucrose gradient ultracentrifugation.33,37 To compare different anti-CD20/22 bsAbs, TF3, which consists of 2 Fabs of veltuzumab covalently linked to one Fab of epratuzumab with the same binding activity as veltuzuamb IgG and epratuzuamb Fab,31 was included. Figure 5 shows the distributions of CD22 and phospho-CD22 in the soluble lysates and the lipid raft fractions prepared from D1-7 cells treated with veltuzumab, epratuzumab, hA20IgG-(LL2scFv)2, TF3, or α-IgM, as revealed by Western blotting. Only nonphosphorylated CD22 was detected in the soluble fraction in untreated (lanes 1,5) and veltuzumab-treated (lane 7) cells, confirming that cell-surface CD22 molecules normally are excluded from lipid rafts. In contrast, treatment with epratuzumab (lanes 2,6), hA20IgG-(LL2scFv)2 (lane 3), or TF3 (lane 4) resulted in rapid CD22 phosphorylation and redistribution into lipid rafts. The difference between the events initiated by epratuzumab or the anti-CD20/22 bsAbs appears to be translocation but not phosphorylation.
Figure 5.
Subcellular localization of CD22 in D1-7 cells. The cells were similarly treated as in Figure 4. The cell lysates were fractionated by sucrose density-gradient ultracentrifugation. The lipid rafts were collected from the interface of 5% to 30% sucrose, and soluble fractions were from 40% sucrose at the bottom of the tubes. Distribution of CD22 and phospho-CD22 in the soluble fractions and lipid rafts were analyzed by Western blotting with HRP-anti-CD22 mAb (top 4 panels) and HRP-4G10 (bottom 4 panels), respectively. Labels CD22 and pCD22 on the right indicate the positions of CD22 and phospho-CD22, respectively. Lanes 3 and 4 are samples treated with hA20IgG-(LL2scFv)2 (labeled as α-CD20/22-L) and TF3 (labeled as α-CD20/22-DNL), respectively. Lane 8 is the sample treated with α-IqM Ab.
It has been known that CD22 is a coreceptor for the BCR complex, and that phosphorylation of CD22 can be induced by BCR ligation.38 Considering that the antiproliferation effect of anti-CD20/22 bsAbs was synergistic with α-IgM, we compared the phosphorylation and translocation of CD22 induced by α-IgM and anti-CD20/22 bsAbs. Although treating D1-7 cells with α-IgM to ligate BCR induced rapid phosphorylation of CD22 (Figure 5 lane 8), it did not result in colocalization of phospho-CD22 into lipid rafts with BCR. These results indicate that (1) the association of CD22 with lipid rafts only results from its binding to epratuzumab or a bsAb derived from epratuzumab and veltuzumab, and (2) phosphorylation of CD22 induced by mechanisms other than ligation of CD22 by anti-CD22 antibodies is not sufficient for relocating CD22 into lipid rafts. Moreover, phosphorylation of CD22 alone does not appear to correlate with cell growth-inhibition, because although anti-CD20/22 bsAbs are antiproliferative, epratuzumab under the same conditions is not.
Cotranslocation of BCR with CD22 into lipid rafts by anti-CD20/22 bsAbs
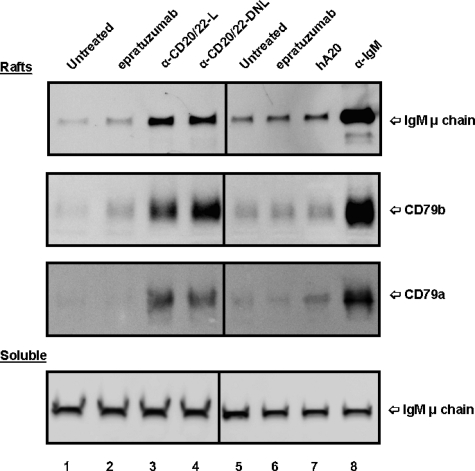
The effect of anti-CD20/22 bsAbs on the membrane location of BCR also was evaluated. In untreated Daudi cells, only a trace of BCR (basal level) was found in lipid rafts (Figure 6 lanes 1,5), in agreement with the results of Cheng et al.39 Treating the cells with α-IgM for 15 minutes resulted in a substantial increase of BCR in the lipid raft fraction (lane 8) as compared with the untreated control (lane 5). When soluble fractions of cells treated with different agents were examined and compared with those of the untreated control, there was no significant difference (Figure 6 bottom panel). However, treating Daudi cells with hA20IgG-(LL2scFv)2 or TF3 appeared to translocate BCR into lipid rafts (lanes 3,4), but not with either parental mAb (lanes 2,6,7).
Figure 6.
Localization of BCR in lipid rafts of D1-7 cells. The cells were treated and the soluble and lipid raft fractions prepared similarly as in Figure 5. The Western blots were probed with mAbs specific for human IgM μ chain, CD79a, and CD79b, to reveal the distribution of BCR complexes in the lipid rafts (top 3 panels) and soluble (bottom panel) fractions. The positions of these proteins are indicated on the right. Lane labels are the same as those in Figure 5. Samples loaded per lane for lipid rafts and soluble fractions were prepared from approximately 4 × 106 and 2 × 105 cells, respectively.
We also examined the distribution of CD20 molecules in a similar study and the results are shown in Figure S3. The relative amounts of CD20 in soluble and lipid raft fractions observed for untreated cells were similar to those observed for cells treated with epratuzumab or α-IgM. Veltuzumab resulted in translocation of CD20 into lipid rafts, as reported for rituximab.40 Both hA20IgG-(LL2scFv)2 and TF3 similarly induced efficient translocation of CD20 in Daudi cells.
In vivo effects
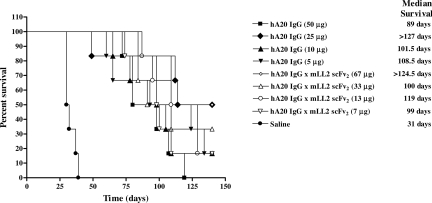
The therapeutic efficacy of multiple administrations of hA20IgG-(LL2scFv)2 at various doses was compared with that of hA20 IgG in SCID mice inoculated with Daudi cells (Figure 7). All the saline control mice were killed by day 39 with a median survival time (MST) of 31 days. Every treatment group was significantly better than this saline control (P = <.001). However, except for the highest doses of veltuzumab (50 μg) and the hA20IgG-(LL2scFv)2 (67 μg), there were no significant differences between any of the other treatment regimens. The group of mice treated with 67 μg hA20IgG-(LL2scFv)2 had a significantly improved survival when compared with mice treated with 50 μg veltuzumab (MST > 124.5 days versus 89 days, respectively; P = .023). Moreover, this treatment group was the only group of mice that exhibited cures (2 of 6 mice tumor-free at end of study, day 140).
Figure 7.
Survival curves of SCID mice bearing disseminated Burkitt lymphoma (Daudi) treated with hA20IgG-(LL2scFv)2 and compared with those treated with the parental hA20 IgG (veltuzumab). Mice were administered intraperitoneally twice weekly for 3 weeks 1 day after Daudi inoculation (1.5 × 107 cells intravenously) with one of 4 different doses of hA20IgG-(LL2scFv)2 or the molar equivalent doses of hA20 IgG.
Discussion
Our aim was to construct and evaluate the functions of recombinant CD20/CD22 bsAbs in comparison to their parental mAbs, and to explore their antiproliferative properties against B-cell lymphoma cell lines in vitro and, preliminarily, in vivo. In these studies, we investigated several forms of anti-CD20/22 bsAbs with more emphasis on an IgG-like construct, hA20IgG-(LL2scFv)2. We chose hA20 (veltuzumab) for the IgG component because many studies in both animals and humans implicated effector functions as critical for an anti-CD20 mAb to be therapeutically effective.41,42 We found hA20IgG-(LL2scFv)2 exhibit somewhat higher ADCC, but lack CDC, despite binding to C1q. Although both hA20IgG-(LL2scFv)2 and hA20IgG-(RFB4scFv)2 contain 2 anti-CD22 scFv moieties, neither showed binding affinity for CD22 comparable to its parental anti-CD22 mAb. This is likely due to the inherent instability of LL2scFv and RFB4scFv, which may be improved by reengineering the VH and VL chains.43 An alternative approach is to combine 3 Fab fragments (as in TF3) or to link one IgG with 4 Fab fragments using the DNL method.30,31,44 In fact, hexavalent IgG-like complexes that are either monospecific or bispecific and contain 6 Fab moieties have been made by the DNL method, and these constructs have been shown to have high binding affinity to both CD20 and CD22, as well as potent antiproliferative activity against B-cell lymphoma cells in vitro and in vivo.44 Hence, these may be more suitable bsAbs for clinical development.
The relatively weak binding avidity to CD22 antigen compared with epratuzumab or RFB4 IgG did not prevent hA20IgG-(LL2scFv)2 and hA20IgG-(RFB4scFv)2 to function as potent inhibitors of cell proliferation in vitro when tested at a range of 1 to 100 nM. This is in contrast to the parental veltuzumab and epratuzumab or RFB4 mAbs, which require cross-linking or immobilization to show antiproliferative effects in most B-lymphoma cell lines.8,12 In addition, the bsAbs prepared by chemical conjugation (hA20xhLL2) or by the DNL method (TF3), both showing similar binding affinity for CD22 as epratuzumab Fab, also were potent inhibitors, while a mixture of veltuzumab and epratuzumab was not. Therefore, the in vitro antiproliferative activity requires merely a covalent linkage between these 2 mAbs. Furthermore, the antiproliferative effect of anti-CD20/22 bsAbs was synergistically enhanced by α-IgM, indicating complementary functions between these 2 agents.
To better understand the mechanism of action of hA20IgG-(LL2scFv)2 against B-lymphoma cells, we investigated the cellular events immediately after stimulation by the bsAb, the parental mAbs, or α-IgM, resulting in several interesting observations. First, phosphorylation of CD22 was induced by epratuzumab, hA20IgG-(LL2scFv)2 and other anti-CD20/22 bsAbs, or α-IgM, but not by veltuzumab. Second, translocation and accumulation of CD22 into lipid rafts was induced by hA20IgG-(LL2scFv)2, but not by α-IgM or veltuzumab. Epratuzumab also induced translocation of CD22 into lipid rafts, but to a lesser extent than the anti-CD20/22 bsAbs, probably due to rapid internalization of the immune complex formed with epratuzumab. Third, translocation of BCR into lipid rafts was induced by α-IgM or hA20IgG-(LL2scFv)2, but not by veltuzumab or epratuzumab. Finally, translocation of CD20 into lipid rafts was induced by veltuzumab and hA20IgG-(LL2scFv)2, but not by epratuzumab or α-IgM. Thus, the distinct property of hA20IgG-(LL2scFv)2, in contrast to parental veltuzumab and epratuzumab, appears to be its ability to translocate CD22 and cotranslocate BCR into lipid rafts, which may account for the observed synergism.
Earlier studies have noted the absence of CD22 in lipid rafts both in unstimulated spleen cells45 and in Raji or tonsil B cells cross-linked with a CD22-specific mAb.46 Therefore, the association of CD22 with lipid rafts induced by epratuzumab or hA20IgG-(LL2scFv)2 is an unexpected finding. During the review of this manuscript, evidence for the trafficking of CD22 along with BCR to lipid rafts was published,47 and we expect that the role of CD22 in lipid rafts will become an area of research interest. Lipid rafts in B cells are known to be a route of receptor-mediated endocytosis for a number of proteins, which include BCR,38 and now probably also CD22. However, because internalization of CD22 induced by anti-CD22 mAbs is very rapid, the Ab-antigen complex might localize in the lipid rafts only transiently before internalization, which could explain why this has not been reported previously. The anti-CD20/22 bsAbs appear to induce the translocation of CD22 in a similar fashion as the parental epratuzumab, but the bsAb-antigen complex could not internalize because it was simultaneously complexed with the noninternalizng CD20.
Another intriguing finding is that translocation and accumulation of BCR in lipid rafts induced by the anti-CD20/22 bsAbs resembles the effects of α-IgM. Similar to antigen stimulation, anti-BCR Abs can rapidly initiate 2 important events: (1) a phosphorylation cascade that results in the production of secondary signaling intermediaries, and (2) the internalization of Ab-BCR complexes. In some cases, these events have been shown to occur in lipid rafts, leading to growth inhibition46 or apoptosis.48 Therefore, the lymphoma proliferation-inhibitory property of anti-CD20/22 bsAbs could be attributed to their ability to bind to both CD20 and CD22 antigens simultaneously, and subsequently to (1) induce a stronger interaction of CD22 and BCR, (2) more efficiently translocate CD22 into lipid rafts due to being complexed with CD20, (3) cotranslocate BCR with CD22 to lipid rafts, and (4) prolong the residence time of CD22 and BCR in lipid rafts for CD22- as well as BCR-mediated signaling to occur. These interpretations are also consistent with epratuzumab showing growth inhibition only when it is immobilized,8 where Ab-bound CD22 is not internalized and could associate with the lipid rafts like the anti-CD20/22 bsAb-bound CD22, exerting similar effects on cell proliferation. Clearly, studies of downstream BCR-signaling related to growth-inhibition, both in normal and malignant human B cells, are indicated.
In these studies, we did not observe any correlation between phosphorylation of CD22 and its translocation into lipid rafts, nor a direct relationship to cell growth-inhibition. However, phosphorylation of CD22 induced by epratuzumab or hA20IgG-(LL2scFv)2 could be critical to the interaction of CD22 and BCR, resulting in cotranslocation of BCR. It is known that CD22 and the BCR complex are physically associated in established lymphoma cell lines, such as Ramos and Daudi, as well as primary B cells from fresh human tonsils.49,50 The association of CD22 with BCR normally would have a low affinity, which could increase upon phosphorylation of CD22 in the cytoplasmic tail induced by epratuzumab binding. It is interesting that although α-IgM induced phosphorylation of CD22 in Daudi and Ramos cells, this did not seem to increase the interaction of CD22 with BCR; thus, no cotranslocation of CD22 with BCR in lipid rafts was observed. This could be due simply to the low level of CD22 associated with BCR, and the translocation of BCR complex to lipid rafts induced by α-IgM ligation being independent of its interaction with CD22. Or, CD22 could cotranslocate with BCR into lipid rafts but then would quickly internalize and segregate from the lipid rafts. Alternatively, it is possible that interaction of CD22 with BCR requires phosphorylation of CD22 at specific sites within the immunoreceptor tyrosine-based motif as well as the immunoreceptor tyrosine-based activation motif.38,49
In contrast to the enhanced inhibition of the growth of lymphoma cell lines in vitro and despite its somewhat higher ADCC, hA20IgG-(LL2scFv)2 failed to show consistent superiority over the parental veltuzumab at most equimolar doses given to mice bearing the Daudi Burkitt lymphoma line. However, a significant survival improvement over veltuzumab was found for the highest dose tested of the bsAb. Thus, although the animal studies indicate that the bsAb has strong antitumor activity, it did not replicate the relative improvement observed in vitro compared with the parental mAbs in the one tumor xenograft tested, because only the highest dose tested proved to be more efficacious than the parental mAb. Hence, other bsAb structures, other tumors with various expression of CD20 and CD22, and even higher test doses need to be evaluated.
In conclusion, anti-CD20/22 bsAbs have been found to possess properties that are distinct from those of the parental mAbs, either alone or as mixtures. They are more potent in inhibiting lymphoma cell proliferation in vitro, despite the constructs composed of the scFv form contributing to a lower binding avidity than the parental CD22 mAb. These findings raise the question of whether bsAbs with other structures and properties may be preferred for clinical development.44
Supplementary Material
Acknowledgments
We thank Dr Edmund A. Rossi for providing TF3 and advice on DNL constructs; Diana M. Delaney, Meiyu Loo, and Li Zeng for excellent technical assistance; and Dr Rhona Stein for editorial comments.
This work was supported in part by US Public Health Service grant CA110297 from the National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
Presented in part at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 9-12, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Z.Q. designed research, performed experiments, made figures, analyzed results, and wrote the paper; D.M.G. propsed the project, designed research, analyzed results, and wrote the paper; T.M.C. performed experiments and made figures; V.S. performed experiments and made figures; H.J.H. designed research; and C.-H.C. designed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: All authors are or have been employees and stockholders of Immunomedics, whose products or potential products were studied in this paper.
Correspondence: David M. Goldenberg, Garden State Cancer Center, CMMI, 520 Belleville Avenue, Belleville, NJ 07109; e-mail: dmg.gscancer@att.net.
References
- 1.Hiddemann W, Buske C, Dreyling M, et al. Treatment strategies in follicular lymphomas: current status and future perspectives. J Clin Oncol. 2005;23:6394–6399. doi: 10.1200/JCO.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Carter P, Smith L, Ryan M. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocr Relat Cancer. 2004;11:659–687. doi: 10.1677/erc.1.00766. [DOI] [PubMed] [Google Scholar]
- 3.Leonard JP, Coleman M, Ketas JC, et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003;21:3051–3059. doi: 10.1200/JCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 4.Leonard JP, Coleman M, Ketas JC, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther. 2006;6:1341–1353. doi: 10.1586/14737140.6.10.1341. [DOI] [PubMed] [Google Scholar]
- 6.Leonard JP, Furman RR, Ruan J, Coleman M. New developments in immunotherapy for non-Hodgkin's lymphoma. Curr Oncol Rep. 2005;7:364–371. doi: 10.1007/s11912-005-0063-4. [DOI] [PubMed] [Google Scholar]
- 7.Press OW, Leonard JP, Coiffier B, Levy R, Timmerman J. Immunotherapy of non-Hodgkin's lymphomas. Hematology Am Soc Hematol Educ Program. 2001:221–240. doi: 10.1182/asheducation-2001.1.221. [DOI] [PubMed] [Google Scholar]
- 8.Carnahan J, Stein R, Qu Z, et al. Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol Immunol. 2007;44:1331–1341. doi: 10.1016/j.molimm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Leonard JP, Coleman M, Ketas J, et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 10.Strauss SJ, Morschhauser F, Rech J, et al. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:3880–3886. doi: 10.1200/JCO.2006.05.6291. [DOI] [PubMed] [Google Scholar]
- 11.Emmanouilides C, Leonard JP, Schuster SJ, et al. Multi-center, phase 2 study of combination antibody therapy with epratuzumab plus rituximab in recurring low-grade NHL. Blood. 2003;102:69a. Abstract 233. [Google Scholar]
- 12.Stein R, Qu Z, Chen S, et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin's lymphoma. Clin Cancer Res. 2004;10:2868–2878. doi: 10.1158/1078-0432.ccr-03-0493. [DOI] [PubMed] [Google Scholar]
- 13.Leung SO, Goldenberg DM, Dion AS, et al. , Construction and characterization of a humanized, internalizing, B-cell (CD22)-specific, leukemia/lymphoma antibody, LL2. Mol Immunol. 1995;32:1413–1427. doi: 10.1016/0161-5890(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 14.Mallender WD, Voss EWJ. Construction, expression, and activity of a bivalent bispecific single-chain antibody. J Biol Chem. 1994;269:199–206. [PubMed] [Google Scholar]
- 15.Pack P, Kujau M, Schroeckh V, et al. Improved bivalent miniantibodies, with identical activity as whole antibodies, produced by high cell density fermentation of Escherichia coli. Biotechnology (NY) 1993;11:1271–1277. doi: 10.1038/nbt1193-1271. [DOI] [PubMed] [Google Scholar]
- 16.Kostelny SA, Cole MS, Tso JY. Formation of a bispecific antibody by the use of leucine zippers. J Immunol. 1992;148:1547–1553. [PubMed] [Google Scholar]
- 17.Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perisic O, Webb PA, Holliger P, Winter G, Williams RL. Crystal structure of a diabody, a divalent antibody fragment. Structure. 1994;2:1217–1226. doi: 10.1016/s0969-2126(94)00123-5. [DOI] [PubMed] [Google Scholar]
- 19.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–163. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 20.Alt M, Muller R, Kontermann RE. Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin gamma1 Fc or CH3 region. FEBS Lett. 1999;454:90–94. doi: 10.1016/s0014-5793(99)00782-6. [DOI] [PubMed] [Google Scholar]
- 21.Park SS, Ryu CJ, Kang YJ, Kashmiri SV, Hong HJ. Generation and characterization of a novel tetravalent bispecific antibody that binds to hepatitis B virus surface antigens. Mol Immunol. 2000;37:1123–1130. doi: 10.1016/s0161-5890(01)00027-x. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Jimenez X, Zhang H, et al. Di-diabody: a novel tetravalent bispecific antibody molecule by design. J Immunol Methods. 2003;279:219–232. doi: 10.1016/s0022-1759(03)00251-5. [DOI] [PubMed] [Google Scholar]
- 23.Perotti B, Doshi S, Chen D, et al. Pharmacokinetics of epratuzumab administered as a single agent or in combination with rituximab in patients with B-cell NHL. Proc Am Soc Clin Oncol. 2003;22:575. Abstract 2311. [Google Scholar]
- 24.Morschhauser F, Leonard JP, Fayad L, et al. Rituximab-relapsing patients with non-Hodgkin lymphoma respond even at lower doses of humanized anti-CD20 antibody, IMMU-106 (hA20): Phase I/II results. Blood. 2006;108:769a. Abstract 2719. [Google Scholar]
- 25.Pawlak-Byczkowska EJ, Hansen HJ, Dion AS, Goldenberg DM. Two new monoclonal antibodies, EPB-1 and EPB-2, reactive with human lymphoma. Cancer Res. 1989;49:4568–4577. [PubMed] [Google Scholar]
- 26.Li JL, Shen GL, Ghetie MA, et al. The epitope specificity and tissue reactivity of four murine monoclonal anti-CD22 antibodies. Cell Immunol. 1989;118:85–99. doi: 10.1016/0008-8749(89)90359-6. [DOI] [PubMed] [Google Scholar]
- 27.Stein R, Belisle E, Hansen HJ, Goldenberg DM. Epitope specificity of the anti-(B cell lymphoma) monoclonal antibody, LL2. Cancer Immunol Immunother. 1993;37:293–298. doi: 10.1007/BF01518451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tijssen P, Kurstak E. Highly efficient and simple methods for the preparation of peroxidase and active peroxidase-antibody conjugates for enzyme immunoassays. Anal Biochem. 1984;136:451–457. doi: 10.1016/0003-2697(84)90243-4. [DOI] [PubMed] [Google Scholar]
- 29.Hayden MS, Linsley PS, Gayle MA, et al. Single-chain mono- and bispecific antibody derivatives with novel biological properties and antitumor activity from a COS cell transient expression system. Ther Immunol. 1994;1:3–15. [PubMed] [Google Scholar]
- 30.Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang C-H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang C, Losman M, Loo M, Qu Z, Rossi EA, Goldenberg DM. A new method of constructing CD20/CD22 bispecific antibody fusion proteins with improved direct lymphoma cytotoxicity compared to rituximab. J Clin Oncol. 2006;24(June 20 Supplement) Abstract 2536. [Google Scholar]
- 32.Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture methods. Mol Med. 2005;110:173–183. doi: 10.1385/1-59259-869-2:173. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Ayer LM, Polyak MJ, et al. The CD20 calcium channel is localized to microvilli and constitutively associated with membrane rafts. J Biol Chem. 2004;279:19893–19901. doi: 10.1074/jbc.M400525200. [DOI] [PubMed] [Google Scholar]
- 34.Shih LB, Lu HH, Xuan H, Goldenberg DM. Internalization and intracellular processing of an anti-B-cell lymphoma monoclonal antibody, LL2. Int J Cancer. 1994;56:538–545. doi: 10.1002/ijc.2910560413. [DOI] [PubMed] [Google Scholar]
- 35.Michel RB, Mattes MJ. Intracellular accumulation of the anti-CD20 antibody 1F5 in B-lymphoma cells. Clin Cancer Res. 2002;8:2701–2713. [PubMed] [Google Scholar]
- 36.Carnahan J, Wang P, Kendall R, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;9(suppl):3982s–3990s. [PubMed] [Google Scholar]
- 37.Deans JP, Robbins SM, Polyak MJ, Savage JA. Rapid redistribution of CD20 to a low density detergent-insoluble membrane compartment. J Biol Chem. 1998;273:344–348. doi: 10.1074/jbc.273.1.344. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto M, Kuwano Y, Watanabe R, et al. B cell antigen receptor and CD40 differentially regulate CD22 tyrpsine phosphorylation. J Immunol. 2006;176:873–879. doi: 10.4049/jimmunol.176.2.873. [DOI] [PubMed] [Google Scholar]
- 39.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janas E, Priest R, Wilde JI, White JH, Malhotra R. Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin Exp Immunol. 2005;139:439–446. doi: 10.1111/j.1365-2249.2005.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson D, Grillo-Lopez A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin's B-cell lymphoma. Biochem Soc Trans. 1997;25:705–708. doi: 10.1042/bst0250705. [DOI] [PubMed] [Google Scholar]
- 42.Vugmeyster Y, Howell K. Rituximab-mediated depletion of cynomolgus monkey B cells in vitro in different matrices: possible inhibitory effect of IgG. Int Immunopharmacol. 2004;4:1117–1124. doi: 10.1016/j.intimp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Arndt MA, Krauss J, Schwarzenbacher R, Vu BK, Greene S, Rybak SM. Generation of a highly stable, internalizing anti-CD22 single-chain Fv fragment for targeting non-Hodgkin's lymphoma. Int J Cancer. 2003;107:822–829. doi: 10.1002/ijc.11451. [DOI] [PubMed] [Google Scholar]
- 44.Rossi EA, Losman ML, Nordstrom DL, et al. Multivalent anti-CD20/anti-CD22 bispecific antibody fusion proteins made by the DNL method show potent lymphoma cytotoxicity. Blood. 2006;108:707a. Abstract 2495. [Google Scholar]
- 45.Weintraub BC, Jun JE, Bishop AC, Shokat KM, Thomas ML, Goodnow CC. Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J Exp Med. 2000;191:1443–1448. doi: 10.1084/jem.191.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrie RJ, Schnetkamp PP, Patel KD, Wasthi-Kalia M, Deans JP. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Sawada T, Adachi T, et al. Synthetic glycan ligand excludes CD22 from antigen receptor-containing lipid rafts. Biochem Biophys Res Commun. 2007;360:759–764. doi: 10.1016/j.bbrc.2007.06.110. [DOI] [PubMed] [Google Scholar]
- 48.Malissein E, Verdier M, Ratinaud MH, Troutaud D. Activation of Bad trafficking is involved in the BCR-mediated apoptosis of immature B cells. Apoptosis. 2006;11:1003–1012. doi: 10.1007/s10495-006-6713-7. [DOI] [PubMed] [Google Scholar]
- 49.Leprince C, Draves KE, Geahlen RL, Ledbetter JA, Clark EA. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc Natl Acad Sci U S A. 1993;90:3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peaker CJ, Neuberger MS. Association of CD22 with the B cell antigen receptor. Eur J Immunol. 1993;3:1358–1363. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.