Abstract
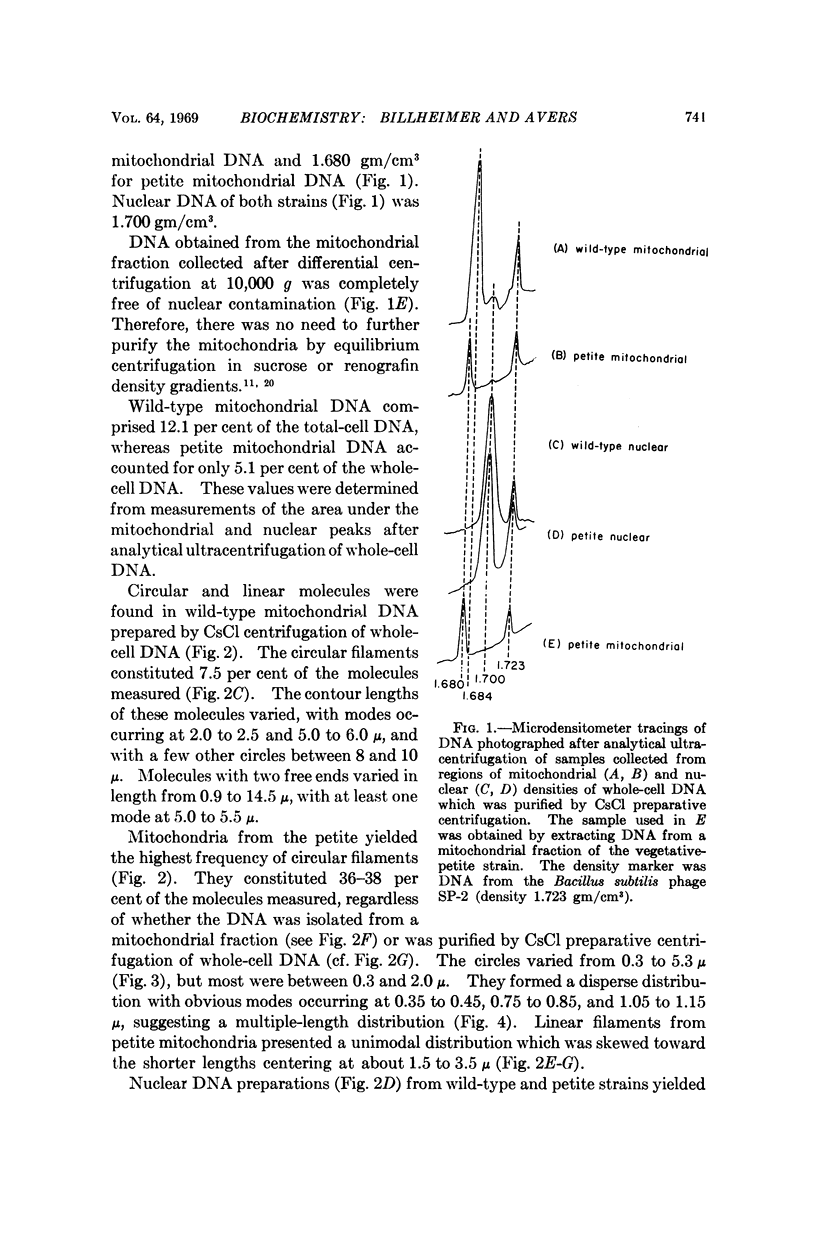
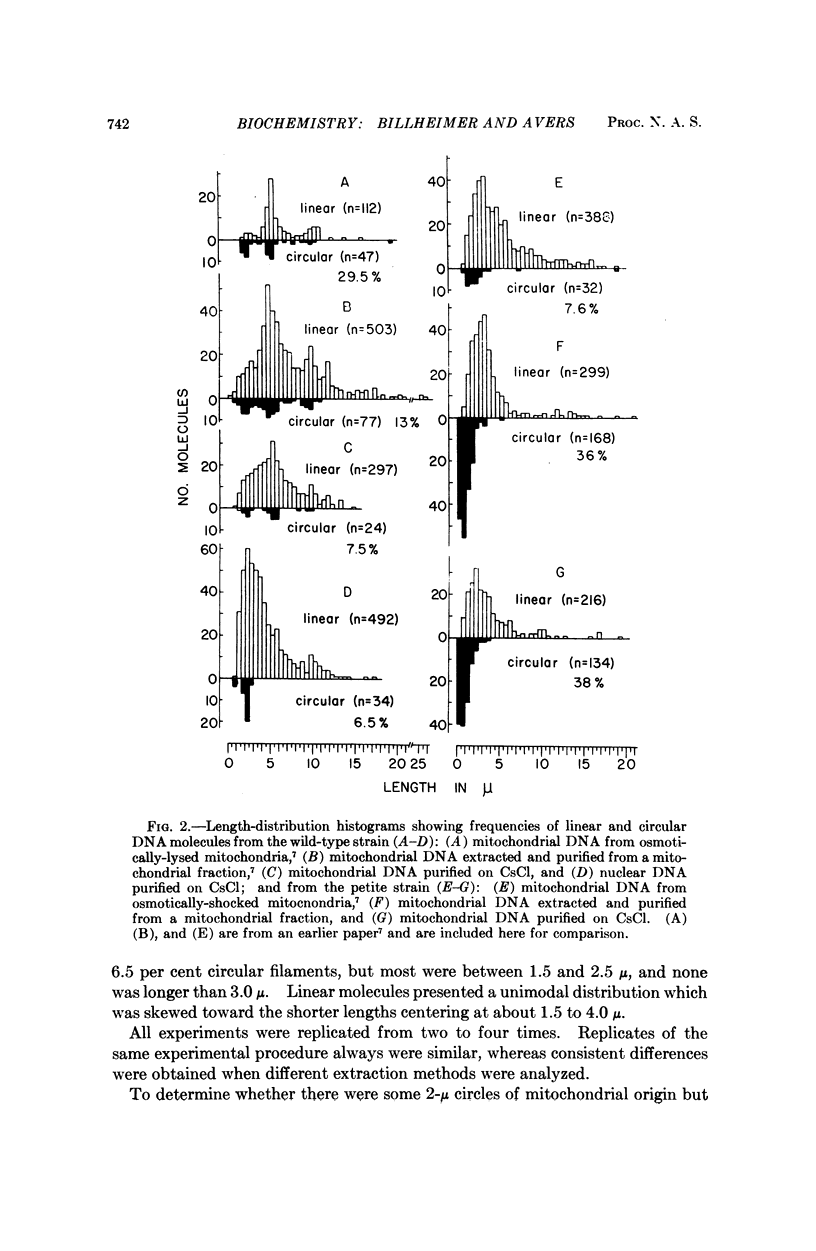
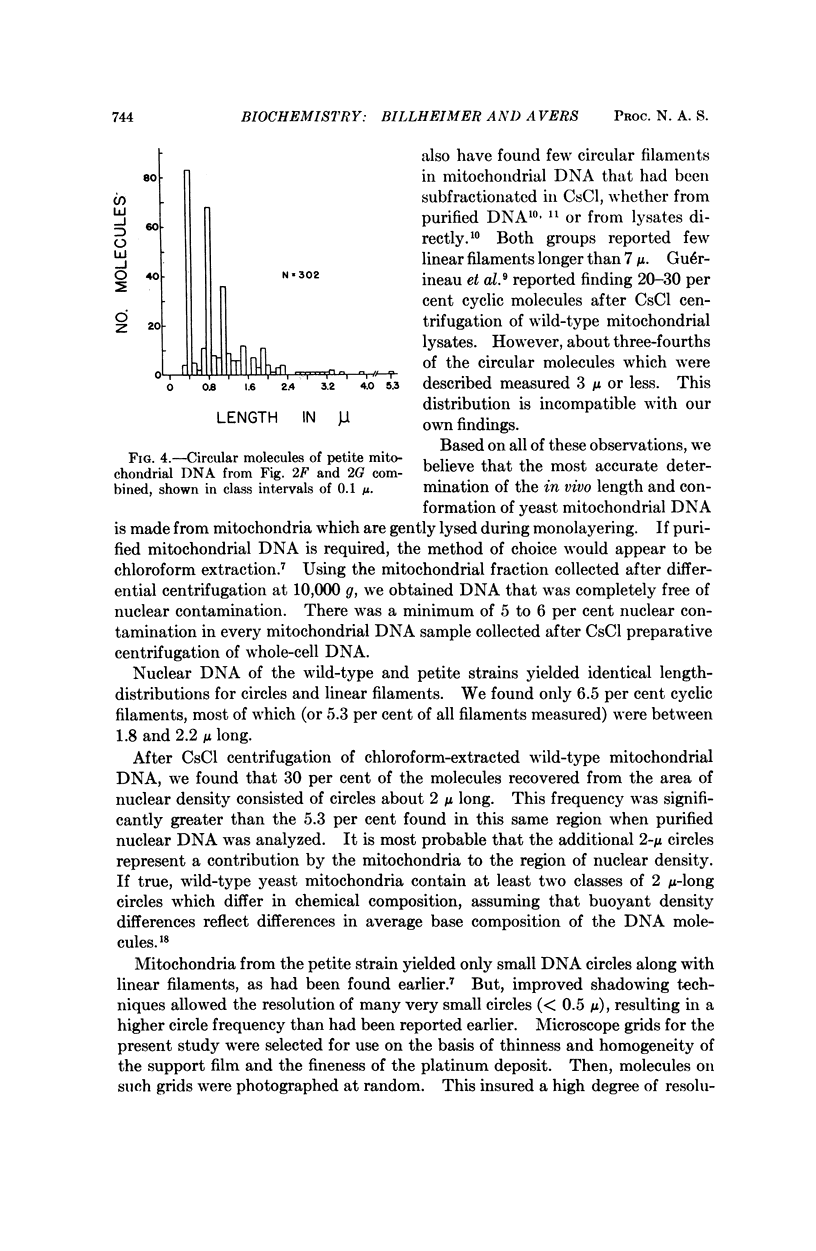
Purified mitochondrial and nuclear DNA from diploid isogenic wild-type and vegetative-petite baker's yeast were analyzed by electron microscopy and by analytical ultracentrifugation in CsCl gradients. The buoyant densities in CsCl of nuclear DNA were identical for the two strains (ρ = 1.700), but there was a difference between mitochondrial DNA from the wild type (ρ = 1.684) and the petite (ρ = 1.680). Electron microscopy revealed both circular and linear filaments for nuclear and for mitochondrial DNA of both strains. Nuclear DNA molecules included 6.5 per cent cyclic filaments principally measuring 2 μ or less in contour length, and linear filaments showing a unimodal, disperse length-distribution centered at about 2 to 3 μ, for both strains. Mitochondrial DNA for wild type varied depending upon the method used to extract and purify the molecules; showing only 7.5 per cent circular molecules from CsCl-subfractionated samples, as compared with 15 per cent circles from chloroform-extracted DNA not subjected to CsCl and up to 50 per cent circles from osmotically-lysed mitochondira, as reported in an earlier study. Modal lengths of circles occurred at about 2, 5, and 10 μ Increasing shear degradation also was evident in comparisons of the length-distribution patterns of linear molecules using the three preparative methods. Petite mitochondrial DNA contained 36-38 per cent circular molecules which measured 0.3-5.3 μ, but principally in the range of 0.3 to 2.0 μ whether from chloroform-extracted populations or from ones subfractionated in CsCl. A previous study of osmotically lysed mitochondria had shown a maximum of 8 per cent circles, which we now attribute to a failure, at that time, to detect circles measuring less than 1 μ, a substantial component encountered in the purified DNA samples in the present study. Linear filaments presented a unimodal length distribution in every case.
Despite the variation in molecule populations derived from the three different preparative methods, there were consistent differences between mitochondrial DNA from wild-type and petite yeast in frequencies and size of circular molecules, as well as in length distribution patterns.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERS C. J., LIN F. H., PFEFFER C. R. HISTOCHEMICAL STUDIES OF MITOCHONDRIAL VARIATION DURING AEROBIC GROWTH OF RESPIRATION-NORMAL BAKER'S YEAST. J Histochem Cytochem. 1965 May-Jun;13:344–349. doi: 10.1177/13.5.344. [DOI] [PubMed] [Google Scholar]
- AVERS C. J., PFEFFER C. R., RANCOURT M. W. ACRIFLAVINE INDUCTION OF DIFFERENT KINDS OF "PETITE" MITOCHONDRIAL POPULATIONS IN SACCHAROMYCES CEREVISIAE. J Bacteriol. 1965 Aug;90:481–494. doi: 10.1128/jb.90.2.481-494.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avers C. J., Billheimer F. E., Hoffmann H. P., Pauli R. M. Circularity of yeast mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):90–97. doi: 10.1073/pnas.61.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avers C. J. Distribution of cytochrome c peroxidase activity in wild-type and petite cells of bakers' yeast grown aerobically and anaerobically. J Bacteriol. 1967 Oct;94(4):1225–1235. doi: 10.1128/jb.94.4.1225-1235.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avers C. J. Heterogeneous length distribution of circular DNA filaments from yeast mitochondria. Proc Natl Acad Sci U S A. 1967 Aug;58(2):620–627. doi: 10.1073/pnas.58.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avers C. J., Rancourt M. W., Lin F. H. Intracellular mitochondrial diversity in various strains of Saccgarintces cerevisiae. Proc Natl Acad Sci U S A. 1965 Aug;54(2):527–535. doi: 10.1073/pnas.54.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G., Carnevali F., Nicolaieff A., Piperno G., Tecce G. Separation and characterization of a satellite DNA from a yeast cytoplasmic "petite" mutant. J Mol Biol. 1968 Nov 14;37(3):493–505. doi: 10.1016/0022-2836(68)90117-4. [DOI] [PubMed] [Google Scholar]
- Goebel W., Helinski D. R. Generation of higher multiple circular DNA forms in bacteria. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1406–1413. doi: 10.1073/pnas.61.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérineau M., Grandchamp C., Yotsuyanagi Y., Slonimski P. P. Examen au microscope électronique du DNA mitochondrial de la levure: molécules à deux extrémités libres. C R Acad Sci Hebd Seances Acad Sci D. 1968 Apr 29;266(18):1884–1887. [PubMed] [Google Scholar]
- Hudson B., Vinograd J. Catenated circular DNA molecules in HeLa cell mitochondria. Nature. 1967 Nov 18;216(5116):647–652. doi: 10.1038/216647a0. [DOI] [PubMed] [Google Scholar]
- Mehrotra B. D., Mahler H. R. Characterization of some unusual DNAs from the mitochondria from certain "petite" strains of Saccharomyces cerevisiae. Arch Biochem Biophys. 1968 Dec;128(3):685–703. doi: 10.1016/0003-9861(68)90078-7. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Grossman L. I., Marmur J., Kleinschmidt A. K. Physical studies on the structure of yeast mitochondrial DNA. J Mol Biol. 1968 May 14;33(3):907–922. doi: 10.1016/0022-2836(68)90327-6. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Dawid I. B. A size difference between mitochondrial DNA molecules of urodele and anuran amphibia. J Cell Biol. 1968 Oct;39(1):222–228. doi: 10.1083/jcb.39.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Gross N. J. The form and size of mitochondrial DNA of the red bean, Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1968 Sep;61(1):245–252. doi: 10.1073/pnas.61.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bruggen E. F., Runner C. M., Borst P., Ruttenberg G. J., Kroon A. M., Stekhoven F. M. Mitochondrial DNA. 3. Electron microscopy of DNA released from mitochondria by osmotic shock. Biochim Biophys Acta. 1968 Jul 23;161(2):402–414. [PubMed] [Google Scholar]



