Abstract
Imatinib mesylate (imatinib) is highly effective in the treatment of chronic myeloid leukemia (CML) but is less effective in eliminating CML stem cells. We investigated whether SKI-606, a potent Bcr-Abl and Src kinase inhibitor without anti-PDGF or c-Kit activity, could effectively target primitive CML progenitors. CML and normal progenitors were cultured with SKI-606 or imatinib. SKI-606 effectively inhibited Bcr-Abl kinase activity in CML CD34+ cells and inhibited Src phosphorylation more potently than imatinib. However, SKI-606 and imatinib resulted in similar suppression of CML primitive and committed progenitor proliferation and growth in CFC and LTC-IC assays. Exposure to either agent alone or in combination resulted in only modest increase in apoptosis. Evaluation of downstream signaling pathways indicated that Akt and STAT5 activity was not changed, but a delayed increase in MAPK activity was seen at high concentrations of SKI-606. SKI-606 inhibited normal progenitor proliferation to a lesser extent than imatinib. SKI-606 effectively inhibits Bcr-Abl and Src kinase activity and inhibits CML progenitor growth with relatively little effect on normal progenitors. However, SKI-606 does not demonstrate increased ability to eliminate primitive CML progenitors by apoptosis compared with imatinib, emphasizing the need for additional strategies besides Bcr-Abl kinase inhibition for curative therapy of CML.
Introduction
Treatment with the Bcr-Abl kinase inhibitor imatinib mesylate (imatinib) has markedly improved the outcome for patients with chronic myeloid leukemia (CML). High response rates have been reported for CML patients in chronic phase (CP) of the disease, whereas patients with later stages of CML (accelerated phase and blast crisis) are prone to develop imatinib resistance.1 Several mechanisms of resistance have been identified, including mutations in the BCR-ABL kinase domain, BCR-ABL amplification, and overexpression and abnormal, constitutive activation of growth stimulatory signaling pathways independent of Bcr-Abl.2–5
Mathematical modeling of in vivo kinetics of response to imatinib, based on analysis of quantitative polymerase chain reaction (Q-PCR) data, suggests that imatinib inhibits production of differentiated leukemia cells but does not deplete leukemia stem cells.6,7 Analysis of bone marrow samples from CML patients in complete remission on imatinib treatment confirms the persistence of leukemia stem and progenitor cells in this patient population.8 Reports of recurrence occurring after discontinuation of imatinib therapy indicate that residual CML cells remaining after imatinib therapy possess pathogenic potential.9,10 The eradication of malignant stem and progenitor cells thus appears to be necessary to improve the treatment outcome for these patients.
We have shown that suppression of CML progenitor growth by imatinib is primarily accomplished through inhibition of abnormally increased proliferation rather than through induction of apoptosis, and that nondividing primitive progenitors are insensitive to imatinib-induced apoptosis.11,12 Our studies indicate that inherent resistance of quiescent CML progenitors to apoptosis plus microenvironmental activation of signaling pathways that contribute to maintenance of viability in imatinib-treated CML progenitors may be potential mechanisms underlying the persistence of malignant progenitors despite imatinib treatment. Other possible mechanisms, such as reduced drug uptake or increased drug efflux by leukemia stem cells, increased Bcr-Abl expression levels in stem cells, and the presence of Bcr-Abl kinase mutant clones among residual leukemia stem cells, are additional mechanisms that may contribute to imatinib resistance.13,14
The substantial progress made in understanding the molecular basis of imatinib-resistance has led to the discovery of a new generation of drugs for treatment of CML that inhibit the Abl kinase much more potently than imatinib and inhibit several Abl kinase mutants that are resistant to imatinib. These drugs are being tested in clinical trials in patients who fail imatinib treatment. One of these agents, Dasatinib, has received FDA approval for treatment of imatinib-resistant CML. Dasatinib, in addition to inhibiting Abl, is a potent inhibitor of Src kinases. Src kinase activation is involved in Bcr-Abl downstream signaling,15 and there is experimental evidence that myeloid-specific Src kinases maintain leukemic cell survival.16 Overexpression of Src family kinases has been identified among the known mechanisms of resistance to imatinib in CML. Therefore, it is possible that dual inhibitors of Bcr-Abl and Src kinases may demonstrate increased efficacy against CML cells. However, the extended spectrum of kinase inhibition may also be associated with increased toxicity toward normal cells. Indeed, clinical experience with Dasatinib indicates significant hematopoietic suppressive effects.17
SKI-606 is an orally bioavailable tyrosine kinase (TK) inhibitor that demonstrates potent activity against the Bcr-Abl and Src kinases. Furthermore, SKI-606 also exerts activity against a variety of clinically relevant imatinib-resistant Abl domain mutations.18–21 As a result, SKI-606 is currently under evaluation in phase 1/2 trials in CML patients resistant to or intolerant of imatinib.22 However, the effects of SKI-606 on primary CML or normal primitive progenitor cells have not been described. It is possible that increased inhibition of Bcr-Abl TK activity in CML progenitors by a more potent, dual Src/Abl kinase inhibitor could result in enhanced targeting of malignant primitive leukemia progenitors. Therefore, in this study, we examined the effect of SKI-606 on the growth of CML primitive and committed progenitor cells as well as normal progenitor cells. We also investigated the effects of SKI-606 on Bcr-Abl and Src kinase activity as well as on downstream signaling mechanisms in CML CD34+ cells. The effects of imatinib on the same populations were studied for comparison.
Methods
Subjects
Peripheral blood samples from newly diagnosed CML chronic phase patients, and umbilical cord blood samples from healthy donors were obtained under Institutional Review Board guidelines. Approval was obtained from the City of Hope National Medical Center Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Inhibitors
Stock solutions of SKI-606 (10 mM, kindly provided by Wyeth Research, Pearl River, NY) and imatinib (Novartis Pharmaceuticals, East Hanover, NJ) were prepared in DMSO and stored at −20°C. SKI-606 was added to cell cultures at concentrations ranging between 0.05 and 0.5 μM. Imatinib was added at a concentration of 5 μM.
Selection of CD34+ progenitors
Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO) density gradient centrifugation (specific gravity, 1.077) for 30 minutes at 400g. CD34+ cells were selected by immunomagnetic column separation (Miltenyi Biotech, Auburn, CA) following the manufacturer's instructions. The purity of selected CD34+ cells, as assessed by fluorescent-activated cell sorting (FACS) analysis, was 97.8% plus or minus 1.1%.
Cell culture and exposure to inhibitors
CD34+ cells were cultured in tissue-culture plates (Falcon; BD Biosciences, San Jose, CA) at 37°C in a humidified atmosphere with 5% CO2 in serum-free medium (Stem Cell Technologies, Vancouver, BC,) supplemented with growth factors (GFs) at concentrations similar to that found in stroma-conditioned medium from long-term bone marrow cultures (200 pg/mL granulocyte-macrophage colony-stimulating factor, 1 ng/mL granulocyte colony-stimulating factor, 200 pg/mL stem-cell factor, 50 pg/mL leukemia inhibitory factor, 200 pg/mL macrophage-inflammatory protein-1, and 1 ng/mL interleukin-6). SKI-606 or imatinib was added at the indicated concentrations. Cells were harvested after 96 hours and assayed in progenitor, proliferation, and apoptosis assays.
Progenitor assays
Colony-forming cells (CFCs).
CD34+ cells were cultured for 96 hours in the presence or absence of inhibitors in triplicate. Cells were plated in methylcellulose progenitor culture and burst forming unit-erythroid and colony forming unit-granulocyte and macrophage were counted after 14 days.
Long-term culture-initiating cells (LTC-ICs).
Cells were plated in long-term bone marrow culture medium on M2-10B4 murine fibroblast feeders previously subcultured in 96-well plates. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2 and fed at weekly intervals. After 6 weeks, half of the medium was removed and the wells were overlaid with CFC-growth-supporting medium. After 2 weeks, wells were scored as positive or negative for the presence of CFC. The frequency of LTC-IC was calculated with L-Calc software (Stem Cell Technologies). Results from the CFC and LTC-IC were reported as percentage of growth inhibition versus control. Cell proliferation curves were derived from these data and regression lines were generated in Microsoft Excel. The doses required for 50% inhibition of proliferation (IC50 values) were analyzed by hand from the dose-response plot.
Apoptosis assay
CD34+CD38+ and CD34+CD38− progenitor cells were cultured for 96 hours in the presence or absence of inhibitors, labeled with annexin V-PE (BD Biosciences PharMingen, San Diego, CA) according to the manufacturer's instruction, and analyzed by flow cytometry. Apoptotic cells were defined as annexin V-PE+.
Proliferation assay
To assess proliferation, a 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) labeling assay was performed as described previously.11 CFSE labeled CD34+CD38+ and CD34+CD38− progenitor cells were cultured for 96 hours in the presence or absence of inhibitors. At the end of the culture period, proliferation was analyzed by flow cytometry (FACScalibur; BD Biosciences). To fit the data, to determine the percentage of cells in each generation, and to generate a proliferation index, ModFit software (Verity, Topsham, ME) was used. The position of the parent generation was set on the basis of the fluorescence profile of an aliquot of cells treated with paraformaldehyde immediately after sorting.
Western blot analysis
CD34+ cells (3 × 105 cells per condition) were cultured in medium containing low concentrations of GFs, in the presence or absence of inhibitors, at indicated concentrations for 16 hours. Cells were lysed in buffer containing 0.5% Nonidet P-40 (Sigma Diagnostics) and 0.5% sodium deoxycholate supplemented with phenylmethylsulfonyl fluoride (1 mM), protease inhibitor mixture, and phosphatase inhibitors (50 mM sodium fluoride, 1 mM sodium vanadate) (all from Sigma Diagnostics). Proteins were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membrane. Primary antibodies used were as follows: anti–Crk-like (anti-CrkL) rabbit polyclonal antibody (sc-319), antiphosphorylated p42/44 MAPK mouse monoclonal antibody (sc-7383), anti-p42/44 MAPK rabbit polyclonal antibody (sc-94), anti-STAT5 rabbit polyclonal antibody (sc-835) (all from Santa Cruz Biotechnology, Santa Cruz, CA), antiphosphorylated STAT5 (Tyr694) rabbit polyclonal antibody, antiphosphorylated Akt (Ser473) rabbit polyclonal antibody, anti-Akt rabbit polyclonal antibody, antiphosphorylated Src Family (Tyr416) rabbit polyclonal antibody, anti-Src rabbit polyclonal antibody, antiphosphorylated MEK1/2 (Ser217/221) rabbit polyclonal antibody, anti-MEK1/2 polyclonal antibody, antiphosphorylated c-Raf (Ser338) rabbit monoclonal antibody (all from Cell Signaling Technology, Danvers, MA), anti–c-Abl (Ab-3) mouse monoclonal antibody (Calbiochem, San Diego, CA), antiphosphotyrosine, clone 4G10, mouse monoclonal antibody, anti-Raf-1 rabbit polyclonal antibody (all from Upstate Biotechnology, Charlottesville, VA) and antiactin mouse monoclonal antibody (AC-15; Sigma). Horseradish peroxidase- or alkaline phosphatase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (Westgrove, PA). Antibody detection was performed using the Superfemto kit (Pierce Biotechnology, Rockford, IL). The protein expression levels were determined by densitometry using Image-Quant software (GE Healthcare, Little Chalfont, United Kingdom).
Fluorescent in situ hybridization analysis
Fluorescent in situ hybridization (FISH) analysis was performed as previously described.23 Briefly, cells derived from colonies resulting from CFC assays were harvested and pooled. After resuspension in hypotonic KCl solution, cells were centrifuged and fixed using Carnoy fixative. The fixed cells were resuspended in 5 to 10 μL Carnoy fixative. Hybridization using the LSI dual labeled BCR-ABL DNA probe was performed in accordance with the manufacturer's instructions (Vysis, Downers Grove, IL). Lymphocytes from a healthy individual served as a BCR-ABL negative control; SD-1 cell lines, derived from an acute lymphoblastic leukemia patient, served as a BCR-ABL positive control. A total of 200 nuclei were scored for each sample.
Statistical analysis
Data obtained from independent experiments were reported as the mean plus or minus SEM. Significance levels were determined by Student t test analysis.
Results
Effect of SKI-606 treatment on CML LTC-IC and CFC growth
We investigated the effects of SKI-606 on CML progenitor cells and compared these effects with those of imatinib on the same populations. We also studied normal progenitor cells as controls to assess selectivity and detect “off-target” effects of the inhibitors. CML CD34+ cells were exposed to SKI-606 (0 to 0.5 μM) or imatinib (5 μM) as single agents for 96 hours under low GF conditions. After drug exposure, the cells were washed to remove the drug, and assays for primitive (LTC-IC) and committed (CFC) progenitors were performed in the absence of further drug exposure. The effects of SKI-606 and imatinib on CML and normal LTC-IC are depicted in Figure 1A,B. Exposure to SKI-606 for 96 hours resulted in a dose-dependent inhibition of CML LTC-IC compared with untreated cells (from 30.5% ± 9.6% suppression with 0.05 μM SKI-606 to 72.3% ± 2.3% suppression with 0.5 μM SKI-606, P = .02 and P < .001, respectively). Comparable effects were observed with 5 μM imatinib treatment. The IC50 for suppression of CML LTC-IC was 0.09 μM for SKI-606. High concentrations of both SKI-606 and imatinib resulted in modest levels of inhibition of normal LTC-IC (35.4% ± 17.7% inhibition with 0.5 μM SKI-606, P = .1; and 35.4% ± 10.3% inhibition with 5 μM imatinib, P = .02).
Figure 1.
Inhibition of primitive and committed progenitor growth after exposure to SKI-606. CD34+ cells from CML patients or healthy donors were exposed to SKI-606 or imatinib at the concentrations indicated for 96 hours. Cells were then assayed for primitive progenitors (LTC-IC) or committed progenitors (CFC) as described in “Progenitor assays.” (A,B) The effects of SKI-606 and imatinib on CML and normal primitive progenitor growth, respectively. (C,D) The effects on CML and normal committed progenitor growth, respectively. The percentage inhibition of primitive and committed progenitor growth after SKI-606 and imatinib treatment relative to untreated controls is shown. Results represent the mean plus or minus SEM based on replicate experiments (CML, n = 4; normal n = 3). (A-D) Concentrations of imatinib resulting in significant CML or normal progenitor growth suppression compared with untreated controls (†††P < .001; ††P < .01; †P < .05). Concentrations of SKI-606 inducing significant CML or normal progenitor growth suppression compared with untreated controls (***P < .001; **P < .01; *P < .05). (E) Inhibition of CML committed progenitor growth after exposure to SKI-606 (0.5 μM) and imatinib (5 μM) in combination (n = 3). The percentage of growth inhibition compared with untreated controls is shown for each condition. Significant suppression of progenitor growth in treated cells compared with controls (ns, not significant; ***P < .001; **P < .01; *P < .05). Error bars represent SEM.
SKI-606 treatment resulted in a significant, dose-dependent suppression of CML CFC. Exposure to 0.5 μM SKI-606 inhibited CML CFC by 59.3% plus or minus 8.1% compared with untreated cells, whereas 5 μM imatinib treatment inhibited CML CFC by 69.9% plus or minus 8.2%. The IC50 for CML CFC suppression for SKI-606 was 0.08 μM (Figure 1C). The degree of CML CFC suppression observed with 5 μM imatinib treatment was consistent with our previous results.11 Normal CFC showed less growth inhibition (0.5 μM SKI-606: 23.3% ± 4.0%, 5 μM imatinib 20.1% ± 3.7%), and the IC50 was not reached within the dose range tested (IC50 > 0.5 μM of SKI-606 and > 5 μM of imatinib, respectively; Figure 1D). As shown in Figure 1E, the combination of SKI-606 (0.5 μM) and imatinib (5.0 μM) did not inhibit CML CFC growth to a significantly greater extent than treatment with either agent alone (63.4% ± 8.6% inhibition with the combination compared with 55.8% ± 5.5% inhibition with 5 μM imatinib and 43.5% ± 2.1% inhibition with 0.5 μM SKI-606 alone). FISH analysis revealed that the CML CFCs were predominantly of malignant origin, with FISH analysis of colonies generated in CFC culture revealing that 98.3% of cells derived from untreated cells were BCR-ABL positive (mean, 98.3%). However CFCs remaining after SKI-606 or imatinib treatment were also predominantly BCR-ABL positive (0.5 μM SKI-606: 92.3%, 5 μM imatinib: 93.5%), indicating that incomplete inhibition of progenitor growth by SKI-606 is not explained by selective persistence of BCR-ABL negative cells.
Effect of SKI-606 treatment on apoptosis and cell division of CML primitive and committed progenitors
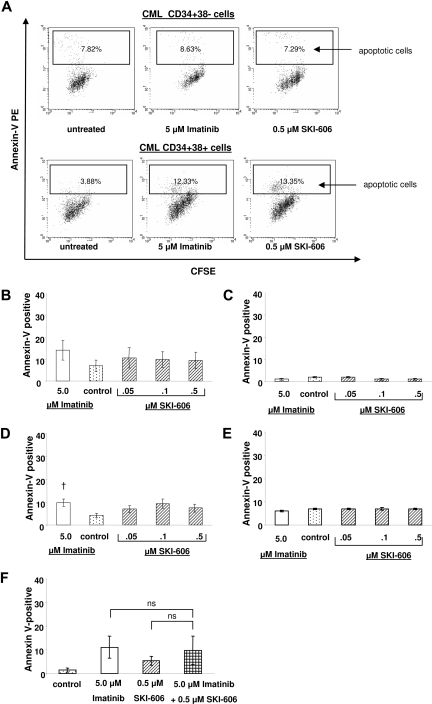
To investigate whether the growth inhibitory effects of SKI-606 on CML progenitors represented inhibition of proliferation or induction of apoptosis or both, CD34+38− and CD34+38+ cells labeled with CFSE were selected using flow cytometry and incubated in a range of concentrations of SKI-606 or 5 μM imatinib under low GF conditions for 96 hours. Cells were subsequently labeled with annexin V-PE and analyzed by flow cytometry for apoptosis (annexin-positive cells) and cell division (reduction in CFSE fluorescence) (Figures 2A, 3A).
Figure 2.
SKI-606 does not specifically induce apoptosis in CML primitive and committed progenitors. CML and normal CD34+CD38− primitive and CD34+CD38+ committed progenitors were exposed to SKI-606 or imatinib at indicated concentrations for 96 hours. Apoptosis was analyzed by FACS as the percentage of cells positively labeled by Annexin V-PE. (A) Representative data for apoptosis of CML CD34+CD38− primitive and CML CD34+CD38+ committed progenitors. (B,C) Compiled data (mean ± SEM) for apoptosis of CML (n = 4) and normal (n = 3) primitive progenitors, respectively. (D,E) Apoptosis of CML (n = 5) and normal (n = 3) committed progenitors, respectively. Significant increase in the percentage of apoptotic cells seen in CML committed progenitors in response to imatinib is indicated (†P < .05). (F) Apoptosis in CD34+CD38+ cells after exposure to SKI-606 (0.5 μM) and imatinib (5 μM) in combination. Results represent the mean plus or minus SEM of replicate experiments (n = 7); ns, not significant.
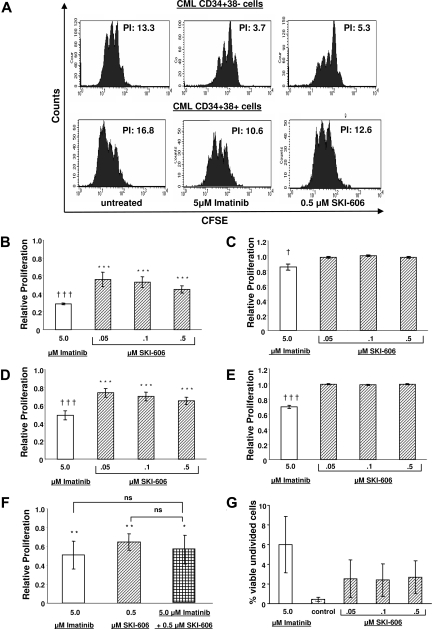
Figure 3.
Proliferation of CML primitive and committed progenitors is suppressed in cells exposed to SKI-606. CFSE labeling assays were used to measure effects of SKI-606 or imatinib on cell division of CML progenitors. CFSE-labeled cells were sorted for CD34 and CD38 expression to yield primitive (CD34+CD38−) and committed (CD34+CD38+) progenitors. Cells were analyzed by FACS for CFSE fluorescence after exposure to inhibitors at the indicated concentrations for 96 hours. (A) Representative CFSE plots for SKI-606 (0.5 μM) and imatinib (5 μM) treated CML CD34+38− primitive and CD34+CD38+ committed progenitors. The calculated proliferation index (PI) for each plot is indicated. (B,C) Compiled data for proliferation of CML and normal primitive progenitors, respectively. (D,E) CML and normal committed progenitors, respectively. Proliferation of inhibitor-treated cells is expressed relative to proliferation in the absence of inhibitor treatment. The mean plus or minus SEM values of replicate experiments are shown (n = 4, CML primitive progenitors; n = 5, CML committed progenitors; n = 3, normal primitive and committed progenitors). (B-E) Significant changes in proliferation in response to imatinib (†††P < .001; †P < .05) or SKI-606 (***P < .001) are indicated. (F) Inhibition of CD34+CD38+ cell proliferation after exposure to imatinib and SKI-606 in combination. Results represent the mean plus or minus SEM of replicate experiments (n = 7). Significant suppression of progenitor proliferation in treated cells compared with controls (**P < .01; *P < .05). (G) The percentage of nondividing (CFSE bright) CML primitive progenitors remaining after exposure to SKI-606 (0.05 to 0.5 μM) or imatinib (5 μM) for 96 hours as determined by flow cytometry.
Treatment of CML primitive progenitors with SKI-606 did not result in significant increase in apoptosis compared with untreated cells (7.0% ± 2.6% in untreated cells and 9.4% ± 3.8% with 0.5 μM SKI-606). Apoptosis was also not significantly increased in CML primitive progenitors treated with 5 μM imatinib (14.0% ± 4.4%, P = .2; Figure 2B). SKI-606 treatment resulted in a similar increase of apoptosis in CML-committed progenitors (from 4.2% ± 0.9% in untreated cells to 7.4% ± 1.7% with 0.5 μM SKI-606). Treatment of the same populations with 5 μM imatinib increased the fraction of apoptotic cells to 9.6% plus or minus 1.5% (P = .01; Figure 2D). In contrast, treatment of normal progenitors with SKI-606 or imatinib did not result in induction of apoptosis in the tested dose range (Figure 2C,E). These results indicate that SKI-606 induces apoptosis in CML progenitor cells only to a modest extent, to a similar extent as seen with imatinib treatment. The combination of SKI-606 and imatinib did not result in significantly enhanced apoptosis of CML progenitors compared with either agent alone (1.4% ± 0.8% apoptosis in untreated cells, 11.1% ± 4.6% with 5 μM imatinib, 5.4% ± 1.9% with 0.5 μM SKI-606, and 9.9% ± 6.0% with the combination; Figure 2F).
We analyzed the effect of SKI-606 on proliferation of CML and normal CD34+CD38+ committed and CD34+CD38− primitive progenitors by tracking cell division of CFSE-labeled cells using flow cytometry and generating a proliferation index (PI) using ModFit Software (Figure 3A). Untreated CML primitive and committed progenitors were significantly more proliferative compared with the corresponding normal population (PI of CML committed progenitors of 18.3 ± 2.5 vs PI of normal committed progenitors of 5.0 ± 0.8, P = .007; PI of CML primitive progenitors of 10.2 ± 2.5 vs PI of normal primitive progenitors of 2.5 ± 0.1, P = .04). Treatment with SKI-606 resulted in a significant, dose-dependent reduction in proliferation of CML CD34+CD38− primitive progenitors (Figure 3B). Treatment with imatinib resulted in similar inhibition of CML CD34+CD38− cell division. Proliferation of CD34+CD38+ committed progenitors also significantly decreased in response to SKI-606 and imatinib treatment (Figure 3D). The proliferation of normal progenitor cells was significantly reduced with 5 μM of imatinib but was not significantly reduced at the highest concentration of SKI-606 (0.5 μM; Figure 3C,E). The IC50 for inhibition of CML primitive progenitor proliferation for SKI-606 was 0.18μM. The IC50 for inhibition of CML committed progenitors for SKI-606 was not reached with the dose range tested. At the highest dose of 0.5 μM, relative proliferation averaged 0.65 plus or minus 0.04 (P < .001, n = 5). Combined treatment with SKI-606 and imatinib did not inhibit CML progenitor proliferation to a greater extent than the individual drugs (relative proliferation compared with controls with 5 μM imatinib was 0.5 ± 0.1, and with 0.5 μM SKI-606 was 0.7 ± 0.1, and with the combination was 0.6 ± 0.2; Figure 3F).
We have shown that nondividing CML progenitors are relatively resistant to imatinib treatment. Inhibition of progenitor proliferation by imatinib, coupled with resistance of nondividing progenitors to imatinib-induced apoptosis, may contribute to retention of nondividing malignant progenitors. We investigated whether SKI-606 treatment resulted in improved targeting of undivided CML CD34+ cells compared with imatinib. As shown in Figure 3G, an increased proportion of undivided progenitors were seen in the cells remaining after SKI-606 treatment compared with untreated controls, although this difference did not reach statistical significance. As seen in previous studies, the proportion of undivided progenitors was increased after imatinib treatment. The proportion of undivided progenitors remaining after combined treatment with SKI-606 and imatinib did not significantly differ from that seen with either agent alone (data not shown).
Our results indicate that SKI-606 inhibits proliferation of CML but not normal CD34+ cells. SKI-606 showed similar efficacy to imatinib in inhibiting CML committed and primitive progenitor growth in CFC and LTC-IC assays, in inhibiting progenitor proliferation and in inducing apoptosis. As with imatinib, SKI-606 appears to suppress CML primitive and committed progenitors by inhibition of proliferation and to a lesser extent by inducing apoptosis. SKI-606 treatment resulted in reduced suppression of normal LTC-IC growth and normal progenitor proliferation compared with imatinib.
Effects of SKI-606 on Bcr-Abl and Src TK activity
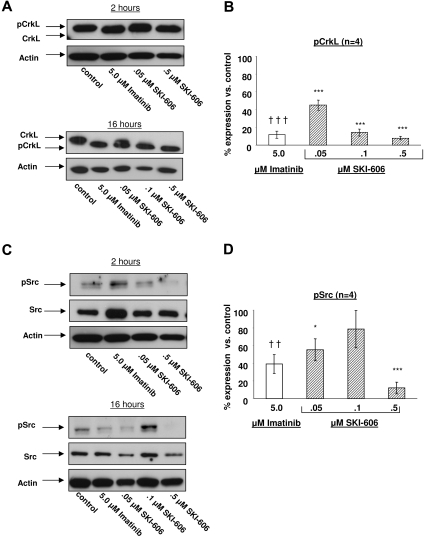
We sought to investigate the effects of SKI-606 on CML progenitor cells and to compare with those of imatinib on the same populations. CML CD34+ cells were exposed to SKI-606 (0 to 0.5 μM) or imatinib (5 μM) as single agents in low GF conditions for 2 hours and 16 hours. After drug exposure CD34+ cell lysates were prepared and Western blotting was performed to detect CrkL and Src phosphorylation to assess the effects of SKI-606 and imatinib on Bcr-Abl and Src TK activity in CML progenitors. Phosphorylated CrkL (P-CrkL) is distinguishable from nonphosphorylated CrkL by its slower migration. As shown in Figure 4A,B, P-CrkL protein levels were high in the untreated control and were effectively suppressed after treatment with SKI-606 for both 2 hours and 16 hours at doses as low as 0.05 μM (44.9% ± 5.6% residual P-CrkL, n = 4, P < .001). Increasing the SKI-606 concentration up to 0.5 μM resulted in further suppression of P-CrkL levels (7.8% ± 1.9%, n = 4, P < .001). Treatment with imatinib at 5 μM resulted in similar suppression of P-CrkL levels (11.8% ± 4.2%, n = 4, P < .001). Similarly, both SKI-606 and imatinib effectively inhibited Bcr-Abl autophosphorylation, whereas total Bcr-Abl levels were not altered (data not shown). P-Src levels were effectively suppressed in response to treatment with SKI-606 (12.1% ± 6.5% residual P-Src compared with untreated controls with 0.5 μM SKI-606, n = 4, P < .001). P-Src levels were also suppressed, but to a lesser extent after treatment with 5 μM imatinib (39.3% ± 10.7%, n = 4, P = .001, Figure 4C,D).
Figure 4.
Effects of SKI-606 treatment on Bcr-Abl and Src kinase activity in CML CD34+cells. CML CD34+ cells were incubated with SKI-606 or imatinib for 2 hours and 16 hours in low GF containing medium as described in “Western blot analysis.” Protein extracts were prepared as described in “Western blot analysis.” (A) Representative results of Western blotting using anti-CrkL and antiactin antibodies. Densitometry analysis was performed and the phosphorylated CrkL/total CrkL ratio was calculated. (B) Results represent mean plus or minus SEM percentage of phosphorylated CrkL normalized to CrkL phosphorylation in the absence of inhibitors after a 16-hour incubation period based on replicate experiments (n = 4). (C) Representative results of Western blotting using anti-pSrc, anti-Src, and antiactin antibodies. The ratio of phosphorylated Src: total Src was calculated. (D) Results represent mean plus or minus SEM percentage of phosphorylated Src after a 16-hour incubation period normalized to Src phosphorylation in the absence of inhibitors based on replicate experiments (n = 4). (B,D) Significant differences for treated cells compared with untreated controls are indicated for imatinib (†††P < .001; ††P < .01) and SKI-606 (***P < .001; *P < .05). Error bars represent SEM.
Effects of SKI-606 on downstream targets of Bcr-Abl
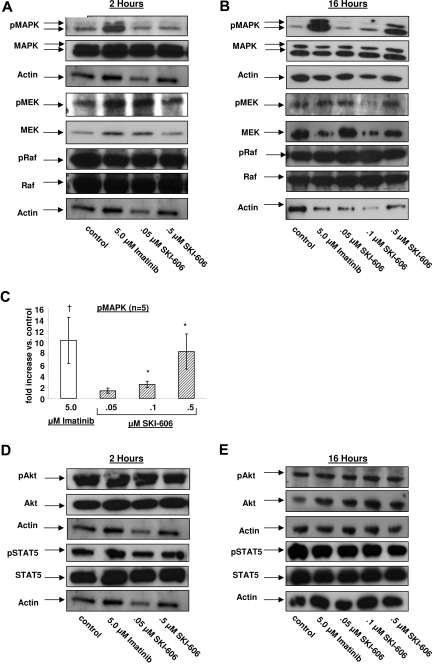
We evaluated the effect of SKI-606 on the MAPK, Akt, STAT5, signaling pathways that are activated downstream of Bcr-Abl and potentially play important roles in abnormal proliferation and survival of CML progenitors. We assessed intracellular signaling in CML CD34+ cells after 2 hours as well as 16 hours of exposure to imatinib and SKI-606 (Figure 5). As seen in previous studies, treatment with 5 μM imatinib resulted in increased MAPK activity in CML CD34+ cells. We observed a similar increase in MAPK activity in CML CD34+ cells after exposure to imatinib for 2 hours or 16 hours (Figure 5A,B). In contrast, increased MAPK activity was only seen after 16 hours of SKI-606 treatment but was not seen after 2 hours of treatment. Significant changes in MAPK activity were only observed with higher concentrations of SKI-606 (0.1 and 0.5 μM) (Figure 5C). These observations suggest that changes in MAPK activity after SKI-606 treatment occur as a compensatory response to Bcr-Abl and Src kinase inhibition. Raf and MEK1/2 are positioned downstream of Bcr-Abl and upstream of MAPK in the Bcr-Abl-MAPK cascade. Western blotting with anti-phospho-MEK1/2 and anti-phospho-Raf antibodies indicated that Raf and MEK-1/2 activity were not altered in CML CD34+ progenitors treated with either drug (Figure 5A,B). These observations suggest that the increased MAPK activity after imatinib and SKI-606 treatment results from modulation of signaling at the level of p42/44 MAPK itself. Incubation of CML CD34+ cells with either SKI-606 or imatinib for 2 hours or 16 hours in the presence of GF did not lead to a significant change in phospho-Akt and phospho-STAT5 levels (Figure 5D,E). These results suggest that GF dependent signaling is maintained (Akt, STAT5) or even enhanced (MAPK) in SKI-606 treated cells.
Figure 5.
Effects of SKI-606 treatment on MAPK, Akt, STAT5, MEK1/2, and Raf signaling pathways in CML CD34+ cells. CML CD34+ cells were incubated with SKI-606 or imatinib for 2 hours and 16 hours as described in “Western blot analysis.” Cell lysates were prepared and Western blotting was performed as indicated in the figure. Results of Western blotting for phospho-MAPK, MAPK, phospho-MEK1/2, MEK1/2, phospho-Raf, Raf, and actin after 2 hours (A) and 16 hours (B) of SKI-606 and imatinib treatment, respectively. (C) Results of densitometric analysis of the ratio of phosphorylated MAPK to total MAPK. Results are presented as fold change after inhibitor treatment relative to untreated controls. Significant differences in phosphorylated MAPK expression levels for treated cells compared with untreated controls are indicated for imatinib (†P < .05) and SKI-606 (*P < .05). Results of Western blotting for phospho-Akt, Akt, phospho-STAT5, STAT5, and actin after 2 hours (D) and 16 hours (E) of SKI-606 and imatinib treatment, respectively. Results shown represent mean plus or minus SEM fold increase of phosphorylated protein compared with the untreated control based on replicate experiments (n = 5).
Discussion
SKI-606 exerts potent antiproliferative activity against imatinib-sensitive and imatinib-resistant CML cell lines, and is being investigated in clinical trials in CML patients who have failed imatinib therapy.22 Because SKI-606 is a more potent inhibitor of the Bcr-Abl kinase than imatinib and additionally inhibits the Src kinase, we investigated whether SKI-606 could effectively inhibit primitive CML hematopoietic cells, a population that is relatively resistant to elimination by imatinib. Our results indicate that SKI-606 effectively inhibited Bcr-Abl and Src kinase activity in CML progenitor cells and suppressed the growth of CML primitive and committed progenitor cells in a dose responsive manner. SKI-606-induced suppression occurred at drug concentrations that are clinically achievable. However, treatment of CML progenitors with SKI-606 did not result in increased cell kill or reduction in residual nonproliferating cells compared with imatinib. SKI-606 inhibited normal progenitors to a lesser extent compared with imatinib.
Exposure to SKI-606 resulted in significant inhibition of CML LTC-IC and CFC growth in a dose-dependent manner. However, at concentrations such as could be achieved with therapeutic exposures, SKI-606 resulted in similar degrees of progenitor suppression as were seen with imatinib. Malignant CFCs or LTC-ICs were not completely eliminated by either drug. Analyzing the mechanisms underlying growth suppression mechanisms, we confirmed that primitive and committed CML progenitors had increased proliferation compared with the samples derived from healthy donors. CML primitive and committed progenitor cell division was markedly inhibited by SKI-606. We observed only a moderate increase in apoptosis in both CML primitive and committed progenitor cells after SKI-606 exposure. Therefore, the effects of SKI-606 on progenitor proliferation and apoptosis were observed to be similar to those of imatinib under similar conditions. We have shown that nondividing CML progenitors are resistant to imatinib-induced apoptosis. Indeed, the antiproliferative effects of imatinib may further enhance numbers of nondividing progenitors. As was seen with imatinib, SKI-606 treatment did not significantly inhibit nondividing CML primitive progenitors, which is consistent with the similar antiproliferative and antiapoptotic effects of the 2 drugs.
SKI-606 is much more effective in inhibiting Bcr-Abl kinase activity than equimolar concentrations of imatinib. SKI-606 significantly inhibited Bcr-Abl TK activity in CML progenitor cells, as measured by inhibition of CrkL phosphorylation, at drug concentrations as low as 0.05 μM, with almost complete inhibition at a concentration of 0.5 μM. However, maximal inhibition of CrkL phosphorylation by SKI-606 and imatinib was similar. Experiments with kinase-defective Src mutants and Src kinase inhibitors indicate a role for Src family kinases in oncogenic signaling downstream of Bcr-Abl. We observed decreased levels of Src kinase phosphorylation in CML progenitors after imatinib treatment, which is likely explained by a decrease in Bcr-Abl mediated Src activation. Partial inhibition of Src kinase activity has also been observed in the setting of imatinib treatment of Bcr-Abl expressing murine leukemia cells. The mechanism of residual Src activation in imatinib-treated CML progenitors is unclear but suggests that signals other than those mediated by Bcr-Abl kinase activity may contribute to Src activation in these cells. These may include signals generated by GF receptors or kinase-independent Bcr-Abl mediated signals. Src kinase phosphorylation levels were decreased in a dose-dependent manner in CD34+ cells treated with SKI-606, and higher concentrations of SKI-606 resulted in greater inhibition of Src kinase phosphorylation than that seen with imatinib.
The finding that SKI-606 is a more potent inhibitor of the Bcr-Abl and Src kinase activity in CML progenitor cells contrasts with the equipotent growth inhibitory effects of SKI-606 and imatinib on these cells. Indeed, a combination of SKI-606 and imatinib did not show improved efficacy in targeting CML progenitors compared with either agent alone, consistent with our observations that both agents by themselves can effectively inhibit Bcr-Abl kinase activity in progenitor cells from newly diagnosed CML patients at clinically achieved drug concentrations. These results indicate that inhibition of Bcr-Abl kinase activity, and indeed Src kinase activity, is not sufficient for elimination of CML progenitors and suggests the need for additional interventions to enhance elimination of these cells. Our previous studies indicate that signaling through GFs may allow persistent signaling through the Ras/MAPK, PI-3K/Akt, and STAT5 signaling pathways in imatinib-treated cells, thereby circumventing the effects of Bcr-Abl TK inhibition.24,25 Consistent with these studies, we found increased p42/44 MAPK activity and persistent STAT5 and Akt activity in CML CD34+ cells treated with SKI-606. Our results suggest that increased MAPK activity after imatinib and SKI-606 treatment is related to modulation of this pathway at the level of p42/44 MAPK itself. This may be mediated by alteration in genes that directly regulate p42/44 MAPK, such as MAPK phosphatases.26 Enhanced MAPK activity after SKI-606 treatment may occur as a compensatory response to Bcr-Abl and Src kinase inhibition, and the delay in the MAPK response suggests a possible role for alteration in gene expression in this effect. It is also possible that inhibition of Bcr-Abl and Src kinase activity in CML progenitors by SKI-606 does not completely inhibit protein function, and that kinase-independent effects of these oncogenes may contribute to continued progenitor survival. In either case, continued activity of these important signaling mechanisms that contribute to cell viability could explain why primitive hematopoietic cells persist despite effective Bcr-Abl and Src kinase inhibition by SKI-606.
Copland et al have shown that another dual Src/Abl kinase inhibitor dasatinib, which is also considerably more potent than imatinib in inhibiting Bcr-Abl kinase activity in CML primitive progenitors, is not more effective in eliminating primitive quiescent CML hematopoietic cells than imatinib.27 These results are consistent with our own observations that SKI-606, too, does not show enhanced activity against CML progenitors compared with imatinib. Although SKI-606 may not have an advantage over dasatinib in elimination of primary CML CP progenitors, there is still a strong rationale for exploring the development of alternative dual Src/Abl kinase inhibitor with favorable toxicity profiles compared with dasatinib and with potential activity against dasatinib-resistant cells. SKI-606 concentrations that resulted in significant inhibition of CML primitive progenitors resulted in only minimal suppression of progenitor cells derived from healthy donors, indicating a good therapeutic index. Dasatinib treatment has been associated with frequent and severe peripheral blood cytopenias. Preliminary clinical assessment suggests that SKI-606 is well tolerated and does not demonstrate similar hematologic toxicity.22 Unlike imatinib and dasatinib, SKI-606 has a more restricted kinase inhibitory profile and does not inhibit platelet-derived growth factor or c-Kit.22 The lack of inhibition of these kinases may contribute to reduced toxicity of SKI-606 toward normal progenitors. Although a combination of SKI-606 and imatinib was not more effective in targeting CML progenitors compared with either agent alone, it remains possible that the combination of imatinib and SKI-606 may be more effective in inhibiting the growth of cells from patients with imatinib-resistant or advanced phase disease compared with either agent alone.
In conclusion, our results indicate that SKI-606 potently inhibits Bcr-Abl and Src kinase activity in CML progenitor cells and results in significant inhibition of CML CFC and LTC-IC growth. The more limited spectrum of kinase activity of SKI-606 appears to be associated with reduced toxicity toward normal progenitors. SKI-606, like imatinib, exerted only moderate effects in inducing apoptosis of CML progenitors, and inhibition of cell division was the predominant mode of action. In addition, SKI-606 did not demonstrate improved targeting of primitive, nondividing progenitors compared with imatinib. These results suggest that CP CML progenitors may demonstrate limited oncogene “addiction” to Bcr-Abl kinase activity and that additional strategies to activate apoptosis in these cells need to be identified. In addition, the results of the current study do not support a role for targeting Src activity in enhancing elimination of primary CML progenitor cells. Other mechanisms and agents that may be capable of inducing apoptosis in primitive quiescent leukemia stem cells may be of greater promise in this regard.28,29 Our work strongly supports the further investigation of specific pathways required for survival of CML stem cells and evaluation of agents targeting these critical survival mechanisms for curative therapy of CML.
Acknowledgments
The authors thank Frank Boschelli, Wyeth Research, Pearl River, NY, for providing us with SKI-606 and for his helpful comments regarding the manuscript, Simran Sindhu for her excellent technical assistance, Lucy Brown and Alex Spalla from the Analytical Cytometry Core for excellent technical support in performing flow cytometry sorting, and Dr Marilyn Slovak and Victoria Bedell in the Cytogenetics Core laboratory for performing the FISH analysis. The authors also thank StemCyte for their generous gift of cord blood samples for these studies.
This work was supported by the National Institutes of Health (grant R01 CA95684, R.B.). R.B. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.K. designed and performed research, collected and analyzed data, and wrote the paper; T.L.H. contributed materials and interpreted data; R.B. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ravi Bhatia, Department of Hematopoietic Stem Cell and Leukemia Research, City of Hope National Medical Center, Duarte, CA 91010; e-mail: rbhatia@coh.org.
References
- 1.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 3.Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104:3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Rahmani M, Corey SJ, Dent P, Grant S. A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. J Biol Chem. 2004;279:34227–34239. doi: 10.1074/jbc.M402290200. [DOI] [PubMed] [Google Scholar]
- 5.Donato NJ, Wu JY, Stapley J, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64:672–677. doi: 10.1158/0008-5472.can-03-1484. [DOI] [PubMed] [Google Scholar]
- 6.Wodarz D. Targeted cancer treatment: resisting arrest. Nat Med. 2006;12:1125–1126. doi: 10.1038/nm1006-1125. [DOI] [PubMed] [Google Scholar]
- 7.Michor F, Hughes TP, Iwasa Y, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, O'Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 10.Higashi T, Tsukada J, Kato C, et al. Imatinib mesylate-sensitive blast crisis immediately after discontinuation of imatinib mesylate therapy in chronic myelogenous leukemia: report of two cases. Am J Hematol. 2004;76:275–278. doi: 10.1002/ajh.20096. [DOI] [PubMed] [Google Scholar]
- 11.Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–3800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- 12.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 13.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 14.Hochhaus A, La Rosee P. Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance. Leukemia. 2004;18:1321–1331. doi: 10.1038/sj.leu.2403426. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi P, Reddy MV, Reddy EP. Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene. 1998;16:1749–1758. doi: 10.1038/sj.onc.1201972. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 18.Manley PW, Cowan-Jacob SW, Mestan J. Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia. Biochim Biophys Acta. 2005;1754:3–13. doi: 10.1016/j.bbapap.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Boschelli DH, Wu B, Barrios Sosa AC, et al. Identification of 7-phenylaminothieno- [3,2-b]pyridine-6-carbonitriles as a new class of Src kinase inhibitors. J Med Chem. 2004;47:6666–6668. doi: 10.1021/jm049237m. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J, Kantarjian H. New targeted approaches in chronic myeloid leukemia. J Clin Oncol. 2005;23:6316–6324. doi: 10.1200/JCO.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Martinelli G, Soverini S, Rosti G, Baccarani M. Dual tyrosine kinase inhibitors in chronic myeloid leukemia. Leukemia. 2005;19:1872–1879. doi: 10.1038/sj.leu.2403950. [DOI] [PubMed] [Google Scholar]
- 22.Cortes J, Baccarani M, Brummendorf TH, et al. A Phase 1/2 study of SKI-606, a dual inhibitor of Src and Abl kinases, in adult patients with Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) or acute lymphocytic leukemia (ALL) relapsed, refractory or intolerant of imatinib [Abstract]. Blood. 2006;108:168a. [Google Scholar]
- 23.Bhatia R, Munthe HA, Williams AD, Zhang F, Forman SJ, Slovak ML. Chronic myelogenous leukemia primitive hematopoietic progenitors demonstrate increased sensitivity to growth factor-induced proliferation and maturation. Exp Hematol. 2000;28:1401–1412. doi: 10.1016/s0301-472x(00)00545-2. [DOI] [PubMed] [Google Scholar]
- 24.Chu S, Holtz M, Gupta M, Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood. 2004;103:3167–3174. doi: 10.1182/blood-2003-04-1271. [DOI] [PubMed] [Google Scholar]
- 25.Konig H, Modi H, Manley P, Holyoake TH, Forman SJ, Bhatia R. Niltonib inhibits Bcr-Abl kinase activity in CML progenitor cells more effectively than imatinib but is equipotent in inducing growth inhibition [Abstract]. Blood. 2006;108:223a. [Google Scholar]
- 26.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 27.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 28.Guzman ML, Rossi RM, Karnischky L, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copland M, Hamilton A, Allan EK, Melo JV, Holyoake TL. BMS-214662 eliminates CML stem cells and is active against blast crisis CML and cells expressing Bcr-Abl kinase mutations. Blood. 2006;108:739. [Google Scholar]