Abstract
The Drosophila Slowpoke calcium-dependent potassium channel (dSlo) binding protein Slob was discovered by a yeast two-hybrid screen using the carboxy-terminal tail region of dSlo as bait. Slob binds to and modulates the dSlo channel. We have found that there are several Slob proteins, resulting from multiple translational start sites and alternative splicing, and have named them based on their molecular weights (in kD). The larger variants, which are initiated at the first translational start site and are called Slob71 and Slob65, shift the voltage dependence of dSlo activation, measured by the whole cell conductance–voltage relationship, to the left (less depolarized voltages). Slob53 and Slob47, initiated at the third translational start site, also shift the dSlo voltage dependence to the left. In contrast, Slob57 and Slob51, initiated at the second translational start site, shift the conductance–voltage relationship of dSlo substantially to more depolarized voltages, cause an apparent dSlo channel inactivation, and increase the deactivation rate of the channel. These results indicate that the amino-terminal region of Slob plays a critical role in its modulation of dSlo.
Keywords: dSlo, Slob, BK channel, potassium channel, channel modulation
INTRODUCTION
The Drosophila Slowpoke channel (dSlo) is a large conductance voltage-gated, calcium-dependent potassium channel (Atkinson et al., 1991; Adelman et al., 1992). dSlo has many tissue-specific splice variants with different properties (Becker et al., 1995; Brenner et al., 1996; Atkinson et al., 1998), and plays important roles in neuronal excitability, neurotransmitter release, and muscle contraction (Warbington et al., 1996; Atkinson et al., 2000). Unlike most other potassium channels, dSlo channels have a large carboxy-terminal tail region. This domain participates in calcium binding (Bian et al., 2001) and is the target of phosphorylation and binding of other regulatory proteins that modulate channel activity (Xia et al., 1998; Schopperle et al., 1998; Wang et al., 1999).
The dSlo binding protein Slob from Drosophila melanogaster was discovered by a yeast two-hybrid screen using the carboxy-terminal tail region of dSlo as bait (Schopperle et al., 1998). It binds to and modulates the dSlo channel (Schopperle et al., 1998; Zhou et al., 1999, 2003). Slob was originally described as a 511–amino acid protein that contains a protein kinase–like domain, and in vitro assays indicated that Slob exhibits weak but regulatable protein kinase activity (Zeng et al., 2004). Slob mRNA (McDonald and Rosbash, 2001; Claridge-Chang et al., 2001; Ceriani et al., 2002; Lin et al., 2002; Ueda et al., 2002) and protein (Jaramillo et al., 2004) cycle in a circadian manner in vivo, and may participate directly or indirectly in circadian pathways (Jaramillo et al., 2004).
Previous studies of the modulation of dSlo by Slob used an epitope-tagged Slob construct, HA-Slob57, in which an HA tag was fused to the amino-terminal region of Slob. We reported that coexpression of dSlo with this construct shifts the voltage dependence of dSlo activation to less depolarized voltages (Zhou et al., 1999). Recently, we have found that there are several Slob proteins, resulting from alternative splicing and multiple translational start sites (unpublished data), and have named them based on their molecular weights (in kD). We have now studied the modulation of dSlo by each Slob protein. The results indicate that Slob57 (the originally characterized variant) and Slob51 shift the voltage dependence of dSlo activation to more depolarized voltages, cause an apparent inactivation of dSlo, and make the channel close faster. This is in contrast to the hyperpolarizing shift we originally reported with an amino-terminal HA-tagged version of Slob57 (Zhou et al., 1999). All other Slobs shift the conductance–voltage relationship of dSlo to less depolarized voltages and have no significant effect on dSlo kinetics. The difference between Slob57/51 and other Slobs (including HA-Slob57) is in their amino termini. Thus the amino-terminal region of Slob57/51 appears to play a critical role in modulating the voltage dependence and deactivation kinetics of dSlo.
MATERIALS AND METHODS
Constructs
All Slob cDNAs were subcloned into the pIRES2-EGFP vector (CLONTECH Laboratories, Inc.) for mammalian expression and electrophysiological studies. Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit, following the manufacturer's instructions (Stratagene). All Slob constructs and site-directed mutations were confirmed by DNA sequencing. The Slowpoke channel used in this study is the alternative splice variant A1C2E1G3 (Genbank/EMBL/DDBJ accession no. M96840), which was subcloned into the pRc/CMV vector (Invitrogen).
Cell Culture and Western Blot
Chinese hamster ovary (CHO) cells were maintained in 75-cm2 culture dishes with Ham's F-12 nutrient mixture (Invitrogen), supplemented with 10% FBS (Invitrogen) and 100 U/ml penicillin and streptomycin (Invitrogen). Plasmids were transfected into CHO cells using Lipofectamine 2000 reagent, according to the manufacturer's specifications (Invitrogen).
Cells were harvested the second day after transfection. In brief, after harvest and wash with PBS, cells were resuspended in 500 μl lysis buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 50 mM NaF, 50 mM KCl, 1% CHAPS, 1 mM phenylmethylsulfonyl fluoride, 2 mM DTT, 1 mg/ml each aprotinin, leupeptin, and pepstatin A) with rotation at 4°C for 30 min. Lysates were centrifuged at 16,000 g for 10 min, and the supernatants were used for Western blot analysis of expression.
Cell lysates were loaded onto 4–15% gradient SDS polyacrylamide gels (Bio-Rad Laboratories, Inc.). Proteins were separated by gel electrophoresis and transferred to nitrocellulose membranes for Western blotting. Membranes were blocked in Tris-HCl buffered saline with 0.1% Tween 20 (TBST) containing 5% nonfat milk. Membranes were incubated with primary antibody (Schopperle et al., 1998) for 1 h at room temperature. Following washes with TBST, membranes were incubated with an appropriate secondary antibody (horseradish peroxidase–conjugated donkey anti-rabbit antibody) at room temperature for 1 h. Labeled proteins were detected using an enhanced chemiluminescence reagent according to the manufacturer's specifications (Amersham Biosciences).
Electrophysiology
CHO cells were transiently transfected with dSlo and different Slob cDNAs in a 1:1 molar ratio using Lipofectamine as described above. 1 d after transfection, whole cell currents were recorded from cells exhibiting green fluorescence, using an Axiovert 25 inverted fluorescence microscope (Carl Zeiss MicroImaging, Inc.) and an Axopatch 200A amplifier (Axon). Glass pipettes with 2–4 MΩ resistance were pulled from thinwall glass (WPI) using a two-stage vertical puller (Narishige). Both the bath and pipette solutions contained (in mM) 100 KCl, 0.5 HEDTA, 2 MgCl2, and 10 HEPES, pH 7.2 (symmetrical conditions). The pipette solution also contained different amounts of free Ca2+ (4 μM, 20 μM, 40 μM, or 110 μM). Total CaCl2 required to generate the desired free Ca2+ was calculated (WEBMAXC; http://www.stanford.edu/~cpatton/webmaxcS.htm), and the appropriate amount of CaCl2 from a 100 mM CaCl2 solution (Orion) was added to the pipette solution. The final concentration of free Ca2+ was measured with a Fisher accumet calcium-sensitive electrode, and determined against a standard curve of free Ca2+ generated with the calcium-sensitive electrode and calcium standards (WPI). Currents were elicited by depolarizing test pulses to different voltages from a holding potential of −80 mV, followed by a hyperpolarizing step to −120 mV to measure tail currents. Currents were filtered at 2 kHz, digitized at 10 kHz with a Digidata 1322A digitizer (Axon), and acquired with pCLAMP9 software (Axon). Peak tail currents were normalized to the maximum peak tail current of the patch and fitted with a Boltzmann function to generate the conductance–voltage curves. Data were analyzed and illustrated using pCLAMP9, Origin, and GraphPad Prism 4.
RESULTS
Slob protein was discovered by a yeast two-hybrid screen using the carboxy-terminal tail region of dSlo as bait (Schopperle et al., 1998). The Slob variant that we initially described contains 511 amino acid residues. It binds to dSlo and modulates the channel activity (Schopperle et al., 1998; Zhou et al., 1999).
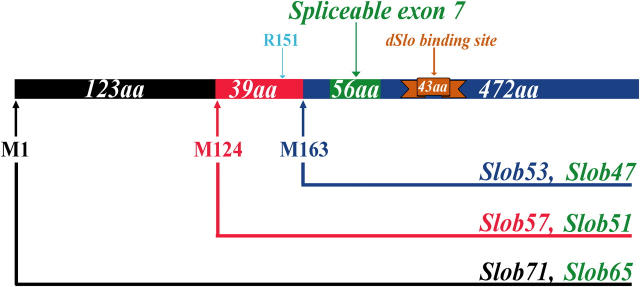
Recently we found that at least four different slob transcripts are present in the fly (unpublished data). These transcripts can initiate translations at either Met1 or Met124 and contain or exclude a spliceable exon, exon 7 of the slob gene. Therefore, at least four different Slob proteins can be produced (Fig. 1). We named them based on their molecular weights. Slob71, the largest variant, starts at Met1 and contains 634 amino acid residues, including exon 7 (Fig. 1, green), which encodes the region from Ser197 to Thr252 (all numbering throughout the text is relative to Met1, the first amino acid residue in Slob71; Fig. 1). The Slob that also starts at Met1, but excludes exon 7, is a 65-kD protein called Slob65 (Fig. 1). The smaller Slob, which initiates at Met124 and contains exon 7, is the Slob variant isolated in the yeast two-hybrid screen; it is now renamed Slob57. Slob51 also starts at Met124, but excludes exon 7 (Fig. 1). Initially, an HA-tagged version of Slob57 (HA-Slob57) was used in our electrophysiological analysis (Zhou et al., 1999). The HA epitope was appended to an arginine residue (Arg151) near the amino terminus of Slob57 (Fig. 1).
Figure 1.
Six Slob proteins from a single Slob gene. There are at least four different transcripts of the slob gene in vivo. These transcripts can initiate translations at either Met1 (M1) or Met124 (M124), and contain or exclude the spliceable exon 7 (green). Therefore, at least four different Slob proteins can be produced. We named them Slob71, Slob65, Slob57, and Slob51 based on their molecular weights. A third Met (M163) can be recognized by the ribosome to translate two more Slobs: Slob53 and Slob47. The epitope-tagged Slob used in our previous study was Slob57, with an amino-terminal HA epitope appended to Arg151 (R151). All Slobs contain the dSlo binding region (brown). All amino acid numbering is relative to the first Met (M1) in Slob71.
To facilitate in vitro electrophysiological analysis of Slobs, we subcloned the cDNAs of different Slobs into the pIRES2-EGFP vector, which expresses GFP, in order to express Slobs in CHO cells. To prevent multiple initiations of translation, the atg (encoding Met124) in the Slob71 and Slob65 constructs was mutated to ctg (encoding Leu). We first tested the heterologous expression of these Slob constructs in CHO cells using Western blot with anti-Slob antibody that recognizes all Slobs. To our surprise, we found multiple bands in these Western blots (Fig. 2 A). For each Slob, in addition to the band with the expected molecular weight (the top bands in lanes 1–4 of Fig. 2 A), there is another major band with a lower molecular weight.
Figure 2.
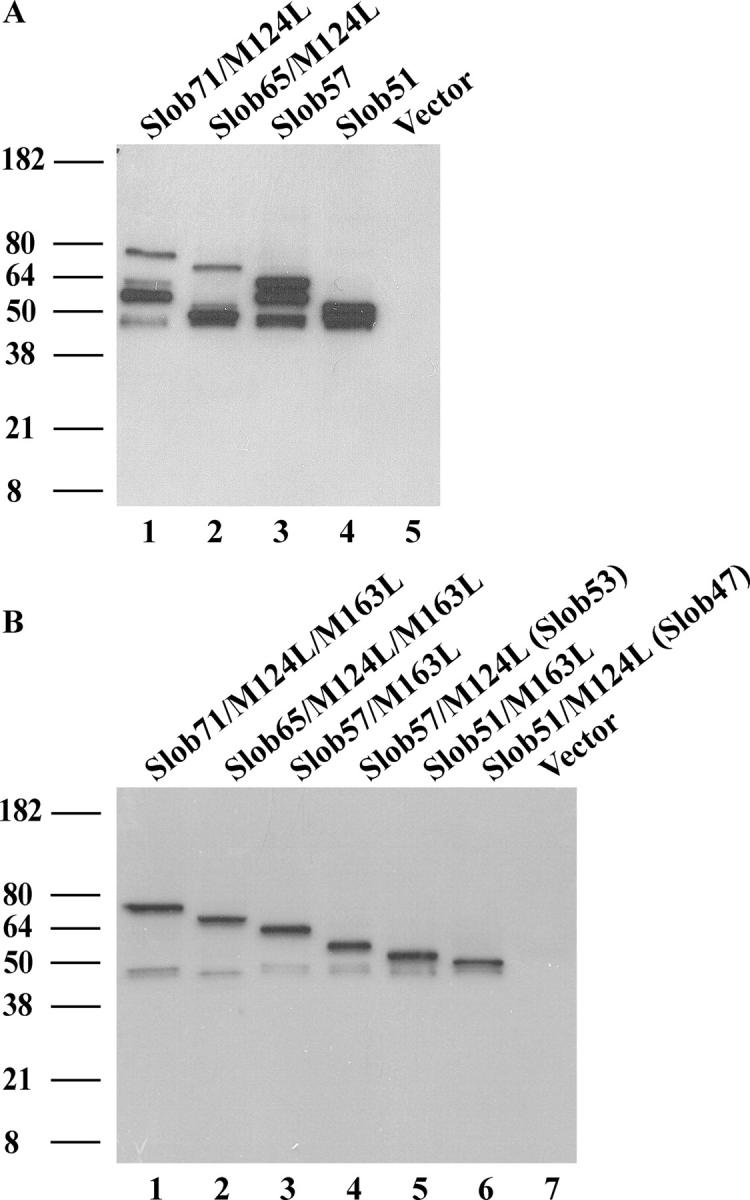
Heterologous expression of Slobs. (A) Different Slobs were transiently transfected into CHO cells. On the second day, proteins in cell lysates were separated by gel electrophoresis, transferred onto nitrocellulose membranes, and blotted with anti-Slob antibody. Lane 1, Slob71 construct with M124L mutation; lane 2, Slob65 construct with M124L mutation; lane 3, Slob57 construct; lane 4, Slob51 construct; lane 5, pIRES2-EGFP vector only. (B) Individual Slob variants can be expressed by mutating one or more translational start sites (see Fig. 1). Lane 1, Slob71 construct with M124L and M163L mutations; lane 2, Slob65 construct with M124L and M163L mutations; lane 3, Slob57 construct with M163L mutation; lane 4, Slob57 construct with M124L mutation, which expresses only Slob53; lane 5, Slob51 construct with M163L mutation; lane 6, Slob51 construct with M124L mutation, which expresses only Slob47; lane 7, pIRES2-EGFP vector only.
One possible explanation for these multiple bands is Slob degradation. However, there are no smaller fragments in the Western blot (Fig. 2 A), as might be expected with proteolytic degradation. A closer examination of the cDNA sequence of Slob reveals that around Met163, the cDNA sequence is ATTatgAAG. There is an adenine (bold) at the −3 position of this atg (encoding Met163), which is critical for the ribosome to recognize that particular atg and initiate translation (Kozak, 1991, 2002). Therefore, we suspected that the lower bands are two smaller Slobs initiated at Met163 (Fig. 1). To test this hypothesis, we mutated the atg (encoding Met163) to ctg (encoding Leu) in our original constructs of Slob71, Slob65, Slob57, and Slob51. When these constructs are expressed, only the top bands with the expected molecular weights remain (compare lanes 1–3 and 5 in Fig. 2 B with lanes 1–4 in Fig. 2 A). Furthermore, when we mutated the atg (encoding Met124) in the original constructs of Slob57 and Slob51 to ctg (encoding Leu), but kept the atg of Met163 intact, we saw two new bands on the Western blot (Fig. 2 B, lanes 4 and 6), corresponding to the lower bands in lanes 1–4 of Fig. 2 A. Therefore two additional Slobs, Slob53 and Slob47, can be expressed in this heterologous expression system. These two Slobs lack the first 39 amino acid residues at the amino-terminal region of Slob57 and Slob51 (Fig. 1). There is also an unidentified band observed in all Slob-expressing cells that migrates at an apparent molecular weight slightly smaller than Slob47 (Fig. 2, A and B).
Previous studies indicated that Slob57 can coimmunoprecipitate with dSlo in a heterologous expression system and in vivo (Schopperle et al., 1998; Zhou et al., 1999). The dSlo binding region in Slob is from Asn314 to Glu356 (Zhou et al., 2003). Since all six Slobs contain the dSlo binding region (Fig. 1), we predicted that all of them can bind dSlo. As expected, anti-Slob antibody was able to coimmunoprecipitate dSlo when it was coexpressed with any of the Slobs (unpublished data).
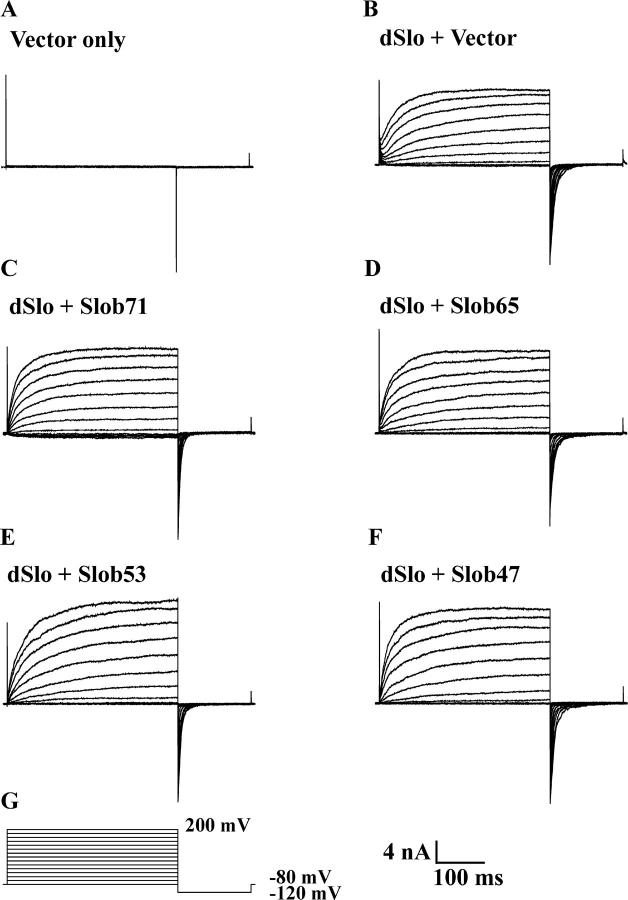
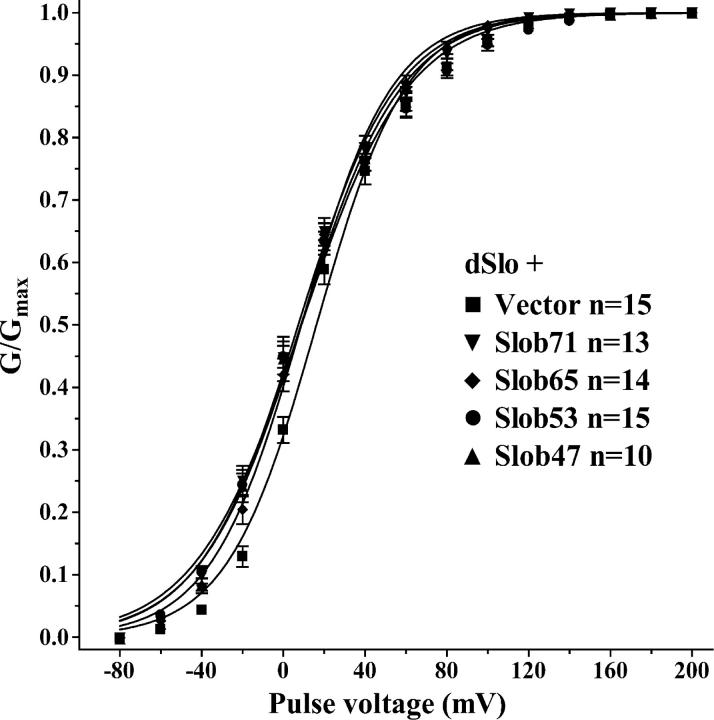
We examined the effects of all the Slobs on dSlo channel function by cotransfecting each with dSlo in CHO cells, which exhibit very low levels of endogenous potassium current (Fig. 3 A). In preliminary experiments, we found that a high concentration of free Ca2+ is required to achieve full dSlo activation and tail current saturation in the presence of Slob57. Therefore we chose to study the modulatory effects of the different Slobs in the presence of 110 μM free Ca2+. Whole cell outward currents were elicited by a 350-ms test pulse to different voltages from a holding potential of −80 mV, followed by hyperpolarization to −120 mV to measure tail currents (Fig. 3 G). A comparison of the current traces in the absence (Fig. 3 B) or presence (Fig. 3, C–F) of various Slobs (Slob71, Slob65, Slob53, and Slob47) indicates that there is no change in activation or deactivation kinetics. However there is a small but significant hyperpolarizing shift in the dSlo conductance–voltage relationship in the presence of each of these Slob variants (Fig. 4 and Table I). Such shifts are reminiscent of (although smaller than) those reported previously with HA-tagged Slob57 (Zhou et al., 1999; unpublished data).
Figure 3.
Modulation of dSlo by Slob71, Slob65, Slob53, and Slob47. Whole cell currents evoked by depolarizing voltage steps (G) with 110 μM free Ca2+, in cells transfected with vector alone (A), dSlo alone (B), or together with different Slobs as indicated (C–F). Currents were elicited by a 350-ms test pulse to different voltages from a holding potential of −80 mV, followed by hyperpolarization to −120 mV to measure tail currents.
Figure 4.
Conductance–voltage relationship for dSlo. The conductance–voltage relationships for dSlo transfected alone or together with Slob71, Slob65, Slob53, or Slob47 were measured from tail currents with 110 μM free Ca2+, as shown in Fig. 3. The conductance–voltage relationship was generated from peak tail currents as described in materials and methods.
TABLE I.
Modulation of the dSlo Conductance–Voltage Relationship by Slob71, 65, 53, and 47
| Transfection condition | V1/2 ± SEM (mV) | Slope ± SEM |
|---|---|---|
| dSlo + Vector (n = 15) | 16.2 ± 0.7 | 21.9 ± 0.6 |
| dSlo + Slob71 (n = 13) | 7.3 ± 0.7a | 24.1 ± 0.6 |
| dSlo + Slob 65 (n = 14) | 8.8 ± 0.6a | 22.2 ± 0.6 |
| dSlo + Slob53 (n = 15) | 8.8 ± 0.7a | 26.1 ± 0.6 |
| dSlo + Slob47 (n = 10) | 8.6 ± 0.9a | 24.7 ± 0.8 |
The V1/2 for dSlo activation and the slope of the conductance–voltage relationships were obtained from the curves shown in Fig. 4.
Significantly different from dSlo + Vector (P < 0.001; Student's t test).
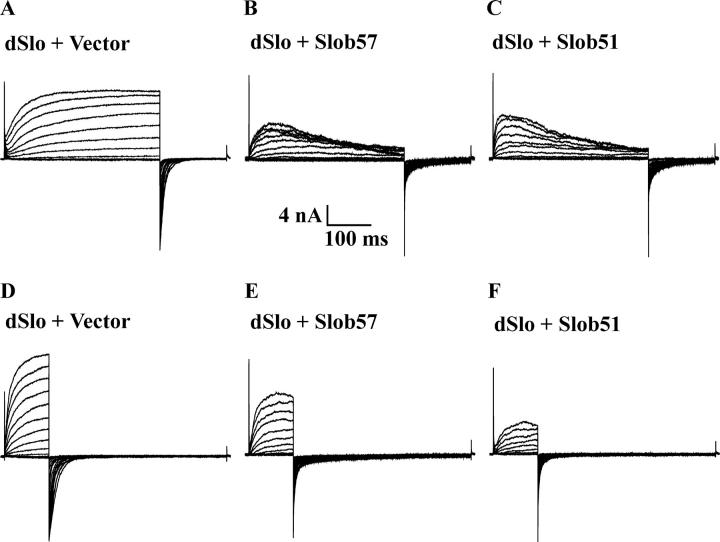
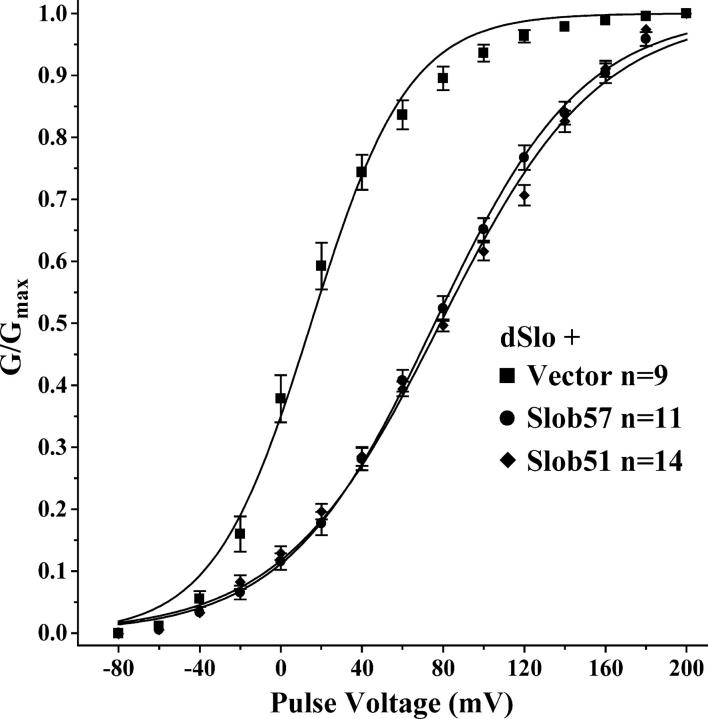
The pattern of modulation is very different when Slob57 or Slob51 is cotransfected with dSlo, as shown in Fig. 5 (B and C). Coexpression with these Slobs causes an apparent inactivation of the dSlo current during the 350-ms depolarizing pulse. Because this apparent inactivation compromises our ability to measure the conductance–voltage relationship from tail currents, we repeated this experiment using a shorter depolarizing pulse, during which the channel does not have time to inactivate. As shown in Fig. 5 (E and F), Slob57 and Slob51 cause a decrease in current compared with dSlo cotransfected with control vector (Fig. 5 D). The channel also appears to close faster in the presence of these two Slobs, as indicated by the more rapid deactivation of the tail currents. As shown in Fig. 6 and Table II, Slob57 and Slob51 shift the conductance–voltage relationship of the channel substantially in the depolarizing direction. In addition, there is a significant decrease in the slope of the conductance–voltage curve. The V1/2 and the slope values for the conductance–voltage relationships are summarized for Slobs71, 65, 53, and 47 in Table I, and for Slobs57 and 51 in Table II.
Figure 5.
Modulation of dSlo by Slob57 and Slob51. Whole cell currents, with 110 μM free Ca2+, were evoked by either 350 ms (A–C) or 100 ms (D–F) depolarizing pulses, followed by hyperpolarization to −120 mV to measure tail currents. dSlo was expressed either alone (A and D) or together with either Slob57 (B and E) or Slob51 (C and F).
Figure 6.
Short pulse conductance–voltage relationship for dSlo. Same as Fig. 4, except the effects of Slob57 and Slob51 were measured using the shorter pulse protocol in Fig. 5 (D–F).
TABLE II.
Modulation of the dSlo Conductance–Voltage Relationship by Slob57 and 51
| Transfection condition | V1/2 ± SEM (mV) | Slope ± SEM |
|---|---|---|
| dSlo + Vector (n = 9) | 14.9 ± 1.1 | 24.1 ± 1.0 |
| dSlo + Slob57 (n = 11) | 75.8 ± 1.0a | 36.6 ± 0.9a |
| dSlo + Slob51 (n = 14) | 78.6 ± 0.9a | 38.9 ± 0.8a |
The V1/2 for dSlo activation and the slope of the conductance–voltage relationships were obtained from the curves shown in Fig. 6 (short pulse protocol).
Significantly different from dSlo + Vector (P < 0.001; Student's t test).
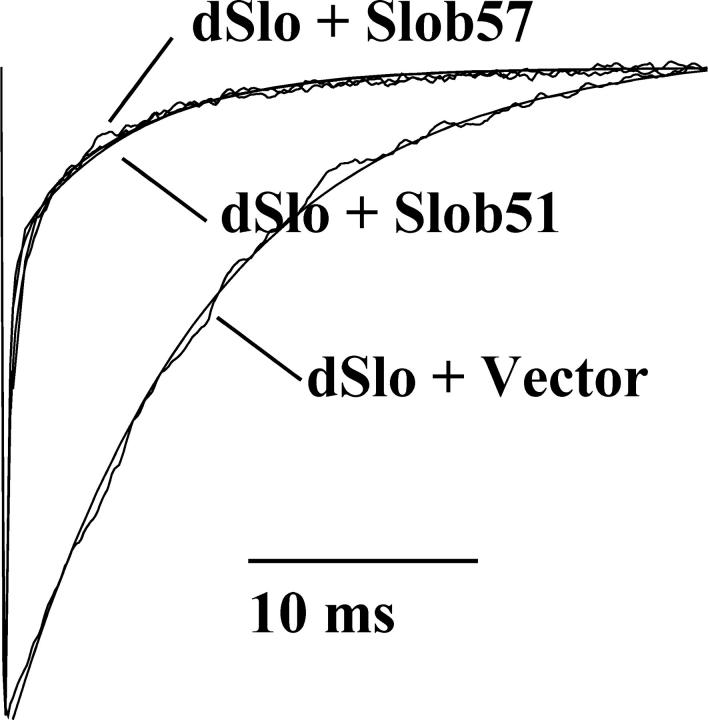
We fitted the current traces with standard exponential functions to obtain the activation and deactivation time constants of dSlo when it is expressed with vector alone or with one of the Slob variants. We found that the time course of dSlo deactivation was very different in the presence of Slob57 or Slob51 (Fig. 7). There were no differences in deactivation time constants in the presence of the other Slobs, and there were no differences in activation time constants of dSlo in the presence of any Slob (unpublished data). When dSlo is expressed alone, the time constant of dSlo deactivation can be well fitted using a single exponential function with a mean τ value of 8.4 ms. In contrast, when dSlo is coexpressed with Slob57, two exponential functions are required to fit the deactivation time course of dSlo. These time constants are voltage independent. The mean fast time constant τf is 3.3 ms with a weight of 72%, while the mean slow time constant τs is 26 ms and contributes 28%. Both the τf and the τs are significantly different (P < 0.001) from the single τ of dSlo when it is expressed alone. A similar result is seen when dSlo is coexpressed with Slob51.
Figure 7.
Slob57 and Slob51 change the deactivation rate of dSlo. Deactivating tail currents following a 100-ms test pulse to +40 mV, with 110 μM free Ca2+, in the absence or presence of Slob57 or Slob51, were normalized and superimposed. The deactivation time constants for dSlo expressed alone, or together with Slob57 or Slob51, were obtained by fitting the tail currents with exponential functions. Both the current traces themselves and the exponential fits to the data (smooth lines) are shown in the figure. When dSlo is expressed without Slobs, in the voltage range from −20 to +40 mV, the tail currents can be fitted with a single exponential function with a mean, voltage-independent τ of 8.4 ± 0.5 ms. In the presence of Slob57 or Slob51, the tail currents can only be fitted, in the voltage range from +20 to +180 mV, with two voltage-independent exponential functions. Both the τf and the τs of dSlo in the presence of Slob57 or Slob51 are significantly different (P < 0.001) from the τ of dSlo expressed alone.
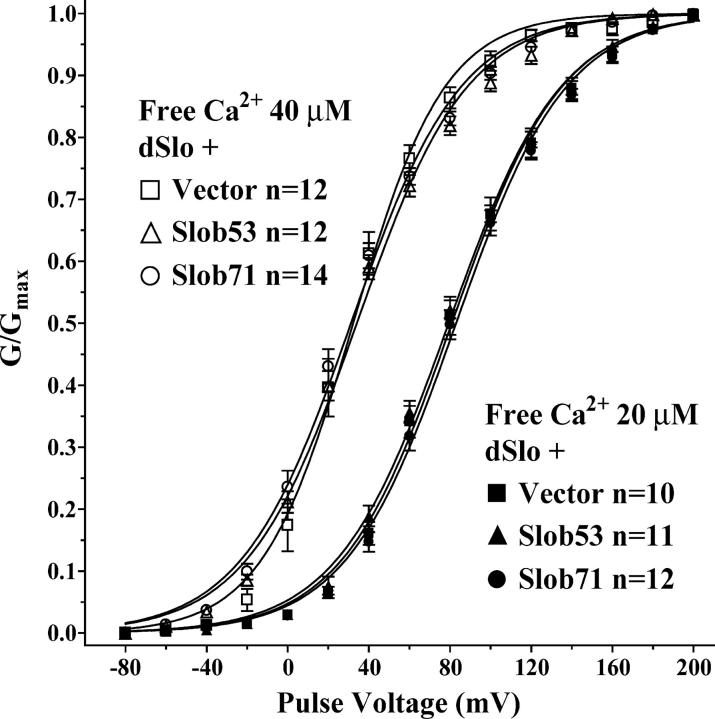
To determine whether the modulation by various Slobs is influenced by the intracellular free Ca2+ concentration, we examined the effect of Slob53 and Slob71 in the presence of 40 or 20 μM free Ca2+ (the latter is the lowest Ca2+ concentration at which we could achieve tail current saturation in the voltage range of our amplifier). As shown in Fig. 8, under these conditions, neither Slob53 nor Slob71 produces the small leftward shift in the conductance–voltage relationship that is seen at 110 μM free Ca2+ (Fig. 4 and Table I), suggesting that this modulatory response is calcium dependent. Although the lack of tail current saturation prevented us from measuring the effects of Slob57 and Slob51 on the conductance–voltage relationship at these lower free Ca2+ concentrations, we did examine whether the apparent inactivation of dSlo caused by Slob57 is Ca2+ dependent. As shown in Fig. 9, Slob57 causes a similar inactivation of the dSlo current during a 350-ms depolarizing pulse, over a wide range of free Ca2+ concentrations.
Figure 8.
Conductance–voltage relationship for dSlo with lower concentrations of free Ca2+. Same as Fig. 4, except the effects of Slob53 and Slob71 were measured with 20 μM (filled symbols) or 40 μM (open symbols) free Ca2+.
Figure 9.
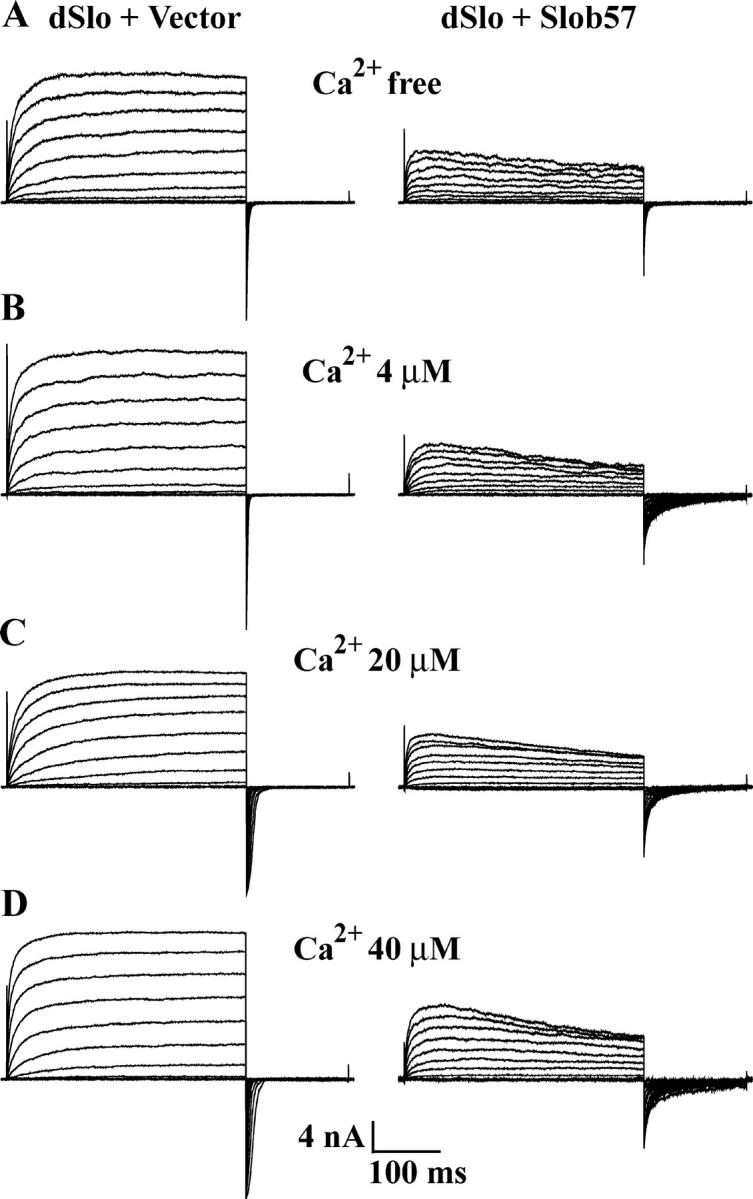
The apparent inactivation of dSlo caused by Slob57 is Ca2+ independent. Whole cell currents, with (A) Ca2+-free solution (no added Ca2+), (B) 4 μM free Ca2+, (C) 20 μM free Ca2+, (D) 40 μM free Ca2+, were evoked by the same pulse protocol as shown in Fig. 3 G. dSlo was expressed either alone or together with Slob57 as indicated.
DISCUSSION
We have found a total of six Slob proteins, resulting from alternative splicing and multiple translational start sites from a single slob gene. At least four of these Slobs are expressed in adult fly heads in vivo (unpublished data), and it is conceivable that the other two may be expressed in other tissues and/or at other developmental stages. All of these Slobs bind dSlo and modulate the voltage dependence of the Slowpoke channel. Slob57 and Slob51 cause an apparent inactivation of dSlo, significantly shift the voltage dependence of dSlo activation to more depolarized voltages, greatly decrease the slope of the conductance–voltage relationship, and increase the deactivation rate of the channel. In contrast, all other Slobs, including the amino-terminal HA-tagged HA-Slob57, do not cause this apparent inactivation, do not change the deactivation rate of dSlo, and shift the conductance–voltage relationship of dSlo slightly, but significantly, to less depolarized voltages.
Although we have found independent mRNA transcripts in fly heads coding for Slob71, Slob65, Slob57, and Slob51 using RT-PCR (unpublished data), we do not yet have direct evidence for the existence of Slob53 and Slob47 in vivo. However, ribosomes can recognize the atg of Met163 and initiate translation there, and the efficiency for the ribosome to recognize this start site appears to be high, as indicated by the following: (a) an adenine at the −3 position of this atg site makes this region a Kozak sequence-like region, which is efficiently recognized by the ribosome to start translation (Kozak, 1991, 2002); (b) in the Slob57 construct, when the atg for Met163 is intact, the yield of Slob57 and Slob53 is almost equal (Fig. 2 A); (c) in the same Slob57 construct, when the start site (Met124) for Slob57 is mutated, Slob53 can still be well expressed (Fig. 2 B). Therefore, it seems likely that the atg of Met163 is an independent start site of translation of slob mRNA, and Slob53 and Slob47 are likely to exist in vivo. The fact that the ribosome can translate both Slob57 and Slob53 from a single slob mRNA is not an isolated example, as many studies have shown that ribosomes can initiate translation at different start sites in a single message (Stacey et al., 2000; Xiong et al., 2001; Takeda et al., 2004; Wang and Rothnagel, 2004; Yang et al., 2004). Since both Slob53 and Slob47 contain the dSlo binding region (Zhou et al., 2003), it is not a surprise that they both bind dSlo (unpublished data).
When Slob71 is coexpressed with dSlo, it slightly but significantly shifts the voltage dependence of dSlo activation to less depolarized voltages at a high concentration of free Ca2+. The V1/2 is reduced by ∼9 mV, and the slope of the conductance–voltage curve is unchanged. However, at lower concentrations of free Ca2+, Slob71 has little effect on dSlo, suggesting that Slob71 activates dSlo in a Ca2+-dependent manner. We have not yet explored the mechanism of the Ca2+ dependence of this modulatory phenomenon. Slob65, Slob53, and Slob47 have a similar effect on the voltage dependence of dSlo activation. HA-Slob57, the Slob construct used in our earlier studies, has a similar but larger effect on dSlo (Zhou et al., 1999). In contrast, the untagged Slob57 and Slob51 modulate dSlo in an entirely different way. Under our experimental conditions, they cause an apparent inactivation of dSlo that is Ca2+ independent (Fig. 9), while dSlo expressed alone or coexpressed with other Slobs does not inactivate during a 350-ms depolarizing pulse. To circumvent this apparent inactivation, a shorter test pulse was used to study the effect of Slob57 and Slob51 on the voltage dependence of dSlo activation; a similar procedure has been used in other studies that involve channel inactivation (Jerng et al., 1999; Xia et al., 2000). The conductance–voltage relationship of dSlo, when expressed alone, is the same whether the short or long pulse is used (Tables I and II). Studies using the short pulse show that Slob57 and Slob51 significantly shift the conductance–voltage relationship of dSlo to more depolarized voltages, and greatly decrease the slope of the conductance–voltage curve. Besides right shifting the conductance–voltage relationship, Slob57 and Slob51 also cause dSlo to close faster, as indicated from an examination of the deactivating tail currents (Fig. 7). The fitting of the deactivation time constant requires two exponential functions, indicating that in the presence of Slob57 or Slob51, dSlo needs two steps, a dominant fast one and a minor slow one, to close. dSlo expressed alone in CHO cells can close in a single step under our experimental conditions, although dSlo expressed in Xenopus oocytes needs two steps to close (DiChiara and Reinhart, 1995). Since Slob57 and Slob51 produce very similar effects, it seems that the spliceable region (from Ser197 to Thr252) of Slob plays little role in this modulation of dSlo.
The modulatory effects of Slob57 and Slob51 on dSlo are totally different than those of other Slobs. The difference in amino acid sequence between Slob57/Slob51 and the other Slobs is at the amino-terminal region. Slob71/Slob65 have a 123-residue extension in front of the amino terminus of Slob57/Slob51, whereas Slob53/Slob47 lack the amino-terminal 39 amino acid residues of Slob57/Slob51. In the case of HA-Slob57, the 10-residue HA epitope was appended to Arg151, the 28th amino acid residue of Slob57 (Fig. 1). The correlation between the different amino termini and the different modulatory effects indicates that the amino-terminal 39-residue region of Slob57/Slob51 plays a critical role in the modulation of dSlo activity. It must form part or all of the functional domain that causes the apparent inactivation of dSlo, shifts the voltage dependence of dSlo to more depolarized voltages, and makes dSlo close faster. When this 39-residue region is removed, the strong inhibitory effect of Slob on dSlo is eliminated, and the remaining protein (i.e., Slob53 and Slob47) slightly activates dSlo. In the larger Slobs, Slob71 and Slob65, the extra 123-residue region that precedes this critical region may mask its effects, such that the larger Slobs have the same minimal effects on dSlo as Slob53 and Slob47.
dSlo belongs to the large conductance, voltage-gated, and calcium-dependent potassium (BK) channel family. Although dSlo inactivation has not been reported previously, some mammalian BK channels do exhibit inactivation (Solaro and Lingle, 1992; Li et al., 1999; Pyott et al., 2004). This trypsin-sensitive (Solaro and Lingle, 1992) and Ca2+-dependent (Hicks and Marrion, 1998; Ding and Lingle, 2002) inactivation is caused by auxiliary β2 or β3 subunits (Wallner et al., 1999; Xia et al., 1999, 2000; Uebele et al., 2000). The amino-terminal residues 2–4 of the β2 subunit may directly block the BK channel to cause inactivation (Xia et al., 2003), and the amino-terminal 33 residues of the β3 subunit also play a critical role in causing BK inactivation (Xia et al., 1999). Therefore, it seems possible that Slob57 and Slob51 may function like these β subunits to cause dSlo inactivation, and this inactivation depends on the amino-terminal 39-residue region of these Slobs. Interestingly, the Slowpoke channel from Periplaneta americana, which is ∼90% identical to dSlo, does not inactivate when it is heterologously expressed, but does so in native cells (Derst et al., 2003). Such inactivation of dSlo by Slob may have important physiological roles in native tissues.
It will be interesting to determine whether the various Slobs bind to and modulate dSlo in a dynamic way, particularly in view of the finding that Slob57 protein cycles in a circadian manner in vivo (Jaramillo et al., 2004). For example, if the amount of Slob57 cycles, and different Slobs compete for dSlo within a cell, then the activity of the dSlo channel in the cell may change as a function of time of day. Further in vivo studies will be required to investigate this intriguing possibility.
Slob57 is the major Slob expressed widely in flies (Jaramillo et al., 2004). We show here that it strongly inhibits dSlo activity. Studies of the downstream, physiological in vivo effects of this inhibition are ongoing. The amino-terminal 39-residue region of Slob57/Slob51 plays a critical role in this regulation of dSlo. Our data reveal that simply by masking or removing this amino-terminal region, Slob can modulate dSlo in very different ways. Thus alternative splicing and/or choice of different translational start sites in Slob may have profound effects on neuronal excitability in vivo.
Acknowledgments
We thank Drs. Tanya S. Ferguson and Mohammad Shahidullah and other members of the Levitan lab for helpful discussions and critical comments on the manuscript.
This work was supported by a grant from the National Institutes of Health to Irwin B. Levitan.
Olaf S. Andersen served as editor.
T.M. Weiger's permanent address is Division of Animal Physiology, University of Salzburg, Department of Cell Biology, Hellbrunnerstrasse 34, A-5020 Salzburg, Austria.
A.M. Jaramillo's present address is Princeton University, Department of Molecular Biology, Lewis Thomas Laboratory, Washington Road, Princeton, NJ 08544.
Abbreviation used in this paper: CHO, Chinese hamster ovary.
References
- Adelman, J.P., K.-Z. Shen, M.P. Kavanaugh, R.A. Warren, Y.-N. Wu, A. Lagrutta, C.T. Bond, and R.A. North. 1992. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 9:209–216. [DOI] [PubMed] [Google Scholar]
- Atkinson, N.S., R. Brenner, R.A. Bohm, J.Y. Yu, and J.L. Wilbur. 1998. Behavioral and electrophysiological analysis of Ca-activated K-channel transgenes in Drosophila. Ann. NY Acad. Sci. 860:296–305. [DOI] [PubMed] [Google Scholar]
- Atkinson, N.S., R. Brenner, W. Chang, J. Wilbur, J.L. Larimer, and J. Yu. 2000. Molecular separation of two behavioral phenotypes by a mutation affecting the promoters of a Ca-activated K channel. J. Neurosci. 20:2988–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, N.S., G.A. Robertson, and B. Ganetzky. 1991. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 253:551–555. [DOI] [PubMed] [Google Scholar]
- Becker, M.N., R. Brenner, and N.S. Atkinson. 1995. Tissue-specific expression of a Drosophila calcium-activated potassium channel. J. Neurosci. 15:6250–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, S., I. Favre, and E. Moczydlowski. 2001. Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation. Proc. Natl. Acad. Sci. USA. 98:4776–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, R., T.O. Thomas, M.N. Becker, and N.S. Atkinson. 1996. Tissue-specific expression of a Ca2+-activated K+ channel is controlled by multiple upstream regulatory elements. J. Neurosci. 16:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani, M.F., J.B. Hogenesch, M. Yanovsky, S. Panda, M. Straume, and S.A. Kay. 2002. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J. Neurosci. 22:9305–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang, A., H. Wijnen, F. Naef, C. Boothroyd, N. Rajewsky, and M.W. Young. 2001. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 32:657–671. [DOI] [PubMed] [Google Scholar]
- Derst, C., S. Messutat, C. Walther, M. Eckert, S.H. Heinemann, and D. Wicher. 2003. The large conductance Ca2+-activated potassium channel (pSlo) of the cockroach Periplaneta americana: structure, localization in neurons and electrophysiology. Eur. J. Neurosci. 17:1197–1212. [DOI] [PubMed] [Google Scholar]
- DiChiara, T.J., and P.H. Reinhart. 1995. Distinct effects of Ca2+ and voltage on the activation and deactivation of cloned Ca2+-activated K+ channels. J. Physiol. 489:403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, J.P., and C.J. Lingle. 2002. Steady-state and closed-state inactivation properties of inactivating BK channels. Biophys. J. 82:2448–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, G.A., and N.V. Marrion. 1998. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J. Physiol. 508:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo, A.M., X. Zheng, Y. Zhou, D.A. Amado, A. Sheldon, A. Sehgal, and I.B. Levitan. 2004. Pattern of distribution and cycling of SLOB, Slowpoke channel binding protein, in Drosophila. BMC Neurosci. 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng, H.H., M. Shahidullah, and M. Covarrubias. 1999. Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore. J. Gen. Physiol. 113:641–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak, M. 1991. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 266:19867–19870. [PubMed] [Google Scholar]
- Kozak, M. 2002. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 299:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z.W., J.P. Ding, V. Kalyanaraman, and C.J. Lingle. 1999. RINm5f cells express inactivating BK channels whereas HIT cells express noninactivating BK channels. J. Neurophysiol. 81:611–624. [DOI] [PubMed] [Google Scholar]
- Lin, Y., M. Han, B. Shimada, L. Wang, T.M. Gibler, A. Amarakone, T.A. Awad, G.D. Stormo, R.N. Van Gelder, and P.H. Taghert. 2002. Influence of the period-dependent circadian clock on diurnal, circadian, and aperiodic gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 99:9562–9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, M.J., and M. Rosbash. 2001. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 107:567–578. [DOI] [PubMed] [Google Scholar]
- Pyott, S.J., E. Glowatzki, J.S. Trimmer, and R.W. Aldrich. 2004. Extrasynaptic localization of inactivating calcium-activated potassium channels in mouse inner hair cells. J. Neurosci. 24:9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopperle, W.M., M.H. Holmqvist, Y. Zhou, J. Wang, Z. Wang, L.C. Griffith, I. Keselman, F. Kusinitz, D. Dagan, and I.B. Levitan. 1998. Slob, a novel protein that interacts with the slowpoke calcium-dependent potassium channel. Neuron. 20:565–573. [DOI] [PubMed] [Google Scholar]
- Solaro, C.R., and C.J. Lingle. 1992. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 257:1694–1698. [DOI] [PubMed] [Google Scholar]
- Stacey, S.N., D. Jordan, A.J. Williamson, M. Brown, J.H. Coote, and J.R. Arrand. 2000. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J. Virol. 74:7284–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, M., K. Obayashi, A. Kobayashi, and M. Maeda. 2004. A unique role of an amino terminal 16-residue region of long-type GATA-6. J. Biochem. (Tokyo). 135:639–650. [DOI] [PubMed] [Google Scholar]
- Uebele, V.N., A. Lagrutta, T. Wade, D.J. Figueroa, Y. Liu, E. McKenna, C.P. Austin, P.B. Bennett, and R. Swanson. 2000. Cloning and functional expression of two families of β-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 275:23211–23218. [DOI] [PubMed] [Google Scholar]
- Ueda, H.R., A. Matsumoto, M. Kawamura, M. Iino, T. Tanimura, and S. Hashimoto. 2002. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J. Biol. Chem. 277:14048–14052. [DOI] [PubMed] [Google Scholar]
- Wallner, M., P. Meera, and L. Toro. 1999. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc. Natl. Acad. Sci. USA. 96:4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Y. Zhou, H. Wen, and I.B. Levitan. 1999. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J. Neurosci. 19:RC4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Q., and J.A. Rothnagel. 2004. 5′-untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation. Nucleic Acids Res. 32:1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbington, L., T. Hillman, C. Adams, and M. Stern. 1996. Reduced transmitter release conferred by mutations in the slowpoke-encoded Ca2+-activated K+ channel gene of Drosophila. Invert. Neurosci. 2:51–60. [DOI] [PubMed] [Google Scholar]
- Xia, X.M., J.P. Ding, and C.J. Lingle. 2003. Inactivation of BK channels by the NH2 terminus of the β2 auxiliary subunit: an essential role of a terminal peptide segment of three hydrophobic residues. J. Gen. Physiol. 121:125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X.-M., J.P. Ding, and C.J. Lingle. 1999. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X.-M., J.-P. Ding, X.-H. Zeng, K.-L. Duan, and C.J. Lingle. 2000. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel β subunit. J. Neurosci. 20:4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X.-M., B. Hirschberg, S. Smolik, M. Forte, and J.P. Adelman. 1998. dSlo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J. Neurosci. 18:2360–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, W., C.C. Hsieh, A.J. Kurtz, J.P. Rabek, and J. Papaconstantinou. 2001. Regulation of CCAAT/enhancer-binding protein-β isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucleic Acids Res. 29:3087–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L., J. Chen, C.C. Chang, X.Y. Yang, Z.Z. Wang, T.Y. Chang, and B.L. Li. 2004. A stable upstream stem-loop structure enhances selection of the first 5′-ORF-AUG as a main start codon for translation initiation of human ACAT1 mRNA. Acta Biochim. Biophys. Sin. (Shanghai). 36:259–268. [DOI] [PubMed] [Google Scholar]
- Zeng, H., H. Fei, and I.B. Levitan. 2004. The slowpoke channel binding protein Slob from Drosophila melanogaster exhibits regulatable protein kinase activity. Neurosci. Lett. 365:33–38. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., H. Fei, and I.B. Levitan. 2003. An interaction domain in Slob necessary for its binding to the slowpoke calcium-dependent potassium channel. Neuropharmacology. 45:714–719. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., W.M. Schopperle, H. Murrey, A. Jaramillo, D. Dagan, L.C. Griffith, and I.B. Levitan. 1999. A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 22:809–818. [DOI] [PubMed] [Google Scholar]