Early in the last century, J.S. Haldane observed, “It is probable indeed that in some way or other the air supply is proportioned to the blood supply, whether by regulation through the muscular coats of the bronchioles or regulation of blood distribution” (Haldane, 1922). Haldane was expressing the opinion that in order to fulfill its essential function of gas exchange (the addition of oxygen and removal of carbon dioxide from the blood), the lung must optimally adjust ventilation and perfusion, requiring the constant regulation of air and blood flows through alterations in the diameter of the supplying conduits. Air and blood are brought in close proximity through separate, highly branched distribution systems that feed gas exchange units (alveoli and alveolar capillaries), whose gas composition is determined by air and blood flow. As the alveoli and pulmonary capillaries consist of millions of exchange units, the individual feeding systems must deliver a balanced supply (of air and blood) to the exchange units. That is, ventilation and perfusion must be matched in the terminal units to achieve efficient overall gas transfer. At the same time, this supply (and matching) of air and blood must be achieved at a minimum of supply resistance and deadspace—significant demands indeed!
The regulation of ventilation and pulmonary blood flow is a function of the diameter of the supply conduits and thus the degree of contraction of the smooth muscle lining these tubes, as determined by bronchomotor and vasomotor nerves, circulating hormones, and local factors such as gas composition. The business end of both systems is the small conduits just upstream of the exchange units, which regulate blood and gas distribution through “small” changes in diameter. How this job is performed (which factors predominate in specific regulatory contexts, what roles do the epithelium and endothelium play in the process, and what goes wrong in, for example, asthma and pulmonary hypertension) is difficult to determine, due in no small measure to the lack of preparations in which these dynamic interactions can be observed. The millions of air/water interfaces within the lung present an overwhelming obstacle to most imaging modalities. Consequently, much of our knowledge of bronchial/pulmonary vascular control derives from isolated airways or vessels, devoid of their surrounding tissues and neural input; much less is known about the critically important small, regulatory bronchioles and arterioles. Moreover, until recently, the application of sophisticated, high speed imaging methods has been largely confined to single cells obtained from large airways or vessels.
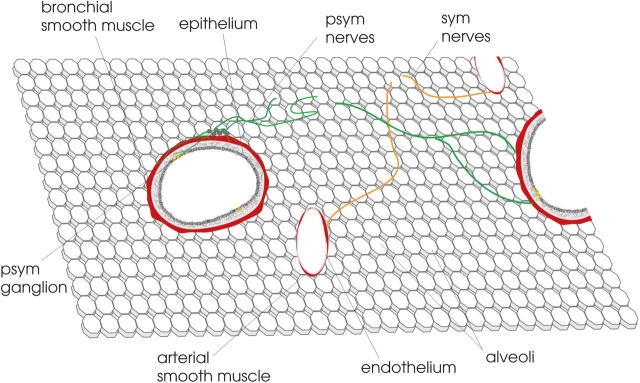
Multicellular preparations that preserve dynamic interactions between cells of different lineages have markedly advanced the understanding of complex biological processes; studies in brain slices, for example, have been of great value in illuminating neural processing mechanisms, nerve/glia interactions, and dendritic branching processes, among others. The usefulness of these preparations also has been expanded by the combination of deep fluorescent imaging by multiphoton microscopy and the use of genetic markers that identify specific lineages or even report cell signaling events (Mainen et al., 1999; Feng et al., 2000; Ji et al., 2004). In this issue of the Journal of General Physiology (Perez and Sanderson, 2005a,b), the laboratory of Michael Sanderson reports a marked improvement in the preparation of lung slices and their use in examining airway and vascular biology in two articles. The importance of this work is that the early lung slice preparations were relatively thick preparations, which were not suitable for high resolution optical imaging (Dandurand et al., 1993), whereas the previous lung slice preparation developed by the Sanderson lab did not preserve vascular spaces (Bergner and Sanderson, 2002). Sanderson and colleagues now show how it is possible, by separately perfusing the pulmonary arterial system with gelatin and the airspaces with agarose (followed by cooling the lungs to solidify these materials), to cut 100-μm-thick lung slices that are ideal for optical imaging (Fig. 1). The choice of “filler materials” is critical for their success, as the gelatin dissolves when the lung slice is warmed to room temperature, thereby maintaining vascular integrity, while the agarose remains in the alveolar spaces, thereby preserving lung volume and the parenchymal forces that are critical determinants of airway caliber in vivo. This new preparation therefore is ideal for the study of the relationship between small airways and pulmonary arterioles and the slices reveal a novel view of the dynamic regulation of bronchiolar and arteriolar diameter; cilia beat, vessels twitch, and airways contract in a complex and captivating interplay, and distinct regulatory mechanisms underlying bronchiolar and arteriolar smooth muscle become apparent.
Figure 1.
Lung slice with preserved architecture. The companion manuscripts by Perez and Sanderson (2005a)(b) describe the use of 100 μm lung sections cut on a vibratome after the airspaces and pulmonary arteries were separately filled. Agarose remains in the alveolar spaces, maintaining the distension of the lung parenchyma. Shown are paired bronchioles and pulmonary arterioles, as well as neural control elements.
For their present studies, Perez and Sanderson load these slices with a fluorescent Ca2+ reporter and use laser scanning confocal microscopy to examine bronchiolar and arteriolar Ca2+ signals in the smooth muscle layers of these tissues. They separately report the pattern of Ca2+ oscillations and contractions in bronchiolar and pulmonary arterial smooth muscle, demonstrating different patterns of Ca2+ responses and distinct contractile behavior. The experiments reveal, to a surprising degree, the distinct pattern of Ca2+ responses that arise from depolarizing versus spasmogenic stimuli, as well as the contrasting pattern of contraction of air and blood conduits. For example, phospholipase C–linked agonists produce homogenous oscillations in cytosolic Ca2+ in myocytes from airways and vessels, whereas depolarization of the preparation with extracellular K+ results in increases in the localized Ca2+ release, or Ca2+ sparks, that likely reflect SR overload.
As important as these results are, what is perhaps most important (for the future) is the development of a preparation in which the physiological relationships between individual lineages and structures in the lung are preserved in all their complexity, as well as the resolution and quality of the data obtained. One can easily imagine how, by careful orientation of sectioning and perhaps the use of slightly thicker sections, key neural elements, such as parasympathetic preganglionic or postganglionic sympathetic nerves, can be preserved within the slice. Genetic tagging of these and other cell lineages with GFP-based reporter transgenes would further facilitate such studies. Moreover, the preparation will be highly valuable also for many studies of complex lung biology in which the full panoply of tools provided by mouse genetics are exploited. Thus one can envision a host of experiments in which specific hypotheses about dynamic regulatory mechanisms are examined through defined manipulations of the mouse genome, and detailed analysis of their effects in dynamic studies within the lung slice. The preparation may also be useful for studies of lung immunology, as tagged immunocytes can be injected and dynamically traced through vascular, interstitial, and airway compartments, using deep fluorescence imaging. Studies of critical airway epithelial cell functions such as coordinated cilia beating and mucous secretion similarly should be facilitated. Thus we can look forward to many laboratories, in addition to the Sanderson laboratory, exploiting this preparation to make important mechanistic advances in our understanding of airway and vascular physiology.
References
- Bergner, A., and M.J. Sanderson. 2002. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J. Gen. Physiol. 119:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandurand, R.J., C.G. Wang, N.C. Phillips, and D.H. Eidelman. 1993. Responsiveness of individual airways to methacholine in adult rat lung explants. J. Appl. Physiol. 75:364–372. [DOI] [PubMed] [Google Scholar]
- Feng, G., R.H. Mellor, M. Bernstein, C. Keller-Peck, Q.T. Nguyen, M. Wallace, J.M. Nerbonne, J.W. Lichtman, and J.R. Sanes. 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 28:41–51. [DOI] [PubMed] [Google Scholar]
- Haldane, J.S. 1922. Respiration. Yale University Press, New Haven, CT. 427 pp.
- Ji, G., M. Feldman, K.Y. Deng, K.S. Greene, J. Wilson, J. Lee, R. Johnston, M. Rishniw, Y. Tallini, J. Zhang, W.G. Wier, M.P. Blaustein, H.B. Xin, J. Nakai, and M.I. Kotlikoff. 2004. Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle. J. Biol. Chem. 279:21461–21468. [DOI] [PubMed] [Google Scholar]
- Mainen, Z.F., M. Maletic-Savatic, S.H. Shi, Y. Hayashi, R. Malinow, and K. Svoboda. 1999. Two-photon imaging in living brain slices. Methods. 18:231–239, 181. [DOI] [PubMed] [Google Scholar]
- Perez, J.F., and M.J. Sanderson. 2005. a. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J. Gen. Physiol. 125:535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, J.F., and M.J. Sanderson. 2005. b. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. J. Gen. Physiol. 125:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]