Abstract
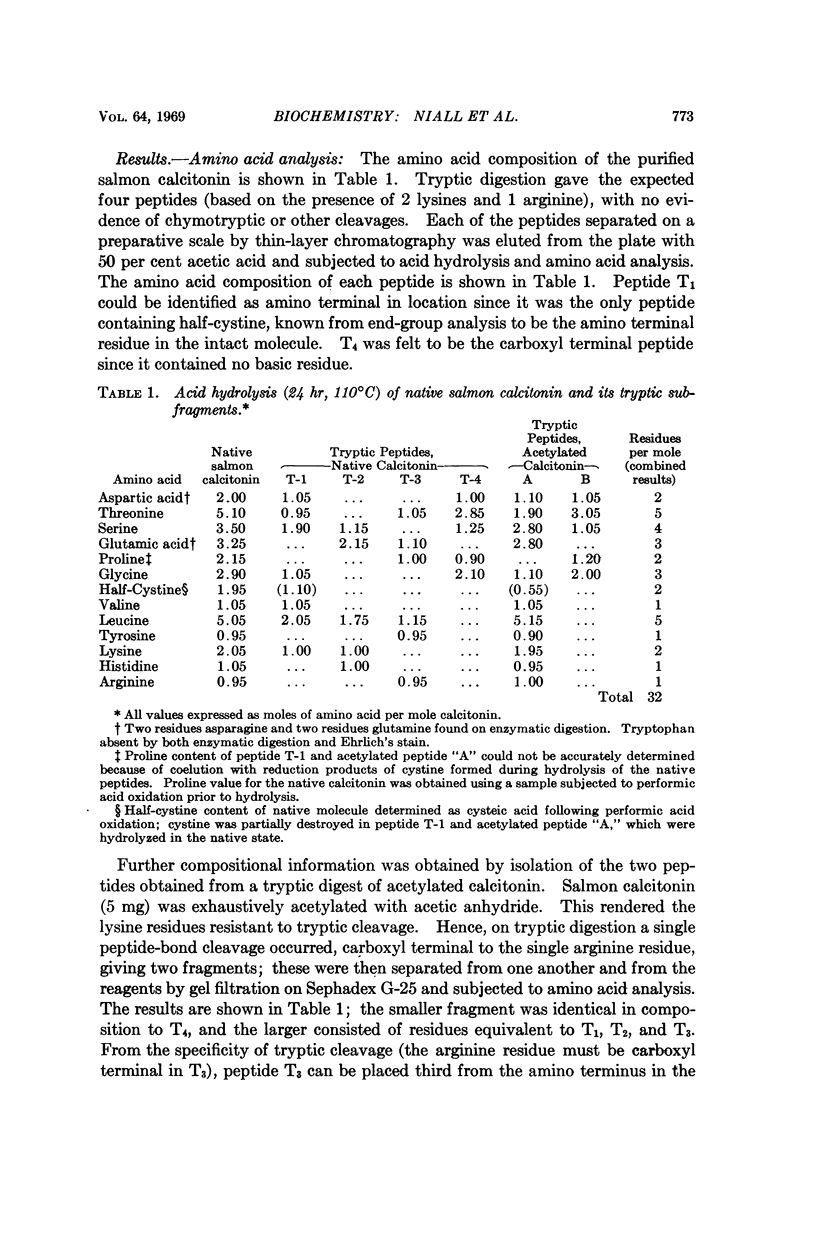
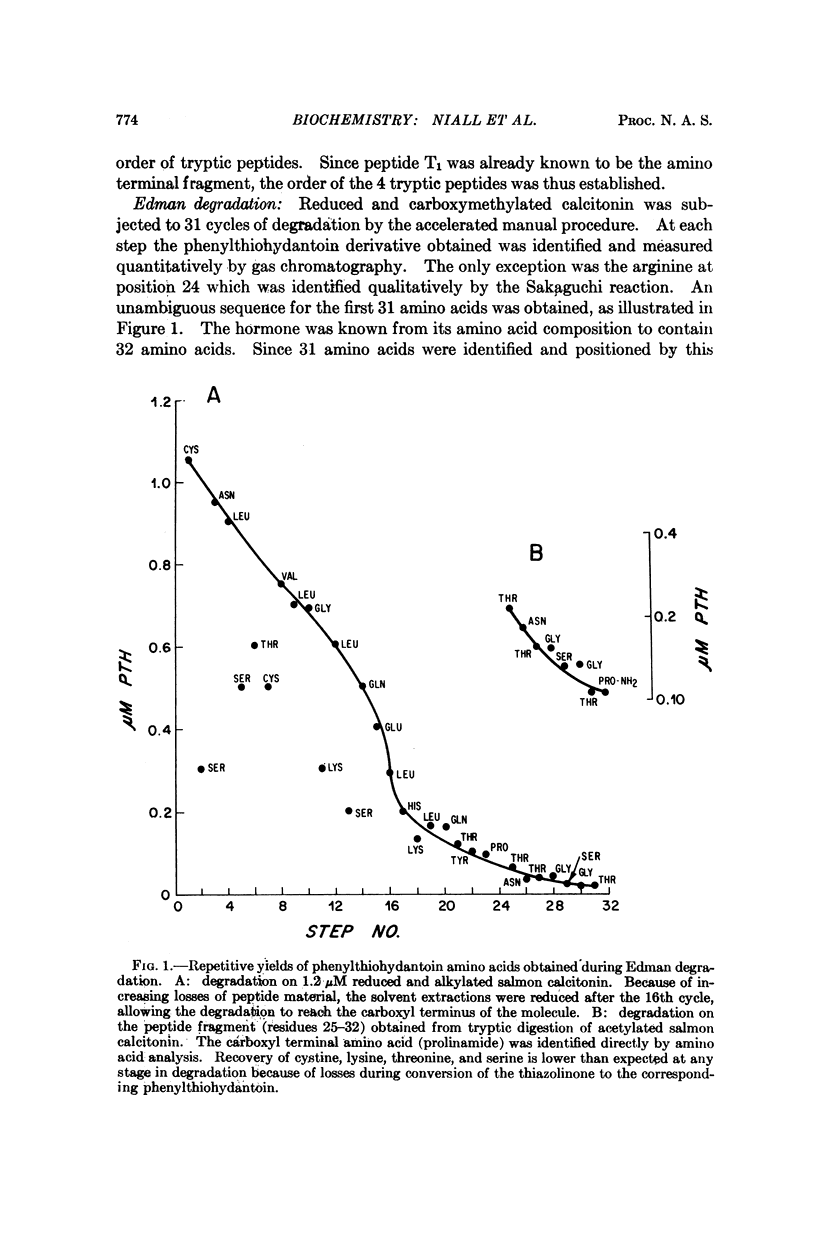
Salmon ultimobranchial calcitonin has been isolated and rendered pure, as demonstrated by several chemical criteria. Its amino acid sequence was determined by means of manual Edman degradation of the intact molecule and of several peptide subfragments. Results of automated degradation provided confirmation of the structure. The salmon molecule possesses, in common with other calcitonins, a 32-amino acid peptide chain terminating in prolinamide and containing half-cystine residues at positions 1 and 7. Although the sequence of the salmon hormone differs considerably from that of the porcine, bovine and human calcitonins, the four hormones are homologous in 9 of 32 positions. The much higher biological potency possessed by the salmon calcitonin makes it of particular interest for future structure function studies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E. Studies on the reduction and re-formation of protein disulfide bonds. J Biol Chem. 1961 May;236:1361–1363. [PubMed] [Google Scholar]
- Bell P. H., Barg W. F., Jr, Colucci D. F., Davis M. C., Dziobkowski C., Englert M. E., Heyder E., Paul R., Snedeker E. H. Purification and structure of porcine calcitonin-1. J Am Chem Soc. 1968 May 8;90(10):2704–2706. doi: 10.1021/ja01012a050. [DOI] [PubMed] [Google Scholar]
- Copp D. H., Cockcroft D. W., Kueh Y. Ultimobranchial origin of calcitonin. Hypocalcemic effect of extracts from chicken glands. Can J Physiol Pharmacol. 1967 Nov;45(6):1095–1099. doi: 10.1139/y67-127. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Lee M. R., Potts J. T., Jr A radioimmunoassay for thyrocalcitonin. Proc Natl Acad Sci U S A. 1968 May;60(1):293–299. doi: 10.1073/pnas.60.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMAN P. Phenylthiohydantoins in protein analysis. Ann N Y Acad Sci. 1960 Aug 31;88:602–610. doi: 10.1111/j.1749-6632.1960.tb20056.x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- FOSTER G. V., MACINTYRE I., PEARSE A. G. CALCITONIN PRODUCTION AND THE MITOCHONDRION-RICH CELLS OF THE DOG THYROID. Nature. 1964 Sep 5;203:1029–1030. doi: 10.1038/2031029a0. [DOI] [PubMed] [Google Scholar]
- Gray W. R. Protein structure: a new strategy for sequence analysis. Nature. 1968 Dec 28;220(5174):1300–1304. doi: 10.1038/2201300a0. [DOI] [PubMed] [Google Scholar]
- Gudmundsson T. V., Woodhouse N. J., Osafo T. D., Galante L., Matthews E. W., MacIntyre I., Kenny A. D., Wiggins R. C. Plasma-calcitonin in man. Lancet. 1969 Mar 1;1(7592):443–446. doi: 10.1016/s0140-6736(69)91482-2. [DOI] [PubMed] [Google Scholar]
- Hubbard R. W., Kremen D. M. Increased sensitivity of accelerated amino acid ion-exchange chromatography. Anal Biochem. 1965 Sep;12(3):593–602. doi: 10.1016/0003-2697(65)90227-7. [DOI] [PubMed] [Google Scholar]
- Hubbard R. W. Studies in accelerated amino acid analysis. Biochem Biophys Res Commun. 1965 Jun 9;19(6):679–685. doi: 10.1016/0006-291x(65)90310-4. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Keutmann H. T., Potts J. T., Jr Improved recovery of methionine after acid hydrolysis using mercaptoethanol. Anal Biochem. 1969 May;29(2):175–185. doi: 10.1016/0003-2697(69)90300-5. [DOI] [PubMed] [Google Scholar]
- Munson P. L., Hirsch P. F., Brewer H. B., Reisfeld R. A., Cooper C. W., Wästhed A. B., Orimo H., Potts J. T., Jr Thyrocalcitonin. Recent Prog Horm Res. 1968;24:589–650. doi: 10.1016/b978-1-4831-9827-9.50017-4. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Rittel W., Zuber H. Menschliches Calcitonin. 3. Struktur von Calcitonin M un. Helv Chim Acta. 1968;51(8):1900–1905. doi: 10.1002/hlca.19680510811. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Zuber H., Rittel W., Kahnt F. W. Thyrocalcitonin. II. Struktur von alpha-Thyrocalcitonin. Helv Chim Acta. 1968 May 31;51(4):917–924. doi: 10.1002/hlca.660510430. [DOI] [PubMed] [Google Scholar]
- Potts J. T., Jr, Niall H. D., Keutmann H. T., Brewer H. B., Jr, Deftos L. J. The amino acid sequence of porcine thyrocalcitonin. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1321–1328. doi: 10.1073/pnas.59.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]



