Abstract
Abasic (AP) sites represent one of the most frequently formed lesions in DNA, and they present a strong block to continued synthesis by the replicative DNA polymerases (Pols). Here we determine the mutational specificity and the genetic control of translesion synthesis (TLS) opposite an AP site in yeast by using a double-stranded plasmid system that we have devised in which bidirectional replication proceeds from a replication origin. We find that the rate, the genetic control, and the types and frequencies of nucleotides inserted opposite the AP site are very similar for both the leading and the lagging DNA strands, and that an A is predominantly inserted opposite the AP site, whereas C insertion by Rev1 constitutes a much less frequent event. In striking contrast, in studies that have been reported previously for AP bypass with gapped-duplex and single-stranded plasmids, it has been shown that a C is the predominant nucleotide inserted opposite the AP site. We discuss the implications of our observations for the mechanisms of TLS on the leading versus the lagging DNA strand and suggest that lesion bypass during replication involves the coordination of activities of the replicative Pol with that of the lesion-bypass Pol.
Keywords: DNA replication, translesion DNA synthesis
Abasic (AP) sites arise in DNA as a result of spontaneous depurination and from the action of base excision-repair processes, where the removal of a damaged base by a DNA glycosylase produces an AP site (1, 2). It has been estimated that a mammalian cell loses as many as 104 purines per day (3). Although AP sites are removed from DNA by the action of AP endonucleases and by nucleotide excision repair, AP sites that remain in DNA undergoing replication present a block to continued synthesis by the replicative polymerases (Pols). Replication through the AP sites, however, can be mediated by the action of translesion synthesis (TLS) DNA Pols or could occur by other means, such as a copy-choice type of DNA synthesis or recombination.
Because an AP site lacks a base, it is the ultimate noninstructional DNA lesion, and any replication through it would be highly mutagenic. In both prokaryotes and mammalian cells, an A is predominantly inserted opposite the AP site, and biochemical studies have shown that replicative Pols from prokaryotes as well as eukaryotes preferentially insert an A opposite it (4–10). In addition, NMR studies have indicated that DNA containing an A opposite the AP site retains all aspects of B-form DNA, and both the unpaired A and AP residue lie inside the helix (11–13). These observations have led to the formulation of an A rule, which posits that DNA Pols preferentially insert an A opposite a noninstructional DNA lesion, such as an AP site, because that confers the least amount of helix distortion (14).
In striking contrast to the observations that have been made with prokaryotes and mammalian cells and with their replicative Pols, genetic studies in Saccharomyces cerevisiae have indicated that a C is preferentially incorporated opposite an AP site, and it has been suggested that yeast cells differ from others in the adoption of a C rule instead (15). The experiments supporting such a conclusion for yeast have used a gapped-duplex plasmid, in which an AP site was located within a 28-nt single-stranded region (15, 16). More recently, experiments with a single-stranded vector containing a site-specific AP site have provided additional support for this conclusion (17). Because C incorporation opposite the AP site is abrogated in rev1Δ cells, Rev1 carries out the incorporation in these plasmid systems (16, 17).
However, because the means by which TLS operates opposite the AP site carried on a gapped-duplex or a single-stranded plasmid versus that in replicating DNA could differ in important ways, we have devised a double-stranded plasmid system where bidirectional replication ensues from a yeast replication origin and where the mutational specificity and the genetic control of TLS can be analyzed for the leading versus the lagging DNA strand. By using this plasmid system, we show here that an A is preferentially incorporated opposite the AP site on both the DNA strands and that C insertion by Rev1 is a much less frequent event. Rev1, however, plays an important structural role in the TLS process. Additionally, we provide evidence for the requirement of Polζ and Pol32 and for ubiquitination of PCNA at its lysine 164 residue. These observations have an important bearing on the means by which TLS operates during replication, and they suggest the existence of underlying differences in the mechanisms that control TLS during replication versus that which takes place during the nonreplicative modes of synthesis.
Results
A Double-Stranded Plasmid System for the Study of TLS in Yeast Cells.
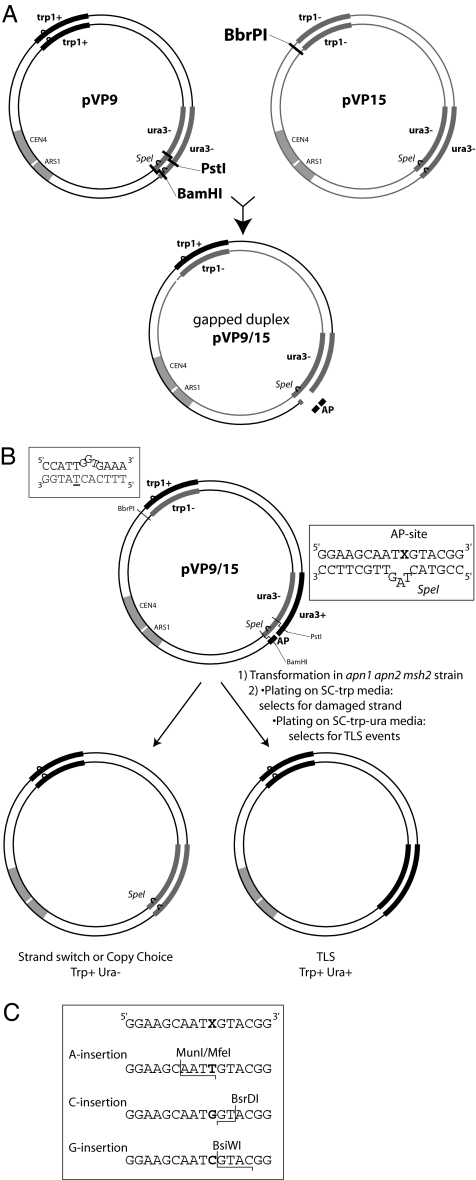
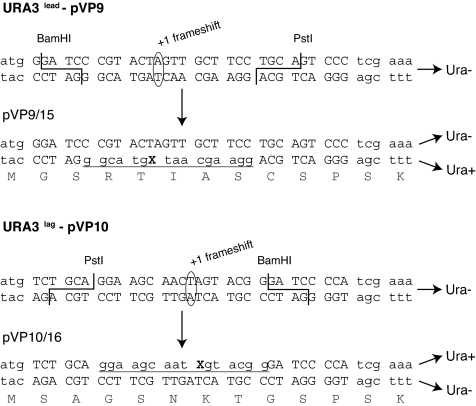
Rev1's DNA Pol activity has been shown to make a major contribution to TLS opposite an AP site carried on a single-stranded plasmid or on a gapped circular plasmid. In these studies, the frequency of a C insertion opposite the AP site has varied from 60–90%, with the remainder being A insertions, and all of the C insertions were found to be Rev1-dependent (15–17). However, because DNA synthesis on the gapped and single-stranded plasmids could differ in important ways from bidirectional synthesis originating from a replication origin, we constructed the double-stranded plasmids, pVP9/15 and pVP10/16, with which we could analyze TLS through a site-specific AP site in the leading and the lagging DNA strands, respectively (Figs. 1 and 2). Both plasmids carry a yeast replication origin, autonomously replicating sequence (ARS1), and the TRP1 gene that selects for transformants that replicated the AP site-containing DNA strand, regardless of whether AP bypass occurred by TLS or by any other mechanism, such as a copy-choice type of synthesis or recombination. The AP site is present in a heteroduplex leader sequence that we inserted in the URA3 gene at its 5′ end in two different orientations, thus presenting the AP site on the leading or the lagging DNA strand during replication (Fig. 2). Because the AP site-containing strand of the heteroduplex is in frame with the URA3 gene, cells harboring plasmid resulting from replication through the AP site by TLS are Ura plus (Fig. 2). The DNA strand opposite from the AP site, however, harbors an SpeI site and a plus 1 frameshift in the leader sequence of the URA3 gene that inactivates the URA3 gene (Figs. 1B and 2). Hence, cells harboring plasmid resulting from replication of the SpeI-containing strand or resulting from any non-TLS-mediated replication of the AP site-containing strand, such as by copy-choice DNA synthesis using the SpeI strand, will not be recovered when cells are plated on medium lacking uracil. Thus, the plating of transformants on synthetic complete medium lacking tryptophan (SC-trp) selects for all of the plasmids resulting from the replication of damaged plasmid, whereas plating transformants on SC-trp lacking uracil (SC-trp-ura) selects for plasmids that underwent TLS through the AP site (Fig. 1B).
Fig. 1.
Plasmids used for TLS assays. (A) Construction of an AP site-containing plasmid. Plasmid pVP15 is linearized by BbrPI digestion. The PstI-BamHI fragment of pVP9 is removed by digestion and purification on a microcon YM-30 column. Both linearized plasmids are denaturated and hybridized together, leading to the formation of two linear forms and two gapped-duplex structures. A 16-mer oligonucleotide containing the AP lesion is ligated into the 17-nt gap of the pVP9/15 duplex, leading to a circular double-stranded plasmid that is purified by centrifugation on a CsCl/ethidium bromide gradient. Because the AP-containing oligonucleotide cannot be inserted into the other gapped-duplex plasmid, this plasmid as well as the two linear forms are not recovered. The resulting plasmid, pVP9/15, has the AP site located on the leading strand. The other plasmid, pVP10/16 (data not shown), was constructed in the same way; in this plasmid, however, the AP site is located on the lagging strand (see Fig. 2). (B) In vivo TLS assay. The plasmid pVP9/15 or pVP10/16 was introduced into yeast cells by electroporation. A portion of the resulting transformants was plated on SC-trp to select for the events resulting from the replication of the damaged strand (including TLS and copy-choice mechanisms). Another portion of the transformants was plated on SC-trp-ura to select for clones arising only from TLS events. The TRP1 gene was changed by introducing a −1 frameshift and a stop codon (underlined T) at position plus 39 of the TRP1 ORF as shown, and the sequence of the AP-containing 16-mer also is shown. (C) Analysis of TLS events by restriction enzyme analyses. Ura plus clones were analyzed by PCR amplification of the URA3 gene and by digestion of the PCR product with restriction enzymes. A, C, and G insertions generated, respectively, MfeI, BsrDI, and BsiWI restriction sites. If none of these enzymes was able to cut, in that case, the PCR product was sequenced.
Fig. 2.
Modification of the URA3 gene by the addition of a 31-mer oligonucleotide leading to URA3lead or URA3lag. The sequence of the URA3 gene in the plasmid (shown by lowercase letters) where the 31-mer oligonucleotide (shown by uppercase letters) was inserted is shown. These modified URA3 genes in plasmids pVP9 or pVP10 are inactive because of the introduction of a plus 1 frameshift mutation. After annealing and ligation of a 16-mer oligonucleotide containing an AP site (X) in the gapped-duplex structures shown in Fig. 1, the URA3lead and URA3lag genes are in frame and active. In pVP9/15, the AP-containing oligonucleotide is inserted in the bottom strand (indicated by underline), which corresponds to the leading strand. In pVP10/16, the AP-containing oligonucleotide is inserted in the top strand (indicated by underline), which corresponds to the lagging strand.
To limit the possibility of the AP site present on the plasmid from being removed by the AP endonucleases before going through the replication fork, we used an apn1Δ apn2Δ yeast strain where both the AP endonuclease genes have been deleted. Additionally, the strain carries the msh2Δ mutation to prevent the removal of the mismatch loop created by the SpeI site (see Fig. 1B). Because mismatch repair proteins act in the removal of mismatched nucleotides from the newly synthesized DNA strand and will be ineffective opposite an AP site, we expect the mismatch repair deficiency to have no effect on TLS opposite this DNA lesion. All of the experiments described here with various yeast strains were done in this apn1Δ apn2Δ msh2Δ genetic background. For the sake of simplicity, the apn1Δ apn2Δ msh2Δ mutant strain is referred to as wild type because it is wild type with respect to TLS. Yeast cells were transformed with the AP site-containing heteroduplex plasmid, and the ratio of Trp plus Ura plus versus Trp plus transformants, which reflects the frequency of TLS events, was determined.
TLS on the Leading and the Lagging DNA Strands in Wild-Type Yeast Cells.
For both the DNA strands, we find that TLS opposite the AP site in wild-type cells occurs with a frequency of ≈6%, which would suggest that the large majority of bypass events opposite the AP site are carried out by processes other than TLS, such as those involving template switching and a copy-choice type of DNA synthesis or by recombination. Interestingly, we find that an A is predominantly inserted opposite the AP site on both the leading and the lagging DNA strands. Overall, the pattern and frequencies with which different nucleotides are inserted opposite the AP site are very similar for the two strands, with A insertion being 66%, C insertion 25%, and G and T insertions being much less frequent, accounting for 8% and 1% of the TLS events, respectively (Table 1).
Table 1.
Frequencies of TLS and types and frequencies of nucleotides incorporated opposite an AP site in the various mutant strains
| Strain | DNA strand | No. sequenced | Nucleotides inserted in TLS, no. (%) |
TLS (% of WT)* | |||
|---|---|---|---|---|---|---|---|
| A | C | G | T | ||||
| WT† | Leading | 64 | 46 (72) | 13 (20) | 4 (6) | 1 (2) | 100 |
| Lagging | 66 | 40 (61) | 19 (29) | 6 (9) | 1 (1) | 100 | |
| Total | 130 | 86 (66) | 32 (25) | 10 (8) | 2 (1) | 100 | |
| rev3Δ | Leading | 21 | 18 (86) | 3 (14) | 0 (0) | 0 (0) | 9 |
| Lagging | 33 | 19 (58) | 14 (42) | 0 (0) | 0 (0) | 7 | |
| Total | 54 | 37 (69) | 17 (31) | 0 (0) | 0 (0) | 8 | |
| rev7Δ | Leading | 22 | 12 (55) | 10 (45) | 0 (0) | 0 (0) | 8 |
| Lagging | 28 | 14 (50) | 14 (50) | 0 (0) | 0 (0) | 10 | |
| Total | 50 | 26 (52) | 24 (48) | 0 (0) | 0 (0) | 9 | |
| rev1Δ | Leading | 24 | 20 (83) | 3 (13) | 1 (4) | 0 (0) | 41 |
| Lagging | 38 | 31 (82) | 3 (8) | 3 (8) | 1 (2) | 43 | |
| Total | 62 | 51 (82) | 6 (10) | 4 (6) | 1 (2) | 41 | |
| rev1-C mut‡ | Leading | 44 | 41 (93) | 1 (2) | 2 (5) | 0 (0) | 79 |
| Lagging | 42 | 35 (83) | 0 (0) | 5 (12) | 2 (5) | 137 | |
| Total | 86 | 76 (89) | 1 (1) | 7 (8) | 2 (2) | 117 | |
| rad30Δ | Leading | 30 | 20 (67) | 7 (23) | 1 (3) | 2 (7) | 107 |
| Lagging | 41 | 28 (68) | 9 (22) | 3 (7) | 1 (3) | 119 | |
| Total | 71 | 48 (68) | 16 (22) | 4 (6) | 3 (4) | 115 | |
| rad30Δ rev7Δ | Leading | 2 | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 4 |
| Lagging | 13 | 4 (31) | 9 (69) | 0 (0) | 0 (0) | 7 | |
| Total | 15 | 6 (40) | 9 (60) | 0 (0) | 0 (0) | 4 | |
| pol30–119§ | Leading | 16 | 14 (88) | 2 (12) | 0 (0) | 0 (0) | 18 |
| Lagging | 29 | 27 (93) | 2 (7) | 0 (0) | 0 (0) | 16 | |
| Total | 45 | 41 (91) | 4 (9) | 0 (0) | 0 (0) | 17 | |
*The frequency of TLS in the WT strain, which was ≈6% for both the DNA strands, has been set at 100%, and the observed TLS frequencies in the various mutant strains are expressed as percentage relative to WT.
†The apn1Δ apn2Δ msh2Δ strain is designated as wild type (WT).
‡rev1-Cmut represents the catalytically inactive Rev1 Pol.
§In pol30–119, lysine 164 of PCNA is changed to arginine.
Contributions of DNA Pols ζ, Rev1, and η to TLS.
The frequency of TLS for both the leading and the lagging DNA strands declines in the rev3Δ and rev7Δ strains to ≈10% of that observed for the wild-type strain, indicating that Polζ is indispensable for AP bypass. The requirement for Rev1, however, is not as absolute as for Polζ because the TLS frequency for both the strands was reduced by ≈60% in the rev1Δ strain compared with the ≈90% reduction seen in the rev3Δ or rev7Δ strains. Polη, in contrast, appears to make little contribution to TLS opposite the AP site because the incidence of TLS was not reduced in the rad30Δ strain (Table 1).
Because Polζ is expected to function at the extension step of TLS (18–21) and because this yeast Pol is the only one known to efficiently extend from the nucleotides inserted opposite the AP site (18), the residual TLS of ≈10% in the rev3Δ or rev7Δ cells would suggest that some other Pol can carry out extension in the absence of Polζ. Also, because an A or a C is still incorporated in the rev3Δ or rev7Δ cells, we presume that this other Pol can extend from an A or a C opposite from the AP site. Although Polη is highly inefficient at both the insertion and extension steps of AP bypass (22), nevertheless, we examined whether, in the absence of Polζ, Polη could support the extension reaction. However, because an A or a C was still incorporated opposite the AP site in the rad30Δ rev7Δ strain, we assume that a Pol other than Polη performs the extension reaction in the absence of Polζ (Table 1).
Previous studies indicating that the incidence of methyl methanesulfonate (MMS)-induced can1r mutations in apn1Δ apn2Δ yeast cells was greatly reduced in the absence of Rev1 as well as Polζ suggested that Rev1 was required for Polζ-dependent TLS opposite the AP sites (23). This requirement of Rev1 for TLS, however, is likely to have resulted from a noncatalytic role of Rev1 because the inactivation of Rev1 DNA Pol function was found to have no significant effect on MMS-induced mutagenesis in the apn1Δ apn2Δ strain (18). With our duplex plasmid system, it is now possible to directly examine the contributions of the catalytic versus the noncatalytic roles of Rev1 in AP bypass. We find that the frequency of a C insertion is reduced in the rev1Δ strain to ≈10% compared with ≈25% in the wild-type strain (Table 1). Importantly, and by contrast to the large reduction in TLS frequency seen in the rev1Δ mutation, we find that the inactivation of Rev1 DNA Pol function has no significant effect on the TLS rate, although the frequency of a C insertion is reduced to ≈1% (Table 1). From these observations, we infer that Rev1 contributes to TLS opposite an AP site primarily in a noncatalytic manner, and although its DNA Pol activity is used, it is not required. Furthermore, although C insertion is almost completely inhibited upon the inactivation of Rev1 DNA Pol function, but the TLS frequency is not affected, we interpret this observation to further suggest that Rev1 acts primarily as an indispensable structural element for promoting Polζ function in TLS (24).
Requirement of Pol32.
Pol32, a nonessential subunit of Polδ, is indispensable for Polζ-dependent, damage-induced mutagenesis. Thus, the level of UV-induced mutations in the pol32Δ strain and the level of MMS-induced mutations in the apn1Δ apn2Δ pol32Δ strain are greatly reduced (18, 25). With the plasmid system, we find that on both the leading and the lagging DNA strands, TLS in the pol32Δ strain was reduced to ≈8% of the wild-type level, indicating that the requirement of Pol32 is as critical as that for Polζ.
Although TLS was affected to the same degree in the pol32Δ strain as in the rev3Δ or rev7Δ strains, the total number of Trp plus plasmids that were recovered from the pol32Δ strain was greatly reduced compared with that in the rev3Δ or rev7Δ strains. For example, compared with a total of ≈3,800 Trp plus plasmids that were recovered from the rev3Δ strain, of which ≈150 were Ura plus (≈0.4%), only ≈140 Trp plus plasmids were recovered from the pol32Δ strain, of which only 6 were Ura plus (≈0.4%). Thus, although the pol32Δ mutation retains viability, we assume that it has an adverse effect on plasmid replication, and such an inference is further supported from our observation of the greatly reduced recovery of nondamaged control plasmid from pol32Δ cells. Because of the small numbers of Ura plus plasmids, we have been unable to deduce with any reasonable certainty the frequencies with which nucleotides other than an A are inserted opposite the AP site in the pol32Δ strain (data not shown).
Requirement of PCNA Ubiquitination.
In DNA-damaged yeast cells, proliferating cell nuclear antigen (PCNA) becomes monoubiquitinated at its lysine 164 residue in a Rad6–Rad18-dependent manner. Subsequently, this PCNA residue is polyubiquitinated in a reaction that depends on the Mms2-Ubc13 ubiquitin-conjugating enzyme and on Rad5, which functions as a ubiquitin ligase (26). From genetic studies in yeast, it has been inferred that PCNA monoubiquitination is a prerequisite for TLS mediated by Polη and Polζ, and PCNA polyubiquitination is important for Rad5/Mms2/Ubc13-dependent postreplication repair (26–28).
To determine the requirement of PCNA ubiquitination for TLS opposite the AP site, we examined the effect of the pol30–119 mutation, in which the lysine 164 residue of PCNA has been changed to arginine (27) and which thus lacks the ability for ubiquitination at this residue. For both the leading and the lagging DNA strands, in the absence of PCNA ubiquitination, the TLS frequency was reduced by ≈85%, and the A and C residues were inserted opposite the AP site with a frequency of ≈90% and 10%, respectively (Table 1).
Discussion
Here, we have devised a plasmid system in which TLS opposite an AP site during bidirectional replication can be examined for both the leading and the lagging DNA strands. By using this plasmid system, we have made the following observations: (i) the rate, genetic control, and types and frequencies of nucleotides inserted opposite the AP site are very similar for the leading versus the lagging DNA strand; (ii) PCNA ubiquitination is indispensable for TLS on both the DNA strands; (iii) an A and not a C is the predominant nucleotide inserted opposite the AP site on both of the DNA strands; (iv) although Polζ as well as Rev1 are required for TLS, the DNA Pol activity of Rev1 is dispensable for TLS opposite the AP sites; and (v) Pol32 is required for TLS on both of the DNA strands. We consider the possible implications of these observations for the coordination of TLS with the progression of the replication fork on both of the DNA strands.
In eukaryotes, there is little information available on how DNA lesions on the leading versus the lagging strand template are handled by the different lesion-bypass processes. From studies of replication products of SV40 DNA in UV-irradiated monkey and human cells (29, 30), it has been inferred that a UV lesion located on the template for the leading strand severely blocks replication fork movement, whereas a UV lesion on the lagging strand template does not significantly impede the growing fork, but only inhibits the completion of the nascent Okazaki fragment (29). Analyses of replication products from human cell-free extracts replicating SV40-derived plasmids have added further evidence that a site-specific T–T dimer when placed in the template of the leading strand presents a severe block to the synthesis of the leading strand, whereas a T–T dimer on the lagging strand causes little inhibition of replication fork progression, but a small gap is left in the lagging strand, which presumably results from the inhibition of Okazaki fragment completion (31–33). UV lesions in yeast cells also inhibit the synthesis of the leading strand, whereas the lagging strand synthesis is not inhibited (34).
On the bases of these noted studies with yeast and human cells, one might have expected the contribution of TLS mechanisms to lesion bypass on the two strands to differ. Because a lesion on the leading strand template is highly inhibitory to replication fork progression, TLS might have been primarily consigned to promoting lesion bypass on this strand because the coordination of a proficient TLS mechanism with the replication ensemble could conceivably be more effective in maintaining the continued progression of the replication fork than the alternative processes of template switching and copy-choice type of synthesis. For a lesion on the lagging strand template, however, the gap remaining in the Okazaki fragment opposite the lesion site could subsequently be filled in by processes other than TLS, such as by transient template switching and a copy-choice type of synthesis, without adversely affecting the fork progression. Hence, one might have expected TLS to occur more frequently on the leading strand than on the lagging strand. Our observations, however, indicate that TLS occurs with nearly equal frequencies on the two DNA strands. Furthermore, our observation that PCNA ubiquitination is equally important for TLS on the leading and the lagging DNA strands implies that this PCNA modification is necessary for promoting the access of the TLS Pols to the replication ensemble on both the DNA strands. Such a requirement of PCNA ubiquitination for TLS on the lagging strand, however, is difficult to reconcile with the model where the filling-in of the gap via TLS occurs after the replication ensemble has moved away from the damage site. Ubiquitination of PCNA in the absence of the replication ensemble would then serve no purpose unless one were to assume that this PCNA modification was needed for the binding of TLS Pols to PCNA at the gap site, which clearly is not the case (35, 36). Hence, from our observations of the similar rates and genetic control of TLS on the leading and the lagging DNA strands, we suggest that, during replication, similar TLS mechanisms operate on both of the DNA strands.
Also, we note that the requirement of PCNA ubiquitination for TLS opposite from a single lesion site in our plasmid system in the absence of any DNA-damaging treatment strongly suggests that, during replication, the stalling of the replicative Pol at a lesion site is sufficient to generate the signal for Rad6–Rad18-dependent PCNA ubiquitination and that a threshold level of DNA damage is not necessary for this PCNA modification to occur.
Whereas C is the predominant nucleotide inserted by Rev1 opposite the AP site located on a gapped-duplex or a single-stranded plasmid, we find that an A is the predominant nucleotide inserted opposite the AP site. From these observations, we infer that TLS operates by different means during replication versus that occurring during the synthesis reactions on gapped plasmids or on single-stranded plasmids. Although Polζ is indispensable for TLS opposite the AP site during replication as well as during repair synthesis and other nonreplicative synthesis reactions, the Rev1 polymerase activity is dispensable for TLS during replication, but Rev1 protein is still needed for TLS as a structural element for Polζ. In the paragraph below, we consider the possible implications of different nucleotide-insertion patterns that occur in TLS during the replicative versus the nonreplicative modes of DNA synthesis.
Yeast cells have three major TLS Pols: η, Rev1, and ζ. Of these Pols, Polη is highly inefficient at both inserting a nucleotide opposite the AP site and extending from the nucleotide inserted (22). In keeping with these biochemical observations, we find no significant contribution of Polη to AP bypass on either of the DNA strands. Although Rev1 can proficiently insert a C opposite an AP site (37, 38), it does not extend from there (18). In contrast, Polζ is a proficient extender of nucleotides inserted opposite an AP site, but it is highly inefficient at inserting a nucleotide opposite the AP site, and therefore it requires Rev1 or another Pol for the insertion reaction (18). Because the yeast-replicative Pols predominantly insert an A opposite the AP site (ref. 18 and data not shown) and because an A is the predominant nucleotide inserted in TLS during replication, we surmise that, during replication, the A nucleotide is inserted opposite the AP site by the replicative Pol and is then extended by Polζ. We suggest that the different nucleotide-insertion patterns seen during the replicative versus the nonreplicative modes of synthesis reflect the underlying dissimilarities in the TLS mechanisms that operate during these cellular processes. The predominance of an A insertion during replication could result if the action of the replicative Pol were somehow coordinated with that of the TLS Pol so that the nucleotide inserted opposite the AP site by the replicative Pol is efficiently extended by Polζ. In contrast, TLS on a gapped or single-stranded plasmid may lack such a coordination and, hence, the requirement of Rev1 DNA Pol activity for C insertion opposite the AP site.
In summary, all our observations–the similar genetic control of TLS on the two DNA strands, including the requirement of PCNA ubiquitination and the predominance of an A insertion–lead us to suggest that, during replication, similar TLS mechanisms operate on the two DNA strands and that on both of the DNA strands the action of the TLS Pol is coordinated with that of the replicative Pol. Such a coordination of activity could provide not only for a more efficient way to replicate through the DNA lesion, but it also would avoid the possibility of nicking the ssDNA template opposite from the gap in the newly synthesized DNA, which could be the case if TLS were to occur by “postreplicative” gap filling, as was suggested previously (34).
Lastly, our observation that Pol32 is as indispensable for TLS on both DNA strands as Polζ raises questions about the possible role of Pol32 in TLS. Because Pol32 is a subunit of Polδ, its requirement for TLS has been thought to result from a role in promoting the assembly of Polζ with Polδ (39). However, because recent evidence suggests that during replication Polε functions on the leading strand (40), synthesis by Polδ could be limited to the lagging strand. In that case, the requirement of Pol32 on both DNA strands might result from a more general role of this Polδ subunit in TLS.
Materials and Methods
Yeast Strains.
The apn1Δ apn2Δ msh2Δ strain was constructed from EMY74.7 (MAT a his3Δ-1 leu2–3,-112, trp1Δ ura3–52), and the various genomic-deletion and other mutations were introduced into the apn1Δ apn2Δ msh2Δ strain.
Plasmid Construction.
Double-stranded, closed circular monoadducted plasmids were generated by using the gapped-duplex method (41), which utilized two similar linear DNAs that when hybridized resulted in a circular duplex containing a nick in one strand and a small gap in the other strand, in which a short oligonucleotide containing the lesion could be annealed (Fig. 1A). Ligation of the nicked DNA strand and of the lesion-containing oligonucleotide resulted in a closed circular DNA containing a single lesion at a specific site. We generated two pairs of precursor plasmids, pVP9 and pVP10, and pVP15 and pVP16 that allowed for a lesion-containing oligonucleotide to be ligated into either the leading or the lagging strand of the resultant heteroduplex (Fig. 2).
Plasmids pVP9, pVP10, pVP15, and pVP16 were generated as follows: All plasmids were derived from YCplac133 (42), which carried the yeast URA3 gene, an ARS1 origin of replication, and the centromeric CEN4 region. The SpeI restriction site located in the CEN4 region was removed by digestion with SpeI, followed by filling in the overhang and religation. The TRP1 gene was cloned into the multicloning site.
To generate a region in which a modified oligonucleotide could be annealed in either the leading or the lagging strand of the vectors, a 31-bp duplex DNA containing the sequence 5′-GGA TCC CGT ACT AGT TGC TTC CTG CAG TCC C-3′ (see pVP9 in Fig. 2) or the sequence 5′-TCT GCA GGA AGC AAC TAG TAC GGG ATC CCC A-3′ (see pVP10 in Fig. 2) was introduced next to the ATG initiation codon at the 5′ end of the URA3 gene by PCR mutagenesis, generating the URA3lead and the URA3lag genetic markers, respectively (Fig. 2). These modified ura3 genes were inactive because of the presence of a plus 1 frameshift in the 31-bp insert. To determine that the introduction of the insert did not alter URA3 function, we verified that plasmids carrying the 30-nt insert without the plus 1 frameshift were able to complement the ura3–52 mutation.
Monoadducted heteroduplex plasmids were generated as follows: Plasmids pVP9 and pVP10 were digested with BamHI and PstI (Fig. 1A), and the vector portion was separated from the 25-bp insert by centrifugation in a YM-30 microcon filter unit (Millipore). The trp1 counterparts pVP15 and pVP16, respectively, were digested with BbrPI; 25 μg each of linearized pVP9 and pVP15 or pVP10 and pVP16 were mixed in a final volume of 5 ml of 1X GM buffer [10 mM Tris (pH 7.5), 10 mM NaCl, and 1 mM EDTA]. The DNAs were denaturated in boiling water for 3 min with constant shaking and rapidly chilled on ice. After the addition of 40 μl of 5 M NaCl to reach a final concentration of 50 mM, the mixtures were incubated for 2 h at 55°C and slowly cooled to room temperature to allow the annealing of the ssDNA molecules, leading to the formation of the gapped-duplex structures (Fig. 1A). The pVP9/15 and pVP10/16 gap duplexes contained a 17-nt gap in which a 16mer oligonucleotide 5′-GGAAGCAATXGTACGG-3′, where X denoted a tetrahydrofurane-type AP site, was ligated (Fig. 1B). The 16mer oligonucleotide sequence was in frame with the URA3 gene, leading to a Ura3 plus phenotype, and thus could be distinguished from the nonlesion-containing strand, which was Ura3 minus because of the plus 1 frameshift (Fig. 2). The 16mer oligonucleotide was PAGE-purified, and 12 pmol was added to the gapped duplex, along with 10 mM MgCl2 and 10 mM DTT. After incubation at 65°C for 2 min, 50 μl of 0.1 M ATP and 25 μl of 10 mg/ml BSA were added, and the mixtures were shifted to 16°C before the addition of 2,000 units of T4 DNA ligase (NEB). Ligation was carried out for 3 min, and the closed circular plasmid was purified by centrifugation in a CsCl/ethidium bromide gradient. The resulting pVP9/15 and pVP10/16 plasmids contained the AP site in the leading versus the lagging strand, respectively (Fig. 2), and the AP site was located 500 bp downstream of ARS1 (Fig. 1B).
Yeast Transformation.
Plasmids carrying the AP site were introduced into yeast cells by electroporation. Yeast cells were grown overnight in 200 ml of yeast extract/peptone/dextrose (YPD) until they reached a density of 106-107 cells per ml. Cells were harvested by centrifugation; washed once with 100 ml of 1 M sorbitol, twice with 100 ml of 1 M sorbitol/1 mM MgCl2, and once with 20 ml of 1 M sorbitol; and then resuspended in 200 μl of 1 M sorbitol. Then 20 ng of plasmid DNA was added to 50 μl of cell suspension and electroporated in a BioRad gene pusler (1,500 V–400 Ω–25 μF). Finally, 1 ml of YPD was added after electroporation, and after incubation at 30°C for 40 min, the cell suspension was washed with water before plating on selective media.
For each transformation, a portion of cells was plated on SC-trp, and another portion was plated on SC-trp-ura. In both cases, by plating on −trp media, we were selecting for the cells that had replicated the damaged strand. Because the nondamaged strand yielded a trp− phenotype, its replication would not produce colonies on SC-trp plates. Thus, the number of colonies that grew on −trp plates indicated the transformation efficiency of the damaged strand, which included colonies arising from TLS and copy-choice mechanisms. If TLS occurred, the phenotype would be Ura plus because the lesion containing strand was Ura plus. Hence, the colonies that grew on −trp-ura plates reflected the TLS events, and the ratio of Ura plus/Trp plus colonies indicated the TLS frequency.
Identification of TLS Products.
To identify the nucleotide inserted opposite the AP site during TLS, a 1.6-kb portion of the URA3lead/lag gene, which contains the 5′ 31-bp insertion, was amplified by PCR from Ura plus colonies by using the oligos LP752 (5′-AGGGAATAAGGGCGACACGGAAATG-3′) and LP753 (5′-TATAAAAATAGGCGTATCACGAGGC-3′). The PCR fragments were then analyzed by restriction endonuclease digestion, followed by gel analysis. A, C, and G insertions generated MfeI, BsrDI, and BsiWI restriction sites, respectively (Fig. 1C). PCR products that were not able to be digested with these enzymes were sequenced to determine whether a T had been inserted or whether another kind of mutation had occurred.
ACKNOWLEDGMENTS.
This work was supported by National Institutes of Health Grant CA107650.
Footnotes
The authors declare no conflict of interest.
References
- 1.Seeberg E, Eide L, Bjorås M. The base excision repair pathway. Trends Biochem Sci. 1995;20:391–397. doi: 10.1016/s0968-0004(00)89086-6. [DOI] [PubMed] [Google Scholar]
- 2.Wallace S. In: Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Scandalios JG, editor. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 49–90. [Google Scholar]
- 3.Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3617. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 4.Avkin S, Adar S, Blander G, Livneh Z. Quantitative measurement of translesion replication in human cells: Evidence for bypass of abasic sites by a replicative DNA polymerase. Proc Natl Acad Sci USA. 2002;99:3764–3769. doi: 10.1073/pnas.062038699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence CW, Borden A, Banerjee SK, LeClerc JE. Mutation frequency and spectrum resulting from a single abasic site in a single-stranded vector. Nucleic Acids Res. 1990;18:2153–2157. doi: 10.1093/nar/18.8.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozzherin DJ, Shibutani S, Tan C-K, Downey KM, Fisher PA. Proliferating cell nuclear antigen promotes DNA synthesis past template lesions by mammalian DNA polymerase δ. Proc Natl Acad Sci USA. 1997;94:6126–6131. doi: 10.1073/pnas.94.12.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagher D, Strauss BS. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: Uniqueness of adenine nucleotides. Biochemistry. 1983;22:4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- 8.Sagher D, Strauss B. Abasic sites from cytosine as termination signals for DNA synthesis. Nucleic Acids Res. 1985;13:4285–4298. doi: 10.1093/nar/13.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibutani S, Takeshita M, Grollman AP. Translesional synthesis on DNA templates containing a single abasic site. J Biol Chem. 1997;272:13916–13922. doi: 10.1074/jbc.272.21.13916. [DOI] [PubMed] [Google Scholar]
- 10.Takeshita M, Eisenberg W. Mechanism of mutatiion on DNA templates containing synthetic abasic sites: Study with a double stranded vector. Nucleic Acids Res. 1994;22:1897–1902. doi: 10.1093/nar/22.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuniasse P, et al. An abasic site in DNA. Solution conformation determined by proton NMR, molecular mechanics calculations. Nucleic Acids Res. 1987;15:8003–8022. doi: 10.1093/nar/15.19.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuniasse P, Fazakerley GV, Guschlbauer W, Kaplan BE, Sowers LC. The abasic site as a challenge to DNA polymerase. A nuclear magnetic resonance study of G, C, and T opposite a model abasic site. J Mol Biol. 1990;213:303–314. doi: 10.1016/S0022-2836(05)80192-5. [DOI] [PubMed] [Google Scholar]
- 13.Kalnik MW, Chang C-N, Grollman AP, Patel DJ. NMR studies of abasic sites in DNA duplexes: Deoxyadenosine stacks into the helix opposite the cyclic analogue of 2-deoxyribose. Biochemistry. 1988;27:924–931. doi: 10.1021/bi00403a013. [DOI] [PubMed] [Google Scholar]
- 14.Strauss BS. The “A-rule” of mutagen specificity: A consequence of DNA polymerase bypass of non-instructional lesions? BioEssays. 1991;13:79–84. doi: 10.1002/bies.950130206. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs PEM, Lawrence CW. Novel mutageneic properties of abasic sites in Saccharomyces cerevisiae. J Mol Biol. 1995;251:229–236. doi: 10.1006/jmbi.1995.0430. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs PEM, McDonald J, Woodgate R, Lawrence CW. The relative roles in vivo of Saccharomyces cerevisiae Polη, Polζ, Rev1 protein and Pol32 in the bypass and mutation inductiion of an abasic site, T-T (6–4) photoadduct and T-T cis-syn cyclobutane dimer. Genetics. 2005;169:575–582. doi: 10.1534/genetics.104.034611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, Xie Z, Shen H, Wang Z. Role of DNA polymerase η in the bypass of abasic sites in yeast cells. Nucleic Acids Res. 2004;32:3984–3994. doi: 10.1093/nar/gkh710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haracska L, et al. Roles of yeast DNA polymerases δ and ζ and of Rev1 in the bypass of abasic sites. Genes Dev. 2001;15:945–954. doi: 10.1101/gad.882301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 20.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Hoogsteen base pair formation promotes synthesis opposite the 1,N6-ethenodeoxyadenosine lesion by human DNA polymerase ι. Nat Struct Mol Biol. 2006;13:619–625. doi: 10.1038/nsmb1118. [DOI] [PubMed] [Google Scholar]
- 21.Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: A one- or two-polymerase affair. Genes Dev. 2002;16:1872–1883. doi: 10.1101/gad.1009802. [DOI] [PubMed] [Google Scholar]
- 22.Haracska L, Washington MT, Prakash S, Prakash L. Inefficient bypass of an abasic site by DNA polymerase η. J Biol Chem. 2001;276:6861–6866. doi: 10.1074/jbc.M008021200. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RE, et al. Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev. 1998;12:3137–3143. doi: 10.1101/gad.12.19.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharya N, Johnson RE, Prakash S, Prakash L. Complex formation with Rev1 enhances the proficiency of yeast DNA polymerase ζ for mismatch extension and for extension opposite from DNA lesions. Mol Cell Biol. 2006;26:9555–9563. doi: 10.1128/MCB.01671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerik KJ, Li X, Pautz A, Burgers PMJ. Characterization of the two small subunits of Sacharomyces cerevisiae DNA polymerase δ. J Biol Chem. 1998;273:19747–19755. doi: 10.1074/jbc.273.31.19747. [DOI] [PubMed] [Google Scholar]
- 26.Hoege C, Pfander B, Moldovan G-L, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 27.Haracska L, Torres-Ramos CA, Johnson RE, Prakash S, Prakash L. Opposing effects of ubiquitin conjugation and SUMO modification of PCNA on replicational bypass of DNA lesions in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24:4267–4274. doi: 10.1128/MCB.24.10.4267-4274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 29.Mezzina M, Mench CFM, Courtin P, Sarasin A. Replication of simian virus 40 DNA after UV irradiation: Evidence of growing fork blockage and single-stranded gaps in daughter strands. J Virol. 1988;62:4249–4258. doi: 10.1128/jvi.62.11.4249-4258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarasin AR, Hanawalt PC. Replication of ultraviolet-irradiated simian virus 40 in monkey kidney cells. J Mol Biol. 1980;138:299–319. doi: 10.1016/0022-2836(80)90288-0. [DOI] [PubMed] [Google Scholar]
- 31.Cordeiro-Stone M, Zaritskaya LS, Price LK, Kaufmann WK. Replication fork bypass of a pyrimidine dimer blocking leading strand DNA synthesis. J Biol Chem. 1997;272:13945–13954. doi: 10.1074/jbc.272.21.13945. [DOI] [PubMed] [Google Scholar]
- 32.Cordeiro-Stone M, Makhov AM, Zaritskaya LS, Griffith JD. Analysis of DNA replication forks encountering a pyrmidine dimer in the template to the leading strand. J Mol Biol. 1999;289:1207–1218. doi: 10.1006/jmbi.1999.2847. [DOI] [PubMed] [Google Scholar]
- 33.Svoboda DL, Vos J-MH. Differential replication of a single, UV-induced lesion in the leading or lagging strand by a human cell extract: Fork uncoupling or gap formation. Proc Natl Acad Sci USA. 1995;92:11975–11979. doi: 10.1073/pnas.92.26.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes M, Foiani M, Sogo JM. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Acharya N, Brahma A, Haracksa L, Prakash L, Prakash S. Mutations in the ubiquitin binding UBZ motif of DNA polymerase η do not impair its function in translesion synthesis during replication. Mol Cell Biol. 2007;27:7266–7272. doi: 10.1128/MCB.01196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haracska L, Unk I, Prakash L, Prakash S. Ubiquitylation of yeast proliferating cell nuclear antigen and its implications for translesion DNA synthesis. Proc Natl Acad Sci USA. 2006;103:6477–6482. doi: 10.1073/pnas.0510924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haracska L, Prakash S, Prakash L. Yeast Rev1 protein is a G template-specific DNA polymerase. J Biol Chem. 2002;277:15546–15551. doi: 10.1074/jbc.M112146200. [DOI] [PubMed] [Google Scholar]
- 38.Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 39.Johansson E, Garg P, Burgers PMJ. The Pol32 subunit of DNA polymerase δ contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J Biol Chem. 2004;279:1907–1915. doi: 10.1074/jbc.M310362200. [DOI] [PubMed] [Google Scholar]
- 40.Pursell ZF, Isoz I, Lundstrom E-B, Johansoon E, Kunkel TA. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broschard TH, Koffel-Schwartz N, Fuchs RP. Sequence-dependent modulation of frameshift mutagenesis at NarI-derived mutation hot spots. J Mol Biol. 1999;288:191–199. doi: 10.1006/jmbi.1999.2667. [DOI] [PubMed] [Google Scholar]
- 42.Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]