Abstract
Although prairie voles (Microtus ochrogaster) are socially monogamous, males vary in both sexual and spatial fidelity. Most males form pairbonds, cohabit with one female, and defend territories. Wandering males, in contrast, have expansive home ranges that overlap many males and females. In the laboratory, pairing is regulated by arginine vasopressin and its predominant CNS receptor, vasopressin 1a receptor (V1aR). We investigated individual differences in forebrain V1aR expression of male prairie voles in mixed-sex seminatural enclosures. Individual differences in V1aR were compared with space use measured by radio telemetry and paternity determined with microsatellite markers. Animals engaging in extra-pair fertilizations (EPFs) as either wanderers or paired residents overlapped significantly more in same- and opposite-sex home ranges. Surprisingly, neither social fidelity measured by space use nor sexual fidelity measured by paternity was associated with V1aR expression in the ventral pallidum (VPall) or lateral septum, areas causally related to pairbond formation. In contrast, V1aR expression in the posterior cingulate/retrosplenial cortex (PCing) and laterodorsal thalamus (LDThal), areas implicated in spatial memory, strongly covaried with space use and paternity. Animals engaging in EPFs either as wanderers or paired residents exhibited low levels of LDThal and PCing V1aR expression. Individual differences in brain and behavior parallel differences between prairie voles and promiscuous congeners. The concordance among space use, paternity, and V1aR in spatial circuits suggests a common link between the mechanisms of spatial behaviors and success at EPF. The combined data demonstrate how organismal biology can inform our understanding of individual and species differences in behavioral mechanisms.
Keywords: posterior cingulate cortex, retrosplenial cortex, extra-pair fertilization, monogamy, neurobiology
The origin and nature of phenotypic variation has long been a core concern of evolutionary biology (1–3). Recent research has demonstrated how variation in small numbers of genes can cause profound differences within and between species (4–6). Although species differences must have their roots in individual differences, few studies have examined how molecular mechanisms relate to individual variation in natural settings, and fewer still have focused on phenotypes as complex as vertebrate social behavior (e.g., 7, 8). In the current study, we investigate the contributions of a neuroendocrine gene, the vasopressin 1a receptor (V1aR), to individual differences in male reproductive tactics and mating success among socially monogamous prairie voles (Microtus ochrogaster).
Behavioral diversity emerges from an underlying variation in the function of neural circuits; the contribution of V1aR to vole mating systems is a particularly interesting example of such variation. Prairie voles differ from promiscuous congeners in that they form long-term pairbonds, cohabit with a single partner, defend territories, and exhibit paternal care (9). Among males, these species differences in behavior are caused in part by stable differences in V1aR expression within the forebrain (10, 11). Injections of vasopressin (AVP) antagonists into either the lateral septum (LS) or the ventral pallidum (VPall) disrupt pairbonding in male prairie voles but do not alter mating (12, 13). Viral overexpression of V1aR in the VPall of normally promiscuous meadow voles (M. pennsylvanicus) increases the propensity to form partner preferences (14). Within prairie voles, individual differences in social behaviors and brain V1aR expression are predicted by variation in a microsatellite adjacent to the prairie vole V1aR promoter (15). Together, these data demonstrate that V1aR function contributes to both intra- and interspecific differences in a complex social behavior. Such findings serve as a foundation from which to explore the nature and the consequences of variation in brain and behavior in more natural environments.
Although prairie voles are considered socially monogamous, in natural settings, a significant number of males and females are single at any given time (16). Most males behave monogamously and adopt a “resident” tactic, but some males assume a nonterritorial “wandering” tactic characterized by large home ranges that overlap multiple males and females. Based on available field data, males may switch between these tactics (16). In parallel to this behavioral variation, we have documented extraordinary diversity in the regional expression of V1aR (17). For example, most prairie voles have high levels of V1aR expression in both the posterior cingulate/retrosplenial cortex (PCing) and dorsal thalamus (mediodorsal and laterodorsal nuclei, MDThal and LDThal), but a significant minority lack expression in these regions altogether (17). Through a combination of functional and neuroanatomical studies, these brain regions have been implicated in spatial memory (e.g., 18–22) and are thus well poised to contribute to individual differences in male reproductive tactics. Does such neural variation relate to patterns of natural behavior? Do measures of mating success provide insights into the persistence of these individual differences?
In the current study, we assess the relationships among male reproductive tactics, neural V1aR expression, and mating success. To do so, we placed six naive male and female adult prairie voles into an outdoor seminatural enclosure with eight replicate enclosures in total (n = 48 of each sex). We used radio telemetry to measure patterns of space use. After ≈3 weeks, we removed animals from the enclosures, determined the paternity of developing embryos, and assessed V1aR expression in the forebrains of males. Together, these data allowed us to ask whether individual differences in V1aR expression in the VPall or LS predicted whether males adopted resident or wandering tactics or were likely to engage in extra-pair fertilizations (EPFs). We next assayed V1aR expression in the PCing and LDThal and asked whether either was related to patterns of male space use or sexual fidelity. By comparing the associations among space use, offspring paternity, and forebrain V1aR, we hope to clarify the nature and persistence of neural and behavioral variation in this species.
Results
Animals.
We recovered 43 males and 38 females serving in this experiment (5 males and 10 females died before recovery). Of the surviving animals, two females shed their radio collars and were therefore excluded from analysis involving space use because we were unable to properly assess their home range size or overlap.
Pairing and Paternity.
A majority of males and females were classified as members of a pair [males, 32 of 43 (74.4%); females, 31 of 36 (86.1%)] (see Methods) (23).
We assessed the paternity of 99 embryos gathered from 27 pregnant females (72.3%). We were unable to assess the pairing status of one pregnant female who shed her radio collar during radio tracking, and we therefore excluded her from our analyses. We determined whether the embryos for the remaining 26 females were the result of an in-pair fertilization (IPF) or an EPF. The methods and results for paternity analyses are reported in detail elsewhere (23). Briefly, a significantly greater proportion of resident males sired offspring than did wandering males (Fisher's exact test; successful vs. unsuccessful residents, 23:9, wanderers, 3:8; P = 0.01). Of the 23 successful resident males, 19 sired offspring with their partner alone, and 4 sired offspring outside of the pair.
In our subsequent analyses, we define successful males as those who sired offspring during the course of our experiment. We define an EPF male as one who sired offspring with a female who was not a partner, which included both successful wanderers and “unfaithful” residents. IPF males are defined as resident males who sired offspring only with a partner. In total, we found 7 EPF and 19 IPF males.
Morphological Correlates of Reproductive Tactics and Mating Success.
Males who adopted a resident or wandering tactic were not significantly different with respect to age, body length, initial body mass, or weight change, nor did these variables differ with mating success (P > 0.10) (Table 1), indicating that these measures do not explain the behavioral or paternity variation we observed. Successful resident and wandering males showed no weight change, whereas unsuccessful wanderers lost weight and unsuccessful residents gained weight, resulting in a trend toward an interaction [F(1,36) = 3.56, P = 0.07] (Table 1). For completeness, we also regressed body mass against body length and took the residual mass as a measure of male condition. We found no differences between residents and wanderers in either initial condition [Student's unpaired t test, two tailed; t(42) = 1.37, P = 0.18] or final condition [t(42) = 1.13, P = 0.26].
Table 1.
Means table for successfully and unsuccessfully breeding resident and wandering male prairie voles*
| Resident |
Wanderer |
|||
|---|---|---|---|---|
| Unsuccessful | Successful | Unsuccessful | Successful | |
| Age, days | 86.1 ± 10.74 | 87.7 ± 5.60 | 79.0 ± 12.88 | 109.3 ± 20.76 |
| Total length, mm | 109.2 ± 2.10 | 110.3 ± 1.30 | 107.3 ± 1.17 | 111.0 ± 2.67 |
| Weight, g | 33.9 ± 1.74 | 38.1 ± 1.64 | 37.9 ± 3.44 | 33.0 ± 1.53 |
| Δ Weight, g | 5.86 ± 1.65 | 0.70 ± 1.23 | −3.29 ± 3.66 | 1.33 ± 1.33 |
| Home range, m2 | 46.0 ± 18.12 | 30.6 ± 3.82 | 59.8 ± 11.53 | 66.22 ± 18.01 |
| VPall, dpm TE | 1,958.5 ± 258.77 | 1,921.3 ± 143.19 | 1,609.9 ± 98.45 | 1,597.5 ± 232.30 |
| LS.dpm TE | 943.7 ± 204.50 | 874.9 ± 100.40 | 927.6 ± 146.27 | 515.5 ± 373.95 |
| PCing, dpm TE | 661.6 ± 251.13 | 660.2 ± 148.93 | 985.6 ± 333.66 | −0.8 ± 42.77 |
| LDThal, dpm TE | 2,616.0 ± 502.09 | 3,323.2 ± 253.92 | 3,400.0 ± 245.28 | 2,376.7 ± 341.72 |
| MDThal, dpm TE | 1,516.5 ± 391.77 | 1,944.1 ± 206.17 | 1,745.1 ± 375.51 | 1,640.0 ± 364.36 |
*Means (±SE) for 10 dependent variables: age at introduction, total body length (total length) at recovery, weight at introduction, weight change (Δ Weight) at recovery, core home range size, and V1aR expression [125I-linear-AVP-specific binding disintegrations per minute (dpm) in tissue equivalence (TE)] in the VPall, LS, PCing, LDThal, and MDThal.
Reproductive Tactics and Space Use.
Core home range size ranged from 8.1 to 188.5 m2 (mean ± SE: 43.7 ± 5.38) (Table 1) for males and from 5.8 to 109.4 m2 (35.4 ± 3.98) for females. Using a nested ANOVA, we found a nonsignificant trend for resident males to have smaller home ranges than wandering males [F(1,39) = 3.85, P = 0.06].
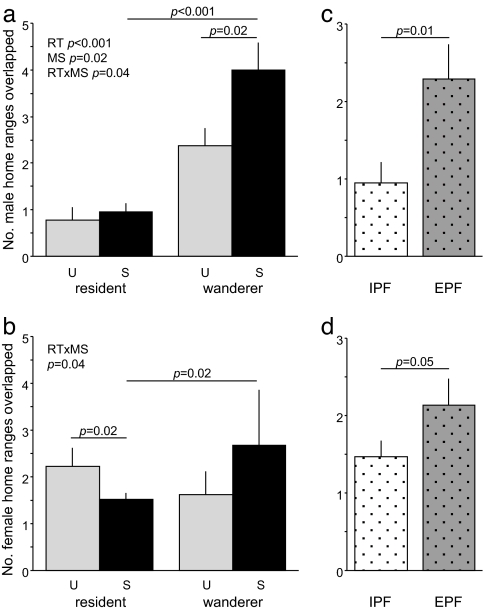
Core home ranges of resident males overlapped fewer male home ranges than did wandering males [F(1,39) = 42.31, P < 0.0001] (Fig. 1a) (for discussion of core home range uses in this context, see ref. 23). Overall, successful males overlapped fewer males than did unsuccessful males [F(1,39) = 6.39, P = 0.02]. These main effects, however, were accompanied by a significant interaction between reproductive tactic (RT) and mating success [MS; RT × MS interaction F(1,39) = 4.11, P = 0.04]. Successful wanderers overlapped significantly more males than did unsuccessful wanderers [Student's t test, one tailed; t(9) = 2.29, P = 0.02]. Similarly, successful wanderers overlapped more males than did successful residents [t(24) = 5.58, P < 0.001]. Successful and unsuccessful residents did not differ [t(30) = 0.53, P = 0.30].
Fig. 1.
Patterns of space use and paternity. (a) Mean (+SE) number of male home ranges overlapped by residents and wanderers who were unsuccessful (U, gray) or successful (S, black) at siring young. (b) Number of female home ranges overlapped by successful and unsuccessful residents and wanderers. (c) Number of males overlapped by IPF (white stipple) and EPF males (gray stipple). (d) Number of females overlapped by IPF and EPF males. Significant (P < 0.05) ANOVA and post hoc t test results are reported. RT, reproductive tactic (resident/wanderer); MS, mating success (U/S); IPF, residents mating with a partner only; EPF, successful wanderers plus residents who sired offspring outside the pair.
To describe how males use space relative to females, we also measured the number of females a male could encounter. Although neither reproductive tactic [F(1,39) = 0.44, P = 0.51] nor mating success [F (1, 39) = 0.17, P = 0.68] was associated with the number of female home ranges that males overlapped, there was a significant interaction between these factors [RT × MS, F(1,39) = 4.41, P = 0.04] (Fig. 1b). Successful residents overlapped significantly fewer females than unsuccessful residents [Student's t test, one tailed; t(30) = 2.12, P = 0.02]. In contrast, successful wanderers overlapped significantly more females than did successful residents [t(24) = 2.13, P = 0.02].
Similar patterns emerged when we compared space use split by sexual fidelity. EPF males overlapped more male home ranges than did IPF males [t(24) = 2.53, P = 0.01] (Fig. 1c), and there was a similar pattern in opposite-sex overlaps [t(24) = 1.68, P = 0.05] (Fig. 1d). In principle, this relationship between extra-pair paternity and space use could be driven by the fact that our definition of EPF males includes both unfaithful residents and successful wanderers, whereas IPF males are always residents. To examine this possibility, we calculated the Z scores of male–male overlaps within a reproductive tactic (resident or wanderer), including animals that did not mate. We then used these Z scores to ask whether EPF males had more male–male overlaps after correcting for overall differences between resident and wanderer tactics. We repeated this analysis for male–female overlaps. As with the untransformed data, this effect is strong for male–male overlaps (P = 0.003) and exhibits a trend in male–female overlaps (P = 0.06). Because of space constraints, we present only the untransformed data (Fig. 1 c and d).
V1aR Expression.
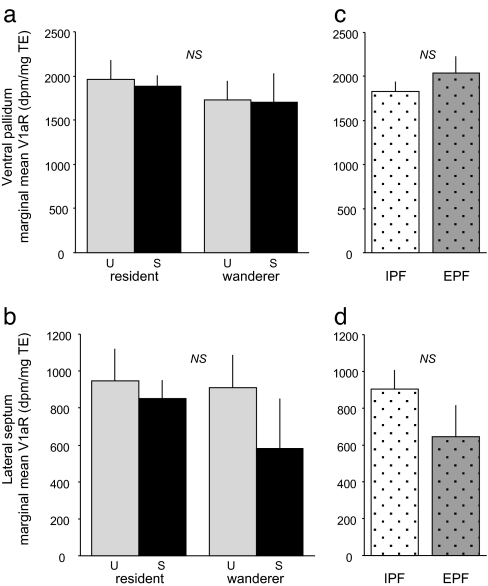
Our data demonstrate low levels of V1aR variation in both the VPall and LS (Fig. 2). Because of their important roles in pairbond formation, we expected to find individual differences in V1aR expression between residents and wanderers in the VPall and LS. Our data did not support this prediction [MANOVA with average specific binding as a covariate; covariate F(2,34) = 6.63, P = 0.004; reproductive tactic, multivariate F(2,34) = 0.80, P = 0.46] (Fig. 3 a and b). Furthermore, there was no significant influence of mating success [F(2,34) = 0.66, P = 0.52], nor was there an interaction between reproductive tactic and mating success [F(2,34) = 0.19, P = 0.83]. Similarly, variation in the VPall and LS did not predict whether males exhibited sexual fidelity to a partner. Comparing males that sired offspring only with their partner (IPF males) to males that sired offspring at least once outside of a pair (EPF males) revealed no differences in these brain structures [multivariate F(2,22) = 0.99, P = 0.39] (Fig. 3 c and d).
Fig. 2.
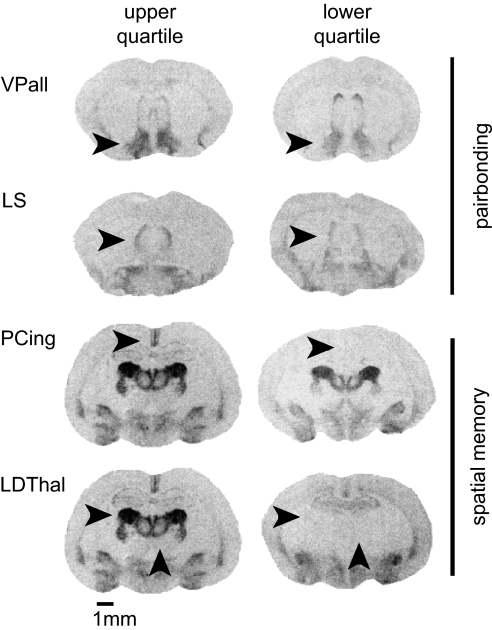
Variation in V1aR expression reflected in ligand binding. Autoradiograms of upper and lower quartile 125I-AVP binding in VPall, LS, PCing, and LDThal, indicated by horizontal arrows. The MDThal (vertical arrow) exhibits expression that covaries strongly with the LDThal and less strongly but significantly with PCing and so was used as a covariate in multivariate analyses of PCing/LDThal. (Scale bar: 1 mm.)
Fig. 3.
V1aR in ventral palladium (VPall) and LS does not predict pairing, mating success, or paternity type. (a) Marginal mean (+SE) V1aR expression in VPall measured as tissue equivalent (TE) disintegrations per minute (dpm TE) of 125I-AVP binding for animals varying in reproductive tactic (RT, resident/wanderer) and mating success (MS; U, unsuccessful; S, successful). (b) Marginal mean (+SE) V1aR expression in LS for successful and unsuccessful residents and wanderers. (c) Marginal mean (+SE) V1aR expression in VPall for IPF and EPF males. (d) Marginal mean (+SE) V1aR expression in LS for IPF and EPF males. Marginal means are adjusted for variation in average specific binding, a measure of tissue quality (see Methods). Raw means and standard errors are provided in Table 1. None of the multivariate comparisons (RT× MS MANOVA or IPF/EPF MANOVA) was significant (P > 0.10).
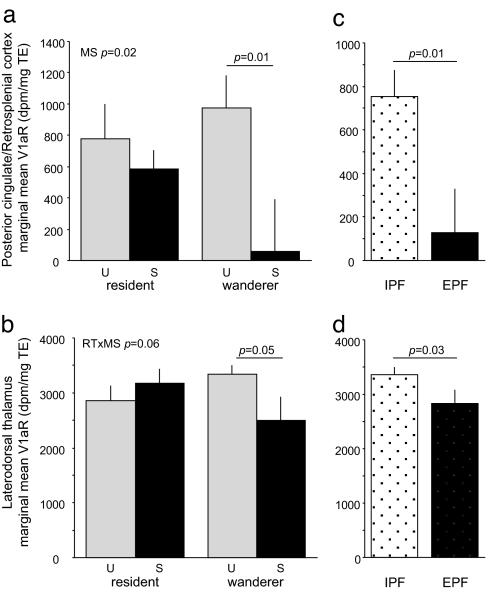
As other studies (15, 17) have reported, V1aR expression in the PCing and LDThal were highly variable (Fig. 2). Although there was no main effect of reproductive tactic [MANOVA with MDThal as covariate; covariate P < 0.001; reproductive tactic F(2,35) = 0.35, P = 0.71] (Fig. 4 a and b), there was a significant main effect of mating success [F(2,35) = 3.87, P = 0.03]. This main effect was accompanied by a significant interaction between reproductive tactic and mating success [RT × MS, F(2,35) = 3.98, P = 0.03] as attributable to PCing binding [F(1,40) = 5.69, P = 0.02; LDThal F(1,40) = 0.80, P = 0.38]. Both regions showed trends toward significant interactions in univariate tests [PCing RT × MS, F(1,40) = 2.43, P = 0.13; LDThal RT × MS, F(1,40) = 3.84, P = 0.06], but these differences reached significance only when the regions were considered simultaneously. The significant multivariate interaction between reproductive tactic and mating success thus reflects V1aR binding across both areas. Post hoc tests reveal that successful wanderers have significantly lower binding than unsuccessful wanderers in both the PCing and LDThal [Student's t test on V1aR binding adjusted for MDThal, one tailed; PCing t(24) = 2.68, P = 0.01; LDThal t(24) = 1.85, P = 0.049] (Fig. 4 a and b).
Fig. 4.
V1aR expression in spatial circuits predicts space use and paternity. (a) Marginal mean (±SE) V1aR expression in PCing for animals varying in reproductive tactic (RT) and mating success (MS). (b) V1aR expression in LDThal for successful and unsuccessful residents and wanderers. (c) V1aR expression in PCing for IPF and EPF males. (d) V1aR expression in LDThal for IPF and EPF males. Marginal means are adjusted for variation in MDThal (see Methods). Raw means and standard errors are provided in Table 1. MANOVA comparing V1aR expression as a function of RT and MS (a and b) produced a significant main effect of MS (P = 0.03) and a significant RTxMS interaction (P = 0.03). MANOVA comparing IPF and EPF males across brain regions also was significant (P < 0.001). Significant ANOVA and post hoc t test results are reported.
As in the space-use data, we find parallels between successful wanderers and the broader group of EPF males. EPF males have significantly less binding in these spatial circuits than do IPF males [MANOVA, MDThal covariate F(2,22) = 41.05, P < 0.001; multivariate F(2,22) = 6.80, P = 0.005] (Fig. 4 c and d). This effect is pronounced in the PCing [t(24) = 2.61, P = 0.01] and is also present in the LDThal [t(24) = 1.90, P = 0.03].
Discussion
In both behavioral and neuronal phenotypes, we find that our laboratory-reared animals living in seminatural enclosures exhibited variation comparable to wild, free-living animals (16, 17). Other researchers report similar patterns of V1aR variation in animals with controlled sexual experience (15, 24), suggesting that the neural differences we report are not caused by the varied experiences of males in these enclosures.
In terms of space use, males adopted the resident and wanderer tactics previously reported (16). On average, successful resident males overlapped few males and just one female (Fig. 1). Wanderers overlapped significantly more males than did residents, and this behavior was associated with a trend toward larger home range sizes. Of the two tactics, resident males are much more likely to fertilize females (23). Because resident males are likely to gain additional fitness advantages through paternal care or infanticide deterrence (25–27), it seems that residency is the favored tactic, whereas wandering makes the best of a bad situation (23). In our data, tactic was not associated with variation in body size, weight, or condition (Table 1) (also see ref. 28). Residents have a longer anogenital distance (AGD) (29), however, which suggests exposure to a more masculinizing environment in utero (30). In prairie voles, long AGD males are preferred by females and have higher sperm counts (29). These traits limit the ability of short AGD males to become successful residents, and thus may favor wandering as an alternative tactic.
Given that a particular male adopted a resident or wandering tactic, the behavioral attributes that predict mating success within those tactics differ substantially (Fig. 1 a and b). Resident males maximize mating success by processes that facilitate mate guarding, such as reducing the size of the area defended, excluding other males, and centering space use around a single female. Wandering males, in contrast, were only successful when they overlapped many male and female home ranges, presumably increasing the likelihood of opportunistic mating. Although paired males generally succeeded by mate guarding, a subset was able to obtain EPFs. Combining space-use data from promiscuous residents and successful wanderers reveals that EPF males as a group are characterized by high same- and opposite-sex overlap (Fig. 1c). This pattern persists even after correcting for differences in overlap associated with wandering or residency. Although EPFs are less common than IPFs, they seem to be important in maintaining diverse patterns of space use.
How is such behavioral variation reflected in neuronal phenotypes? Is there a pattern of forebrain V1aR expression that characterizes individual differences in monogamy? We first examined the expression of V1aR in the LS and the VPall, two limbic regions in which V1aR function coordinates pairbond formation in the laboratory (13, 14). Based on such causal data, as well as previously documented species differences, we hypothesized that resident and wandering males would differ in the abundance of V1aR expressed in either the LS or the VPall. Despite substantial numbers of resident (n = 32) and wandering (n = 11) males, we found no significant differences in either structure (Fig. 3 a and b). Variation in these regions also was unable to predict sexual fidelity (Fig. 3 c and d). Given that resident males have much greater mating success than wandering males, one interpretation of these findings is that selection favors males who have the ability to form pairbonds (23) and has thus eliminated much of the variation in these structures. This interpretation is supported by the low levels of variation in the VPall and the LS (Fig. 2) (15, 17). Interestingly, Hammock and Young (15) find significantly correlated variation in laboratory pairbonding, LS V1aR, and the length of a microsatellite at the prairie vole V1aR locus. Because we did not find a similar relationship between LS and residency, our data indicate that other factors play a greater role in determining individual differences in pairbond formation and sexual fidelity in natural settings. These factors may include variation in mating frequency and its putative effects on vasopressin release (e.g., see ref. 31) or environmental influences on other regulators of male attachment, such as cortisol (32) and corticotrophin-releasing factor (33, 34).
Although V1aR expression in the VPall and the LS was relatively stable across individuals, expression in the PCing and LDThal was highly variable (Fig. 2) (also see refs. 15 and 17). We found a significant interaction between reproductive tactic and mating success that mirrored patterns of space use (Figs. 1 and 4). Successful wanderers were defined by a low level of V1aR expression in the PCing and LDThal (Fig. 4 a and b). Indeed, V1aR in these regions was a good predictor of sexual fidelity in general, particularly in PCing (Fig. 4 c and d). Interestingly, V1aR expression in PCing also predicts sexual fidelity across vole species. PCing V1aR expression is especially robust in pine voles (24), a species thought to be genetically monogamous (35). Promiscuous meadow and montane voles, like EPF prairie voles, lack PCing expression altogether (Table 1) (24).
The strong concordance between space-use measures and V1aR expression in spatial circuits suggests a common contribution of these phenotypes to EPF. PCing and LDThal are strongly connected to one another and to the hippocampus (22, 36), which is central to spatial memory (37–38). Neuronal activity and postlesion deficits confirm the role of PCing in spatial learning (18–19, 21, 39), whereas some studies associate it with social and emotional memory as well (40, 41). The role of LDThal in spatial memory is less studied (20), but our data suggest that its contribution to space-use and extra-pair paternity is weaker than that of PCing. Last, we note that vasopressin regulates aggression, dominance learning, and social memory in a number of rodents (42–44). These data lead us to suggest that V1aR expression in PCing and perhaps LDThal modulates memory for the spatial location of aggressive interactions, which influences rates of extra-pair paternity.
Wandering, less competitive males should encounter estrus females more often if they intrude into more territories. Similarly, paired males seeking EPFs must leave their own territories to intrude on neighbors. Among voles and many other taxa, local residents have substantial advantages over intruders (45–47), and entering the territory of another carries significant risk of defeat. We hypothesize that the increase in same-sex home range overlap by successful wanderers, and by EPF males in general (Fig. 1 a and c), results from an inability to link social defeats with contextual cues. Thus, the reduced V1aR in PCing and LDThal could represent a mechanism for adaptive forgetting that facilitates EPF by promoting intrusion. If this hypothesis is correct, animals with naturally or experimentally reduced V1aR will be less able to form associations between place and defeat. Such experiments would aid in distinguishing this hypothesis from plausible alternatives, including memory for sites of sexual interactions and nonspatial functions of these brain regions.
By integrating measures of natural behaviors, mating success, and receptor expression, we arrive at an enriched understanding of individual and species differences in brain and behavior. In a sense, we find evidence for a monogamous brain, but the nature of this phenotype comes as a surprise. Brain regions responsible for the capacity of prairie voles to pairbond (VPall and LS) do not predict intraspecific variation in sexual or social fidelity in the field. In contrast, regions implicated in spatial navigation (PCing and LDThal) effectively predict space use and extra-pair paternity. We suggest that among prairie voles, EPFs enable the persistence of individual differences in space use and its neural substrates; this diversity in turn reveals relationships between individual and species differences in social behavior and its mechanisms. Together these data highlight how evolutionary and ecological approaches contribute to a deeper understanding of the origins and consequences of natural variation in brain and behavior.
Methods
Test Animals.
We used 48 male and 48 female prairie voles to investigate how individual differences in brain phenotype related to space use and sexual fidelity. At weaning, we grouped all animals into same-sex littermates and maintained them under a 14:10 light:dark cycle in 29 × 18 × 13-cm polycarbonate cages. Food and water were provided ad libitum, and the temperature was maintained at 21 ± 2°C. All animals used were reared with at least one other littermate in their home cage.
Animals were distributed into eight groups, each consisting of six nulliparous females and six adult, sexually mature males. All individuals were ear-tagged and weighed, and a tail clipping was taken before introduction to field enclosures. All animals were of similar age and weight. Further details on field and paternity methods can be found in Ophir et al. (23).
Field Enclosures.
The study was conducted in four field enclosures located on the University of Memphis South Campus (for details, see refs. 23, 49, and 50). Each enclosure measured 20 × 30 m and consisted primarily of mixed pasture grasses (e.g., rye, fescue, and brome) and dicots. Densities were within the range of natural densities reported elsewhere (16, 57).
Radio Telemetry and Trapping.
We outfitted each vole with a 1.9-g transmitter and collar (BD-2C; Holohil Systems) 2 days before introduction to the field. Animals were tracked with an LA12 radio telemetry receiver (AVM Instruments) to within 1 m of their actual location.
To initiate a trial, we placed six female and six male voles in an enclosure. All animals were standardized for age and body mass across enclosures at the start of each trial. We ran a series of four trial blocks each consisting of two simultaneous trials over the 2004 breeding season.
We recorded telemetry readings twice daily for at least 12 days, varying time of day and enclosure order. On day 18, we began trapping animals and removing them from the enclosure, allowing enough time for fertilization but not parturition (gestation is 21 days). All animals were trapped and removed from enclosures within 4 days and before any births occurred. We recorded AGD, body mass and length, male testis size, and size and number of embryos (per female). We collected tissue from all animals and the embryos of females for genetic parentage analysis (reported in ref. 23).
Home Range Size and Space Use.
We used the software package Ranges V (Anatrack) to calculate minimum convex polygons (MCP) with 75% fixes from the assembled x and y coordinates to estimate the size of each core home range. We focused on the central 75% of data points to estimate the core home ranges without resorting to more complex statistical kernel methods (refs. 51 and 52; also see ref. 23 for more discussion). From these MCPs, we calculated the percent of home range overlap between pairs of individuals.
Pair Determination.
We estimated encounter rates between each pair of individuals by taking the product of the proportion of home range area one individual overlapped another and vice versa. To determine which individuals should be considered pairs, we calculated relative encounter rates (RER) for each possible pair (23). The RER is defined as the encounter rate for a given pair divided by the sum of encounter rates for all opposite-sexed individuals. An RER of ≥0.5 indicates that a given male encountered a given female more frequently than all other females combined. We defined a pair as a male–female couple in which both animals encountered one another more frequently than all other opposite-sexed individuals combined. For this study, we equate a paired male with a resident and a single male with a wanderer.
Tissue Extraction and Autoradiography.
We killed subjects with CO2, followed by rapid decapitation to collect brains from subjects serving in the field enclosures. Once dissected, the brains were frozen on powdered dry ice and stored at −70°C until sectioning. Four sets of 20-μm-thick coronal slices at 100-μm intervals were mounted on Superfrost slides (Fisher Scientific) and stored at −70°C. To visualize and quantify V1aR binding (24, 53), we used standard protocols for autoradiography by using 125I-linear AVP (PerkinElmer). Briefly, sections were lightly fixed, incubated with 50 pM 125I-linear AVP, washed in Tris buffer, and air dried. Sections were then exposed to film for 72 h alongside radiographic standards. High expression of V1aR results in high binding of radioactive ligand and can be measured by the optical density of film exposed to the tissue sections. We investigated forebrain V1aR by digitizing films on a Microtek ScanMaker 5900 and quantifying the standardized scans by using NIH ImageJ software. We estimated nonspecific binding from background levels of cortical binding on the same sections (53).
Data Analysis.
Before beginning a full analysis, we determined whether the enclosures differed significantly in the frequency of pairing or extra-pair paternity by using a replicated G test (54). When examining male residency, for example, we calculated the proportion of all males who were residents or wanderers, and we used these proportions to determine the expected numbers of residents and wanderers in each enclosure. We used the heterogeneity G statistic to quantify enclosure effects (54) and estimated its null distribution by using Monte Carlo methods (10,000 replicates). We found no significant enclosure effects on male pairing (G = 6.18, P = 0.78) and EPF/IPF frequencies (G = 11.42, P = 0.30), and thus we pooled data across enclosures for the ANOVA methods described immediately below.
We compared home range size and same- and opposite-sex home range overlap by using ANOVA. Specifically, we used two-factor ANOVAs to investigate differences between RT (resident or wanderer) and MS (successful or unsuccessful) for males for each of these measures of space use. (We define a successful male as any individual who sired offspring.) We used Student's t tests to compare variation in these measures across animals who mated with a partner (IPF) to those who mated with a female who was not a partner (EPF includes successful wanderers as well as residents who mated outside the pair).
When examining neural variation, we performed a MANOVA on data from regions previously implicated in pair bonding (VPall and LS) by using the average specific V1aR binding across all V1aR-expressing forebrain regions as a covariate to control for variation in tissue quality. We present graphical data as “marginal” means and standard errors, a term that indicates that the data have been adjusted to correct for variation in the covariate, average specific binding (Fig. 3). The uncorrected data are given in Table 1. Including a covariate improved our statistical power, but did not alter the overall patterns. In all MANOVAs, we report F statistics and associated P values by using Roy's Largest Root.
We performed a separate MANOVA on data from brain regions implicated in spatial memory (PCing and LDThal). Variation in the LDThal is strongly correlated with the developmentally related but functionally distinct MDThal (in current data, r = 0.77, P < 0.001) (also see Fig. 2) (17). To separate the contributions of the PCing and LDThal from the contributions of variation in MDThal, we initially used both average specific and MDThal binding as covariates. However, entering MDThal in our model as a covariate rendered average specific binding uninformative [MDThal covariate F(2,35) = 23.59, P < 0.001; average specific binding covariate F(2,35) = 0.02, P = 0.98], so average specific binding was removed from the model. Marginal means (Fig. 4) have thus been corrected for variation in MDThal; uncorrected data are given in Table 1. Omitting average specific binding did not alter our pattern of significant results.
We used MANOVAs to compare brain V1aR expression between EPF and IPF individuals in the VPall and the LS (average specific binding as covariate) and in the PCing and LDThal (MDThal as covariate). When performing post hoc tests in analyses with covariates, we used Student's t tests on residuals after adjusting for the covariate.
When performing t tests, we used one-tailed tests when expectations for the direction of effects had clear precedents. We expected phenotypes of successful wanderers or EPF males to resemble those of promiscuous species (increased conspecific overlap and lower V1aR expression in VPall, PCing, and LDThal). Similarly, we expected the reverse of successful residents and IPF males. Because of conflicting patterns in the LS (antagonists reduce pairbond formation, but species differences indicate lower LS expression in prairie voles), we did not have a priori predictions for individual differences in this brain region. In practice, all reported t tests were one tailed except those comparing condition of residents and wanderers.
ACKNOWLEDGMENTS.
We thank Dr. Anna Bess Sorin for involvement in paternity analyses and Dr. Polly Campbell, Dr. Walt Wilczinski, and three anonymous reviewers for useful critiques of this manuscript. This work was supported by National Science Foundation Grants 0316631 (to J.O.W.) and 0316451 (to S.M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 2.Dobzhansky T. Cold Spring Harbor Group Symp Quant Biol. 1955;20:1–15. doi: 10.1101/sqb.1955.020.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Lott DF. Intraspecific Variation in the Social Systems of Wild Vertebrates. Cambridge, UK: Cambridge Univ Press; 1991. [Google Scholar]
- 4.Orr HA. Nature Rev Gen. 2005;6:119–127. doi: 10.1038/nrg1523. [DOI] [PubMed] [Google Scholar]
- 5.Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP. Science. 2006;313:101–104. doi: 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- 6.Prud'homme B, Gompel N, Rokas A, Kassner VA, Williams TM, Yeh SD, True JR, Carroll SB. Nature. 2006;440:1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- 7.Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ. Behav Gen. 2006;36:163–172. doi: 10.1007/s10519-005-9017-8. [DOI] [PubMed] [Google Scholar]
- 8.Howell S, Westergaard G, Hoos B, Chavanne TJ, Shoaf SE, Cleveland A, Snoy PJ, Suomi SJ, Higley JD. Am J Primatol. 2007;69:851–865. doi: 10.1002/ajp.20369. [DOI] [PubMed] [Google Scholar]
- 9.Getz LL, Carter CS. Am Sci. 1996;84:56–62. [Google Scholar]
- 10.Young LJ, Wang Z. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Young LJ, Liu Y, Insel TR. J Comp Neurol. 1997;378:535–546. [PubMed] [Google Scholar]
- 12.Winslow JT, Hastings N, Cater CS, Harbaugh CR, Insel TR. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Curtis JT, Wang ZX. Behav Neurosci. 2001;155:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 14.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 15.Hammock EAD, Young LJ. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- 16.Getz LL, McGuire B, Hofmann J, Pizzuto T, Frase B. J Mammal. 1993;74:44–58. [Google Scholar]
- 17.Phelps SM, Young LJ. J Comp Neurol. 2003;466:564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- 18.Cooper BG, Mizumori SJY. J Neurosci. 2001;21:3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire EA. Scan J Psycol. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- 20.van Groen T, Kadish I, Wyss JM. Behav Brain Res. 2002;136:329–337. doi: 10.1016/s0166-4328(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 21.Harker KT, Whishaw IQ. Neurosci Biobehav Rev. 2004;28:485–496. doi: 10.1016/j.neubiorev.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Shinkai M, Yokofujita J, Oda S, Murakami K, Igarashi H, Kuroda M. Anat Embryol. 2005;210:317–326. doi: 10.1007/s00429-005-0047-z. [DOI] [PubMed] [Google Scholar]
- 23.Ophir AG, Phelps SM, Sorin AB, Wolff JO. Anim Behav. 2008 in press. [Google Scholar]
- 24.Insel TR, Wang ZX, Ferris CF. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZX, Novak MA. J Comp Psychol. 1992;106:163–171. [Google Scholar]
- 26.Gubernick DJ, Teferi T. Proc R Soc London B. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff JO, Macdonald DW. Trends Ecol Evol. 2004;19:127–134. doi: 10.1016/j.tree.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Solomon NG, Jacquot JJ. Can J Zool. 2002;80:951–955. [Google Scholar]
- 29.Ophir AG, DelBarco-Trillo J. Physiol Behav. 2007;92:533–540. doi: 10.1016/j.physbeh.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Vandenbergh JG. Am Sci. 2003;91:218–225. [Google Scholar]
- 31.Insel TR, Preston S, Winslow JT. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 32.DeVries AC, DeVries MB, Taymans S, Carter CS. Proc Natl Acad Sci USA. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 34.Lim MM, Liu Y, Ryabinin AE, Bai YH, Wang ZX, Young LJ. Horm Behav. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marfori MA, Parker PG, Gregg TG, Vandenbergh JG, Solomon NG. J Mammal. 1997;78:715–724. [Google Scholar]
- 36.Kobayashi Y, Amaral DG. J Comp Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- 37.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 38.Sherry DF, Jacobs LF, Gaulin SJC. Trends Neurosci. 1992;15:298–303. doi: 10.1016/0166-2236(92)90080-r. [DOI] [PubMed] [Google Scholar]
- 39.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 40.Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. Brain. 2001;124:804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- 41.Maddock RJ. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 42.Albers HE, Bamshad M. In: Advances in Brain Vasopressin (Progress in Brain Research. Urban IJA, Burbach JPH, DeWied D, editors. Amsterdam: Elsevier Science; 1998. pp. 395–408. [Google Scholar]
- 43.Danzer R, Bluthe RM, Koob GF, Le Moal M. Psychopharmacology. 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 44.Goodson JL, Bass AH. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 45.Archer J. The Biology of Aggression. Cambridge, UK: Cambridge Univ Press; 1987. [Google Scholar]
- 46.Maynard Smith J, Parker GA. Nature. 1976;24:159–175. [Google Scholar]
- 47.Wolff JO, Freeberg MH, Dueser RD. Behav Ecol Sociobiol. 1983;12:237–242. [Google Scholar]
- 48.Yoder JM, Dooley JL, Zawacki JF, Bowers MA. Am Midl Nat. 1996;135:1–8. [Google Scholar]
- 49.Ophir AG, Phelps SM, Sorin AB, Wolff JO. J Mammal. 2007;88:989–999. [Google Scholar]
- 50.Mahady SJ, Wolff JO. Behav Ecol Sociobiol. 2002;52:31–37. [Google Scholar]
- 51.White GC, Garrott RA. Analysis of Wildlife Radio-Tracking Data. New York: Academic; 1990. [Google Scholar]
- 52.Row JR, Blouin-Demers G. Copeia. 2006;4:797–802. [Google Scholar]
- 53.Young LJ, Winslow JT, Nilsen R, Insel TR. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- 54.Sokal RR, Rohlf FJ. Biometry. San Francisco: Freeman; 1981. [Google Scholar]