Abstract
We previously reported that mast cells express renin, the rate-limiting enzyme in the renin–angiotensin cascade. We have now assessed whether mast cell renin release triggers angiotensin formation in the airway. In isolated rat bronchial rings, mast cell degranulation released enzyme with angiotensin I-forming activity blocked by the selective renin inhibitor BILA2157. Local generation of angiotensin (ANG II) from mast cell renin elicited bronchial smooth muscle contraction mediated by ANG II type 1 receptors (AT1R). In a guinea pig model of immediate type hypersensitivity, anaphylactic mast cell degranulation in bronchial rings resulted in ANG II-mediated constriction. As in rat bronchial rings, bronchoconstriction (BC) was inhibited by a renin inhibitor, an AT1R blocker, and a mast cell stabilizer. Anaphylactic release of renin, histamine, and β-hexosaminidase from mast cells was confirmed in the effluent from isolated, perfused guinea pig lung. To relate the significance of this finding to humans, mast cells were isolated from macroscopically normal human lung waste tissue specimens. Sequence analysis of human lung mast cell RNA showed 100% homology between human lung mast cell renin and kidney renin between exons 1 and 10. Furthermore, the renin protein expressed in lung mast cells was enzymatically active. Our results demonstrate the existence of an airway renin–angiotensin system triggered by release of mast-cell renin. The data show that locally produced ANG II is a critical factor governing BC, opening the possibility for novel therapeutic targets in the management of airway disease.
Keywords: angiotensin II, angiotensin II type 1 receptors, asthma, hypersensitivity, lung
The renin–angiotensin system (RAS) has been traditionally viewed as a circulating axis, whereby renin is released into the circulation from the kidneys in response to decreased renal perfusion pressure, decreased delivery of NaCl at the macula densa, and/or increased renal sympathetic nerve activity (1). The proteolytic cleavage of angiotensinogen (aogen) by renin constitutes the rate-limiting step of the RAS cascade.
In addition to this classical view, it is believed that many tissues, including the lung, may possess the capacity to generate angiotensin (ANG II) locally (2, 3). This is supported by experiments demonstrating that ANG II persists in lung of nephrectomized rats (4) and findings showing that ANG II can be elevated in the lungs in the absence of an elevated systemic RAS (5). Components of RAS have been identified in lung tissue, including aogen mRNA (6, 7), and angiotensin-converting enzyme (ACE), the pulmonary endothelium being the primary source for ACE in the body (8). ANG II receptors are also expressed in lung tissue, with the ANG II type 1 receptor (AT1R) subtype found on bronchial smooth muscle cells (9) and ANG II type 2 receptor (AT2R) observed on the bronchial epithelial cell brush border (10). An intrapulmonary source of renin protein has not yet been identified.
We previously reported that mast cells synthesize, store, and release active renin capable of triggering ANG II formation (11). In addition, we demonstrated that renin release from cardiac mast cells triggers a local cardiac RAS that causes arrhythmias in a model of ischemia/reperfusion (12). In that the upper and lower airways are populated by mast cells (13), we undertook the present study to determine whether the release of renin from bronchial mast cells triggers the activation of a local RAS in the airway. Because ANG II is a potent bronchoconstricting agent in vivo (14), we focused on the relationship between mast cell renin release, ensuing formation of ANG II, and bronchial smooth muscle contraction.
Our findings in bronchus demonstrate that renin is released with mast cell degranulation, triggering ANG II formation, activation of AT1R on smooth muscle, and contraction. Furthermore, we show that human lung mast cells express enzymatically active renin. Nucleotide analysis of total RNA extracted from human lung mast cells reveals renin transcript that is homologous to human kidney renin. Thus, our results are consistent with the existence of a local airway RAS playing a crucial role in bronchoconstriction (BC).
Results
We tested the hypothesis that mast cell degranulation is the pivotal event in local RAS activation, initiation of ANG II formation, and activation of ANG II receptors on bronchial smooth muscle, causing BC. Functional assays were carried out by using individual microdissected rat bronchial rings to determine whether renin is released from mast cells, and whether it is active, i.e., able to cleave endogenous aogen, present in the interstitial fluid, eventually forming ANG II, thus eliciting BC. Representative tracings of isometric contractile responses of individual bronchial rings to K+ and the mast cell degranulating agent compound 48/80 (C48/80) are shown in Fig. 1 A–F. Contractile responses to C48/80 (300 μg/ml) were expressed as percentage of the response to K+ (80 mM). The mast cell degranulating agent caused smooth muscle contraction that was about one-half the amplitude of contraction seen with maximal K+ depolarization. When rings were pretreated with the mast cell stabilizer cromolyn (300 μM), the contractile response to C48/80 was reduced by ≈80% (Fig. 1 B and G), suggesting that mast cell mediators released by C48/80 are responsible for the generated contraction. To assess the contribution of renin, and subsequently ANG II, to the bronchial contractile response, we tested the response of isolated bronchial rings to C48/80 in the presence of the highly selective renin inhibitor BILA2157 (1 μM) (15) or the AT1R antagonist EXP3174 (100 nM), the active metabolite of losartan (16). BILA2157 and EXP3174 each significantly inhibited the contractile response to C48/80 by ≈60–65% (Fig. 1 D, F, and G).
Fig. 1.
C48/80 elicits release of mast cell renin, triggering ANG II-induced contraction of rat bronchial rings. (A–G) Representative experimental traces of the C48/80-induced contractile responses in rat bronchial rings. (A, C, and E) Administration of C48/80 (300 μg/ml) elicits release of mast cell renin and induces smooth muscle contraction in bronchial rings (n = 12). The effect of C48/80 is attenuated by the mast cell stabilizer cromolyn (300 μM; n = 6) (B); the renin inhibitor BILA2157 (1 μM; n = 12) (D); and an AT1R antagonist, EXP3174 (100 nM; n = 12) (F). y axis, Contractile responses to C48/80 were expressed as percentage of the response to K+ (80 mM). (G) Bar graph summarizing data from all experiments represented by traces in A–F. Bars indicate means ± SEM. **, Significantly different from own control; P < 0.01 by one-way ANOVA with Dunnett's procedure. W, wash.
These results suggested that mast cell-derived renin triggers the local formation of ANG II, which then acts on AT1R on bronchial smooth muscle eliciting contraction. We then compared the bronchial contractile response to ANG II to that of methacholine, a prototypical bronchoconstricting agent [positive control (17, 18)] and histamine, another bronchoconstricting agent (17), to which the rat is insensitive [negative control (19, 20)]. As shown in Fig. 2, ANG II elicited a concentration-dependent increase in contractile force ranging between ≈10 and 140% of the response to K+ (80 mM) at concentrations between 6 nM and 10 μM, compared with an increase of ≈40 and 220% with methacholine concentrations between 100 nM and 100 μM. As expected, rat bronchial rings did not contract in response to histamine in concentrations up to 100 μM.
Fig. 2.
Contractile responses of isolated rat bronchial rings to ANG II and methacholine. The administration of ANG II (open circles) and methacholine (solid circles) elicits a concentration-dependent contraction. Histamine (solid triangles; negative control) has no contractile effect. Contractile responses are expressed as percentage of the response to K+ (80 mM). Points are means (±SEM; n = 3).
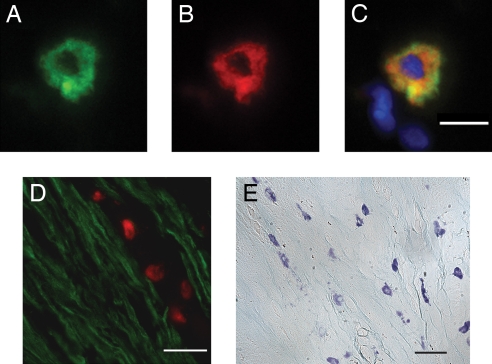
To substantiate these functional findings, we immunoscreened sections of rat bronchus for renin. Mast cells were identified by immunostaining with avidin (conjugated to fluorescein) (21). Avidin binds to the negatively charged heparin proteoglycans of the mast cell granules (22) as we have previously demonstrated (12). A typical example of an avidin-stained bronchial mast cell is shown in Fig. 3A. This section was costained with the polyclonal anti-renin Ab, raised in rabbit to human recombinant renin (11, 23) and conjugated to Alexa Fluor 594 donkey anti-rabbit IgG (Fig. 3B). The overlay of Fig. 3 A and B shows that renin and avidin are colocalized to the same cell (Fig. 3C), confirming our previous results in mouse heart (12).
Fig. 3.
Mast cells are closely apposed to smooth muscle cells expressing AT1R in rat bronchus. (A–C) Rat bronchial mast cell colabeled with avidin-FITC (A) and an anti-renin Ab (B). Color overlay is shown in C with the nuclear stain, DAPI (blue). (Scale bar, 5 μm.) (D) Representative section of rat bronchus costained with avidin-rhodamine (to identify mast cells, red) and an anti-AT1R Ab conjugated to Alexa Fluor 488. Smooth muscle cells stained positive for AT1R (green). (Scale bar, 15 μm.) (E) Toluidine blue-stained mast cells adjacent to smooth muscle cells in rat bronchus. (Scale bar, 30 μm.)
We also analyzed the spatial relationship between mast cells and bronchial smooth muscle. Fig. 3D is a section of rat bronchus stained both with avidin-conjugated rhodamine and an AT1R Ab conjugated to Alexa Fluor 488 donkey anti-rabbit IgG. As shown, mast cells are in close proximity to bronchial smooth muscle expressing AT1R. A lower power transmitted light view of bronchus stained with toluidine blue also shows the close association of mast cells with smooth muscle (Fig. 3E).
Collectively, the functional and immunohistochemical findings in rat bronchus support the contention that renin released from mast cells can trigger the activation of an airway RAS. The ensuing ANG II then activates AT1R in bronchial smooth muscle leading to BC.
We next focused on the role of mast cell renin release and local RAS in immediate hypersensitivity.
Inasmuch as the guinea pig is highly sensitive to histamine (24, 25) and easily subjected to anaphylaxis (26, 27), we next used individual bronchial rings microdissected from sensitized guinea pigs and determined whether renin could be released in active form by anaphylactic mast cell degranulation, leading to ANG II formation and, thus, BC. Antigen challenge of bronchial rings excised from sensitized guinea pigs resulted in a smooth muscle contraction comparable in amplitude to that elicited by maximal depolarization with K+ (80 mM) (Fig. 4A, C, E, and G). When presensitized bronchial rings were challenged with antigen in the presence of cromolyn (300 μM), their contractile response was ≈60% less than that of untreated rings (Fig. 4 B and G). Similarly, treatment with BILA2157 (1 μM) or EXP3174 (100 nM) attenuated anaphylactic BC by ≈50–60%, respectively (Fig. 4 D, F, and G).
Fig. 4.
Immediate hypersensitivity elicits the release of mast cell renin, resulting in ANG II-induced smooth muscle contraction in isolated guinea pig bronchial rings. Representative experimental traces of contractile responses measured in bronchial rings obtained from guinea pigs presensitized with rabbit anti-OA cytotropic IgG and challenged with OA (50 μg/ml) in the absence (control; n = 6) (A, C, and E) or presence of cromolyn (300 μM; n = 6) (B), BILA2157 (1 μM; n = 6) (D), or EXP3174 (100 nM; n = 6) (F). y axis, Contractile responses expressed as percentage of the response to K+ (80 mM). (G) Bar graph summarizing data from all experiments represented by traces in A–F. Bars indicate means ± SEM. *, **, Significantly different from control; P < 0.05 and 0.01, respectively, by one-way ANOVA with Dunnett's multiple-comparison test. W, wash.
These functional findings suggested that antigen challenge elicits the release of renin from bronchial mast cells with the consequent activation of a local RAS culminating in ANG II-mediated BC. Indeed, the administration of exogenous ANG II (0.1–100 nM) to isolated guinea pig bronchial rings resulted in a concentration-dependent contractile response that ranged between ≈2 and 30% of the response to 80 mM K+. This response to ANG II was progressively antagonized and eventually abolished by increasing concentrations of EXP3174 (10–100 nM; Fig. 5).
Fig. 5.
Concentration–response curves for the contractile effect of ANG II on sensitized guinea pig bronchial rings. The administration of ANG II (control) elicits concentration-dependent contraction of isolated sensitized guinea pig bronchial rings. The effect of ANG II is progressively attenuated by EXP3174 as a function of its concentration (10–100 nM). Contractile responses are expressed as percentage of the response to K+ (80 mM). Points are means (± SEM; n = 4).
Having demonstrated that ANG II causes BC, and that renin inhibition and AT1 receptor blockade each attenuates anaphylactic BC, we next assayed the releasate of isolated guinea pig lungs to verify that the anaphylactic reaction results in mast cell degranulation and release of active renin. Lungs were excised from guinea pigs presensitized with rabbit anti-ovalbumin cytotropic IgG and challenged with ovalbumin (OA) (i.e., anaphylaxis). As shown in Table 1, isolated guinea pig lung anaphylaxis was characterized by mast cell degranulation, as evidenced by a marked overflow of β-hexosaminidase (β-hex) (a marker of mast cell degranulation) and histamine. Notably, renin was also copiously released. We next explored the relevance of these functional findings to human airway and assessed whether lung mast cells express renin.
Table 1.
Immediate hypersensitivity elicits mast cell renin release in effluent of isolated guinea pig lungs exposed to anaphylaxis ex vivo
| Basal | Anaphylaxis | |
|---|---|---|
| Renin, pg·ml−1·h−1 | 1.08 ± 0.37 | 11.56 ± 1.69*** |
| β-Hex, OD × 100 | 17.03 ± 3.9 | 65.86 ± 8.85*** |
| Histamine, nmol/g | 0.18 ± 0.18 | 16.33 ± 2.25*** |
Shown are means ± SEM (n = 6).
***, P < 0.0001.
Fig. 6A shows a representative image of mast cells isolated from human lung tissue stained with toluidine blue. To determine whether human lung mast cells express renin mRNA, total RNA (1 μg) was extracted from human lung mast cells, reverse transcribed, and amplified by PCR by using sense and antisense primers specific for human renin (exons 4 and 7) (28). Fig. 6B is an ethidium bromide-stained gel showing that the human lung mast cell renin PCR product is ≈300 bp, similar to what we reported for cultured human mast cells, HMC-1 (11). To further characterize the PCR product from human lung mast cells, the DNA band was extracted from the gel and sequenced. The reported nucleotide sequence was then compared with the known sequence of Homo sapiens renin mRNA by BLAST analysis (accession no. BC047752). There was 100% homology between the sequence from human lung mast cells at exons 1–10 of the renin gene and H. sapiens renin establishing a precedent for the presence of renin in native lung mast cells. Human lung mast cells also express renin protein in that the isolated human lung mast cells immunoreact with the anti-renin Ab (Fig. 6C). There was no immunoreactivity in the mast cells when using anti-renin Ab preadsorbed with an excess of human renin (data not shown).
Fig. 6.
Mast cells isolated from human lung express renin. (A) Toluidine-blue stained mast cells isolated from human lung tissue. (Scale bar, 10 μm.) (B) Total RNA was extracted from isolated human lung mast cells and reverse transcribed. cDNA was amplified by PCR using specific primers for human renin gene (exons 4–7). A 100-bp DNA ladder (m) was run on the gel. (C) Mast cells isolated from human lung stained positive for renin protein with the monoclonal anti-renin Ab (1:200). (Scale bar, 10 μm.) (D) Measurement of renin activity [ANG I formed (in picograms per milliliter per hour)] in the lysate from isolated human lung mast cells (±SEM; n = 3).
To ascertain that the renin protein in the human lung mast cells is active, i.e., capable of cleaving aogen, lysate from isolated mast cells was assayed for renin-dependent ANG I activity by RIA as routinely performed in our laboratory (11). To account for a possible contribution to ANG I formation by cathepsin D, which is found in mast cells and able to cleave aogen, although at a rate 105 times slower than renin (29), ANG I activity was measured in the absence and presence of the specific renin inhibitor, BILA2157 (100 nM; IC50, 1 nM) (15). About 70% of the total ANG I formed by the mast cell lysate originates from renin, indicating that mast cell-derived renin is active (Fig. 6D).
Discussion
The identification of tissue-specific RAS, whereby locally generated ANG II acts on resident receptors, has gained considerable attention, especially in heart (30), brain (31), eye (32), and testis (2). Here, we report that the lung also possesses a local RAS driven by non-kidney-derived renin. Our functional studies demonstrate that mast cells in the bronchi express renin, which, when released, triggers local ANG II formation. This locally formed ANG II causes bronchial smooth muscle contraction. Furthermore, we demonstrate that human lung mast cells express renin. In that clinical studies have shown that administration of ANG II to mild asthmatics causes BC (14), our results provide a rationale for targeting mast cell renin and an ensuing local RAS for the treatment of airway constriction.
Mast cells populate the upper and lower respiratory tree (13). They are found predominantly in the submucosal connective tissue of normal human bronchi (13). In asthma, however, mast cells are known to infiltrate the airway. Biopsies from human asthmatics show mast cell hyperplasia at the epithelial surface (33) and in the smooth muscle layer (33, 34). Our results in normal rat demonstrate that mast cells are closely apposed to bronchial smooth muscle expressing the AT1R (Fig. 3), so that ANG II formed from the release of renin from mast cells can activate these receptors and cause smooth muscle contraction. Our functional data confirm the involvement of AT1R in that EXP3174, the active metabolite of losartan (16), blocked the contractile response to mast cell degranulation in rat and guinea pig bronchial rings (Figs. 1, 4, and 5).
Generation of a concentration–response curve with exogenous ANG II demonstrates that the magnitude of the BC elicited by ANG II in rat bronchial rings is comparable to that evoked by the muscarinic receptor agonist, methacholine (Fig. 2). To assess whether the concentration of ANG II necessary to elicit BC is physiologically relevant, the endogenous concentration of ANG II generated by the release of mast cell renin was approximated from the ANG II concentration–response curves generated in rat and guinea pig bronchial rings (Figs. 2 and 5). Choosing an exogenous ANG II concentration that yielded BC of equal magnitude to that obtained with C48/80 or OA in the presence of BILA2157, we estimated that mast cell renin release generates a local ANG II concentration of ≈30 nM. This is also the concentration that elicits BC in human bronchial rings (35, 36). This supports the view that ANG II produced locally from the release of mast cell renin is pathophysiologically significant in the bronchus.
In allergic asthma, mast cells release a variety of mediators such as histamine, tryptase, leukotrienes (C4 and D4), adenosine, cytokines, and kinins, which elicit acute and chronic airway constriction and inflammation (37, 38). Our functional studies demonstrate that renin is released from mast cells and mediates airway constriction via ANG II. Also, renin along with histamine and β-hex was released into the lung effluent in response to anaphylaxis. Although our experiments focused on BC and the role of ANG II derived from mast cell renin, we cannot rule out the contribution of ANG II to the inflammatory response, in that ANG II is a proinflammatory agent (39).
Previously, we have demonstrated the presence of renin in rodent mast cells and in the human mastocytoma cell line, HMC-1 (11). Here, we extend these observations to include native human lung mast cells (Fig. 6). RT-PCR of total RNA from isolated human lung mast cells generated PCR product, which when sequenced was 100% homologous to H. sapiens renin. This finding confirmed that native human mast cells are capable of expressing renin. Immunocytochemical analysis of the isolated human mast cells demonstrates that these cells express renin protein in addition to the mRNA, consistent with our previous findings in rodent heart and in HMC-1 cells. Also, mast cell renin is enzymatically active, thereby capable of cleaving aogen and triggering local ANG II formation (Fig. 6D).
In conclusion, our results advance the concept that renin, released from mast cells, is a significant mediator of BC and acts by triggering the activation of a local airway RAS. Our findings uncover the pathological significance of mast cell renin and ensuing ANG II production in immediate hypersensitivity and allergy. We believe that we have identified a mediator of airway constriction and a potential therapeutic target for treating hyperresponsiveness as occurs in asthma and COPD.
Materials and Methods
Rat Bronchial Rings.
All experiments were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Pathogen-free male Sprague–Dawley rats (Charles River), weighing 200–300 g, were anesthetized with CO2 vapor and killed by exsanguination. Lungs were rapidly removed en bloc and placed in Krebs–Henseleit (KH) buffer. Bronchi were dissected free of parenchymal lung tissue, connective tissue, and fat. Bronchial preparations were cut into small rings (3- to 4-mm length × 3- to 4-mm internal diameter) and used immediately.
Isometric Force Generation.
Isometric force generation by rat bronchial rings was measured according to Ikawati (40). Preparations were mounted onto stainless-steel hooks in double-jacketed, 20-ml glass organ baths (Radnoti) containing KH solution maintained at 37°C, 95% O2–5% CO2. Resting tension was kept at 200–250 mg. Mechanical responses were measured isometrically with force transducers interfaced to a PowerLab/8SP data acquisition system and analyzed with Chart5 for Windows (AD Instruments). Tension was calculated as milligrams and expressed as percentage of response to K+ (80 mM).
Bath fluid was changed at 15-min intervals during a 60- to 90-min equilibration period. Next, high K+ KH solution (containing 80 mM KCl) was added, and after the plateau of the contraction was reached, preparations were washed with normal KH solution. This procedure was repeated twice until a maximal constant contraction value was obtained. Drugs were added 30 min after the high K+ cycle. At the end of each experiment, high K+ KH solution was added again to confirm tissue viability. After blotting onto filter paper, tissue wet weights were determined.
Guinea Pig Sensitization.
Male Hartley guinea pigs (300–350 g; Charles River) were passively sensitized by i.p. administration of 0.8 mg/ml rabbit anti-OA IgG (Rockland Immuno) according to established methods whereby these antibodies sensitize mast cells for subsequent anaphylactic challenge (41, 42). Twenty-four hours later, the animals were anesthetized with CO2 and killed by exsanguination. Lungs were rapidly removed and placed in KH buffer. Bronchial ring preparations were used immediately for the measurement of isometric force generation experiments as described for rat bronchial rings.
Guinea Pig Lung Ex Vivo.
Twenty-four hours after anti-OA sensitization, animals were killed, and the lungs were excised and then cannulated via the pulmonary artery for KH perfusion of the pulmonary circulation. Lungs were then mounted on a modified Langendorff perfusion apparatus (Radnoti), inflated with room air, and perfused with KH solution (95% oxygen, 5% CO2). KH buffer was pumped (Master Flex; Cole–Parmer) at a rate of 15 ml/min at 37°C. Basal perfusion pressure was 15 ± 4 mmHg. Perfusion pressure was recorded on a PowerLab system (AD Instruments). Isolated lungs were perfused with KH buffer for 45 min before antigen challenge (intratracheal injection of OA, 1 mg/0.5 ml). Lung overflow was measured by effluent collections before antigen challenge and up to 30 min thereafter. Samples were assayed for β-hex, histamine, and renin.
β-Hex.
β-Hex was assayed by using a modification of the method of Schwartz et al. (43). A 20-μl sample of lung effluent was placed into a well of a 96-well plate. A total of 50 μl of substrate solution (p-nitrophenyl-N-acetyl-d-glucosamide; 1.3 mg/ml in 0.1 M citrate buffer, pH 4.5) was added to each well and incubated for 90 min at 37°C. The reaction was stopped by adding 150 μl of 0.2 M glycine (pH 10.7). Optical density (OD) was read at 405 nm by using SoftMax Pro 4.8. Mean OD was calculated for each sample, run in quadruplicate.
Histamine.
Histamine content of the collected effluent was assayed by using an enzyme immunoassay kit (Immunotech). Detection limit was ≈0.05 nM.
Renin Activity.
Renin activity was measured in lung effluent and in isolated human lung mast cell lysate, as previously reported (11, 12). Renin activity (ANG I formed) was determined by using a GammaCoat Plasma Renin Activity 125I RIA kit (DiaSorin). Detection limit was ≈0.01 pmol (12).
Immunohistochemistry.
Cryostat sections (10 μm) of paraformaldehyde-fixed rat bronchial tissue were immunoscreened for the presence of renin (11). Rabbit polyclonal anti-renin Ab was applied to bronchial sections at a dilution of 1:400 for 2 h. Sections were then exposed to Alexa Fluor 594 donkey anti-rabbit IgG diluted 1:1200 (Molecular Probes) and avidin-FITC (1:2,500) (Vector Labs) for 1 h at 37°C. Sections were then washed and immersed in DAPI (Molecular Probes) to stain nuclei, followed by fixation with 4% paraformaldehyde. Washed sections were mounted on coverslips in Vectashield antifading solution (Vector Labs) for fluorescent viewing. Mast cells were identified as cells that were triple stained (i.e., renin, FITC-avidin, and DAPI positive). As a negative control, sections were stained with nonimmune rabbit serum (1:400) instead of polyclonal anti-renin Ab.
AT1R immunostaining was performed on paraffin-embedded sections (10 μm) of Bouin's-fixed rat bronchial tissue that were deparaffinized, rehydrated, and washed in distilled water. Sections were placed in target retrieval solution (DakoCytomation) for 15 min at 95°C. After cooling the slides, sections were incubated with 10% FBS for 1 h at 37°C followed by incubation with the polyclonal rabbit anti-AT1R Ab (Santa Cruz) at a dilution of 1:100. For mast cell identification, sections were washed and then incubated with a mixture of Alexa Fluor 488 donkey anti-rabbit IgG (green) diluted 1:600 and avidin-rhodamine (red) diluted 1:2,200.
Isolated human lung mast cells were fixed and permeabilized on glass slides and stained with the monoclonal mouse anti-renin Ab (Swant Scientific) (1:200) conjugated to Alexa Fluor 488 donkey anti-mouse IgG (1:600). Nuclei were stained with DAPI. For all immunofluorescence experiments, tissue sections were examined with an inverted fluorescence microscope (Nikon Eclipse TE 2000-U) interfaced to an electron multiplying charge-coupled device (Hamamatsu) and processed with MetaMorph software (Universal Imaging).
Sections and isolated native mast cells were also stained with toluidine blue (0.5% in acetic acid, pH 0.44) for visualizing mast cells under transmitted light.
Mast Cell Isolation.
Mast cells were isolated from 2–4 g of freshly excised waste tissue specimens of macroscopically normal lung tissue from patients undergoing lobectomy (institutional review board-approved procedure). Samples were minced, filtered, pelleted by centrifugation, and then washed in PBS solution containing BSA. The cell pellet was resuspended in solution containing rabbit polyclonal anti-FcεRI Ab (1:50) (Upstate Cell Signaling) and incubated at 4°C for 25 min. Cells were then pelleted, washed several times, and resuspended in solution containing goat anti-rabbit IgG colloidal microbeads (1:5) (Miltenyi Biotec) for 15 min at 4°C. FcεRI-labeled mast cells were pelleted, washed, and isolated from the total cell population by magnetic cell sorting using MACS magnetic separation columns and units (Miltenyi Biotec). This technique yielded ≈3 × 106 cells per 2 g of tissue, as determined by FACS sorting.
RT-PCR.
Total RNA was extracted from isolated human lung mast cells by using TRIzol reagent (Invitrogen). One microgram of total RNA from each sample was reverse-transcribed and cDNA amplified by RT-PCR using a Qiagen Onestep RT-PCR kit. Sense primers specific for human renin gene at exons 1, 4, and 7 were 5′-ATGGATGGATGGAGAAGGAT-3′, 5′-TCTCAGCCAGGACATCATCA-3′, and 5′-TGGGAGGACAGATTGTGCT-3′, respectively. Antisense primers specific for human renin gene at exons 5, 7, and 10 were 5′-TCTGTGTCACCGTGATTCCA-3′, 5′-AGTGGAAATTCCCTTCGTAA-3′, and 5′-GGCCAAGGCGAAGCCAAT-3′, respectively (PubMed accession no. BC047752; ref. 28). The amplification profile used was as follows: 50°C for 30 min, 95°C for 15 min, then 94°C for 30 s, 55°C for 30 s, 72°C for 1.5 min (40 cycles), and finally 72°C for 10 min. PCR products generated were ≈517, ≈302, and ≈503 bp for renin exons 1 through 5, 4 through 7, and 7 through 10, respectively. PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining. Renin RT-PCR products were extracted from the agarose gel and purified by using QIAquick Gel Extraction kit (Qiagen), and sequenced by Genewiz.
Drugs and Chemicals.
C48/80, ANG II, aogen, cromolyn, histamine, methacholine, p-nitrophenyl-N-acetyl-d-glucosamide, and toluidine blue were obtained from Sigma-Aldrich. BILA2157 was a gift from Boehringer Ingelheim; EXP3174 was a gift from Merck, Sharpe, and Dohme. BILA2157 and EXP3174 were dissolved in DMSO and ethanol, respectively, with further dilutions in the appropriate buffer; at the concentration used, DMSO and ethanol did not affect mediator release.
Statistics.
All values were expressed as means ± SEM. Statistical comparisons among the various experimental conditions were obtained either by Student's paired t test or ANOVA followed by Dunnett's test. P < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS.
We acknowledge Dr. N. K. Altorki (Weill Cornell Medical College, New York, NY) and Dr. D. F. Catanzaro (Weill Cornell Medical College, New York, NY) for providing human lung waste tissue and the polyclonal anti-renin Ab, respectively. These studies were supported by National Institutes of Health Grants HL73400, HL34215, HL47073, HL46403, and DK60726.
Footnotes
Conflict of interest statement: A patent for related technology has been filed with the U.S. Patent and Trademark office by Cornell University. R.B.S. and R.L. are coinventors.
This article is a PNAS Direct Submission.
References
- 1.Valtin H, Schafer JA. Renal Function. Boston: Little, Brown; 1995. pp. 135–138. [Google Scholar]
- 2.Phillips MI, Speakman EA, Kimura B. Regul Pept. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- 3.Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T, McAnulty RJ, Laurent GJ. Am J Physiol. 2004;286:L156–L164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ, Kladis A, Duncan AM. Hypertension. 1993;22:513–522. doi: 10.1161/01.hyp.22.4.513. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJ, Kladis A, Valentijn AJ. J Cardiovasc Pharmacol. 1995;26:233–240. doi: 10.1097/00005344-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DJ, Habener JF. J Clin Invest. 1986;78:31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell DJ, Habener JF. Endocrinology. 1989;124:218–222. doi: 10.1210/endo-124-1-218. [DOI] [PubMed] [Google Scholar]
- 8.Vane JR. Br J Pharmacol. 1969;35:209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Am J Physiol. 1993;264:F989–F995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 10.Bullock GR, Steyaert I, Bilbe G, Carey RM, Kips J, De Paepe B, Pauwels R, Praet M, Siragy HM, de Gasparo M. Histochem Cell Biol. 2001;115:117–124. doi: 10.1007/s004180000235. [DOI] [PubMed] [Google Scholar]
- 11.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. Proc Natl Acad Sci USA. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, Silver RB, Levi R. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyce JA. Prostaglandins Leukot Essent Fatty Acids. 2003;69:195–205. doi: 10.1016/s0952-3278(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 14.Millar EA, Angus RM, Hulks G, Morton JJ, Connell JM, Thomson NC. Thorax. 1994;49:492–495. doi: 10.1136/thx.49.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simoneau B, Lavallee P, Anderson PC, Bailey M, Bantle G, Berthiaume S, Chabot C, Fazal G, Halmos T, Ogilvie WW, et al. Bioorg Med Chem. 1999;7:489–508. doi: 10.1016/s0968-0896(98)00265-x. [DOI] [PubMed] [Google Scholar]
- 16.Wong PC, Price WA, Jr, Chiu AT, Duncia JV, Carini DJ, Wexler RR, Johnson AL, Timmermans PB. J Pharmacol Exp Ther. 1990;255:211–217. [PubMed] [Google Scholar]
- 17.Hargreave FE, Ryan G, Thomson NC, O'Byrne PM, Latimer K, Juniper EF. Eur J Respir Dis Suppl. 1982;121:79–88. [PubMed] [Google Scholar]
- 18.Ward MD, Selgrade MK. Methods. 2007;41:80–90. doi: 10.1016/j.ymeth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Chand N, Eyre P. Agents Actions. 1978;8:171–184. doi: 10.1007/BF01966600. [DOI] [PubMed] [Google Scholar]
- 20.Lulich KM, Paterson JW. Br J Pharmacol. 1980;68:633–636. doi: 10.1111/j.1476-5381.1980.tb10854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tharp MD, Seelig LL, Jr, Tigelaar RE, Bergstresser PR. J Histochem Cytochem. 1985;33:27–32. doi: 10.1177/33.1.2578142. [DOI] [PubMed] [Google Scholar]
- 22.Kokkonen JO, Kovanen PT. Biochem J. 1987;241:583–589. doi: 10.1042/bj2410583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell WG, Gahnem F, Catanzaro DF, James GD, Camargo MJ, Laragh JH, Sealey JE. Hypertension. 1996;27:1121–1133. doi: 10.1161/01.hyp.27.5.1121. [DOI] [PubMed] [Google Scholar]
- 24.Levi R, Capurro N, Lee CH. Eur J Pharmacol. 1975;30:328–335. doi: 10.1016/0014-2999(75)90117-x. [DOI] [PubMed] [Google Scholar]
- 25.Chand N, DeRoth L. Pharmacology. 1979;19:185–190. doi: 10.1159/000137308. [DOI] [PubMed] [Google Scholar]
- 26.Capurro N, Levi R. Circ Res. 1975;36:520–528. doi: 10.1161/01.res.36.4.520. [DOI] [PubMed] [Google Scholar]
- 27.Lewis A, Blumenthal A, Dervinis A. Inflamm Res. 1983;13:269–275. doi: 10.1007/BF01971477. [DOI] [PubMed] [Google Scholar]
- 28.Becker BN, Jacobson LM, Becker YT, Radke NA, Heisey DM, Oberley TD, Pirsch JD, Sollinger HW, Brazy PC, Kirk AD. Transplantation. 2000;69:1485–1491. doi: 10.1097/00007890-200004150-00046. [DOI] [PubMed] [Google Scholar]
- 29.Hackenthal E, Hackenthal R, Hilgenfeldt U. Biochim Biophys Acta. 1978;522:574–588. doi: 10.1016/0005-2744(78)90089-x. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch AT, Pinto YM, Schunkert H, Dzau VJ. Am J Cardiol. 1990;66:22D–30D. doi: 10.1016/0002-9149(90)90473-e. [DOI] [PubMed] [Google Scholar]
- 31.Unger T, Badoer E, Ganten D, Lang RE, Rettig R. Circulation. 1988;77:140–154. [PubMed] [Google Scholar]
- 32.Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Br J Ophthalmol. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laitinen LA, Laitinen A, Haahtela T. Am Rev Respir Dis. 1993;147:697–704. doi: 10.1164/ajrccm/147.3.697. [DOI] [PubMed] [Google Scholar]
- 34.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 35.Millar EA, Nally JE, Thomson NC. Eur Respir J. 1995;8:1838–1841. doi: 10.1183/09031936.95.08111838. [DOI] [PubMed] [Google Scholar]
- 36.Ramsay SG, Clayton RA, Dagg KD, Thomson LJ, Nally JE, Thomson NC. Respir Med. 1997;91:609–615. doi: 10.1016/s0954-6111(97)90007-x. [DOI] [PubMed] [Google Scholar]
- 37.Galli SJ, Gordon JR, Wershil BK. Agents Actions Suppl. 1993;43:209–220. doi: 10.1007/978-3-0348-7324-6_18. [DOI] [PubMed] [Google Scholar]
- 38.Galli SJ. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 39.Luft FC. Curr Hypertens Rep. 2001;3:61–67. doi: 10.1007/s11906-001-0082-y. [DOI] [PubMed] [Google Scholar]
- 40.Ikawati Z, Hayashi M, Nose M, Maeyama K. Eur J Pharmacol. 2000;402:297–306. doi: 10.1016/s0014-2999(00)00482-9. [DOI] [PubMed] [Google Scholar]
- 41.Feigen GA, Prager DJ. Am J Cardiol. 1969;24:474–491. doi: 10.1016/0002-9149(69)90490-1. [DOI] [PubMed] [Google Scholar]
- 42.Levi R. In: Human Inflammatory Disease, Clinical Immunology. Marone G, Lichtenstein LM, Condorelli M, Fauci AS, editors. Toronto: B. C. Decker; 1988. pp. 93–105. [Google Scholar]
- 43.Schwartz LB, Austen KF, Wasserman SI. J Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]